Abstract
Capsule endoscopy is nowadays the diagnostic technique of choice in the study of small bowel pathologies, allowing the non-invasive study of the entire mucosa. This has led, together with new technical advances, to the creation of two new models (PillCam ESO and PillCam Colon) for the study of esophageal and colonic diseases. These two new capsules offer an interesting alternative to conventional endoscopy in the study of the upper and lower digestive tracts, because traditional endoscopy is often unpleasant and uncomfortable for the patient, can be painful, often requires moderate or deep sedation and is not without complications (hemorrhage, perforation, etc.). PillCam Colon is particularly important for its usefulness in the diagnosis of colonic polyps, and is a potentially useful tool in cases of incomplete colonoscopy or in colorectal cancer screening, even more when most patients are reluctant to undergo screening programs due to the said disadvantages of conventional colonoscopy. This article discusses the advantages of capsule endoscopy over conventional endoscopy, its current application possibilities and indications in routine clinical practice. In the various sections of the work, we assess the application of endoscopic capsule in different sections of the digestive tract (esophagus, stomach, and colon) and finally the potential role of panendoscopy with PillCam Colon.
Keywords: Conventional endoscopy, Capsule endoscopy, Esophageal capsule endoscopy, Colon capsule endoscopy, Panendoscopy
Core tip: Upper gastrointestinal endoscopy and colonoscopy are the techniques of choice for the study of the pathologies of the upper and lower digestive tracts. Despite their many advantages, these techniques can be unpleasant and uncomfortable for the patient and may even require sedation, with the potential disadvantages that might imply. In this scenario, capsule endoscopy (PillCam ESO for the study of the esophagus and stomach and PillCam Colon mainly for the study of colonic diseases) is an alternative to conventional endoscopy, as it has demonstrated its usefulness, an adequate diagnostic yield and good tolerance by patients.
INTRODUCTION
Upper gastrointestinal endoscopy (UGE) and colonoscopy represent the gold standard and the preferred endoscopic techniques for the study of diseases of the upper and lower digestive tracts. Their advantages and diagnostic and therapeutic yield have been clearly demonstrated. However, although minimally invasive, they can be unpleasant/painful for the patient, require sedation and are not free of complications; these are why they are not indicated for some patients in many diagnostic procedures. This is particularly important in colorectal cancer screening, where the rate of adherence to such programs is low, given the reluctance of patients to have colonoscopy performed.
In 2000, capsule endoscopy (CE), a new non-invasive method that allowed the complete and direct study of the small bowel (SB), was born[1]. This technique has revolutionized the diagnosis and therapeutic algorithm of intestinal pathologies, so that, today, it is considered the technique of choice to study the diseases of the small bowel[2-8].
Its development contributed later to the birth of other devices. Since October 2004 there has been a new capsule available, the esophageal capsule endoscope (PillCam ESO), capable of studying esophageal diseases in detail.
Years later, as the study of colonic diseases through colonoscopy can be unpleasant/painful for the patient, incomplete in 5%-20% of cases and not free of potential complications in up to 2% of procedures (due to perforations, hemorrhage, infections, vasovagal responses, etc.), a new device, the colonic capsule, was created in order to allow the study of colonic diseases by capsule endoscopy.
The different models of capsule endoscopy mentioned above and currently available on the market (SB capsule, esophageal capsule and colon capsule) are unique in being able to explore different areas of the gastrointestinal tract; they are direct, noninvasive, painless for patients and without need for sedation; they offer also a high diagnostic yield and high reliability, which make them a diagnostic method chosen by many patients refusing conventional endoscopy.
In this article we discuss whether the different models of capsule endoscopy can be an alternative to conventional endoscopy in patients who refuse the latter.
ESOPHAGEAL CAPSULE ENDOSCOPY
Initial studies of the esophagus performed with the SB capsule had a low diagnostic yield[9], with the exception of the study carried out by Ramirez et al[10] with a string-capsule using the PillCam SB, that achieved a high diagnostic yield (close to 100%), although it was not validated by subsequent studies.
For this reason, a new capsule, esophageal capsule endoscope (ECE), was specifically created to study this section of the digestive tract; it was named PillCam ESO, by Given Imaging, a company based in Yokneam, Israel; the dimensions of the capsule are 26 mm × 11 mm, with two lenses and a higher capacity to capture images. Due to the usefulness of the capsule, it was soon recommended to study chronic gastroesophageal reflux disease (GERD) [mainly for diagnosis purposes and for the management of Barrett’s esophagus (BE)] as well as for the screening of esophageal varices in portal hypertension (first using PillCam ESO1, that captured 2 images per second at each end, and later with the use of PillCam ESO2, capturing seven images per second).
Sanchez-Yague et al[11] showed that esophageal capsule could be useful in patients with suspected esophageal diseases who refused conventional endoscopy.
Most published series comparing PillCam ESO to UGE establish a high specificity and negative predictive value of the capsule for the screening of BE. However, the sensitivity was remarkably low, with high interobserver variability and low yield in short-segment BE, making it impossible to recommend this technique for these patients[12,13].
A subsequent meta-analysis of more than 600 patients with GERD concluded that ECE had a moderate sensitivity and specificity for the diagnosis of BE and that UGE should remain the gold standard technique in patients with BE[14].
Later, Chavalitdhamrong et al[15] showed, in a study including more than 500 patients, that esophageal capsule obtained high quality images from patients with symptoms of GERD in a non-invasive way, demonstrating that it could be an alternative screening test for the diagnosis of BE.
The main disadvantages of esophageal capsule in this subgroup of patients are the following: (1) difficulty to fully visualize the Z-line, partially improved with the patient in right lateral decubitus position; (2) inability to use local staining techniques, unlike those used with UGE; and (3) inability to take biopsies and therefore to know the degree of dysplasia associated with intestinal metaplasia.
In short, although patients mostly prefer the PillCam ESO to conventional endoscopy, larger studies are needed with larger number of patients (probably only possible through multicenter studies) to assess the actual role of ECE in patients with BE. Until then, UGE, preferably with magnification techniques and histological examination of the biopsies obtained, should be considered the gold standard technique for these patients[16].
With regard to the diagnostic yield for the screening of esophageal varices in patients with portal hypertension, the initial studies and meta-analysis of esophageal capsule proved its usefulness in the assessment of esophageal varices, in the detection of varices with significant size suggestive of primary prevention of varicose bleeding and in the diagnosis of portal hypertensive gastropathy[17-20].
Subsequently, Ishiguro et al[21] evaluated the role of esophageal capsule in the detection of varices, red spots and high risk varices in Japanese cirrhotic patients; the results showed that the capsule had a higher diagnostic yield than conventional endoscopy, indicating that the capsule is a useful technique for the screening and management of this population.
Table 1 shows the main studies published on the role of PillCam ESO in the screening/management of esophageal varices in cirrhotic patients, its sensitivity, specificity and safety in predicting patients requiring prophylactic treatment. Some representative images of our experience with PillCam ESO are shown in Figures 1 and 2.
Table 1.
Main studies published on PillCam Colon in esophageal varices
| Ref. | n | S | E | Accuracy of treatment1 |
| Eisen et al[17], 2006 | 32 | 100% | 89% | NA |
| de Franchis et al[18], 2008 | 288 | 88% | 84% | 91% |
| Lapalus et al[19], 2009 | 120 | 77% | 86% | 92% |
| Ishiguro et al[21], 2012 | 29 | 95% | 84% | 85% |
Accuracy for indicated prophylactic treatment.
Figure 1.
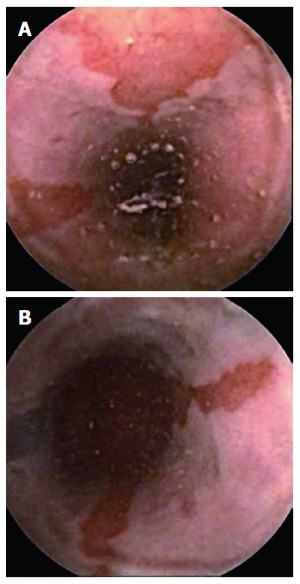
Suspected Barrett’s esophagus seen with PillCam ESO. A: Suspected long Barrett’s esophagus; B: Ectopic tissue mucosa ascending from Z line.
Figure 2.
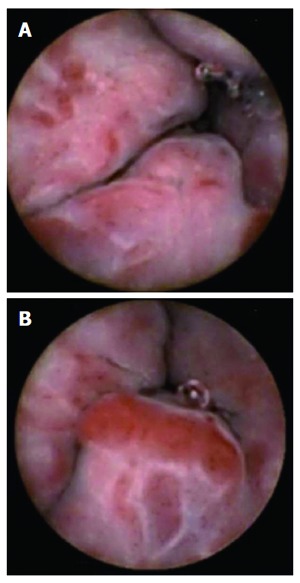
Esophageal varices seen with PillCam ESO. A: Large esophageal varices with red spots; B: Large esophageal varices in distal esophagus.
As a result of the publishing of the previously mentioned studies, nowadays, the only clearly accepted indication for esophageal capsule (until the appearance of new technological advances) is the screening and management of esophageal varices, given its role in patients with GERD being more controversial.
GASTRIC AND ESOPHAGEAL CAPSULE ENDOSCOPY
Regarding the gastric cavity, nowadays, there is no capsule specifically designed to study gastric diseases. While using a capsule at this level could help to obtain many images and to diagnose existing pathologies, the morphology of the gastric cavity as well as its high volume makes it impossible to be sure that all areas of the stomach would be visualized, especially the gastric fundus, and we could be leaving relevant pathologies undiagnosed. Thus, the exploration of the entire stomach using an EC is impossible with the currently available models.
However, if we were able to remotely control the endoscopic capsules available, we would be able to visualize the entire gastric mucosa. This control would allow us to take more pictures, from more angles and at different distances, improving the diagnostic yield of the EC in the stomach[22].
Initial experimental studies performed in animals have shown that the remote control of EC is a possibility that can be developed first in healthy volunteers and then in patients[23,24].
Thus, Swain et al[25] evaluated the effectiveness of remotely controlling a PillCam Colon modified with the inclusion of magnetic materials, in the esophagus and stomach of a healthy volunteer, by means of an external magnetic field repeatedly moved and rotated in different directions, observing that a larger number of images could be obtained. The authors demonstrated that an EC could be externally controlled with a magnetic field in a simple, safe and effective way, controlling its movements by viewing through a flexible gastroscope. There were no complications and the procedure was not painful or unpleasant for the patient.
Later, Rey et al[26] evaluated the diagnostic accuracy of a new magnetically driven EC (Olympus-Siemens) in healthy volunteers and patients with epigastric pain and/or symptoms of reflux. Low level magnetic fields were used to control a dual sensor capsule in the stomach of the patients included in the study with an air-water interface provided by the ingestion of 1300 cc of water one hour before the test time. In analyzing the results, they found that the gastric mucosa could be technically visualized in all cases except one (98%). In the remaining 52 patients, the antrum, body, fundus and cardia could be completely visualized in 98%, 96%, 73% and 75% of cases, respectively. There were no complications.
Similarly, Keller et al[27] studied the gastric mucosa by means of a PillCam Colon modified by the inclusion of magnetic material to allow for remote control in 10 healthy volunteers. The entire gastric mucosa could be observed in 75% of cases. As limitations to this experimental technique, it could be noticed that small amounts of fluid limited the visibility of small areas in the most apical parts of the fundus and gastric distension produced was not enough to evaluate all folds. Therefore, the visualization of the gastric mucosa in the patients included in the said study was good, although not complete in all of them.
Undoubtedly, these studies open the door to new technological developments that will likely succeed in the future to explore the whole of the gastric mucosa in an easy, safe and effective way[28].
Thus, new especially designed capsules could be created to study the esophagus, stomach and the first portions of the duodenum, having in this case a “new PillCam ESO” modified for remote operation.
Recently, two studies have proposed the use of the esophageal capsule for two pathologies previously considered to be “alien” in publications related with the PillCam ESO. Chandran et al[29] assessed its role in stratifying the risk of upper gastrointestinal bleeding, noticing that its major limitation was the low rate of duodenal visualization and the discord between the capsule and conventional endoscopy. However, when the PillCam ESO made proper assessments of the duodenum, the concordance between both tests was excellent. Moreover, Shah et al[30] concluded after studying a small group of patients who were to undergo bariatric surgery that there were no significant differences between the findings made by PillCam ESO and those observed by conventional endoscopy.
COLON CAPSULE ENDOSCOPY
PillCam COLON capsule endoscopy (CCE) (Given Imaging Ltd, Yoqneam, Israel) is a new capsule that has been designed to explore the colon. Two models have been developed: the first generation of CCE (CC1) is similar to the conventional capsule but has two cameras which are able to record video images from both ends. The device measures 31 by 11 mm and acquires images at a rate of 4 frames per second. The pre-programmed “sleep” mode allows recording of images from the esophagus and the stomach for 3 min and after the capsule switches to sleep mode for 1 h 45 min, so that it saves battery. During this period, the capsule is likely to transit most of the small bowel and reaches approximately the level of terminal ileum. Recording and downloading of data are similar to those for small-bowel capsule endoscopy[31].
Recently, a second-generation colon capsule (CC2) has been developed to improve the sensitivity for detection of colonic changes. The new PCC-2 is bigger (11.6 mm × 31.5 mm) and two new characteristics have been introduced: (1) the view angle from both the imagers has been widened to 172 degrees; and (2) in order to further enhance the colon coverage, the capsule is equipped with an adaptable image acquisition rate depending on the speed of progression of the capsule along the colon; CC-2 captures 35 frames per second while it is moving and 4 frames per second when it is stationary. Also, there is a new data recorder that guides the medical staff and the patient through the procedure. In fact, it buzzes and vibrates and displays instructions to alert the patient to continue the preparation according to the protocol previously explained to the patient. The new RAPID software develops a flexible spectral imaging color enhancement (FICE) technology to allow a more detailed analysis of the mucosal surface and also has a polyp size estimation tool.
Colorectal cancer (CRC) is the second most frequent cause of cancer-related death in Western countries. Nevertheless, no more than 25% of compliance has been achieved in screening programs, because of different problems but, without any doubt, because of people′s resistance to conventional colonoscopy[32]. The high-priority objective and indications for CCE are the “screening” of colorectal cancer in the risk population. In addition, it appears to be a promising new modality for colonic evaluation, not only adenomas, and it could be a good alternative in patients refusing conventional colonoscopy, to complete colon examination in patients with no conclusive incomplete colonoscopy, or when it is contraindicated[33].
In order to assess the sensitivity and specificity of CCE1 compared to colonoscopy in screening colorectal cancer, some studies were performed[34-36]. The most important study published about CCE is a prospective, multicenter study comparing capsule endoscopy with colonoscopy in the detection of colorectal polyps or cancer in a group of patients with known or suspected colonic diseases[34]. A total of 328 patients were included in the study. Sensitivity and specificity of CCE to detect polyps of 6 mm in size or larger were 64% and 84%, respectively. It is important to comment that of 19 cancers detected by colonoscopy, 14 were detected by capsule endoscopy. These results are similar to those of the two European studies published[35,36]. In these studies, the sensitivity was 69% and 76%, specificity was 81% and 64%, the positive predictive value (PPV) was 74% and 83% and the negative predictive value (NPV) was 78% and 54% for polyp detection. We recently published our results that are similar to the above mentioned, although specificity is lower[37]. Compared to colonoscopy, the rate of agreement was 75.6%, the sensitivity was 84%, the specificity was 62.5%, PPV was 77.7% and NPV was 71.4%. Two meta-analyses have been recently published and confirm that CCE is a reasonable method for screening asymptomatic individuals for colorectal polyps. It may be particularly useful for patients with “incomplete” colonoscopy, those with contraindications for conventional colonoscopy, and those unwilling to undergo colonoscopy because of its perceived inconvenience and discomfort[38,39].
To date, two studies have evaluated CCE-2. An Israeli multicenter trial was the first one[40]. In this study, CCE-2 was prospectively compared with conventional colonoscopy as the gold standard. Colonoscopy was independently performed after capsule ingestion. A total of 98 patients were enrolled. Patients were considered to have a significant finding when polyps at least 6 mm in size or masses were detected. Sensitivity for polyps at least 6 mm in size was 89%, and at least 10 mm in size was 88%, with specificities of 76% and 89%, respectively. Recently a European, prospective, multicenter trial including eight European sites was published by Spada et al[41]. A total of 109 patients were enrolled. Sensitivity for polyps at least 6 mm in size was 84%, and at least 10 mm in size was 88%, with specificities of 64% and 95%, respectively. CCE-2 correctly classified 35 and 28 of these patients, corresponding to a detection rate of 90% for neoplasia at least 6 mm in size, and 93% for adenomas at least 10 mm in size. Similarly to the Eliakim study, in this study the low specificity for polyps at least 6 mm in size was explained by a substantial rate of false-positive polyps because of size mismatch. It must be considered important that the CCE-2 is able to detect more small lesions than colonoscopy.
Tables 2 and 3 summarize the main studies on PillCam Colon 1 and PillCam Colon 2 in the detection of colonic polyps, respectively.
Table 2.
Results of main colon trials using PillCam Colon 1
| PillCam Colon C1 | Year | n | S | E | PPV | NPV |
| Results for polyps (any size) | ||||||
| Eliakim et al[35] | 2006 | 91 | 69% | 81% | 74% | 78% |
| Schoofs et al[36] | 2006 | 41 | 76% | 64% | 83% | 54% |
| Van Gossum et al[34] | 2009 | 328 | 64% | 84% | 60% | 86% |
| Results for significant polyps (> 6 mm or > 3 polyps > 3 mm) | ||||||
| Eliakim et al[35] | 2006 | 91 | 63% | 94% | 67% | 91% |
| Schoofs et al[36] | 2006 | 41 | 60% | 73% | 46% | 83% |
| Van Gossum et al[34] | 2009 | 328 | 64 | 84 | ||
PPV: Positive predictive value; NPV: Negative predictive value.
Table 3.
Results of main colon trials using PillCam Colon 2
Although the main objective of CCE must be colon cancer screening, CE should be considered a new technique able to detect colonic lesions in patients with special indications for colorectal cancer screening such as ulcerative colitis (UC) or patients who refuse conventional colonoscopy or with incomplete colonoscopy. CCE after incomplete colonoscopy appears to yield significant findings, guide further workup, and have high patient acceptance[42].
UC is a chronic inflammatory condition that causes continuous mucosal inflammation of the colon. Visualization of the mucosa affected in UC is essential for many aspects of disease management (including drug dosing and duration and the decision to deliver intravenous medication or undergo surgery) and can predict recurrence rates and complications related to UC according to the degree of mucosal healing[43]. Also, patients with UC have an elevated risk of developing colon cancer, which can be attributed to the length of time since disease onset and the extent of colon affected by UC[44]. As such, it is recommended in patients who have had UC for 8-10 years (or 15 years of disease in patients with left-sided colitis) that annual or biannual surveillance colonoscopy should be conducted[45,46]. Consequently, CCE could play a role in patients with UC. To date, a few preliminary studies have demonstrated the feasibility of CCE for the management of patients with known UC, suggesting that CCE may be useful to monitor inflammation and to screen for colorectal cancer in patients with UC[47,48]. Nevertheless, Meister et al[49 showed a significantly better assessment of disease activity by standard colonoscopy than by CCE. Furthermore, compared with colonoscopy, the extent of UC was underestimated when evaluated by CCE. In contract, Ye et al[48 reported a good correlation in the severity (k = 0.751) between CCE and colonoscopy but a moderate correlation in the extent of the inflammation, perhaps because it is not easy to determine the precise location of disease by CCE and the experience of the physician with CCE evaluation in this study was limited. We performed a pilot study using CCE-2 and observed that there was a good correlation between CCE and colonoscopy in assessing the disease severity and extent of inflammation[50].
Some representative images of our experience with PillCam colon are shown in Figures 3, 4, 5 and 6.
Figure 3.
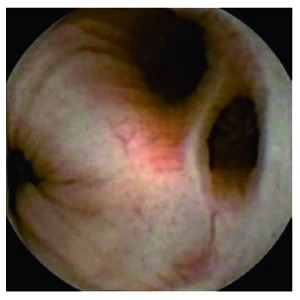
Diverticulosis coli.
Figure 4.
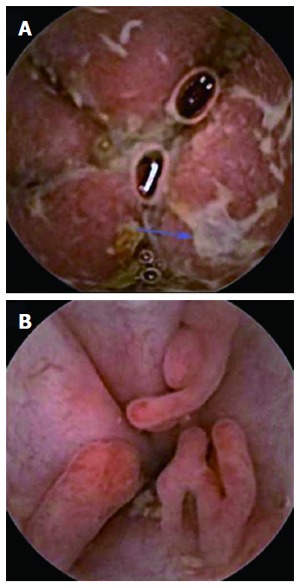
Severe (A) and pseudopolyps in inactive (B) ulcerative colitis.
Figure 5.
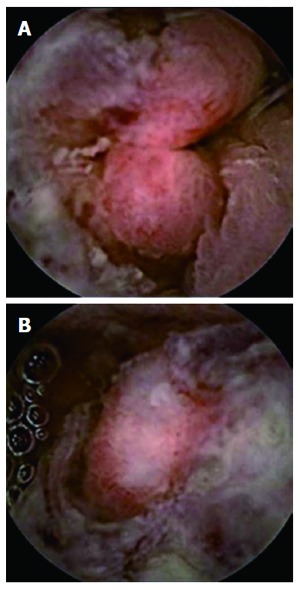
Colorectal cancer. A: Ulcerated sigmoid neoformation; B: Partially stenosing sigmoid neoformation.
Figure 6.
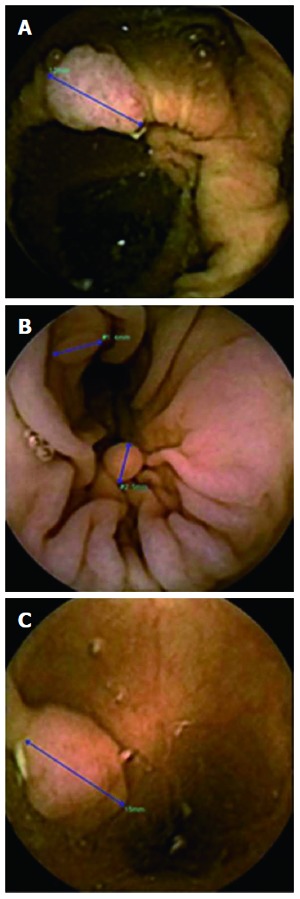
Polyps. PillCam Colon-2 using polyp size estimation tool. PillCam Colon-2 using polyp size estimation tool. A: Senile ascending colon polyp; B: Millimetric descending colon polyps; C: Semi-pediculated polyp greater than 1cm in the sigmoid.
On the other hand, CCE preparation must be exhaustive because all the faecal remains cannot be removed by CCE opposite to colonoscopy. Even small amounts of residual stool may prevent an accurate visualization of the colonic mucosa by CCE. The colon preparation for CCE has two aims: to provide a clean colon and clear images and to promote capsule propulsion first through the entire small bowel and then through the colon to the rectum. Preparation is really important because as Spada et al[51] have demonstrated when an adequate preparation is obtained, accuracy of CCE tends to be higher and comparable with that of colonoscopy.
To address the above issue, a specific preparation for CCE was designed using polyethylene glycol (PEG) and two boosters of sodium phosphate (NaP). The main role of the NaP booster is to accelerate CCE transit through the small and the large bowel so that the colonic mucosa could be seen before the end of the battery. This conventional preparation was first evaluated in two initial pilot studies. In a study by Eliakim et al[35], the overall cleanliness of the colon was rated as excellent or good in 84.4% of the cases. In the second pilot study the results are better; an excellent or good preparation was achieved in 90% of the cases[36]. In a recent study published by our group with the same preparation the grade of cleanliness was good or excellent in 65.6%[37].
In recent times, for CCE-2, some changes of the regimen of CCE have been proposed. Low doses of NaP are now included in the regimen of preparation for CCE-2 to reduce the risk of adverse events (one booster of 30 mL NaP with 1 liter of water when the capsule has entered the small bowel, and a second booster of 15-25 mL NaP with 0.5 liter of water 3 h later if the capsule has not been excreted) or instead of this booster PEG booster has been proposed. Also the volume of PEG has been reduced[52].
PEG solutions are safe and effective, but require consumption of large volumes of fluid, generally 4 liters. The 2 L PEG solution plus ascorbic acid (PEG + Asc) is also effective and safe, and the volume is reduced. Some studies have studied these points. In a study by Ell et al[53], it is concluded that the PEG + Asc bowel preparation reduces the volume patients have to drink, so it was more acceptable to patients, and should, therefore, improve effectiveness in routine practice. In another study PEG + Asc provided effective bowel cleansing, which was equivalent to that of sodium picosulphate + magnesium citrate in terms of grading cleansing as overall success or failure[54]. Nevertheless, it is important to consider the split dose. In this sense the cleansing results are worse if patients receive the full dose PEG + Asc the evening before the procedure compared to the split dose[55]. A colon cleansing procedure using PEG + ascorbic acid for CCE yielded an adequate cleansing level in > 80% of patients, and good accuracy for detecting polyps[56]. This procedure may be considered as an alternative, particularly for patients in whom sodium phosphate-based preparations are contraindicated. Based on these data, we have performed a study that demonstrated the efficacy of 2 L PEG[57]. The main aim was to compare the level of cleansing with two different regimens. It was a prospective and blinded study. In the first group (A) patients were prepared with 2 L of PEG plus ascorbic acid and in the second group (B) 4 L of PEG was used. In group A, “excellent and good” preparation was more frequent than in group B, and also in the cecum, right colon and transverse colon, although there was no significant difference. We can conclude with these preliminary results that PEG 2 L could be better than PEG 4 L in the colonic preparation for patients that will undergo capsule colonoscopy, although more studies must be conducted with more patients.
Table 4 shows the main bowel preparation regimens used nowadays.
Table 4.
Bowel preparation schedule
| Schedule | Intake | |
| Day-2 | Sennosides 80-160 mg | |
| Day-1 | All day | Clear liquid diet |
| Evening | 2 L PEG | |
| or | ||
| 1 L PEG + ascorbic acid | ||
| Exam Day | Morning | 2 L PEG |
| or | ||
| 1 L PEG + ascorbic acid | ||
| Approximately 10 am | Capsule Ingestion | |
| 1st Boost | 30 mL NaP and 1 L water | |
| Small bowel detection | or | |
| 0.5 L PEG | ||
| 2nd boost | 15 mL NaP and 0.5 L water | |
| 3 h after 1st boost | or | |
| 0.5 L PEG | ||
| Suppository | 10 mg bisacodyl | |
| 2 h after 2nd boost | ||
In conclusion, although many studies must be done to develop and improve the sensitivity and specificity of CCE, it is a new endoscopic tool that can be used for screening colon cancer in patients who refuse conventional colonoscopy, or in cases where it is contraindicated or it has been incomplete.
PANENDOSCOPY
As shown above, various studies published on PillCam Colon have demonstrated its usefulness in the detection of colonic polyps, with better sensitivity and specificity levels, as well as better positive and negative predictive values directly related to the increase in the learning curve and with the latest colonic capsule prototype, the PillCam Colon 2.
Moreover, by analyzing the different colonic studies we have been able to realize that thanks to the PillCam Colon ability to produce images of other extracolonic locations, it was able to identify lesions and pathologies at these locations.
By panendoscopy we mean the ability to obtain images of the digestive tract from the esophagus to the hemorrhoidal plexus without interruptions and, therefore, to explore the entire gastrointestinal tract wirelessly and noninvasively.
Our group has conducted a preliminary descriptive study (not yet published) in which, in view of the results obtained and acting cautiously given the absence of previously published data, PillCam Colon allows recording the entire digestive tract in most patients, making it possible to find relevant pathologies in other sections of the digestive tract, especially in the small bowel, although technical and procedural improvements are necessary to achieve the correct visualization of the stomach and esophagus (Figure 7).
Figure 7.
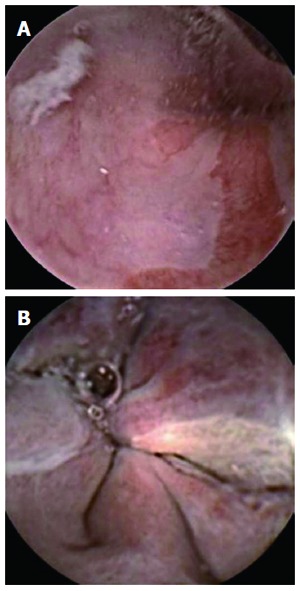
Suspected Barrett’s esophagus (A) and esophageal varices seen (B) with PillCam Colon.
Thus, in patients with indications for colonoscopy for suspected colonic disease who refuse to undergo this technique, the PillCam Colon helps explore not only this intestinal segment but also the whole digestive tract through panendoscopy.
CONCLUSION
In patients refusing conventional endoscopy, capsule endoscopy allows the direct and non-invasive study of the upper and lower digestive tracts. Unlike colonic segments, where PillCam Colon allows visualizing the mucosa with a high diagnostic yield, in esophageal and gastric sections (with the exception of the screening of esophageal varices) a larger number of studies and improvements in the procedures with capsule endoscopy are needed in order to achieve comparable efficacy to conventional UGE.
Footnotes
P- Reviewers: Kato M, Richardson WS, Skrypnyk IN S- Editor: Wen LL L- Editor: Wang TQ E- Editor: Zhang DN
References
- 1.Iddan G, Meron G, Glukhovsky A, Swain P. Wireless capsule endoscopy. Nature. 2000;405:417. doi: 10.1038/35013140. [DOI] [PubMed] [Google Scholar]
- 2.Pennazio M, Santucci R, Rondonotti E, Abbiati C, Beccari G, Rossini FP, De Franchis R. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology. 2004;126:643–653. doi: 10.1053/j.gastro.2003.11.057. [DOI] [PubMed] [Google Scholar]
- 3.Teshima CW, Kuipers EJ, van Zanten SV, Mensink PB. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol. 2011;26:796–801. doi: 10.1111/j.1440-1746.2010.06530.x. [DOI] [PubMed] [Google Scholar]
- 4.Herrerías JM, Caunedo A, Rodríguez-Téllez M, Pellicer F, Herrerías JM. Capsule endoscopy in patients with suspected Crohn’s disease and negative endoscopy. Endoscopy. 2003;35:564–568. doi: 10.1055/s-2003-40241. [DOI] [PubMed] [Google Scholar]
- 5.Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–1248; quiz 1249. doi: 10.1038/ajg.2009.713. [DOI] [PubMed] [Google Scholar]
- 6.Ohmiya N, Nakamura M, Takenaka H, Morishima K, Yamamura T, Ishihara M, Miyahara R, Kawashima H, Itoh A, Hirooka Y, et al. Management of small-bowel polyps in Peutz-Jeghers syndrome by using enteroclysis, double-balloon enteroscopy, and videocapsule endoscopy. Gastrointest Endosc. 2010;72:1209–1216. doi: 10.1016/j.gie.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Caunedo-Alvarez A, Gómez-Rodríguez BJ, Romero-Vázquez J, Argüelles-Arias F, Romero-Castro R, García-Montes JM, Pellicer-Bautista FJ, Herrerías-Gutiérrez JM. Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAID) use as assessed by capsule endoscopy. Rev Esp Enferm Dig. 2010;102:80–85. doi: 10.4321/s1130-01082010000200002. [DOI] [PubMed] [Google Scholar]
- 8.Rondonotti E, Spada C, Cave D, Pennazio M, Riccioni ME, De Vitis I, Schneider D, Sprujevnik T, Villa F, Langelier J, et al. Video capsule enteroscopy in the diagnosis of celiac disease: a multicenter study. Am J Gastroenterol. 2007;102:1624–1631. doi: 10.1111/j.1572-0241.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 9.Neu B, Wettschureck E, Rösch T. Is esophageal capsule endoscopy feasible? Results of a pilot. Endoscopy. 2003;35:957–961. doi: 10.1055/s-2003-43488. [DOI] [PubMed] [Google Scholar]
- 10.Ramirez FC, Shaukat MS, Young MA, Johnson DA, Akins R. Feasibility and safety of string, wireless capsule endoscopy in the diagnosis of Barrett’s esophagus. Gastrointest Endosc. 2005;61:741–746. doi: 10.1016/s0016-5107(05)00322-6. [DOI] [PubMed] [Google Scholar]
- 11.Sánchez-Yagüe A, Caunedo-Alvarez A, García-Montes JM, Romero-Vázquez J, Pellicer-Bautista FJ, Herrerías-Gutiérrez JM. Esophageal capsule endoscopy in patients refusing conventional endoscopy for the study of suspected esophageal pathology. Eur J Gastroenterol Hepatol. 2006;18:977–983. doi: 10.1097/01.meg.0000230094.21911.f8. [DOI] [PubMed] [Google Scholar]
- 12.Sharma P, Wani S, Rastogi A, Bansal A, Higbee A, Mathur S, Esquivel R, Camargo L, Sampliner RE. The diagnostic accuracy of esophageal capsule endoscopy in patients with gastroesophageal reflux disease and Barrett’s esophagus: a blinded, prospective study. Am J Gastroenterol. 2008;103:525–532. doi: 10.1111/j.1572-0241.2007.01233.x. [DOI] [PubMed] [Google Scholar]
- 13.Galmiche JP, Sacher-Huvelin S, Coron E, Cholet F, Soussan EB, Sébille V, Filoche B, d’Abrigeon G, Antonietti M, Robaszkiewicz M, et al. Screening for esophagitis and Barrett’s esophagus with wireless esophageal capsule endoscopy: a multicenter prospective trial in patients with reflux symptoms. Am J Gastroenterol. 2008;103:538–545. doi: 10.1111/j.1572-0241.2007.01731.x. [DOI] [PubMed] [Google Scholar]
- 14.Bhardwaj A, Hollenbeak CS, Pooran N, Mathew A. A meta-analysis of the diagnostic accuracy of esophageal capsule endoscopy for Barrett’s esophagus in patients with gastroesophageal reflux disease. Am J Gastroenterol. 2009;104:1533–1539. doi: 10.1038/ajg.2009.86. [DOI] [PubMed] [Google Scholar]
- 15.Chavalitdhamrong D, Chen GC, Roth BE, Goltzer O, Sul J, Jutabha R. Esophageal capsule endoscopy for evaluation of patients with chronic gastroesophageal reflux symptoms: findings and its image quality. Dis Esophagus. 2011:Epub ahead of print. doi: 10.1111/j.1442-2050.2010.01136.x. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Vázquez J, Jiménez-García VA, Herrerías-Gutiérrez JM. Esophageal capsule endoscopy and Barrett’s esophagus: where are we in 2013? Rev Gastroenterol Mex. 2013;78:55–56. doi: 10.1016/j.rgmx.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Eisen GM, Eliakim R, Zaman A, Schwartz J, Faigel D, Rondonotti E, Villa F, Weizman E, Yassin K, deFranchis R. The accuracy of PillCam ESO capsule endoscopy versus conventional upper endoscopy for the diagnosis of esophageal varices: a prospective three-center pilot study. Endoscopy. 2006;38:31–35. doi: 10.1055/s-2005-921189. [DOI] [PubMed] [Google Scholar]
- 18.de Franchis R, Eisen GM, Laine L, Fernandez-Urien I, Herrerias JM, Brown RD, Fisher L, Vargas HE, Vargo J, Thompson J, et al. Esophageal capsule endoscopy for screening and surveillance of esophageal varices in patients with portal hypertension. Hepatology. 2008;47:1595–1603. doi: 10.1002/hep.22227. [DOI] [PubMed] [Google Scholar]
- 19.Lapalus MG, Ben Soussan E, Gaudric M, Saurin JC, D’Halluin PN, Favre O, Filoche B, Cholet F, de Leusse A, Antonietti M, et al. Esophageal capsule endoscopy vs. EGD for the evaluation of portal hypertension: a French prospective multicenter comparative study. Am J Gastroenterol. 2009;104:1112–1118. doi: 10.1038/ajg.2009.66. [DOI] [PubMed] [Google Scholar]
- 20.Lu Y, Gao R, Liao Z, Hu LH, Li ZS. Meta-analysis of capsule endoscopy in patients diagnosed or suspected with esophageal varices. World J Gastroenterol. 2009;15:1254–1258. doi: 10.3748/wjg.15.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiguro H, Saito S, Imazu H, Aihara H, Kato T, Tajiri H. Esophageal Capsule Endoscopy for Screening Esophageal Varices among Japanese Patients with Liver Cirrhosis. Gastroenterol Res Pract. 2012;2012:946169. doi: 10.1155/2012/946169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swain P. The future of wireless capsule endoscopy. World J Gastroenterol. 2008;14:4142–4145. doi: 10.3748/wjg.14.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ciuti G, Donlin R, Valdastri P, Arezzo A, Menciassi A, Morino M, Dario P. Robotic versus manual control in magnetic steering of an endoscopic capsule. Endoscopy. 2010;42:148–152. doi: 10.1055/s-0029-1243808. [DOI] [PubMed] [Google Scholar]
- 24.Menciassi A, Valdastri P, Quaglia C, Buselli E, Dario P. Wireless steering mechanism with magnetic actuation for an endoscopic capsule. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:1204–1207. doi: 10.1109/IEMBS.2009.5332426. [DOI] [PubMed] [Google Scholar]
- 25.Swain P, Toor A, Volke F, Keller J, Gerber J, Rabinovitz E, Rothstein RI. Remote magnetic manipulation of a wireless capsule endoscope in the esophagus and stomach of humans (with videos) Gastrointest Endosc. 2010;71:1290–1293. doi: 10.1016/j.gie.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 26.Rey JF, Ogata H, Hosoe N, Ohtsuka K, Ogata N, Ikeda K, Aihara H, Pangtay I, Hibi T, Kudo S, et al. Feasibility of stomach exploration with a guided capsule endoscope. Endoscopy. 2010;42:541–545. doi: 10.1055/s-0030-1255521. [DOI] [PubMed] [Google Scholar]
- 27.Keller J, Fibbe C, Volke F, Gerber J, Mosse AC, Reimann-Zawadzki M, Rabinovitz E, Layer P, Schmitt D, Andresen V, et al. Inspection of the human stomach using remote-controlled capsule endoscopy: a feasibility study in healthy volunteers (with videos) Gastrointest Endosc. 2011;73:22–28. doi: 10.1016/j.gie.2010.08.053. [DOI] [PubMed] [Google Scholar]
- 28.Keller J, Fibbe C, Rosien U, Layer P. Recent advances in capsule endoscopy: development of maneuverable capsules. Expert Rev Gastroenterol Hepatol. 2012;6:561–566. doi: 10.1586/egh.12.26. [DOI] [PubMed] [Google Scholar]
- 29.Chandran S, Testro A, Urquhart P, La Nauze R, Ong S, Shelton E, Philpott H, Sood S, Vaughan R, Kemp W, et al. Risk stratification of upper GI bleeding with an esophageal capsule. Gastrointest Endosc. 2013;77:891–898. doi: 10.1016/j.gie.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Shah A, Boettcher E, Fahmy M, Savides T, Horgan S, Jacobsen GR, Sandler BJ, Sedrak M, Kalmaz D. Screening pre-bariatric surgery patients for esophageal disease with esophageal capsule endoscopy. World J Gastroenterol. 2013;19:6188–6192. doi: 10.3748/wjg.v19.i37.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez-Urien I, Carretero C, Borda A, Muñoz-Navas M. Colon capsule endoscopy. World J Gastroenterol. 2008;14:5265–5268. doi: 10.3748/wjg.14.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 33.Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez A, Quintero E. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534–540.e1. doi: 10.1016/j.cgh.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Van Gossum A, Munoz-Navas M, Fernandez-Urien I, Carretero C, Gay G, Delvaux M, Lapalus MG, Ponchon T, Neuhaus H, Philipper M, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264–270. doi: 10.1056/NEJMoa0806347. [DOI] [PubMed] [Google Scholar]
- 35.Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 36.Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971–977. doi: 10.1055/s-2006-944835. [DOI] [PubMed] [Google Scholar]
- 37.Herrerías-Gutiérrez JM, Argüelles-Arias F, Caunedo-Álvarez A, San-Juan-Acosta M, Romero-Vázquez J, García-Montes JM, Pellicer-Bautista F. PillCamColon Capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy. Rev Esp Enferm Dig. 2011;103:69–75. doi: 10.4321/s1130-01082011000200004. [DOI] [PubMed] [Google Scholar]
- 38.Rokkas T, Papaxoinis K, Triantafyllou K, Ladas SD. A meta-analysis evaluating the accuracy of colon capsule endoscopy in detecting colon polyps. Gastrointest Endosc. 2010;71:792–798. doi: 10.1016/j.gie.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 39.Spada C, Hassan C, Marmo R, Petruzziello L, Riccioni ME, Zullo A, Cesaro P, Pilz J, Costamagna G. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps. Clin Gastroenterol Hepatol. 2010;8:516–522. doi: 10.1016/j.cgh.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Eliakim R, Yassin K, Niv Y, Metzger Y, Lachter J, Gal E, Sapoznikov B, Konikoff F, Leichtmann G, Fireman Z, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026–1031. doi: 10.1055/s-0029-1215360. [DOI] [PubMed] [Google Scholar]
- 41.Spada C, Hassan C, Munoz-Navas M, Neuhaus H, Deviere J, Fockens P, Coron E, Gay G, Toth E, Riccioni ME, et al. Second-generation colon capsule endoscopy compared with colonoscopy. Gastrointest Endosc. 2011;74:581–589.e1. doi: 10.1016/j.gie.2011.03.1125. [DOI] [PubMed] [Google Scholar]
- 42.Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc. 2014;79:307–316. doi: 10.1016/j.gie.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 43.Lichtenstein GR, Rutgeerts P. Importance of mucosal healing in ulcerative colitis. Inflamm Bowel Dis. 2010;16:338–346. doi: 10.1002/ibd.20997. [DOI] [PubMed] [Google Scholar]
- 44.Eaden JA, Mayberry JF. Colorectal cancer complicating ulcerative colitis: a review. Am J Gastroenterol. 2000;95:2710–2719. doi: 10.1111/j.1572-0241.2000.02297.x. [DOI] [PubMed] [Google Scholar]
- 45.Biancone L, Michetti P, Travis S, Escher JC, Moser G, Forbes A, Hoffmann JC, Dignass A, Gionchetti P, Jantschek G, et al. European evidence-based Consensus on the management of ulcerative colitis: Special situations. J Crohns Colitis. 2008;2:63–92. doi: 10.1016/j.crohns.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, Eaden JA, Rutter MD, Atkin WP, Saunders BP, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 47.Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44:754–758. doi: 10.1055/s-0032-1309819. [DOI] [PubMed] [Google Scholar]
- 48.Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117–124. doi: 10.1111/1751-2980.12005. [DOI] [PubMed] [Google Scholar]
- 49.Meister T, Heinzow HS, Domagk D, Dortgolz A, Lenze F, Ross M, Domschke W, Lügering A. Colon capsule endoscopy versus standard colonoscopy in assessing disease activity of ulcerative colitis: a prospective trial. Tech Coloproctol. 2013;17:641–646. doi: 10.1007/s10151-012-0965-8. [DOI] [PubMed] [Google Scholar]
- 50.San Juan-Acosta M, Caunedo-Álvarez A, Argüelles Arias F, Castro-Laria L, Gómez-Rodríguez BJ, Romero-Vázquez J, Belda-Cuesta A, Pellicer-Bautista F, Herrerías-Gutiérrez JM. Colon Capsule Endoscopy is a Safe and Useful Tool to Assess Disease Parameters in Patients with Ulcerative Colitis. Eur J Gastroenterol Hepatol. 2014:In press. doi: 10.1097/MEG.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 51.Spada C, Riccioni ME, Hassan C, Petruzziello L, Cesaro P, De Vincentis F, Costamagna G. PillCam Colon Capsule Endoscopy (PCCE): The Quality of Preparation Makes the Difference! Gastrointest Endosc. 2010;5:AB203–AB204. [Google Scholar]
- 52.Spada C, Hassan C, Ingrosso M, Repici A, Riccioni ME, Pennazio M, Pirozzi GA, Pagano N, Cesaro P, Spera G, et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: a pilot study. Dig Liver Dis. 2011;43:300–304. doi: 10.1016/j.dld.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 53.Ell C, Fischbach W, Bronisch HJ, Dertinger S, Layer P, Rünzi M, Schneider T, Kachel G, Grüger J, Köllinger M, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol. 2008;103:883–893. doi: 10.1111/j.1572-0241.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 54.Worthington J, Thyssen M, Chapman G, Chapman R, Geraint M. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin. 2008;24:481–488. doi: 10.1185/030079908x260844. [DOI] [PubMed] [Google Scholar]
- 55.Corporaal S, Kleibeuker JH, Koornstra JJ. Low-volume PEG plus ascorbic acid versus high-volume PEG as bowel preparation for colonoscopy. Scand J Gastroenterol. 2010;45:1380–1386. doi: 10.3109/00365521003734158. [DOI] [PubMed] [Google Scholar]
- 56.Hartmann D, Keuchel M, Philipper M, Gralnek IM, Jakobs R, Hagenmüller F, Neuhaus H, Riemann JF. A pilot study evaluating a new low-volume colon cleansing procedure for capsule colonoscopy. Endoscopy. 2012;44:482–486. doi: 10.1055/s-0031-1291611. [DOI] [PubMed] [Google Scholar]
- 57.Argüelles-Arias F, San Juan-Acosta M, Belda-Cuesta A, Caunedo-Álvarez A, Romero-Vázquez J, Herrerías-Gutiérrez JM. A prospective study comparative of two regimens of preparation: ascorbic acid PEG vs PEG with PillCam colon capsule endoscopy (C2) Eur J Gastroenterol Hepatol. 2014:In press. [Google Scholar]


