Abstract
AIM: To explore the protective effect of bone marrow mesenchymal stem cells (BM MSCs) in the small intestinal mucosal barrier following heterotopic intestinal transplantation (HIT) in a rat model.
METHODS: BM MSCs were isolated from male Lewis rats by density gradient centrifugation, cultured, and analyzed by flow cytometry. The HIT models were divided into a non-rejection group, saline-treated rejection group (via penile vein), and BM MSC–treated group (via penile vein). Intestinal mucosal barrier injury was estimated by diamine oxidase (DAO) and D-lactic acid (D-LA) expression levels. Tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-10 (IL-10), and transforming growth factor-β (TGF-β) were detected by enzyme-linked immunosorbent assay. Ultrastructural change of tight junctions (TJs) was observed under transmission electron microscope. Expression levels of the TJ proteins occludin and zona occludens (ZO)-1, affected by the inflammatory factors, were measured using real-time polymerase chain reaction and Western blotting.
RESULTS: The pathological score at each time point after surgery indicated significantly less serious injury in the BM MSCs-treated group than in the rejection group (P < 0.05). In the former, graft levels of DAO and D-LA were reduced, and TNF-α and INF-γ production was inhibited (at day 7: 10.6473 ± 0.0710 vs 17.2128 ± 0.4991, P < 0.05; 545.1506 ± 31.9416 vs 810.2637 ± 25.1175, P < 0.05). IL-10 and TGF-β production was increased greatly (at day 7: 125.7773 ± 4.7719 vs 80.3756 ± 2.5866, P < 0.05; 234.5273 ± 9.3980 vs 545.1506 ± 31.9416, P < 0.05). There was increased expression of occludin and ZO-1 protein (at day 7: 0.2674 ± 0.0128 vs 0.1352 ± 0.0142, P < 0.05; at day 5: 0.7189 ± 0.0289 vs 0.4556 ± 0.0242, P < 0.05) and mRNA (at day 7: 0.3860 ± 0.0254 vs 0.1673 ± 0.0369, P < 0.05; at day 5: 0.5727 ± 0.0419 vs 0.3598 ± 0.0242, P < 0.05).
CONCLUSION: BM MSCs can improve intestinal barrier permeability, repair TJs, and increase occludin and ZO-1 protein expression. With altered cytokine levels, they can protect the intestinal mucosa after transplantation.
Keywords: Bone marrow mesenchymal stem cells, Small intestinal transplantation, Intestinal mucosal barrier, Occludin, Zona occludens-1
Core tip: Rejection and sepsis after small intestinal transplantation (SITx) is a serious and common complication. The small intestinal mucosal barrier plays an important role in the progression of postoperative complications. This study demonstrated that in rats, implantation of recipient-derived bone marrow mesenchymal stem cells decreased intestinal permeability and preserved intestinal mucosal barrier function after SITx via a mechanism linked to the balance between graft inflammatory cytokine levels and increased expression of the intestinal tight junction proteins occludin and zona occludens-1.
INTRODUCTION
Small intestinal transplantation (SITx) has become the definitive treatment for patients with end-stage intestinal failure who cannot tolerate parenteral nutrition[1]. However, SITx is difficult due to the strong expression of histocompatibility antigens, large numbers of resident leukocytes, and micro-organism colonization. Rejection and sepsis following SITx is a serious and common complication that affects both patient and graft survival[2].
Bone marrow mesenchymal stem cells (BM MSCs) are pluripotent adult stem cells. BM MSCs give rise to mesoderm cells[3,4] and differentiate into osteoblasts, chondrocytes, adipocytes, myocytes, and liver and neural cells[5-7], which have potential for use in treating various diseases. Allogeneic MSCs that were transplanted into primates via an intravenous route and distributed to the gastrointestinal tract proliferated[8]. Due to the secretion of several growth factors, BM MSCs also exhibit immunomodulatory capabilities[9-13]. BM MSCs reduce intestinal ischemia-reperfusion (I/R) injury in rats[14,15] and contribute to significant prolongation of composite tissue allotransplant survival[16] and promotion of graft revascularization[17].
The intestinal mucosa is the physical, chemical, immunological, and biological barrier against toxins and pathogens in the gut lumen. The intestinal mucosal barrier is composed of mucosal fluid, microvilli, epithelial mucosal cell tight junctions (TJs), and other special structures. TJs are the most important structures in the intestinal mucosal barrier. They are composed of multiple proteins, including transmembrane proteins such as occludin, tricellulin, claudins, and junctional adhesion molecule (JAM). The intracellular portions of these transmembrane proteins interact with cytoplasmic peripheral membrane proteins, including zona occludens (ZO)-1, -2, and -3[18-20], and two distinct transmembrane proteins: occludin and claudin[21,22], which are linked to the actin-based cytoskeleton[23]. TJs function as occlusion barriers by maintaining cellular polarity and homeostasis and by regulating paracellular space permeability in the epithelium[24]. Occludin and ZO-1 proteins play crucial roles in TJ assembly and maintenance[25-27]. In this study, we established a heterotopic intestinal transplantation (HIT) model in rats to explore the protective effect of BM MSC transplantation in the small intestinal mucosal barrier and the possible mechanisms thereof.
MATERIALS AND METHODS
Animals and rat HIT models
Seventy-six inbred specific pathogen-free Brown Norway (BN) and 30 Lewis (LEW) male rats weighing 200-220 g were used as donors in the homologous and isologous experimental groups, respectively. One hundred and six LEW male rats weighing approximately 220-250 g were used as recipients. Additionally, 25 male LEW rats weighing 100-120 g were used for BM MSC extraction. All animals were purchased from the Chinese Academy of Military Medical Sciences (Beijing, China). The use of animals and animal experimental procedures in this study was approved by the Ethics Committee of the Chinese Academy of Military Medical Sciences.
All rats were fasted for 12 h with free access to water before surgery and randomly assigned to a non-rejection group (A, LEW-LEW, n = 30); saline-treated rejection group (B, BN-LEW, via penile vein, n = 43); and BM MSC-treated group (C, BN-LEW, via penile vein, n = 33). The operations were performed using standard sterile technique under general anesthesia with 5% chloral hydrate (50 mL/kg).
Donor operation
The small intestine was visualized via a pubis-xiphoid midline incision. The small intestine was retracted to the left and packed in saline-moistened gauze. All tributary branches of the mesenteric artery and vein were visualized, ligated with 5-0 silk, and divided. The small intestine itself was cut just distal to the duodenum and proximal from the cecum. The mesenteric artery was freed from the surrounding tissue by dissecting the colon. The tissue connecting the small intestine to the colon was dissected. Lactated Ringer’s solution (5 mL) containing 125 U heparin was then administered systemically to the donor animal before the graft was removed from the donor and placed in cold lactated Ringer’s solution (0-4 °C) after being flushed via the mesenteric artery with the same solution. Grafts were perfused with cold saline at low pressure.
Recipient operation
The abdomen was opened, and the small intestine of the recipient packed in saline-moistened gauze inside the abdominal cavity, and retracted to the left. The aorta was freed distal to the left renal artery. An oval aperture was cut in the occluded part of the aorta, and an end-to-side anastomosis with the graft aorta attached to the mesenteric artery was created. In the same fashion, an anastomosis was created between the recipient portal vein and the donor postcaval vein. For both anastomoses, a continuous 10-0 silk suture was done under × 20 magnification. The proximal end of the donor small bowel was anastomosed to the stoma on the abdominal wall. Saline (1 mL, 0.9%) or culture media (1 mL) containing 5 × 106 BM MSCs was injected via the penile vein. The abdomen was closed and the animals were allowed to recover with free access to tap water and standard pellet rat chow. Only for surviving animals, HIT was included in the analysis. After transplantation, all animals were euthanized at 1, 3, 5, 7 and 10 d. The graft was removed from each animal and stored at -80 °C until analysis. Intestine samples were fixed for histopathological analysis and transmission electron microscopy.
Isolation and characterization of BM MSCs
BM MSCs were isolated from the femur and tibia of male LEW rats (100-120 g). Red blood cells were lysed using 0.1 mol/L NH4Cl; the remaining cells were washed, resuspended, and cultured for 4 wk in DMEM/F12 (Gibco, Carlsbad, CA, United States) containing 100 U/mL penicillin, 100 mg/mL streptomycin, and 15% fetal bovine serum. BM MSCs were cultured in an incubator at 37 °C in 5% CO2 with saturated humidity. The medium was changed every 72 h. When the third-passage cells reached 80% confluence, cells were trypsinized, washed, centrifuged, and resuspended at 1 × 107/mL in phosphate-buffered saline.
BM MSCs were stained using antibodies against CD29, CD90, RT1A, CD45, RT1B (BioLegend, San Diego, CA, United States), and CD34 (Santa Cruz Biotechnology, Santa Cruz, CA, United States), and analyzed by flow cytometry (FACSCalibur; BD Biosciences, Alaska, MN, United States). The proportion of CD29-, CD90-, and RT1A-positive cells and CD34-, CD45-, and RT1B-negative cells was > 98%. BM MSCs were also confirmed as spindle-shaped, plastic-adherent cells under standard culture conditions by microscopy. The purity of BM MSCs was > 95% (Figure 1).
Figure 1.
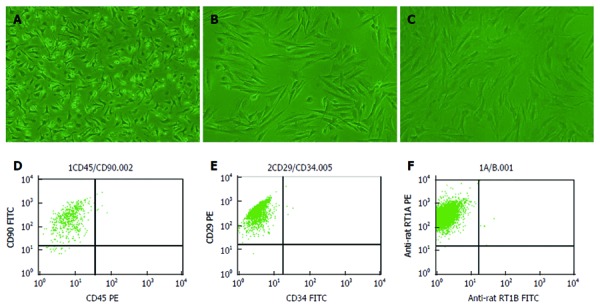
Morphology and flow cytometry results of Lewis-derived bone marrow mesenchymal stem cells. A: First-passage bone marrow mesenchymal stem cells (BM MSCs); B: Second-passage BM MSCs; C: Third-passage BM MSCs (× 200 magnification); D: The proportion of CD90+ and CD450- cells was approximately 95.48%; E: The proportion of CD29+ and CD34- cells was approximately 99.82%; F: The proportion of RT1A+ and RT1B- cells was approximately 99.87%.
Diagnosis and evaluation of rejection
A pathologist blinded to the source analyzed the slides. The degree of histopathological changes was graded semiquantitatively using the histological injury scale described by Chiu et al[28]: 0: normal mucosal villi; 1: development of a subepithelial space, usually at the villi apex, with capillary congestion; 2: extension of the subepithelial space with moderate epithelial lifting from the lamina propria; 3: massive epithelial lifting down the sides of the villi and ulceration at the villous tips; 4: denuded villi with dilated capillaries and increased cellularity of the lamina propria; and 5: degradation and disintegration of the lamina propria, hemorrhage, and ulceration. The summation of six randomly chosen fields from each rat was evaluated and averaged to determine the degree of mucosal injury.
Enzyme-linked immunosorbent assay
The graft levels of diamine oxidase (DAO), D-lactic acid (D-LA), tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-10 (IL-10), and transforming growth factor-β (TGF-β) were determined using the kits from R&D Systems, Minneapolis, MN, United States, according to the manufacturer’s protocol.
Detection and observation of intestinal mucosal ultrastructure
Ultrathin (70-nm) sections were prepared using standard techniques and examined under a transmission electron microscope (Hitachi H-600, Tokyo, Japan).
Western blotting of tissue occludin and ZO-1
Intestinal tissue samples were homogenized and lysed in buffer [50 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl, 100 μg/mL phenylmethylsulfonyl fluoride, 1% Triton X-100] for 30 min on ice. Then, 50 μg protein samples were boiled for 5 min in sample buffer, separated by 10% and 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto nitrocellulose membranes. Nonspecific reactivity was blocked using 5% non-fat dry milk in TBST [10 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 0.05% Tween-20] for 1 h at room temperature. The membrane was then incubated with a rabbit anti-rat polyclonal occludin and ZO-1 antibody (1:500; Santa Cruz Biotechnology) at 4 °C overnight. After three washes in TBST, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2000; Santa Cruz Biotechnology) for 2 h at room temperature. Reactive protein was detected using an ECL chemiluminescence system (Boster, Wuhan, China).
Real-time PCR detection of occludin and ZO-1 mRNA
RNA was isolated with TRIzol (TakaRa, Japan). Reverse transcription (RT) to complementary DNA was performed with 12 μg RNA by Moloney murine leukemia virus-RT at a final volume of 10 μL according to the manufacturer’s instructions. The reaction volume was increased to 20 μL and the dilutions used in the reactions were always performed in accordance with the β-Actin content. Product specificity was determined using 2% agarose gel electrophoresis. The sequences of the primers used are listed in Table 1.
Table 1.
Sequences of primers for occludin, zona occludens-1, and β-actin
| Tested gene product | Primer sequences | Fragment | |
| Occludin | Upstream | 5’-CCTGTTTAGTTAGGTGAAG-3’ | 156 bp |
| Downstream | 5’-TTCCTGAGAAGGGTTATG-3’ | ||
| ZO-1 | Upstream | 5’-GGGGGATTTATAACTTGGG-3’ | 321 bp |
| Downstream | 5’-CTGGTTGGATGTCTGTGG-3’ | ||
| β-Actin | Upstream | 5’-GCGTGACATTAAAGAGAAGCTG-3’ | 500 bp |
| Downstream | 5’-AGAAGCATTTGCGGTGCAC-3’ | ||
ZO-1: Zona occludens-1.
Statistical analysis
SPSS version 13.0 (SPSS, Chicago, IL, United States) was used for statistical analysis. Values reported are the mean ± SD. Different groups of data were compared by analysis of variance. P < 0.05 was considered statistically significant.
RESULTS
BM MSC extraction
Cells were confirmed as BM MSCs based on their spindle-shaped morphology, adherence to plastic, and flow cytometry results (Figure 1). Most of the third-passage adherent cells were CD90-, CD29-, and RT1A-positive and negative for the MSC markers CD45, CD34, and RT1B. Furthermore, the percentage of CD90+ and CD45- cells rapidly increased from 80% to > 98% over the first three passages.
General condition of the rats, graft histopathology, and grade of intestinal mucosal injury after HIT
Following small intestinal transplantation, all rats in group A survived for 10 d; in group B, two rats died by day 5, four rats died by day 7 and six rats died by day 10; and in group C, one rat died by day 7 and two rats died by day 10. The 10-d death rate was 0 (0/30), 30.23% (13/43), 9.09% (3/33) in groups A, B, and C, respectively. We observed no significant abnormal activities; the rats were only slightly sluggish 1 d after surgery. Subsequently, they became frequently irritable, and there was an abnormal increase in secretions from the nose and mouth, reddened ears, and liquid stools at 3 d after surgery. On day 5, there were obvious pathological symptoms of severe rejection, relative sluggishness, weight loss, liquid stools, and abdominal mass; physical examination revealed a macerated peristomal site with surrounding erythema, induration, and serosanguineous drainage. The condition of the rats worsened over time (Figure 2). The general condition improved at each time point in group C, and the pathological scores were mitigated (Table 2).
Figure 2.
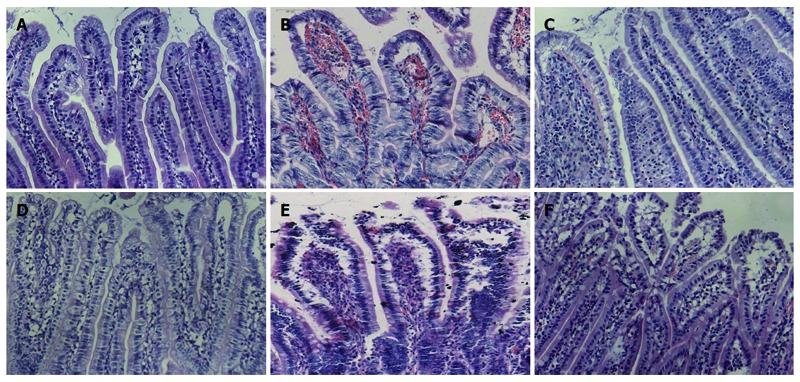
Graft histopathology at different time points after heterotopic intestinal transplantation (HE staining, × 200). A: Normal intestine with normal villous architecture and glands in group A; B: Intestinal mucosa degradation and hemorrhage of the lamina propria, ulceration, decreased ratio of villus height and crypt height, aggravated lymphocyte infiltration, partial gland epithelial necrosis in group B at day 5 after operation; D: There were decreased intestinal mucosal villi with mild deformity and interstitial infiltration of inflammatory cells; E: The condition was aggravated 7 d after heterotopic intestinal transplantation, and there was epithelial degeneration, intestinal wall thinning and necrosis, and interstitial inflammatory cell infiltration in great quantities; C, F: Recovery of the damaged mucosa in group A at (C) day 5 and (F) day 7.
Table 2.
Grade of intestinal mucosal injury following heterotopic intestinal transplantation
| Group | D 1 | D 3 | D 5 | D 7 | D 10 |
| A | 8.26 ± 0.65 | 9.37 ± 0.92 | 10.36 ± 0.78 | 12.65 ± 0.65 | 13.14 ± 0.86 |
| B | 17.4 ± 1.76a | 30.64 ± 3.62a | 41.6 ± 2.27a | 67.2 ± 2.57a | 81.74 ± 3.52a |
| C | 12.17 ± 1.17c | 24.00 ± 1.54c | 30.17 ± 0.41c | 41.83 ± 2.93c | 52.33 ± 1.03c |
All values are mean ± SD (n = 6; the summation of six randomly chosen fields from each rat were evaluated and averaged to determine the degree of mucosal injury). A: Non-rejection group; B: Rejection group; C: Bone marrow mesenchymal stem cells therapy group.
P < 0.05 vs group A;
P < 0.05 vs group B.
Small intestinal mucosal barrier function changes following transplantation
At each time point, the graft DAO levels in group B increased more than twofold compared to that in group A (P < 0.05). However, the graft DAO levels in group C were significantly lower than that in group B at each time point (P < 0.05) (Table 3).
Table 3.
Levels of graft diamine oxidase and D-lactic acid following heterotopic intestinal transplantation
| Group | D 1 | D 3 | D 5 | D 7 | D 10 | |
| DAO (U/mL) | A | 10.15 ± 1.10 | 12.86 ± 1.35 | 15.76 ± 1.33 | 18.55 ± 1.77 | 21.83 ± 1.21 |
| B | 22.36 ± 2.82a | 34.74 ± 5.59a | 44.54 ± 2.77a | 51.61 ± 1.70a | 68.02 ± 2.46a | |
| C | 18.69 ± 2.17c | 29.79 ± 2.49c | 36.15 ± 3.98c | 40.13 ± 1.21c | 59.87 ± 4.34c | |
| D-LA (g/L) | A | 4.12 ± 0.53 | 6.13 ± 0.57 | 8.62 ± 1.67 | 10.78 ± 0.72 | 11.67 ± 1.56 |
| B | 6.32 ± 0.46a | 8.65 ± 0.62a | 10.46 ± 0.98a | 12.74 ± 0.68a | 17.74 ± 1.75a | |
| C | 3.08 ± 0.32c | 4.08 ± 0.30c | 5.031 ±0.18c | 7.25 ± 0.51c | 12.81 ± 0.47c |
All values are mean ± SD (n = 6). A: Non-rejection group; B: Rejection group; C: Bone marrow mesenchymal stem cells therapy group.
P < 0.05 vs group A;
P < 0.05 vs group B. DAO: Diamine oxidase; D-LA: D-lactic acid.
Ultrastructural characteristics of the intestinal mucosa and TJs
In group A, the epithelial cells and TJs remained intact. By contrast, there was TJ and villi disruption, loose microvilli, and swollen organelles with decreased electron density in group B at 5 d after surgery. At the same time point, the TJs and endothelial cell mitochondria and microvilli in group C remained undisrupted (Figure 3).
Figure 3.
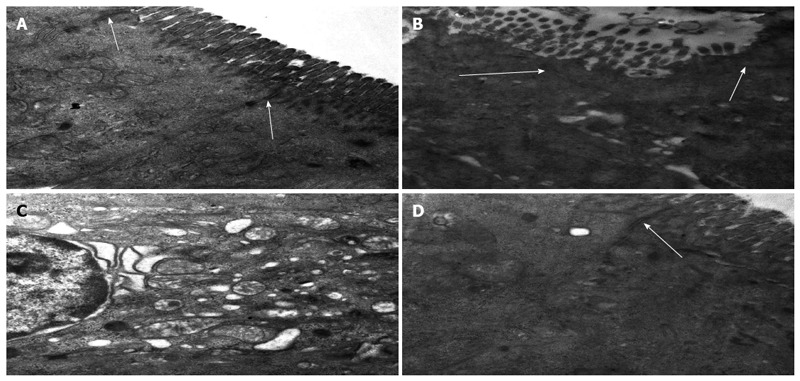
Ultrastructural characteristics of intestinal mucosa and tight junctions following heterotopic intestinal transplantation (magnification × 30000). A: Epithelial cells and tight junctions (TJs) (arrows) were intact in group A; B: Intestinal microvilli and TJs (arrows) were disrupted, and some microvilli were loose; C: Organelles were swollen with reduced electron density at day 5 after transplantation in group B; D: Microvilli and mitochondria of the endothelial cells were almost normal and TJs (arrow) were not disrupted at day 5 after transplantation in group C.
Expression of occludin and ZO-1 protein following HIT
Occludin and ZO-1 expression decreased more significantly in group B than in group A; however, occludin and ZO-1 expression was significantly higher in group C than in group B (Figure 4).
Figure 4.
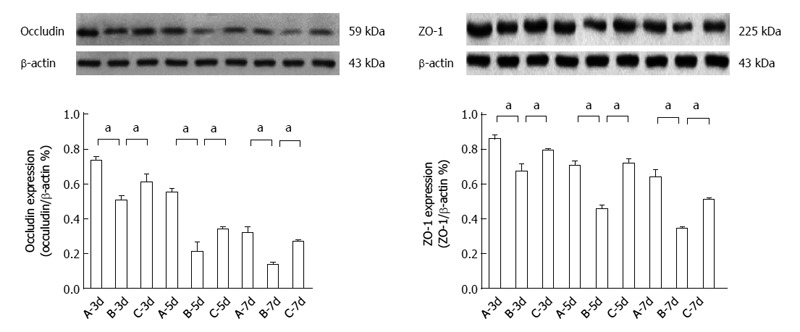
Occludin and zona occludens-1 protein expression after heterotopic intestinal transplantation. Occludin and zona occludens (ZO)-1 protein expression was significantly lower in group B than in group A (aP < 0.05 vs group B at day 5: 0.2082 ± 0.0582 vs 0.5477 ± 0.0284; aP < 0.05 vs group B at day 7: 0.3415 ± 0.0128 vs 0.6387 ± 0.046). Occludin and ZO-1 protein expression was significantly higher in group C than in group B (aP < 0.05 vs group B at day 7: 0.2674 ± 0.0128 vs 0.1352 ± 0.0142; aP < 0.05 vs group B at day 5: 0.7189 ± 0.0289 vs 0.4556 ± 0.0242). A: Group A (non-rejection); B: Group B (rejection); C: Group C (bone marrow mesenchymal stem cells therapy). β-actin was used as the loading control. Values shown are the mean ± SD.
Expression of occludin and ZO-1 mRNA following HIT
The expression of occludin and ZO-1 mRNA decreased more significantly in group B than in group A. Occludin and ZO-1 mRNA expression was significantly higher in group C than in group B (Figure 5).
Figure 5.
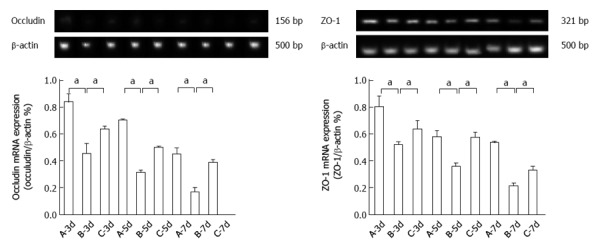
Occludin and zona occludens-1 mRNA expression after heterotopic intestinal transplantation. Occludin and zona occludens (ZO)-1 mRNA expression was significantly lower in group B than in group A (aP < 0.05 vs group B at day 5: 0.3135 ± 0.0168 vs 0.7011 ± 0.0128; aP < 0.05 vs group B at day 7: 0.2101 ± 0.0279 vs 0.5345 ± 0.0136). Occludin and ZO-1 mRNA expression was significantly higher in group C than in group B at day 5 (aP < 0.05 vs group B at day 7: 0.3860 ± 0.0254 vs 0.1673 ± 0.0369; aP < 0.05 vs group B at day 5: 0.5727 ± 0.0419 vs 0.3598 ± 0.0242). A: Group A (non-rejection); B: Group B (rejection); C: Group C (bone marrow mesenchymal stem cells therapy). β-actin was used as the loading control. Values shown are the mean ± SD.
Graft TNF-α, IFN-γ, IL-10, and TGF-β levels
Compared to group B, graft TNF-α, IFN-γ, IL-10, and TGF-β levels were decreased significantly at day 7 in group A (P < 0.05); graft TNF-α and IFN-γ levels were decreased significantly and graft IL-10 and TGF-β levels were increased significantly at day 7 in group C (P < 0.05) (Figure 6).
Figure 6.
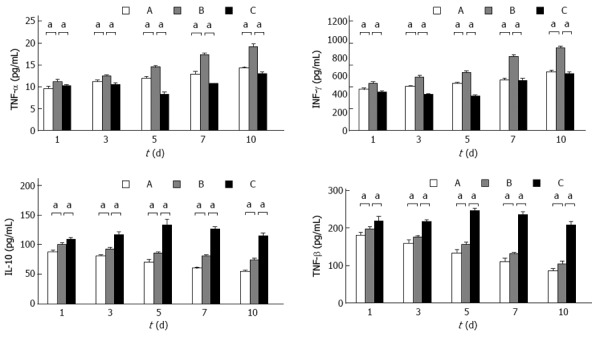
Graft tumor necrosis factor-α, interferon-γ, interleukin-10, and transforming growth factor-β levels. Graft tumor necrosis factor-α (TNF-α), interferon-γ (INF-γ), interleukin-10 (IL-10), and transforming growth factor-β (TGF-β) levels in group B were significantly decreased compared to that in group A at day 7 (17.2128 ± 0.4991 vs 12.8364 ± 0.7131, P < 0.05; 810.2637 ± 25.1175 vs 555.3763 ± 17.9702, P < 0.05; 80.3756 ± 2.5866 vs 59.9878 ± 2.1521, P < 0.05; 130.8756 ± 3.3428 vs 109.9878 ± 9.7968, P < 0.05). Graft TNF-α and IFN-γ levels in group C were significantly decreased compared to that in group B at day 7 (10.6473 ± 0.0710 vs 17.2128 ± 0.4991, P < 0.05; 545.1506 ± 31.9416 vs 810.2637 ± 25.1175, P < 0.05); graft IL-10 and TGF-β levels in group C were significantly increased compared to that in group B at day 7 (125.7773 ± 4.7719 vs 80.3756 ± 2.5866, P < 0.05; 234.5273 ± 9.3980 vs 545.1506 ± 31.9416, P < 0.05). A: Group A (non-rejection); B: Group B (rejection); C: Group C (BM MSC therapy). All values are mean ± SD (n = 6). aP < 0.05 vs group B.
DISCUSSION
Although the HIT model does not represent the physiological state of small intestinal function, it is used for investigating immunological reactions such as rejection. Additionally, the survival rate of the HIT model is higher and it involves a simple technique[29]. In this study, we explored the mechanism of intestinal mucosal barrier protection following HIT in rats through BM MSC implantation. In injured tissues, BM MSC transmigration across the endothelium is a useful tool for cellular therapy. MSCs develop tight cell-cell contacts and integrate into the endothelial wall of the capillary vessel[30]. Based on its simplicity and safety, saline or BM MSCs were injected via the penile vein postoperatively.
Postoperatively, the general condition of the rats worsened over time. At the same time, the grade of histopathological changes increased. The graft levels of DAO and D-LA increased with the rejection reaction, indicating impaired intestinal barrier function. DAO and D-LA are sensitive markers of intestinal permeability[31]. Furthermore, we observed disrupted intestinal microvilli and TJs, some loose microvilli, and swollen organelles with reduced electron density (Figure 3). However, the clinical symptoms improved after the BM MSC injection. The DAO and D-LA levels in group C also decreased more significantly than that in group B.
TJs are multi-protein complexes composed of transmembrane proteins, peripheral membrane (scaffolding) proteins, and regulatory molecules that include kinases. The most important transmembrane protein is occludin, which defines several aspects of TJ permeability. Peripheral membrane proteins such as ZO-1 and ZO-2 are crucial to TJ assembly and maintenance, partly because these proteins contain multiple domains for interaction with other proteins such as claudins, occludin, and actin[32]. Occludin and ZO-1 expression decreased more significantly in group B than in group A, particularly at 5 and 7 d; however, occludin and ZO-1 protein and mRNA expression was significantly higher in group C than in group B, particularly at days 7 and 5 (Figures 4 and 5), and was consistent with the ultrastructural changes. Based on the above results, we concluded that BM MSCs can protect the intestinal mucosal barrier, improve TJ permeability, repair TJ ultrastructure, and promote occludin and ZO-1 protein and mRNA expression.
BM MSCs do not express costimulatory molecules and are potent inhibitors of T cell proliferation in mixed lymphocyte cultures, prolonging allograft survival in rodent models[33-37]. BM MSC treatment favors the re-establishment of cellular homeostasis by both increasing endogenous proliferation processes and inhibiting the apoptosis of small intestinal epithelial cells. The effects of BM MSCs stem from their ability to improve the renewal capability of the small intestinal epithelium[38]. BM MSCs produce growth factors that play a critical role in healing damaged tissues. Growth factor production by BM MSCs in response to the wound microenvironment suggests that they might augment wound healing through the responsive secretion of growth factors that enhance angiogenesis and promote wound repair[39]. TNF-α can increase intestinal epithelial cell paracellular permeability[40], reduce expression of the ZO-1 gene promoter[41] and ZO-1 protein[42], and can cause abnormal distribution of ZO-1 protein. TNF-α can cause TJ relaxation and depolymerization, and exhibits a synergistic effect with IFN-γ[43]. TGF-β can both stimulate and inhibit cell proliferation by a two-way regulatory function[44]. After BM MSC injection, graft TNF-α and IFN-γ levels decreased significantly, and that of graft TGF-β and IL-10 increased significantly. Therefore, we inferred that BM MSCs protect and repair damaged intestinal barrier function following HIT by activating paracrine IL-10 and TGF-β, and that IL-10 and TGF-β exhibit sufficient immunomodulatory capabilities and protect intestinal epithelial cells and TJs, suppressing paracrine TNF-α and INF-γ.
In conclusion, BM MSCs can protect and repair damaged intestinal mucosal barrier function following HIT, and cytokines are involved in this effect. The mechanism of BM MSCs is complex and requires further study.
COMMENTS
Background
Small intestinal transplantation (SITx) has become the definitive treatment for patients with end-stage intestinal failure who cannot tolerate parenteral nutrition. Even after years of development, however, the rate of postoperative infection remains high. According to the Intestinal Transplant Registry, the cause of death after SITx is 51% within one year and remains 41.7% after one year. Under physiological conditions, the small intestinal mucosa is a barrier against toxins and pathogens in the gut lumen. Ischemia-reperfusion (I/R) and rejection damage of the intestinal mucosa after heterotopic intestinal transplantation (HIT) easily lead to bacterial translocation and endotoxemia. Bone marrow mesenchymal stem cells (BM MSCs) reduce intestinal I/R injury in rats and contribute to significant prolongation of graft survival. However, the mechanism is unclear. Although previous studies have provided insight into the molecular structure of tight junctions (TJs), considerably less is known about their functionality under physiological or pathophysiological conditions. Few studies have described the intestinal mucosa ultrastructure or TJ changes after HIT. In this study, the authors used a rat model of HIT to investigate the effect of BM MSCs on intestinal mucosa ultrastructure, with emphasis on the mechanisms of intestinal barrier dysfunction.
Research frontiers
In this study, the authors demonstrated that BM MSC implantation decreased intestinal permeability and preserved intestinal mucosal barrier function after HIT in rats. The mechanism was linked to reduced tumor necrosis factor-α and interferon-γ levels, increased interleukin-10 and transforming growth factor-β levels, and increased protein and mRNA expression of the intestinal TJ proteins occludin and zona occludens (ZO)-1.
Innovations and breakthroughs
This is believed to be the first study to report that BM MSCs reduce rejection after HIT, occludin and ZO-1 downregulation, and TJ disruption via a cytokine-regulated mechanism.
Applications
By understanding how BM MSCs protect the intestinal mucosal barrier, this study may represent a future strategy for therapy or prevention of rejection and sepsis after SITx, which is a serious and common complication.
Terminology
TJs are the most important structures in the mucosal barrier. They are composed of multiple proteins, including transmembrane proteins such as occludin, tricellulin, claudins, and junctional adhesion molecule. The intracellular portions of these transmembrane proteins interact with cytoplasmic peripheral membrane proteins, including ZO-1, -2, -3, and two distinct transmembrane proteins: occludin and claudin, which are linked to the actin-based cytoskeleton. TJs function as occlusion barriers by maintaining cellular polarity and homeostasis and by regulating paracellular space permeability in the epithelium.
Peer review
This paper demonstrates the impact of BM MSCs on rat small intestine after HIT. This study will be of interest and the paper is clearly written.
Footnotes
Supported by The Natural Science Foundation of China, No. 81270528; the Natural Science Foundation of Tianjin, China, No. 08JCYBJC08400, No. 11JCZDJC27800 and No. 12JCZDJC25200; and the Technology Foundation of Health Bureau of Tianjin, China, No. 2011KY11
P- Reviewers: De Ponti F, Iacobellis F S- Editor: Gou SX L- Editor: Ma JY E- Editor: Liu XM
References
- 1.Grant D, Abu-Elmagd K, Reyes J, Tzakis A, Langnas A, Fishbein T, Goulet O, Farmer D. 2003 report of the intestine transplant registry: a new era has dawned. Ann Surg. 2005;241:607–613. doi: 10.1097/01.sla.0000157265.85388.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsai HL, Island ER, Chang JW, Gonzalez-Pinto I, Tryphonopoulos P, Nishida S, Selvaggi G, Tekin A, Moon J, Levi D, et al. Association between donor-specific antibodies and acute rejection and resolution in small bowel and multivisceral transplantation. Transplantation. 2011;92:709–715. doi: 10.1097/TP.0b013e318229f752. [DOI] [PubMed] [Google Scholar]
- 3.Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 4.Kassem M, Kristiansen M, Abdallah BM. Mesenchymal stem cells: cell biology and potential use in therapy. Basic Clin Pharmacol Toxicol. 2004;95:209–214. doi: 10.1111/j.1742-7843.2004.pto950502.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 6.Snykers S, De Kock J, Tamara V, Rogiers V. Hepatic differentiation of mesenchymal stem cells: in vitro strategies. Methods Mol Biol. 2011;698:305–314. doi: 10.1007/978-1-60761-999-4_23. [DOI] [PubMed] [Google Scholar]
- 7.Ni WF, Yin LH, Lu J, Xu HZ, Chi YL, Wu JB, Zhang N. In vitro neural differentiation of bone marrow stromal cells induced by cocultured olfactory ensheathing cells. Neurosci Lett. 2010;475:99–103. doi: 10.1016/j.neulet.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 8.Devine SM, Cobbs C, Jennings M, Bartholomew A, Hoffman R. Mesenchymal stem cells distribute to a wide range of tissues following systemic infusion into nonhuman primates. Blood. 2003;101:2999–3001. doi: 10.1182/blood-2002-06-1830. [DOI] [PubMed] [Google Scholar]
- 9.DelaRosa O, Lombardo E. Modulation of adult mesenchymal stem cells activity by toll-like receptors: implications on therapeutic potential. Mediators Inflamm. 2010;2010:865601. doi: 10.1155/2010/865601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482–1490. doi: 10.1002/eji.200425405. [DOI] [PubMed] [Google Scholar]
- 11.Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516–525. [PubMed] [Google Scholar]
- 12.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, Marschall HU, Dlugosz A, Szakos A, Hassan Z, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397. doi: 10.1097/01.tp.0000214462.63943.14. [DOI] [PubMed] [Google Scholar]
- 13.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 14.Shen ZY, Zhang J, Song HL, Zheng WP. Bone-marrow mesenchymal stem cells reduce rat intestinal ischemia-reperfusion injury, ZO-1 downregulation and tight junction disruption via a TNF-α-regulated mechanism. World J Gastroenterol. 2013;19:3583–3595. doi: 10.3748/wjg.v19.i23.3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Qu L, Li Y, Gu L, Shi Y, Zhang J, Zhu W, Li J. Bone marrow mesenchymal stem cells reduce intestinal ischemia/reperfusion injuries in rats. J Surg Res. 2011;168:127–134. doi: 10.1016/j.jss.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Kuo YR, Goto S, Shih HS, Wang FS, Lin CC, Wang CT, Huang EY, Chen CL, Wei FC, Zheng XX, et al. Mesenchymal stem cells prolong composite tissue allotransplant survival in a swine model. Transplantation. 2009;87:1769–1777. doi: 10.1097/TP.0b013e3181a664f1. [DOI] [PubMed] [Google Scholar]
- 17.Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 18.Morita K, Itoh M, Saitou M, Ando-Akatsuka Y, Furuse M, Yoneda K, Imamura S, Fujimoto K, Tsukita S. Subcellular distribution of tight junction-associated proteins (occludin, ZO-1, ZO-2) in rodent skin. J Invest Dermatol. 1998;110:862–866. doi: 10.1046/j.1523-1747.1998.00209.x. [DOI] [PubMed] [Google Scholar]
- 19.Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720. doi: 10.1016/j.coph.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–G254. doi: 10.1152/ajpgi.2000.279.2.G250. [DOI] [PubMed] [Google Scholar]
- 25.Yu AS, McCarthy KM, Francis SA, McCormack JM, Lai J, Rogers RA, Lynch RD, Schneeberger EE. Knockdown of occludin expression leads to diverse phenotypic alterations in epithelial cells. Am J Physiol Cell Physiol. 2005;288:C1231–C1241. doi: 10.1152/ajpcell.00581.2004. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106:61–66. doi: 10.1073/pnas.0802741106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–1569. doi: 10.1074/jbc.M804783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 29.Nakao A, Tahara K, Inoue S, Tanaka N, Kobayashi E. Experimental models of small intestinal transplantation in rats: orthotopic versus heterotopic model. Acta Med Okayama. 2002;56:69–74. doi: 10.18926/AMO/31697. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt A, Ladage D, Steingen C, Brixius K, Schinköthe T, Klinz FJ, Schwinger RH, Mehlhorn U, Bloch W. Mesenchymal stem cells transmigrate over the endothelial barrier. Eur J Cell Biol. 2006;85:1179–1188. doi: 10.1016/j.ejcb.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Chioléro R. [Consequences of surgery on nutritional status] Ann Fr Anesth Reanim. 1995;14 Suppl 2:39–46. doi: 10.1016/s0750-7658(95)80101-4. [DOI] [PubMed] [Google Scholar]
- 32.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 33.Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228–241. doi: 10.1007/BF02256058. [DOI] [PubMed] [Google Scholar]
- 34.Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, Dazzi F. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–3729. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 35.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 36.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 37.Sbano P, Cuccia A, Mazzanti B, Urbani S, Giusti B, Lapini I, Rossi L, Abbate R, Marseglia G, Nannetti G, et al. Use of donor bone marrow mesenchymal stem cells for treatment of skin allograft rejection in a preclinical rat model. Arch Dermatol Res. 2008;300:115–124. doi: 10.1007/s00403-007-0827-9. [DOI] [PubMed] [Google Scholar]
- 38.Sémont A, Mouiseddine M, François A, Demarquay C, Mathieu N, Chapel A, Saché A, Thierry D, Laloi P, Gourmelon P. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–961. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y, Dulchavsky DS, Gao X, Kwon D, Chopp M, Dulchavsky S, Gautam SC. Wound repair by bone marrow stromal cells through growth factor production. J Surg Res. 2006;136:336–341. doi: 10.1016/j.jss.2006.07.037. [DOI] [PubMed] [Google Scholar]
- 40.Hanada S, Harada M, Koga H, Kawaguchi T, Taniguchi E, Kumashiro R, Ueno T, Ueno Y, Ishii M, Sakisaka S, et al. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 41.Millar AJ, Gupte GL. Small bowel transplantation in children. Br J Hosp Med (Lond) 2007;68:19–23. doi: 10.12968/hmed.2007.68.1.22650. [DOI] [PubMed] [Google Scholar]
- 42.Song HL, Lv S, Liu P. The roles of tumor necrosis factor-alpha in colon tight junction protein expression and intestinal mucosa structure in a mouse model of acute liver failure. BMC Gastroenterol. 2009;9:70. doi: 10.1186/1471-230X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sappington PL, Han X, Yang R, Delude RL, Fink MP. Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther. 2003;304:464–476. doi: 10.1124/jpet.102.043182. [DOI] [PubMed] [Google Scholar]
- 44.Saas P, Bonnefoy F, Kury-Paulin S, Kleinclauss F, Perruche S. Mediators involved in the immunomodulatory effects of apoptotic cells. Transplantation. 2007;84:S31–S34. doi: 10.1097/01.tp.0000269113.59857.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]


