Abstract
Crohn’s disease (CD) is a chronic inflammatory condition that plagues millions all over the world. This debilitating bowel disease can start in early childhood and continue into late adulthood. Signs and symptoms are usually many and multiple tests are often required for the diagnosis and confirmation of this disease. However, little is still understood about the cause(s) of CD. As a result, several theories have been proposed over the years. One theory in particular is that Mycobacterium avium subspecies paratuberculosis (MAP) is intimately linked to the etiology of CD. This fastidious bacterium also known to cause Johne’s disease in cattle has infected the intestines of animals for years. It is believed that due to the thick, waxy cell wall of MAP it is able to survive the process of pasteurization as well as chemical processes seen in irrigation purification systems. Subsequently meat, dairy products and water serve as key vehicles in the transmission of MAP infection to humans (from farm to fork) who have a genetic predisposition, thus leading to the development of CD. The challenges faced in culturing this bacterium from CD are many. Examples include its extreme slow growth, lack of cell wall, low abundance, and its mycobactin dependency. In this review article, data from 60 studies showing the detection and isolation of MAP by PCR and culture techniques have been reviewed. Although this review may not be 100% comprehensive of all studies, clearly the majority of the studies overwhelmingly and definitively support the role of MAP in at least 30%-50% of CD patients. It is very possible that lack of detection of MAP from some CD patients may be due to the absence of MAP role in these patients. The latter statement is conditional on utilization of methodology appropriate for detection of human MAP strains. Ultimately, stratification of CD and inflammatory bowel disease patients for the presence or absence of MAP is necessary for appropriate and effective treatment which may lead to a cure.
Keywords: Mycobacterium paratuberculosis, Crohn’s disease, Culture, PCR, Johne’s disease, Inflammatory bowel disease
Core tip: The review manuscript describes the past, present and predicted future research accomplishments in the area of Crohn’s disease and Mycobacterium avium subspecies paratuberculosis. This is a highly debated area and Dr. Naser’s thoughts described in this review will fuel interest and discussions in inflammatory bowel disease research. The manuscript has been in preparation for a couple of years and it is of high quality.
INTRODUCTION
One of the earliest documented descriptions of Crohn’s disease (CD) was described in 1769 by Giovanni Battista, an Italian physician. He described the results of an autopsy of a man who had suffered from chronic bowel movements throughout his life and subsequently died from diarrhea and fever[1]. This may have been the first account of granulomatous inflammatory bowel disease (IBD). Several years later in 1813, Combe and Saunders observed a patient who suffered from an abnormally narrow and thickened ileum[1] and Abercrombie had reported a case in 1828 whereby a patient suffered from an inflamed ileum (ileitis) as well as skip lesions affecting certain segments of the ascending colon and cecum[1]. There were several medical publications made in the 20th century which provided further insight into the characteristics and features of CD. For example, some such cases were reported by Braun (1901), Koch (1903), Lesniowski (1903), Wilmanns (1905), Moynihan (1907), and Proust (1907)[1]. In 1913 a surgeon by the name of Dalziel (1861-1924) had reported the symptoms of several of his patients that closely resembled clinical manifestations in cattle suffering from Johne’s disease[2]. He is credited as being the first scientist to hypothesize that the causative agent of Johne’s disease, Mycobacterium avium subsp. paratuberculosis (MAP), may in fact be responsible for chronic intestinal inflammation observed in the intestines of humans. In 1923 Moschcowitz and Wilensky[3] had reported four cases of young patients suffering from non-specific granulomata of the intestine. In these patients they observed what appeared to be tumor-like masses that were hard, thick, and associated with all four coats of the large intestine which caused stricture of the lumen. Based on these observations it was originally believed that these structural changes were confined to the colon. However, it was found that such lesions could also be located in the small intestine which was later seen in one patient[4]. In fact, it is interesting to note that all four of the patients had a history of appendicitis and appendectomy.
In 1932 acknowledgement of CD as an official medical entity was as a result of an article published by Drs. Burrill Crohn, Leon Ginzburg, and Gordon G. Oppenheimer, who all worked at Mount Sinai Hospital in New York at the time. Their article entitled “Regional ileitis: A Pathological and Clinical Entity” had appeared in the Journal of the American Medical Association in October 1932[5]. The title “Crohn’s disease,” has been coined after Dr. Burrill B. Crohn, a gastroenterologist who presented the above named paper at the annual American Medical Association in May 1932. The study described a disease that exclusively affected the terminal ileum of 14 patients from a pathological and clinical standpoint[5]. The patients were primarily young adults of ages ranging from 17 to 52 years, but only two of them were older than 40 years[5]. It was expressed that this was a moderately acute disease that was associated with inflammation characterized by rapid necrosis throughout the affected tissue, and by inflammation associated with scar tissue[5]. Furthermore, it was indicated that the disease is clinically represented by certain common symptoms similar to ulcerative colitis-fever, diarrhea, and even weight loss[5]. Dr. Crohn et al[5] also witnessed that the ulcers associated with the mucosa were accompanied by a non-uniform connective tissue reaction of the remaining walls of the involved intestine, a process which frequently leads to the narrowing of the lumen of the intestine, and this has been known to be associated with the formation of multiple fistulas. Other physicians reported of similar concurrent observations, but these reports cited the involvement of a number of different parts of the gastrointestinal (GI) tract. For instance, the first case reporting evidence of inflammation present in the colon and not just in the ileum was by Colp in 1934[6]. His report is considered as the first case detailing of ileocolitis which described that this inflammatory process could also extend to the cecum and the ascending colon[6]. In addition, several years later granulomatous lesions were also found in the skin[7]. As a result, it was becoming quickly apparent that CD is a chronic inflammatory disease that can affect any region of the GI tract ranging from the mouth to the anus, the ileum being the most commonly targeted site. In 1938, Penner and Crohn observed that 8 out of 50 analyzed patients suffering from regional ileitis displayed anal fistulae as a possible complication. They were initially unaware that anal and perianal fistulae could present such a complication of ileitis[8]. In 1952, Wells[9] introduced the term segmental colitis while delivering a lecture at the Royal College of Surgeons of England. According to Wells, this condition is associated with the formation of fibrous tissue on the bowel wall leading to its increased thickening as well as the presence of ulcers of the mucosa. These ulcers have a patchy pattern of spreading and are therefore known as “skip lesions”[9]. Most importantly this condition was observed in patients without lesions present in the ileum or jejunum. Wells presumed that segmental colitis is a form of colonic CD, but this was never acknowledged by Crohn himself[9].
At present, research on CD has partitioned it into three categories: inflammatory, obstructive, and fistulating[10]. The inflammatory and obstructive types tend to occur simultaneously and cause obstructions of the bowel due to thickening of the intestinal wall as a result of inflammation. The fistula types are commonly associated with erosion of the bowel walls including perianal fistulas and enteroenteric fistulas[10]. Depending on the locations of these erosions the disease is called Crohn’s or granulomatous colitis if symptoms occur in the large intestine, Crohn’s enteritis if symptoms occur in the small intestine, or Crohn’s ileitis if symptoms occur in the ileum[11]. Furthermore, it has been documented that some patients suffer with inflammation of fat cells under the skin (erythema nodusum), or in large joints (peripheral arthritis)[10]. It is important to note that CD is quite similar to another IBD known as ulcerative colitis (UC). The latter affects only the colon whereas CD can affect any region of the GI tract[12].
WHO IS AFFECTED BY IBD?
Statistical evidence has indicated that the highest prevalence of CD and UC is in North America, northern Europe, and the United Kingdom. These diseases are beginning to rise in southern Europe, Asia, Africa, and Latin America. In fact, as much as 1.4 million persons in the United States and approximately 2.2 million individuals worldwide cope with IBD on a daily basis[13]. However, in one epidemiological study of CD based on ethnicity, it was revealed that CD is least prevalent in African Americans, Asians, and Hispanics. The rate of prevalence for African Americans was 29.8 per 100000, for Hispanics it was 4.1 per 100000, and for Asians it was 5.6 per 100000[14]. It has also been found that the occurrence of IBD is higher in industrialized countries such as North America and Europe vs under developed or developing countries. Therefore, this suggests that the pathogenesis of IBD may be caused by certain environmental factors[15]. This indicates that genetic susceptibility alone cannot account for the prevalence of CD. In addition, the incidence and prevalence of CD is essentially equal among men and women. Unfortunately, CD is a lifelong debilitating disease which can start in early childhood and continue into late adulthood. Most cases of CD are usually reported or initially diagnosed when the patient is in his or her late teens or early twenties. Recent studies have indicated that in the last few decades the number of CD patients diagnosed before the age of 40 years has increased to 80%[16]. This therefore emphasizes the young adult and adolescent age group as a primary target of this disease. Understanding the etiology of CD may facilitate the development of rapid and cost effective methods for disease diagnosis.
DIAGNOSIS OF CD
The most accurate and effective examination for the diagnosis of CD is a full colonoscopy along with intubation of the ileum[17]. This type of endoscopic examination allows the physician to clearly visualize the colon, ileum, and even certain parts of the lower regions of the small intestine. Physicians can also take multiple biopsies of all the segments of the colon as well as the terminal ileum[17]. Dye-based chromoendoscopy is an advanced imaging technique which allows for the visualization of subtle changes in the lining of the intestine. An alternative imaging method that can be utilized is capsule endoscopy, which is usually selected when there is no evidence of stricture or stenosis[18]. Other technology detects inflammation of the distal ileum such as enhanced gadolinium magnetic resonance imaging which has been proven to be very effective in distinguishing between inflammatory diseases of the GI tract, is non-invasive, and does not produce any radiation[19].
WHAT IS THE ETIOLOGY OF CD?
Unfortunately, the etiology or cause(s) of CD are still unknown. However, there have been several theories that have been proposed to explain this phenomenon. For example, the leading theories suggest that CD can be caused by certain environmental factors or by a dysregulated immune response in a genetically susceptible host. Many believe a milieu of environmental factors such as diet and certain infectious agents may trigger this disease. For example, it has been found that a diet of refined sugars, fatty acids, fast foods, and minimal consumption of fruits, vegetables, and fibers can contribute to triggering the disease[20]. Certain foods play a pivotal role in influencing the microbiome composition of the human gut. In fact, a “Westernized” diet is believed to change the microbiological environment such that there is an increased susceptibility for the development of intestinal bowel disease[20]. Some of the infectious causative agents studied in connection with CD include viruses, yeast, and bacteria including Escherichia coli, Listeria monocytogenes, Chlamydia trachomatis, Pseudomonas maltophilia, Bacteroides fragilis, Mycobacterium kansasii, and MAP. Fortunately, it is almost universally accepted that a host genetic predisposition is critical for development of CD[21].
In recent years, the amount of interest and research data in support of a possible infectious etiology for CD has been well noted. More specifically, the forerunner of the proposed infectious causative agents is MAP. However, there are several critics and skeptics who still discredit this theory. Therefore the goal of this review article is to shed light on this current predicament with the intention to further clarify our understanding of the pathogenesis of CD from the perspective of an infectious agent such as MAP.
MAP AND JOHNE’S DISEASE
It was in 1895 when Johne and Frothingham first identified MAP as the causative agent of chronic inflammation in the gut of a cow[22]. Johne’s disease was later coined after Johne for his work in identifying this chronic inflammatory enteric disease in cattle, but this disease has also been observed over the years in several different animals such as sheep, goats, rabbits, monkeys, and even chimpanzees[22]. MAP belongs to the Mycobacterium avium complex (MAC) which consists of at least M. avium and M. intracellulare[23]. Through DNA sequence analysis it is possible to evaluate the similarities and differences among mycobacterial strains. It has been documented that MAP shares certain sequence similarities with other strains of MAC. For example there is a 16S-23S rDNA internal transcribed spacer (ITS) that is approximately a 280 base paired region located on the rRNA operon of mycobacteria[24]. It was found that ITS sequence analysis of MAP taken from 3 different mammalian species-bovine, primate, and human did not indicate much sequence variation between them and in 17 strains of MAC[24]. Thus, the connection between MAP and other mycobacterial strains is observed through this highly conserved sequence similarity. In addition, mycobacteria can be broadly classified as either an environmental or parasitic species on the basis of their epidemiological and pathological nature[25]. Environmental mycobacteria such as the other strains of MAC can be considered as opportunistic bacteria that are found in a variety of habitats. Some of these environments include wet soil, rivers, agricultural slurry, the intestines of birds, ruminants, humans, and even within protists[26,27].
MAP is an obligate parasitic mycobacterium that causes chronic inflammation in the gut of several mammalian species and is considered to have three major genetic differences that serve to separate it from other non-pathogenic MAC. These differences are the presence of an insertion element designated as IS900, the presence of a genetic element known as “GS”, and the presence of a unique MAP gene (hspX) located in a specific genomic region. MAP contains a highly conserved insertion sequence (IS) or IS element referred to as IS900, which is repeatedly found in its genome approximately 15-20 times[28]. IS900 contains 1451 base pairs and harbors neither terminal inverted repeats nor flanking direct repeats normally found in other classical IS elements[28]. As a result, IS900 is grouped in a family of insertion elements that is specifically found in certain microorganisms. Some of these IS elements include IS901 and IS902 found in Mycobacterium avium subsp. silvaticum[29], IS116 present in Streptomyces clavuligerus[30], and IS1110 located in M. avium[31]. It has been documented that the pathogenic nature of several microorganisms has been linked to the presence of IS elements[32]. IS900 is able to take control of the translational machinery of MAP and thereby affects the expression of certain genes. It achieves this task by encoding for a putative transposase of 399 amino acids in size called p43 on one strand[33]. On the complementary strand IS900 encodes for a very unique gene called the hed (host-expression-dependent) gene[34]. This gene is quite unique in that upon entry into the MAP genome it requires a promoter, termination codon, and ribosome binding site (RBS). Previous studies have indicated that IS900 enters the genome of MAP at a specific consensus target sequence such that it is located between the RBS and start codon of the target gene in one specific direction[33]. As a result of this alignment, the hed ORF comes under the control of the mycobacterium host promoter thereby allowing for the translation of the Hed protein[33]. Thus, this is one probable explanation for how the insertion element IS900 may assist in the pathogenic phenotype of MAP compared with the other strains of MAC.
The second major genetic difference between MAP and other mycobacterial strains of MAC is that MAP contains a genetic element designated as “GS”, which contains a low guanosine and cytosine (G + C) content[35]. GS is a 6496 bp element which possesses six genes-gsa, gsbA, gsbB, gsc, gsd, and mpa[36]. In addition, it has been found that the mpa gene of the GS element in MAP is a putative acetyltransferase, and has mpa homologues present in other microorganisms such as oafA and oac of Salmonella typhimurium and Shigella flexneri, respectively[37-39]. Other virulence regions including “pathogenicity islands” or Pais have been reported on MAP chromosome[37] and have been found to be similar to a few protein-coding genes found in Mycobacterium tuberculosis. These protein-coding genes are drrA, drrB, and drrC, are located at Rv2936-Rv2938, and have been commonly associated with the pathogenic phenotype observed in M. tuberculosis[38].
TRANSMISSION OF MAP TO HUMANS THROUGH COW’S MILK
The real concern for the transmission of MAP from cattle to humans is that MAP-infected cows remain asymptomatic in a lengthy subclinical phase[39]. As a result of this, infected cows are not removed and may continue to be harvested for milk and meat, and the spread of MAP can go unnoticed through fecal matters to the rest of the herd[40]. There have been several cases reporting the culture and isolation of MAP from the milk of subclinical or asymptomatic cows[39].
There has been a plethora of documentation about the number of cases in several countries reporting outbreaks of human illness due to improper ‘heat-treated’ milk and dairy products. It has been observed that certain pathogens such as Campylobacter species, Salmonella species, L. monocytogenes, and even Y. enterocolitica have been found in pasteurized milk, powdered milk, and even cheese, thereby contaminating these products and causing human illness[41]. Thus, it is apparent that milk can serve as a means of transmission of these pathogens. Similarly because MAP is found to a large extent in dairy herds and domestic livestock, it can be inferred that it may be present in raw milk. It is assumed that the pasteurization process will destroy any viable pathogens including MAP. However, there have been numerous case studies indicating the thermal-resistant characteristics of MAP thereby enabling its survival after pasteurization. Chiodini and Hermon-Taylor simulated pasteurization methodologies under laboratory conditions as defined by the Public Health Service, US Food and Drug Administration[42]. They performed the high-temperature, short-time (HTST) method of pasteurization in which the milk samples were heated to 72 °C for 15 s in accordance with commercial pasteurization techniques[43]. The results indicated that approximately 3%-5% of strains of MAP survived this process. Also, the pasteurization of MAP obtained from human tissues suspended in milk showed to have a higher survival rate (38.7% and 26.2%) than the bovine samples (8.7% and 9.0%)[43]. Grant et al[44] reported that MAP was not completely destroyed after pasteurization if it was already present in the milk at a concentration greater than 104 cfu/mL. In other studies, Sung et al[45] were able to statistically determine the D values for various strains of MAP tested which estimated that MAP can survive HTST pasteurization methods when initially present at a concentration greater than 101 organisms/mL of milk. However, there have been some critics who have dismissed the validity of the previous studies because they claimed that the HTST pasteurization method performed in the laboratory setting cannot simulate commercial pasteurization conditions such as the turbulent flow of milk[44]. For example, Stabel et al[46] challenged the validity of these previous studies and reported that there was no evidence indicating the presence of viable MAP after the performance of HTST pasteurization simulated with an Armfield HTST laboratory pasteurizer. However, Grant et al[44] defended the studies previously performed in the field and criticized the methodology selected by Stabel et al[46]. She indicated that Stabel et al[46]. had frozen and sonicated MAP prior to its addition to raw milk. Grant et al[44] also expressed that MAP will not under normal circumstances naturally undergo such treatments prior to contaminating milk samples. Furthermore, freezing and sonicating MAP will only make it more susceptible to heat shock As already indicated by Richards et al[47] in 1977, freezing (-70 °C) of bovine fecal samples contaminated with MAP substantially reduced the viability of MAP. Also, it was Sung et al[45] who reported the decreased thermal resistance of declumped MAP cells compared to clumped MAP cells, thereby highlighting the changes caused by the sonication of MAP cells. It is without a doubt that milk can serve as a vehicle for the transmission of MAP from animals to humans through consumption of diary and meat products from infected animals.
PREVALENCE OF MAP IN THE ENVIRONMENT AND IN WATER
One of the major contributors to the spread of MAP in the environment is through the feces of infected cattle. Both subclinically and clinically infected cows excrete massive amounts of MAP through their feces on various pastures and farmlands[48,49]. This is a serious problem because it has already been documented that MAP can persist in the environment for long durations[50]. MAP is capable of surviving in fecal matter and in the soil for up to 12 wk[51]. Muskens et al[52] conducted a study investigating whether infected cattle could transmit MAP to other animals such as sheep grazing on the same pastures. They reported that 20% (10/50) of sheep showed evidence for the presence of MAP in their tissues. Subsequently, MAP can spread and infect other animals which come in contact with infected cattle. Furthermore, the prevalence of MAP in the environment is not only due to infected cattle, but can be due to other infected animals such as rabbits and deer which can also spread MAP abundantly through their feces[53]. Unfortunately, this is only part of the problem. In most cases the cow’s fecal matter is used to make manure which is subsequently distributed across agricultural lands as fertilizer and thus contaminating ground water, rivers, and other surface bodies of water[36]. It will be just a matter of time before the accidental host (human population) is infected with MAP. MAP has been shown to resist chlorine disinfection treatment at concentrations similar to those used to disinfect public drinking water systems[54]. Clearly, it is apparent that water is a very potent vehicle for the transmission of MAP to humans.
MAP CHALLENGES IN THE LABORATORY
From the outset, MAP is an obligate intracellular bacterium which presents multiple challenges in the laboratory with respect to its cultivation from tissue samples from both CD patients and even Johne’s disease in animals. Unfortunately this fastidious bacterium is very slow-growing and often requires the cultures to be incubated for an extended period as much as 16 wk at a time[55]. As a result it has become quite problematic over the years to isolate and culture it through conventional means. Furthermore, MAP has very specific growth requirements which must be met for its survival. For example, this intracellular bacillus is unable to synthesize iron-chelating compounds, and therefore its host must provide iron for MAP to survive. Furthermore, due to its high mycolic content mycobacteria can easily adapt to intracellular growth in macrophages and may even become drug resistant[56]. In addition, MAP in CD assumes a cell-wall deficient spheroplast-like form which complicate culture requirement and void the use of the golden standard Ziehl-Neelsen mycobacterial staining test. For this reason, MAP in its spheroplastic form cannot be identified by light microscopy which adds to the challenges of confirming its presence in a laboratory setting[57]. Due to these difficulties, MAP-specialized scientists looked towards better techniques for the detection and characterization of microorganisms. This led them to the utilization of IS900 polymerase chain reaction (PCR) for the detection of MAP and later on the development of appropriate culture media. Nevertheless, some challenges remain including standardization of the methodology, and most importantly spreading the awareness to clinicians and scientists that standard methodology is not appropriate for the detection of MAP in humans[58].
INVESTIGATING MAP ASSOCIATION WITH CD
It was in 1913, when Dalziel (1861-1924), a surgeon at Glasgow reportedly characterized 13 cases of chronic intestinal enteritis in humans[2]. Upon histological and clinical examination of nine patients, Dalziel specifically noticed that different parts of the gastrointestinal tract were affected: the jejunum, transverse and sigmoid colon, as well as the mid-ileum[2]. He reported that these symptoms closely paralleled clinical findings observed in cattle suffering from Johne’s disease, a chronic inflammatory disease of the gut. As a result, Dalziel speculated that paratuberculosis, the then known causative agent of Johne’s disease, could be a potential etiological agent responsible for the observed symptoms in his patients[2]. It was not until 1932 when Crohn’s disease was first introduced as a clinical entity was it possible to connect the pathological and clinical findings described in CD to Dalziel’s observations in 1913. However, much skepticism and uncertainty persisted with respect to the etiology of Crohn’s disease. Furthermore, confidence in this mycobacterial hypothesis over the years has suffered tremendously due to the substantial difficulty and failure in culturing mycobacteria from CD tissues and the reliance on methodology which were not appropriate for MAP from humans. MAP association with CD theory was revived when Chiodini et al[59] in 1984 reported the isolation of uncharacterized mycobacteria from tissues of three CD patients. They proposed that the bacterium existed in a cell-wall defective form which was later characterized as MAP[60]. Similar results were reported from studies out of David Graham and John Hermon-Taylor laboratories (discussed below). Advancements in cultural techniques and PCR assays unique to MAP by Naser’s team (discussed below) fueled and renewed interest in investigating a possible etiological connection between MAP and CD.
CULTURE OF MAP FROM CD PATIENTS
In this review, data from a total of 23 peer review studies which investigated the presence of MAP in CD specimens using culture techniques were reviewed. As shown in Table 1, the results from 16 (70%) studies supported the association between MAP and CD. Only 7 (30%) studies did not support such association (Table 2). Much of the difficulty in culturing or isolating MAP stems from the fact that this fastidious organism has very specific nutritional requirements and is a very slow growing bacterium[59,61,62]. Culture of MAP in liquid or agar-based media requires weeks to months of laboratory incubation[22]. The presence of MAP in a cell wall-deficient spheroplastic form in humans adds additional challenges to growing it in the laboratory. Many investigators reported the recovery of MAP in a cell wall-deficient form from the tissues of CD patients at a higher occurrence than control groups consisting of non-IBD patients[59,61-72]. Certainly, the advent of PCR, RT-PCR and nested PCR had facilitated the detection of MAP IS900 in cultures from CD patients[53,57,58,64,66,68,69,72]. Table 1 lists a total of 16 studies which strongly support the association between MAP and CD. The development of mycobacterial growth indicator tube (MGIT) sparked a new wave of interest led by Saleh Naser team who supplemented MGIT media with additives essential for survival of cell wall-deficient in vitro and restoration of the cell wall. Consequently, Schwartz et al[62] reported a higher frequency of MAP in CD patients at 37% (10/27) vs healthy controls at 5.6% (2/36). What is truly insightful in this study is the fact that MAP was found at a higher percentage (86%) in surgically resected tissue samples than in tissue biopsies (20%) taken from CD patients[62]. These results alluded to the supposition that MAP may in fact be located below the mucosal layer instead of found on the apical surface area[62]. Naser et al[69] further employed the same culture condition to study whether or not MAP is present in human milk. They reported the presence of MAP in 100% (2/2) of breast milk samples taken from lactating CD mothers who had just given birth, compared to 0% (0/5) of healthy lactating controls. Thus, this study provides critical evidence to support the similarity between Johne’s disease and MAP infection in CD. MAP was later on detected from breast milk from additional CD patients (data not shown). Most interestingly, Naser et al[58] were able to culture viable MAP from the buffy coat of blood sampled from CD patients at a significant percentage 50% (14/28). These intriguing results are further substantiated based on the fact that there was no evidence for the culture of MAP from the blood of the healthy control groups 0% (0/15). Other scientists reported the presence of MAP in 14/33 (42%) bowel-pinch biopsies of CD patients (14/33) compared to 3/33 (9%) non-IBD controls. It was Kirkwood et al[66] who sought to investigate if there was an association between MAP and CD in children who were symptomatic of this disease at an early stage. They revealed that 40% (4/10) of the cultured mucosal biopsies from the CD patients contained viable MAP, whereas 0% (0/4) of the healthy non-IBD controls showed no evidence for the presence of MAP. Consequently, these findings clearly indicate the possible association between MAP and CD, and according to Kirkwood et al[66] these results imply that MAP maybe implicated with the early-onset of CD in children. Sechi et al[57] also reported a particularly strong association between MAP and CD based on their population study which involved the analysis of people in Sardinia diagnosed with and without CD. According to their results it was found that MAP DNA was detected in intestinal mucosal biopsies of approximately 63% (19/30) of CD patients compared to 10.3% (3/29) of control patients.
Table 1.
Studies supporting mycobacterium avium subspecies paratuberculosis association with Crohn’s disease by Culture n (%)
| Study | Crohn’s disease | Control |
| Bull et al[63] | 14 (33) | 3 (33) |
| Chiodini et al[82] | 16 (26) | 13 (26) |
| Chiodini et al[59] | 3 (100) | NP |
| Collins et al[64] | 15 (19) | 3 (6.3) |
| Gitnick et al[65] | 4 (14.8) | 1 (1.8) |
| Kirkwood et al[66] | 4 (40) | 0 (0) |
| Markesich et al[61] | 12 (50) | 1 (7.7) |
| Mendoza et al[67] | 30 (100) | 0 (0) |
| Moss et al[68] | 6 (33.3) | 1 (16.7) |
| Naser et al[69] | 2 (100) | 0 (0) |
| Naser et al[58] | 14 (50) | 0 (0) |
| Schwartz et al[62] | 10 (37) | 2 (5.6) |
| Sechi et al[57] | 19 (63.3) | 3 (10.3) |
| Singh et al[70] | 4 (80) | 6 (27.3) |
| Singh et al[71] | 29 (50) | 5 (12.5) |
| Wall et al[72] | 6 (20) | 0 (0) |
NP: Not performed.
Table 2.
Studies not supporting mycobacterium avium subspecies paratuberculosis association with Crohn’s disease by Culture n (%)
| Ref. | Crohn’s disease | Control |
| Clarkston et al[83] | 0/21 (0) | NP |
| Dumonceau et al[105] | 0/31 (0) | 0/22 (0) |
| Graham et al[84] | 6/19 (31.5) | 7/17 (41) |
| Kallinowski et al[75] | 0/21 (0) | 0/24 (0) |
| Kreuzpaintner et al[85] | 0/23 (0) | 0/23 (0) |
| Parrish et al[73] | 0/130 (0) | 0/130 (0) |
| Ricanek et al[74] | 2/75 (2.7) | 2/135 (1.5) |
NP: Not performed.
Contrary to the above data, there have been some studies providing evidence for the dismissal of MAP as a causative agent of CD (Table 2). For example, Parrish et al[73] conducted a study analyzing blood samples taken from 260 individuals who consisted of 130 CD patients and 130 healthy individuals. After culturing MAP, the results revealed that none of the CD patients 0% (0/130) as well as the healthy controls 0% (0/130) showed evidence for the presence of MAP[73]. Only one patient was reported having a positive result by PCR[73]. Due to the fact that MAP and MAP DNA are present in the food chain and the fact that MAP DNA has been detected in the blood of patients with CD and type I diabetes mellitus and in less frequency in the blood of healthy controls, most scientists in the field may question the protocol used in this study. In another study, Ricanek et al[74] collected bowel biopsies from 321 individuals, of which 75 of these biopsies were collected from CD patients and 135 were collected from non-IBD patients. After long-term culture of MAP it was reported that only 2.7% (2/75) of CD patients and 1.5% (2/135) of non-IBD patients showed the presence of MAP[74]. Similarly, Kallinowski et al[75] documented the inability to culture MAP from a variety of sources such as stool, sera, and even gut tissue samples. They reported that 0% (0/21) of CD patients and 0% (0/24) of healthy controls had MAP through culture[75]. The results from these studies should not be surprising since MAP is extremely fastidious and requires specialized culture media to grow which is contrary to culture media used in these studies. Other studies which failed to detect MAP in CD have depended on traditional standard methodology designed to culture and detect bacillary MAP from Johne’s disease animals or other Mycobacterium species. It is important that investigators realize that M. avium subspecies paratuberculosis is not the same as M. avium or M. tuberculosis. Moreover, tissue and blood specimens collected from patients with active antibiotic treatment should be used for attempts to culture MAP in the laboratory. Rarely did the studies described in Table 2 allotted to whether the subjects used in their studies had antimicrobial agents prior to submission of the specimens.
DETECTION OF MAP DNA BY PCR
A total of 52 studies investigating MAP DNA in CD have been reviewed. Table 3 lists a total of 27 studies providing evidence in support of MAP association with CD by PCR. On the contrary. Table 4 lists 25 studies which present data in contradiction of MAP-CD association.
Table 3.
Studies supporting mycobacterium avium subspecies paratuberculosis association with Crohn’s disease by polymerase chain reaction n (%)
| Ref. | Crohn’s disease | Control |
| Autschbach et al[76] | 52 (100) | 5 (100) |
| Bentley et al[86] | 122 (33.8) | 43 (21.5) |
| Bull et al[63] | 34 (92) | 9 (26) |
| 14 (42) | 3 (9) | |
| Collins et al[64] | 15 (19) | 3 (6.3) |
| Dell’lsola et al[87] | 13 (72) | 7 (29.2) |
| Erasmus et al[88] | 10 (38) | 4 (11) |
| Fidler et al[89] | 4 (12.9) | 0 (0) |
| Gan et al[90] | 17 (47.2) | 3 (15) |
| Ikonomopoulos et al[91] | 7 (35) | NP |
| Kirkwood et al[66] | 22 (39) | 6 (15) |
| 8 (16) | 0 (0) | |
| Lisby et al[92] | 11 (46) | 3 (11) |
| Mendoza et al[67] | 18 (60) | 0 (0) |
| Mishina et al[78] | 8 (100) | 0 (0) |
| Moss et al[68] | 6 (33.3) | 1 (16.7) |
| Murray et al[93] | 2 (22) | 0 (0) |
| Naser et al[69] | 2 (100) | 0 (0) |
| Naser et al[58] | 13 (46) | 3 (20) |
| Romero et al[77] | 10 (83) | 1 (17) |
| Ryan et al[94] | 6 (50) | 0 (0) |
| Sanderson et al[95] | 26 (65) | 5 (12.5) |
| Scanu et al[96] | 20 (87) | 3 (15) |
| Sechi et al[57] | 25 (83.3) | 3 (10.3) |
| Singh et al[70] | 4 (80) | 5 (22.7) |
| Singh et al[71] | 28 (96.6) | NP |
| Tiveljung et al[97] | 3 (27) | 0 (0) |
| Tuci et al[98] | 21 (68) | 11 (48) |
| Wall et al[72] | 6 (20) | 0 (0) |
NP: Not performed.
Table 4.
Studies not supporting mycobacterium avium subspecies paratuberculosis association with Crohn’s disease by polymerase chain reaction n (%)
| Ref. | Crohn’s disease | Control |
| Al-Shamali et al[99] | 0 (0) | 0 (0) |
| 0 (0) | 0 (0) | |
| Baksh et al[100] | 0 (0) | NP |
| Bernstein et al[101] | 0 (0) | 6 (21.4) |
| Cellier et al[102] | 2 (4) | 2 (10) |
| 0 (0) | 0 (0) | |
| Chiba et al[103] | 0 (0) | 0 (0) |
| Clarkston et al[83] | 1 (4.8) | 0 (0) |
| Dumonceau et al[104] | 17 (47) | 13 (57) |
| 0 (0) | 0 (0) | |
| Domonceau et al[105] | 0 (0) | 0 (0) |
| Ellingson et al[106] | 0 (0) | 0 (0) |
| Frank and Cook[81] | 0 (0) | 0 (0) |
| Gibson et al[107] | 0 (0) | 0 (0) |
| Kallinowski et al[75] | 0 (0) | 0 (0) |
| Kanazawa et al[108] | 0 (0) | 0 (0) |
| Kreuzpaintner et al[85] | 0 (0) | 0 (0) |
| Lozano-Leon et al[109] | 0 (0) | 0 (0) |
| Parrish et al[73] | 0 (0) | 1 (0.77) |
| Ricanek et al[74] | 0 (0) | 1 (0.28) |
| Riggio et al[110] | 0 (0) | 0 (0) |
| Quirke[21] | 0 (0) | 0 (0) |
| Rowbotham et al[80] | 0 (0) | 1 (3.8) |
| Sasikala et al[79] | 0 (0) | 0 (0) |
| Suenaga et al[111] | 10 (100) | 14 (87.5) |
| 10 (100) | 14 (87.5) | |
| Toracchio et al[112] | 1 (5) | NP |
| Tzen et al[113] | 0 (0) | 3 (27.3) |
| Wu et al[114] | 0 (0) | NP |
NP: Not performed.
One of the studies showing a strong connection between MAP and CD has been performed by Autschbach et al[76]. They reported that a staggering 52% (52/100) of tissue from CD patients were found positive for the presence of MAP DNA compared to only 5% (5/100) of the non-IBD patients. Similarly, Romero et al[77] had examined several surgical tissue samples from 20 individuals by performing nested PCR specific for the IS900 sequence. The results from Naser’s lab indicated that a substantially high percentage 83% (10/12) of CD patients were positive for the presence of MAP, while a much smaller percentage 17% (1/6) of non-IBD patients were positive for MAP[77]. In addition, there was another compelling study conducted by Bull et al[63] in 2003 in John Hermon-Taylor’s laboratory, which presented data in support of MAP as a causative agent for CD. Fresh ileocolonic mucosal biopsies were collected and analyzed for the presence of MAP by the performance of PCR specific for IS900. The results revealed that 92% (34/37) of CD patients were positive for the presence of MAP DNA compared to a significantly diminished number of healthy controls 26% (9/34)[63]. In this same study Bull et al[63] had cultivated MAP using MGIT cultures described by Naser et al[58] and Schwartz et al[62]. After twelve weeks of incubation, PCR was performed with these cultures which again indicated a higher frequency of CD patients 42% (14/33) positive for MAP DNA vs only 9% (3/33) of healthy controls[63]. This data strengthens the support of MAP in connection with CD. Mishina et al[78] analyzed mucosal specimens using RT-PCR for the detection of MAP RNA where they found MAP in 100% (8/8) of CD patients and 0% (0/2) in non-IBD. This study is of particular importance because MAP RNA was amplified (without culture) adding more support to the presence of viable MAP in CD[78].
At the same time, many studies based on PCR techniques have failed to detect MAP DNA in CD and concluded the lack of association between MAP and CD (Table 4). For example, Sasikala et al[79] indicated that 0% (0/93) of CD patients showed the presence of MAP and 0% (0/97) of healthy controls were also negative for the presence of MAP. Similarly, Rowbotham et al[80] reported that none (0/68) of CD patients were positive for the presence of MAP and just 3.8% (1/26) of healthy controls had MAP. Lozano-Leon et al[109] indicated the absence of MAP in the blood of 73 CD patients and 73 healthy controls. Frank and Cook in 1996 also reported the absence of MAP in both CD and control subjects[81]. The investigators in these studies should be commended on their interest to question whether or not MAP is associated with CD, and for including impressive numbers of specimens in their studies. Due to the fact that MAP and MAP DNA are found in the food chain including dairy and meat products as well as in drinking water, it is difficult to accept that MAP or MAP DNA is not detected even accidently in some specimens. The methodology used in many of these studies must have lacked essential steps to recover the low abundance of MAP in CD specimens and must have not been able to reduce the laboratory loss of some MAP or MAP derivatives. Whether the loss of MAP occurred at the specimen collection level or during the analysis, it should be avoided. Tissue specimens must be collected appropriately and adequately from active ulcerated sites. Specimens should be transported promptly and appropriately by avoiding freezing and use of anti-microbial solutions. Blood should be withdrawn into tubes with anticoagulants, transported without freezing, and promptly, to avoid lysis of leukocytes and loss of MAP. DNA extraction conditions should be optimized to recover single MAP genome which is also free from PCR inhibitors such as hemoglobin. Earlier study in our laboratory suggested that MAP from CD patients contained limited IS900 copies compared to bovine MAP strains. Nested PCR consisting of two amplification rounds is necessary for sufficient detection of MAP DNA. For reasons mentioned above and other unknown factors, standard PCR based on a single amplification should not be used for detection of MAP in CD.
CONCLUSION
In this review, data has been presented in the form of tables providing evidence for and against an association between MAP and CD by PCR and culture. It was revealed that MAP can be detected and isolated from the tissues, blood, and milk of many CD patients. Based on this information, MAP is definitively involved in the pathogenesis of some CD cases even though other studies have not acknowledged this association as represented in Tables 2 and 4. It must be emphasized that much of the controversy concerning MAP and CD stems from the inconsistent methodologies that have been used in the detection and isolation of MAP, which have questioned the causal relationship between this bacterium and CD. These observed discrepancies result from the fact that the methods that were designed for the detection of MAP in animals with Johne’s disease are inappropriate for the detection of MAP in humans. Consequently, the need for more sophisticated and optimized methodologies are required so that there can be accurate detection and isolation of MAP in CD patients. One such methodology has been developed in our laboratory, and success has been achieved based on key principles shown in Figure 1. Other factors that may also limit the detection of MAP in clinical samples from some CD patients include the stage of the disease, and prior treatment with antibiotics or drugs with antimicrobial activity. For example, negative detection of MAP in peripheral blood samples could be correlated with a localized intestinal CD compared to cases with advanced disease associated with systemic complications. The latter is most likely to lead to the presence of MAP in circulation.
Figure 1.
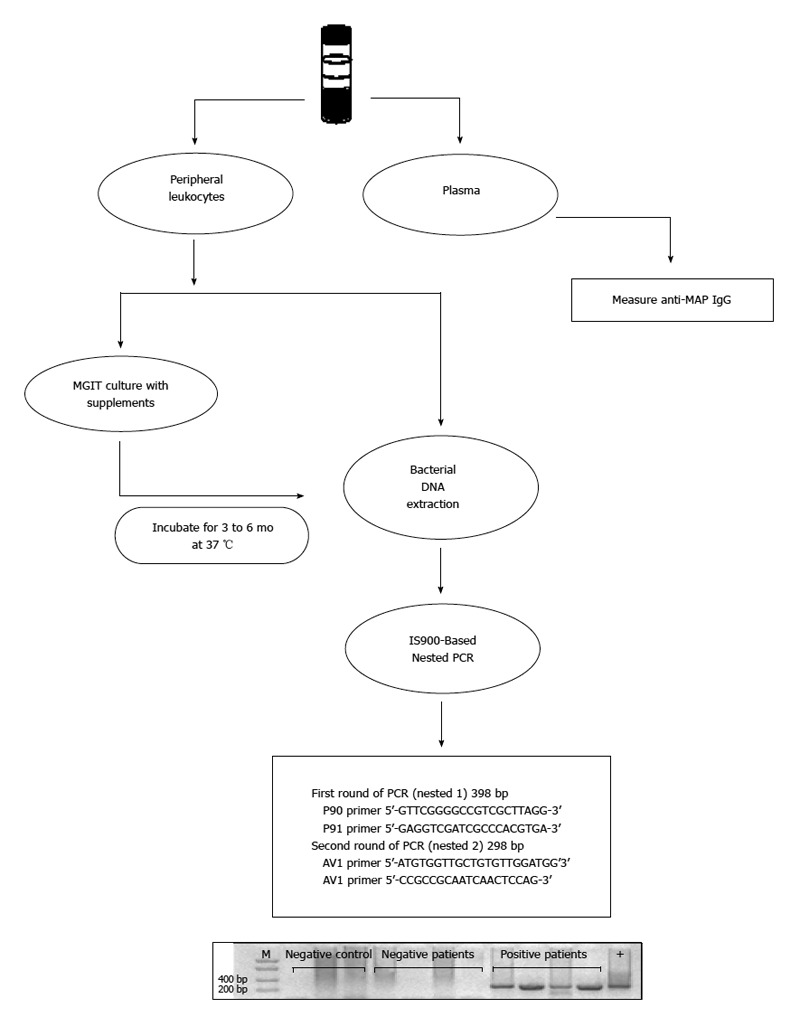
Schematic illustration of successful Mycobacterium avium subspecies paratuberculosis detection in clinical samples. Coded EDTA blood samples were collected from patients for investigating the presence of Mycobacterium avium subspecies paratuberculosis (MAP). Blood plasma was analyzed by measuring the concentration of anti-MAP IgG antibodies. Peripheral leukocytes were analyzed for the presence of MAP. In the first method, DNA was extracted followed by IS900-based nested polymerase chain reaction (PCR) using MAP-specific primers. In the second method a mycobacterium growth indicator tube (MGIT) liquid culture system with supplements was used to culture MAP lacking cell wall followed by 3 to 6 mo incubation and IS900-based nested PCR analysis.
Finally, it is also worth noting that it is a fact that CD is a syndrome with multifactorial etiology. It is very possible that lack of detection of MAP in clinical samples from some CD patients may be due to the absence of MAP role in these patients. The latter statement is conditional on utilization of methodology appropriate for detection of human MAP strains. Stratification of CD and IBD patients for the presence or absence of MAP is necessary for appropriate and effective treatment which may lead to a cure.
Footnotes
P- Reviewers: Chamberlin W, Imaeda H, Teramoto-Matsubara OT, Tsai JF, Xu JM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Brzezinski A. Medical management of the patient with an ostomy. In: Lichtenstein GR, Scherl EJ, editors. Crohn’s Disease: the Complete Guide to Medical Management. NJ: Thorofare; 2011. pp. 417–423. [Google Scholar]
- 2.Dalziel TK. Thomas Kennedy Dalziel 1861-1924. Chronic interstitial enteritis. Dis Colon Rectum. 1989;32:1076–1078. doi: 10.1007/BF02553886. [DOI] [PubMed] [Google Scholar]
- 3.Moschowitz E, Wilensky A. Non-specific granulomata of the intestine. Am J Med Sci. 1923;166:48–66. [Google Scholar]
- 4.Wilensky ME. A Non-specific granulomata of the intestine. Am J Med Sci. 1927;173:374–380. [Google Scholar]
- 5.Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis; a pathologic and clinical entity. Am J Med. 1952;13:583–590. doi: 10.1016/0002-9343(52)90025-9. [DOI] [PubMed] [Google Scholar]
- 6.Aufses AH. The history of Crohn’s disease. Surg Clin North Am. 2001;81:1–11, vii. doi: 10.1016/s0039-6109(05)70270-x. [DOI] [PubMed] [Google Scholar]
- 7.McCallum DI, Gray WM. Metastatic Crohn’s disease. Br J Dermatol. 1976;95:551–554. doi: 10.1111/j.1365-2133.1976.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 8.Penner A, Crohn BB. PERIANAL FISTULAE AS A COMPLICATION OF REGIONAL ILEITIS. Ann Surg. 1938;108:867–873. doi: 10.1097/00000658-193811000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells C. Ulcerative colitis and Crohn’s disease. Ann R Coll Surg Engl. 1952;11:105–120. [PMC free article] [PubMed] [Google Scholar]
- 10.Bandzar S, Gupta S, Platt MO. Crohn’s disease: a review of treatment options and current research. Cell Immunol. 2013;286:45–52. doi: 10.1016/j.cellimm.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Naser SA, Arce M, Khaja A, Fernandez M, Naser N, Elwasila S, Thanigachalam S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J Gastroenterol. 2012;18:412–424. doi: 10.3748/wjg.v18.i5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.What is Crohn’s Disease? 2013 Dec 22. Available from: http://www.ccfa.org.
- 13.Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 14.Kurata JH, Kantor-Fish S, Frankl H, Godby P, Vadheim CM. Crohn’s disease among ethnic groups in a large health maintenance organization. Gastroenterology. 1992;102:1940–1948. doi: 10.1016/0016-5085(92)90317-r. [DOI] [PubMed] [Google Scholar]
- 15.Cho JH. Inflammatory bowel disease: genetic and epidemiologic considerations. World J Gastroenterol. 2008;14:338–347. doi: 10.3748/wjg.14.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman HJ. Long-term natural history of Crohn’s disease. World J Gastroenterol. 2009;15:1315–1318. doi: 10.3748/wjg.15.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beattie RM, Croft NM, Fell JM, Afzal NA, Heuschkel RB. Inflammatory bowel disease. Arch Dis Child. 2006;91:426–432. doi: 10.1136/adc.2005.080481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann H, Mönkemüller K, Günther C, Atreya R, Vieth M, Neurath MF. Advanced endoscopic imaging for diagnosis of Crohn’s disease. Gastroenterol Res Pract. 2012;2012:301541. doi: 10.1155/2012/301541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laghi A, Borrelli O, Paolantonio P, Dito L, Buena de Mesquita M, Falconieri P, Passariello R, Cucchiara S. Contrast enhanced magnetic resonance imaging of the terminal ileum in children with Crohn’s disease. Gut. 2003;52:393–397. doi: 10.1136/gut.52.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burisch J, Pedersen N, Cukovic-Cavka S, Turk N, Kaimakliotis I, Duricova D, Bortlik M, Shonová O, Vind I, Avnstrøm S, et al. Environmental factors in a population-based inception cohort of inflammatory bowel disease patients in Europe - An ECCO-EpiCom study. J Crohns Colitis. 2014;8:607–616. doi: 10.1016/j.crohns.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Quirke P. Antagonist. Mycobacterium avium subspecies paratuberculosis is a cause of Crohn’s disease. Gut. 2001;49:757–760. doi: 10.1136/gut.49.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Zaatari FA, Osato MS, Graham DY. Etiology of Crohn’s disease: the role of Mycobacterium avium paratuberculosis. Trends Mol Med. 2001;7:247–252. doi: 10.1016/s1471-4914(01)01983-9. [DOI] [PubMed] [Google Scholar]
- 23.Thorel MF, Krichevsky M, Lévy-Frébault VV. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 24.Frothingham R. Evolutionary bottlenecks in the agents of tuberculosis, leprosy, and paratuberculosis. Med Hypotheses. 1999;52:95–99. doi: 10.1054/mehy.1997.0622. [DOI] [PubMed] [Google Scholar]
- 25.Wayne LG, Sramek HA. Agents of newly recognized or infrequently encountered mycobacterial diseases. Clin Microbiol Rev. 1992;5:1–25. doi: 10.1128/cmr.5.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rhodes G, Henrys P, Thomson BC, Pickup RW. Mycobacterium avium subspecies paratuberculosis is widely distributed in British soils and waters: implications for animal and human health. Environ Microbiol. 2013;15:2761–2774. doi: 10.1111/1462-2920.12137. [DOI] [PubMed] [Google Scholar]
- 27.Rindi L, Garzelli C. Genetic diversity and phylogeny of Mycobacterium avium. Infect Genet Evol. 2014;21:375–383. doi: 10.1016/j.meegid.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 28.Green EP, Tizard ML, Moss MT, Thompson J, Winterbourne DJ, McFadden JJ, Hermon-Taylor J. Sequence and characteristics of IS900, an insertion element identified in a human Crohn’s disease isolate of Mycobacterium paratuberculosis. Nucleic Acids Res. 1989;17:9063–9073. doi: 10.1093/nar/17.22.9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunze ZM, Wall S, Appelberg R, Silva MT, Portaels F, McFadden JJ. IS901, a new member of a widespread class of atypical insertion sequences, is associated with pathogenicity in Mycobacterium avium. Mol Microbiol. 1991;5:2265–2272. doi: 10.1111/j.1365-2958.1991.tb02157.x. [DOI] [PubMed] [Google Scholar]
- 30.Leskiw BK, Mevarech M, Barritt LS, Jensen SE, Henderson DJ, Hopwood DA, Bruton CJ, Chater KF. Discovery of an insertion sequence, IS116, from Streptomyces clavuligerus and its relatedness to other transposable elements from actinomycetes. J Gen Microbiol. 1990;136:1251–1258. doi: 10.1099/00221287-136-7-1251. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez Perez M, Fomukong NG, Hellyer T, Brown IN, Dale JW. Characterization of IS1110, a highly mobile genetic element from Mycobacterium avium. Mol Microbiol. 1994;12:717–724. doi: 10.1111/j.1365-2958.1994.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 32.Bartlett DH, Silverman M. Nucleotide sequence of IS492, a novel insertion sequence causing variation in extracellular polysaccharide production in the marine bacterium Pseudomonas atlantica. J Bacteriol. 1989;171:1763–1766. doi: 10.1128/jb.171.3.1763-1766.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tizard ML, Moss MT, Sanderson JD, Austen BM, Hermon-Taylor J. p43, the protein product of the atypical insertion sequence IS900, is expressed in Mycobacterium paratuberculosis. J Gen Microbiol. 1992;138 Pt 8:1729–1736. doi: 10.1099/00221287-138-8-1729. [DOI] [PubMed] [Google Scholar]
- 34.Doran T, Tizard M, Millar D, Ford J, Sumar N, Loughlin M, Hermon-Taylor J. IS900 targets translation initiation signals in Mycobacterium avium subsp. paratuberculosis to facilitate expression of its hed gene. Microbiology. 1997;143(Pt 2):547–552. doi: 10.1099/00221287-143-2-547. [DOI] [PubMed] [Google Scholar]
- 35.Tizard M, Bull T, Millar D, Doran T, Martin H, Sumar N, Ford J, Hermon-Taylor J. A low G+C content genetic island in Mycobacterium avium subsp. paratuberculosis and M. avium subsp. silvaticum with homologous genes in Mycobacterium tuberculosis. Microbiology. 1998;144(Pt 12):3413–3423. doi: 10.1099/00221287-144-12-3413. [DOI] [PubMed] [Google Scholar]
- 36.Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng J, Stellakis ML, Sumar N. Causation of Crohn’s disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol. 2000;14:521–539. doi: 10.1155/2000/798305. [DOI] [PubMed] [Google Scholar]
- 37.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 38.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon SV, Eiglmeier K, Gas S, Barry CE, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 39.Sweeney RW, Whitlock RH, Rosenberger AE. Mycobacterium paratuberculosis cultured from milk and supramammary lymph nodes of infected asymptomatic cows. J Clin Microbiol. 1992;30:166–171. doi: 10.1128/jcm.30.1.166-171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sweeney RW. Transmission of paratuberculosis. Vet Clin North Am Food Anim Pract. 1996;12:305–312. doi: 10.1016/s0749-0720(15)30408-4. [DOI] [PubMed] [Google Scholar]
- 41.Sharp JC. Infections associated with milk and dairy products in Europe and North America, 1980-85. Bull World Health Organ. 1987;65:397–406. [PMC free article] [PubMed] [Google Scholar]
- 42.Grade a pasteurization milk ordance. USA: Public Health Service, Food and Drug Administration; 1985. [Google Scholar]
- 43.Chiodini RJ, Hermon-Taylor J. The thermal resistance of Mycobacterium paratuberculosis in raw milk under conditions simulating pasteurization. J Vet Diagn Invest. 1993;5:629–631. doi: 10.1177/104063879300500424. [DOI] [PubMed] [Google Scholar]
- 44.Grant IR, Ball HJ, Neill SD, Rowe MT. Inactivation of Mycobacterium paratuberculosis in cows’ milk at pasteurization temperatures. Appl Environ Microbiol. 1996;62:631–636. doi: 10.1128/aem.62.2.631-636.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sung N, Collins MT. Thermal tolerance of Mycobacterium paratuberculosis. Appl Environ Microbiol. 1998;64:999–1005. doi: 10.1128/aem.64.3.999-1005.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stabel JR, Steadham EM, Bolin CA. Heat inactivation of Mycobacterium paratuberculosis in raw milk: are current pasteurization conditions effective? Appl Environ Microbiol. 1997;63:4975–4977. doi: 10.1128/aem.63.12.4975-4977.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards WD, Thoen CO. Effect of freezing on the viability of Mycobacterium paratuberculosis in bovine feces. J Clin Microbiol. 1977;6:392–395. doi: 10.1128/jcm.6.4.392-395.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.International Association for Paratuberculosis. Fifth International Colloquium on Paratuberculosis, 1997. Rehoboth: Mass; 1997. pp. 333–338. [Google Scholar]
- 49.Abubakar I, Myhill DJ, Hart AR, Lake IR, Harvey I, Rhodes JM, Robinson R, Lobo AJ, Probert CS, Hunter PR. A case-control study of drinking water and dairy products in Crohn’s Disease--further investigation of the possible role of Mycobacterium avium paratuberculosis. Am J Epidemiol. 2007;165:776–783. doi: 10.1093/aje/kwk067. [DOI] [PubMed] [Google Scholar]
- 50.Salgado M, Alfaro M, Salazar F, Troncoso E, Mitchell RM, Ramirez L, Naguil A, Zamorano P, Collins MT. Effect of soil slope on the appearance of Mycobacterium avium subsp. paratuberculosis in water running off grassland soil after application of contaminated slurry. Appl Environ Microbiol. 2013;79:3544–3552. doi: 10.1128/AEM.00610-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whittington RJ, Marsh IB, Reddacliff LA. Survival of Mycobacterium avium subsp. paratuberculosis in dam water and sediment. Appl Environ Microbiol. 2005;71:5304–5308. doi: 10.1128/AEM.71.9.5304-5308.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muskens J, Bakker D, de Boer J, van Keulen L. Paratuberculosis in sheep: its possible role in the epidemiology of paratuberculosis in cattle. Vet Microbiol. 2001;78:101–109. doi: 10.1016/s0378-1135(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 53.Greig A, Stevenson K, Henderson D, Perez V, Hughes V, Pavlik I, Hines ME, McKendrick I, Sharp JM. Epidemiological study of paratuberculosis in wild rabbits in Scotland. J Clin Microbiol. 1999;37:1746–1751. doi: 10.1128/jcm.37.6.1746-1751.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor RH, Falkinham JO, Norton CD, LeChevallier MW. Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl Environ Microbiol. 2000;66:1702–1705. doi: 10.1128/aem.66.4.1702-1705.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Legrand E, Sola C, Rastogi N. [Mycobacterium avium-intracellulare complex: phenotypic and genotypic markers and the molecular basis for interspecies transmission] Bull Soc Pathol Exot. 2000;93:182–192. [PubMed] [Google Scholar]
- 56.Rastogi N, Legrand E, Sola C. The mycobacteria: an introduction to nomenclature and pathogenesis. Rev Sci Tech. 2001;20:21–54. doi: 10.20506/rst.20.1.1265. [DOI] [PubMed] [Google Scholar]
- 57.Sechi LA, Scanu AM, Molicotti P, Cannas S, Mura M, Dettori G, Fadda G, Zanetti S. Detection and Isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn’s disease in Sardinia. Am J Gastroenterol. 2005;100:1529–1536. doi: 10.1111/j.1572-0241.2005.41415.x. [DOI] [PubMed] [Google Scholar]
- 58.Naser SA, Ghobrial G, Romero C, Valentine JF. Culture of Mycobacterium avium subspecies paratuberculosis from the blood of patients with Crohn’s disease. Lancet. 2004;364:1039–1044. doi: 10.1016/S0140-6736(04)17058-X. [DOI] [PubMed] [Google Scholar]
- 59.Chiodini RJ, Van Kruiningen HJ, Merkal RS, Thayer WR, Coutu JA. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn’s disease. J Clin Microbiol. 1984;20:966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshimura HH, Graham DY, Estes MK, Merkal RS. Investigation of association of mycobacteria with inflammatory bowel disease by nucleic acid hybridization. J Clin Microbiol. 1987;25:45–51. doi: 10.1128/jcm.25.1.45-51.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Markesich DC, Graham DY, Yoshimura HH. Progress in culture and subculture of spheroplasts and fastidious acid-fast bacilli isolated from intestinal tissues. J Clin Microbiol. 1988;26:1600–1603. doi: 10.1128/jcm.26.8.1600-1603.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz D, Shafran I, Romero C, Piromalli C, Biggerstaff J, Naser N, Chamberlin W, Naser SA. Use of short-term culture for identification of Mycobacterium avium subsp. paratuberculosis in tissue from Crohn’s disease patients. Clin Microbiol Infect. 2000;6:303–307. doi: 10.1046/j.1469-0691.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- 63.Bull TJ, McMinn EJ, Sidi-Boumedine K, Skull A, Durkin D, Neild P, Rhodes G, Pickup R, Hermon-Taylor J. Detection and verification of Mycobacterium avium subsp. paratuberculosis in fresh ileocolonic mucosal biopsy specimens from individuals with and without Crohn’s disease. J Clin Microbiol. 2003;41:2915–2923. doi: 10.1128/JCM.41.7.2915-2923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Collins MT, Lisby G, Moser C, Chicks D, Christensen S, Reichelderfer M, Høiby N, Harms BA, Thomsen OO, Skibsted U, et al. Results of multiple diagnostic tests for Mycobacterium avium subsp. paratuberculosis in patients with inflammatory bowel disease and in controls. J Clin Microbiol. 2000;38:4373–4381. doi: 10.1128/jcm.38.12.4373-4381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gitnick G, Collins J, Beaman B, Brooks D, Arthur M, Imaeda T, Palieschesky M. Preliminary report on isolation of mycobacteria from patients with Crohn’s disease. Dig Dis Sci. 1989;34:925–932. doi: 10.1007/BF01540280. [DOI] [PubMed] [Google Scholar]
- 66.Kirkwood CD, Wagner J, Boniface K, Vaughan J, Michalski WP, Catto-Smith AG, Cameron DJ, Bishop RF. Mycobacterium avium subspecies paratuberculosis in children with early-onset Crohn’s disease. Inflamm Bowel Dis. 2009;15:1643–1655. doi: 10.1002/ibd.20967. [DOI] [PubMed] [Google Scholar]
- 67.Mendoza JL, San-Pedro A, Culebras E, Cíes R, Taxonera C, Lana R, Urcelay E, de la Torre F, Picazo JJ, Díaz-Rubio M. High prevalence of viable Mycobacterium avium subspecies paratuberculosis in Crohn’s disease. World J Gastroenterol. 2010;16:4558–4563. doi: 10.3748/wjg.v16.i36.4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moss MT, Sanderson JD, Tizard ML, Hermon-Taylor J, el-Zaatari FA, Markesich DC, Graham DY. Polymerase chain reaction detection of Mycobacterium paratuberculosis and Mycobacterium avium subsp silvaticum in long term cultures from Crohn’s disease and control tissues. Gut. 1992;33:1209–1213. doi: 10.1136/gut.33.9.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naser SA, Schwartz D, Shafran I. Isolation of Mycobacterium avium subsp paratuberculosis from breast milk of Crohn’s disease patients. Am J Gastroenterol. 2000;95:1094–1095. doi: 10.1111/j.1572-0241.2000.01954.x. [DOI] [PubMed] [Google Scholar]
- 70.Singh AV, Singh SV, Makharia GK, Singh PK, Sohal JS. Presence and characterization of Mycobacterium avium subspecies paratuberculosis from clinical and suspected cases of Crohn’s disease and in the healthy human population in India. Int J Infect Dis. 2008;12:190–197. doi: 10.1016/j.ijid.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 71.Singh AV, Singh SV, Singh PK, Sohal JS, Singh MK. High prevalence of Mycobacterium avium subspecies paratuberculosis (‘Indian bison type’) in animal attendants suffering from gastrointestinal complaints who work with goat herds endemic for Johne’s disease in India. Int J Infect Dis. 2011;15:e677–e683. doi: 10.1016/j.ijid.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 72.Wall S, Kunze ZM, Saboor S, Soufleri I, Seechurn P, Chiodini R, McFadden JJ. Identification of spheroplast-like agents isolated from tissues of patients with Crohn’s disease and control tissues by polymerase chain reaction. J Clin Microbiol. 1993;31:1241–1245. doi: 10.1128/jcm.31.5.1241-1245.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parrish NM, Radcliff RP, Brey BJ, Anderson JL, Clark DL, Koziczkowski JJ, Ko CG, Goldberg ND, Brinker DA, Carlson RA, et al. Absence of mycobacterium avium subsp. paratuberculosis in Crohn’s patients. Inflamm Bowel Dis. 2009;15:558–565. doi: 10.1002/ibd.20799. [DOI] [PubMed] [Google Scholar]
- 74.Ricanek P, Lothe SM, Szpinda I, Jorde AT, Brackmann S, Perminow G, Jørgensen KK, Rydning A, Vatn MH, Tønjum T. Paucity of mycobacteria in mucosal bowel biopsies from adults and children with early inflammatory bowel disease. J Crohns Colitis. 2010;4:561–566. doi: 10.1016/j.crohns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 75.Kallinowski F, Wassmer A, Hofmann MA, Harmsen D, Heesemann J, Karch H, Herfarth C, Buhr HJ. Prevalence of enteropathogenic bacteria in surgically treated chronic inflammatory bowel disease. Hepatogastroenterology. 1998;45:1552–1558. [PubMed] [Google Scholar]
- 76.Autschbach F, Eisold S, Hinz U, Zinser S, Linnebacher M, Giese T, Löffler T, Büchler MW, Schmidt J. High prevalence of Mycobacterium avium subspecies paratuberculosis IS900 DNA in gut tissues from individuals with Crohn’s disease. Gut. 2005;54:944–949. doi: 10.1136/gut.2004.045526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Romero C, Hamdi A, Valentine JF, Naser SA. Evaluation of surgical tissue from patients with Crohn’s disease for the presence of Mycobacterium avium subspecies paratuberculosis DNA by in situ hybridization and nested polymerase chain reaction. Inflamm Bowel Dis. 2005;11:116–125. doi: 10.1097/00054725-200502000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Mishina D, Katsel P, Brown ST, Gilberts EC, Greenstein RJ. On the etiology of Crohn disease. Proc Natl Acad Sci USA. 1996;93:9816–9820. doi: 10.1073/pnas.93.18.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sasikala M, Reddy DN, Pratap N, Sharma SK, Balkumar PR, Sekaran A, Banerjee R, Reddy DB. Absence of Mycobacterium avium ss paratuberculosis-specific IS900 sequence in intestinal biopsy tissues of Indian patients with Crohn’s disease. Indian J Gastroenterol. 2009;28:169–174. doi: 10.1007/s12664-009-0068-2. [DOI] [PubMed] [Google Scholar]
- 80.Rowbotham DS, Mapstone NP, Trejdosiewicz LK, Howdle PD, Quirke P. Mycobacterium paratuberculosis DNA not detected in Crohn’s disease tissue by fluorescent polymerase chain reaction. Gut. 1995;37:660–667. doi: 10.1136/gut.37.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frank TS, Cook SM. Analysis of paraffin sections of Crohn’s disease for Mycobacterium paratuberculosis using polymerase chain reaction. Mod Pathol. 1996;9:32–35. [PubMed] [Google Scholar]
- 82.Chiodini RJ, Van Kruiningen HJ, Thayer WR, Coutu JA. Spheroplastic phase of mycobacteria isolated from patients with Crohn’s disease. J Clin Microbiol. 1986;24:357–363. doi: 10.1128/jcm.24.3.357-363.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clarkston WK, Presti ME, Petersen PF, Zachary PE, Fan WX, Leonardi CL, Vernava AM, Longo WE, Kreeger JM. Role of Mycobacterium paratuberculosis in Crohn’s disease: a prospective, controlled study using polymerase chain reaction. Dis Colon Rectum. 1998;41:195–199. doi: 10.1007/BF02238248. [DOI] [PubMed] [Google Scholar]
- 84.Graham DY, Markesich DC, Yoshimura HH. Mycobacteria and inflammatory bowel disease. Results of culture. Gastroenterology. 1987;92:436–442. doi: 10.1016/0016-5085(87)90139-9. [DOI] [PubMed] [Google Scholar]
- 85.Kreuzpaintner G, Kirschner P, Wallner A, Kölble R, Hesterberg R, Thomas L, Borchard F. Mycobacteria of Runyon groups I, II and IV do not play an aetiological role in Crohn’s disease. Eur J Gastroenterol Hepatol. 1995;7:1177–1182. doi: 10.1097/00042737-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 86.Bentley RW, Keenan JI, Gearry RB, Kennedy MA, Barclay ML, Roberts RL. Incidence of Mycobacterium avium subspecies paratuberculosis in a population-based cohort of patients with Crohn’s disease and control subjects. Am J Gastroenterol. 2008;103:1168–1172. doi: 10.1111/j.1572-0241.2007.01742.x. [DOI] [PubMed] [Google Scholar]
- 87.Dell’Isola B, Poyart C, Goulet O, Mougenot JF, Sadoun-Journo E, Brousse N, Schmitz J, Ricour C, Berche P. Detection of Mycobacterium paratuberculosis by polymerase chain reaction in children with Crohn’s disease. J Infect Dis. 1994;169:449–451. doi: 10.1093/infdis/169.2.449. [DOI] [PubMed] [Google Scholar]
- 88.Erasmus DL, Victor TC, van Eeden PJ, Falck V, van Helden P. Mycobacterium paratuberculosis and Crohn’s disease. Gut. 1995;36:942. doi: 10.1136/gut.36.6.942-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fidler HM, Thurrell W, Johnson NM, Rook GA, McFadden JJ. Specific detection of Mycobacterium paratuberculosis DNA associated with granulomatous tissue in Crohn’s disease. Gut. 1994;35:506–510. doi: 10.1136/gut.35.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gan H, Ouyang Q, Bu H. [Mycobacterium paratuberculosis in the intestine of patients with Crohn’s disease] Zhonghua Neike Zazhi. 1997;36:228–230. [PubMed] [Google Scholar]
- 91.Ikonomopoulos JA, Gorgoulis VG, Kastrinakis NG, Zacharatos PV, Kokotas SN, Evangelou K, Kotsinas AG, Tsakris AG, Manolis EN, Kittas CN. Sensitive differential detection of genetically related mycobacterial pathogens in archival material. Am J Clin Pathol. 2000;114:940–950. doi: 10.1309/7ABR-E7MJ-18V9-CM4M. [DOI] [PubMed] [Google Scholar]
- 92.Lisby G, Andersen J, Engbaek K, Binder V. Mycobacterium paratuberculosis in intestinal tissue from patients with Crohn’s disease demonstrated by a nested primer polymerase chain reaction. Scand J Gastroenterol. 1994;29:923–929. doi: 10.3109/00365529409094864. [DOI] [PubMed] [Google Scholar]
- 93.Murray A, Oliaro J, Schlup MM, Chadwick VS. Mycobacterium paratuberculosis and inflammatory bowel disease: frequency distribution in serial colonoscopic biopsies using the polymerase chain reaction. Microbios. 1995;83:217–228. [PubMed] [Google Scholar]
- 94.Ryan P, Bennett MW, Aarons S, Lee G, Collins JK, O’Sullivan GC, O’Connell J, Shanahan F. PCR detection of Mycobacterium paratuberculosis in Crohn’s disease granulomas isolated by laser capture microdissection. Gut. 2002;51:665–670. doi: 10.1136/gut.51.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sanderson JD, Moss MT, Tizard ML, Hermon-Taylor J. Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Gut. 1992;33:890–896. doi: 10.1136/gut.33.7.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scanu AM, Bull TJ, Cannas S, Sanderson JD, Sechi LA, Dettori G, Zanetti S, Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis infection in cases of irritable bowel syndrome and comparison with Crohn’s disease and Johne’s disease: common neural and immune pathogenicities. J Clin Microbiol. 2007;45:3883–3890. doi: 10.1128/JCM.01371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tiveljung A, Söderholm JD, Olaison G, Jonasson J, Monstein HJ. Presence of eubacteria in biopsies from Crohn’s disease inflammatory lesions as determined by 16S rRNA gene-based PCR. J Med Microbiol. 1999;48:263–268. doi: 10.1099/00222615-48-3-263. [DOI] [PubMed] [Google Scholar]
- 98.Tuci A, Tonon F, Castellani L, Sartini A, Roda G, Marocchi M, Caponi A, Munarini A, Rosati G, Ugolini G, et al. Fecal detection of Mycobacterium avium paratuberculosis using the IS900 DNA sequence in Crohn’s disease and ulcerative colitis patients and healthy subjects. Dig Dis Sci. 2011;56:2957–2962. doi: 10.1007/s10620-011-1699-6. [DOI] [PubMed] [Google Scholar]
- 99.Al-Shamali M, Khan I, Al-Nakib B, Al-Hassan F, Mustafa AS. A multiplex polymerase chain reaction assay for the detection of Mycobacterium paratuberculosis DNA in Crohn’s disease tissue. Scand J Gastroenterol. 1997;32:819–823. doi: 10.3109/00365529708996540. [DOI] [PubMed] [Google Scholar]
- 100.Baksh FK, Finkelstein SD, Ariyanayagam-Baksh SM, Swalsky PA, Klein EC, Dunn JC. Absence of Mycobacterium avium subsp. paratuberculosis in the microdissected granulomas of Crohn’s disease. Mod Pathol. 2004;17:1289–1294. doi: 10.1038/modpathol.3800184. [DOI] [PubMed] [Google Scholar]
- 101.Bernstein CN, Nayar G, Hamel A, Blanchard JF. Study of animal-borne infections in the mucosas of patients with inflammatory bowel disease and population-based controls. J Clin Microbiol. 2003;41:4986–4990. doi: 10.1128/JCM.41.11.4986-4990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cellier C, De Beenhouwer H, Berger A, Penna C, Carbonnel F, Parc R, Cugnenc PH, Le Quintrec Y, Gendre JP, Barbier JP, et al. Mycobacterium paratuberculosis and Mycobacterium avium subsp. silvaticum DNA cannot be detected by PCR in Crohn’s disease tissue. Gastroenterol Clin Biol. 1998;22:675–678. [PubMed] [Google Scholar]
- 103.Chiba M, Fukushima T, Horie Y, Iizuka M, Masamune O. No Mycobacterium paratuberculosis detected in intestinal tissue, including Peyer’s patches and lymph follicles, of Crohn’s disease. J Gastroenterol. 1998;33:482–487. doi: 10.1007/s005350050119. [DOI] [PubMed] [Google Scholar]
- 104.Dumonceau JM, Van Gossum A, Adler M, Fonteyne PA, Van Vooren JP, Deviere J, Portaels F. No Mycobacterium paratuberculosis found in Crohn’s disease using polymerase chain reaction. Dig Dis Sci. 1996;41:421–426. doi: 10.1007/BF02093838. [DOI] [PubMed] [Google Scholar]
- 105.Dumonceau JM, Van Gossum A, Adler M, Van Vooren JP, Fonteyne PA, De Beenhouwer H, Portaels F. Detection of fastidious mycobacteria in human intestines by the polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 1997;16:358–363. doi: 10.1007/BF01726363. [DOI] [PubMed] [Google Scholar]
- 106.Ellingson JL, Cheville JC, Brees D, Miller JM, Cheville NF. Absence of Mycobacterium avium subspecies paratuberculosis components from Crohn’s disease intestinal biopsy tissues. Clin Med Res. 2003;1:217–226. doi: 10.3121/cmr.1.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gibson J, Riggio M, McCreary C, Lennon A, Toner M. Looking for Mycobacterium paratuberculosis DNA by polymerase chain reaction (PCR) in orofacial granulomatosis (OFG) and oral Crohn’s disease tissue in an Irish population. Ir Med J. 2000;93:218. [PubMed] [Google Scholar]
- 108.Kanazawa K, Haga Y, Funakoshi O, Nakajima H, Munakata A, Yoshida Y. Absence of Mycobacterium paratuberculosis DNA in intestinal tissues from Crohn’s disease by nested polymerase chain reaction. J Gastroenterol. 1999;34:200–206. doi: 10.1007/s005350050244. [DOI] [PubMed] [Google Scholar]
- 109.Lozano-Leon A, Barreiro-de Acosta M, Domínguez-Munoz JE. Absence of Mycobacterium avium subspecies paratuberculosis in Crohn’s disease patients. Inflamm Bowel Dis. 2006;12:1190–1192. doi: 10.1097/01.mib.0000236931.58793.22. [DOI] [PubMed] [Google Scholar]
- 110.Riggio MP, Gibson J, Lennon A, Wray D, MacDonald DG. Search for Mycobacterium paratuberculosis DNA in orofacial granulomatosis and oral Crohn’s disease tissue by polymerase chain reaction. Gut. 1997;41:646–650. doi: 10.1136/gut.41.5.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Suenaga K, Yokoyama Y, Okazaki K, Yamamoto Y. Mycobacteria in the intestine of Japanese patients with inflammatory bowel disease. Am J Gastroenterol. 1995;90:76–80. [PubMed] [Google Scholar]
- 112.Toracchio S, El-Zimaity HM, Urmacher C, Katz S, Graham DY. Mycobacterium avium subspecies paratuberculosis and Crohn’s disease granulomas. Scand J Gastroenterol. 2008;43:1108–1111. doi: 10.1080/00365520802116455. [DOI] [PubMed] [Google Scholar]
- 113.Tzen CY, Wu TY, Tzen CY. Detection of mycobacteria in Crohn‘s disease by a broad spectrum polymerase chain reaction. J Formos Med Assoc. 2006;105:290–298. doi: 10.1016/S0929-6646(09)60120-0. [DOI] [PubMed] [Google Scholar]
- 114.Wu SW, Pao CC, Chan J, Yen TS. Lack of mycobacterial DNA in Crohn’s disease tissue. Lancet. 1991;337:174–175. doi: 10.1016/0140-6736(91)90837-f. [DOI] [PubMed] [Google Scholar]


