Abstract
AIM: To investigate and compare the inhibitory effects of rapamycin in the different stages of liver fibrosis.
METHODS: We performed bile duct ligation (BDL) in male Wistar rats (n = 24). The experimental rats were classified into four groups: the BDL+/Rapa- group (un-treated control, n = 4), the BDL+/Rapa+ group (treated 14 d after BDL, n = 8), the BDL+/Rapa++ group (treated on the day after BDL, n = 8), and the BDL-/Rapa- group (un-treated, sham -operated control, n = 4). The BDL+/Rapa+ and BDL+/Rapa++ groups were administered rapamycin (2 mg/kg) for 28 d. The liver tissues were tested by immunohistochemical staining for α-smooth muscle actin (α-SMA) and cytokeratin.
RESULTS: The liver mRNA levels of transforming growth factor (TGF)-β1 and platelet-derived growth factor (PDGF) were measured using the polymerase chain reaction. The protein levels of liver p70s6K and p-p70s6k were determined using Western blotting. α-SMA expression was lowest in the BDL+/Rapa++group. TGF-β1 and PDGF expression levels in the rapamycin-treated group were lower than those in the un-treated group and higher than those in the control groups (TGF-β1: 0.23 ± 0.00 vs 0.34 ± 0.01, 0.23 ± 0.0 vs 0.09 ± 0.00, P < 0.0001; PDGF: 0.21 ± 0.00 vs 0.34 ± 0.01, 0.21 ± 0.0 vs 0.09 ± 0.00, P < 0.0001). The p70s6k and p-p70s6k levels decreased in the treated groups and were lowest in the BDL+/Rapa++group (p70s6k: 1.05 ± 0.17 vs 1.30 ± 0.56, 0.40 ± 0.01 vs 1.30 ± 0.56, P < 0.0001; p-p70s6k: 1.40 ± 0.5 vs 1.67 ± 0.12, 0.70 ± 0.01 vs 1.67 ± 0.12, P < 0.0001).
CONCLUSION: The results of our study indicate that rapamycin has inhibitory effects on liver fibrosis, and the treatment is most effective in the early stages of fibrosis.
Keywords: Liver cirrhosis, Sirolimus, Transforming growth factor beta, Platelet-derived growth factor, Ribosomal protein S6 kinases
Core tip: Liver cirrhosis is a serious disease causing significant mortality, but a curative treatment has not yet been developed. Therefore, there is great interest within the field of drug development in developing agents capable of inhibiting the progression of hepatic fibrosis. Rapamycin is an immunosuppressive agent that is also expected to attenuate the progression of liver fibrosis. We therefore aimed to investigate the inhibitory effects of rapamycin in the early and late stages of fibrosis, with the goal of contributing to the development of novel fibrosis treatments.
INTRODUCTION
Liver cirrhosis, which is the end result of the fibrosis that accompanies several chronic liver diseases, can yield deadly complications in affected patients.is accompanied by complications that are often the cause of death of cirrhosis patients. Bile duct ligation (BDL) has been widely used as a model of liver fibrosis in animal models[1-4].
Transforming growth factor beta 1 (TGF-β1) and platelet-derived growth factor (PDGF) are key cytokines involved in the healing process that occurs following acute or chronic liver damage[5]. TGF-β1, which is a 25-kDa dimeric protein that is secreted in its latent form, is converted into its active form after liver injury. This molecule is a pro-fibrogenic cytokine, the serum and tissue levels of which increase in models of chronic liver disease. In various tissues, such as the liver, kidney, lung, bone marrow, and skin, TGF-β1 is involved in the recovery of damaged tissues, playing an important role in fibrotic processes[6-8].
PDGF is a potent proliferative cytokine that is involved in the promotion of cell division and angiogenesis. It is associated with fibrosis, atherosclerosis, and malignant disease[9-12].
The 70-kDa ribosomal S6 kinase (p70s6k), which is activated by growth factors and hormones through the phosphatidylinositol 3-kinase (PI3K)-dependent signaling pathway, is a downstream molecule of the mammalian target of rapamycin (mTOR) and is involved in protein synthesis and cell cycle control. Phospho-p706k (p-p70s6k) is the active form of p70s6k and plays an important role in the G1/S cell cycle transition. The role of p70s6k in cell cycle control has been assessed in previous studies in the presence of rapamycin, also known as sirolimus[13].
Rapamycin is a bacterial macrolide antibiotics that blocks cell proliferation by inhibiting the G1/S transition in several cell types. In particular, several studies have shown that rapamycin delays the G1/S transition of fibroblasts, making it of potential use in the treatment of fibrotic diseases. Rapamycin is used clinically as an important immunosuppressive drugs to prevent rejection after organ transplantation. It also inhibits the growth of mammalian cells, such as B and T lymphocytes, by suppressing the activation of p70s6k and inhibiting mTOR[14-18].
The levels of alpha smooth muscle actin (α-SMA), a marker of activated hepatic stellate cells (HSCs) and myofibroblasts, increase after chronic liver damage. Therefore, α-SMA can be used to identify and quantify activated HSCs in liver fibrosis[19-21].
Cytokeratin 19 (CK 19), is involved in cytoskeleton formation, is expressed in bile duct cells and their related carcinomas. Although this protein shows almost no expression in hepatocytes, it has emerged as an important marker of liver stem cells[22].
The aim of our study was to investigate the inhibitory effects of rapamycin in BDL-induced liver fibrosis in rats. We classified the treatment animals into 2 groups, the BDL and control groups, and administered rapamycin to both groups to determine the efficacy of its inhibitory effects according to the degree of liver fibrosis.
MATERIALS AND METHODS
Animals and ethics
Normal male Wistar rats (approximately 100 g body weight) were kept in 12-h light/dark cycles with free access to food and water. All the animal experiments were approved by a state-appointed animal ethics board. All institutional and national guidelines for the care and use of laboratory animals were followed.
Induction of fibrosis and sham surgery
The BDL procedure was performed on 20 rats. Before surgery, the rats were anesthetized using 0.2 mL of a 1:4 mixture of ketamine (Huons Co., Korea) and Rompun (Byer Co., Germany). In the BDL group, the middle part of the bile duct was cut and the 2 ends were tied. The control group (BDL-/Rapa-) rats (n = 4) underwent sham laparotomies.
Rapamycin administration in the treatment groups
Four of the 20 rats that underwent BDL were allocated to the BDL+/Rapa- group(non-treatment group), and the remaining 16 rats were given rapamycin. A sonde was used for the daily oral administration of the drug at a certain time, and the administration occurred at different time points. Eight rats were given with 2 mg/kg Rapamune (Wyeth Co., Puerto Rico, United States) 14 d post- BDL (BDL+/Rapa+, 2 wk post-BDL), and the other 8 rats given the drug immediately after BDL (BDL+/Rapa++, immediately after BDL). The drug administration period was 28 d for all subjects.
Hepatic tissue and blood sample collection
The survival times of the 4 groups were 42 d and all rats were sacrificed after the treatment of the BDL+/Rapa+ group was completed. The hepatic tissue was stored at -70 oC for the polymerase chain reaction (PCR) and Western blot analyses. Blood samples were collected by puncturing the heart under anesthesia and were centrifuged (3000 rpm, 10 min). Aspartate transaminase (AST), alanine transaminase, total protein (TP), albumin (Alb), and total bilirubin (TB) levels were measured using a measuring device (FUJI FILM, DRI-CHEM 4000I, Japan).
Haematoxylin and eosin staining and masson trichrome staining
For the hematoxylin and eosin (HE) staining, the tissues were fixed in formalin, stained with Harris’s hematoxylin for 5 min, washed with tap water for 5 min, treated with 1% acid alcohol for 30 s, and then washed under running water for 1 min. This procedure was followed by staining with eosin for 2 min, dehydrating with alcohol, and sealing with xylene.
For the Masson Trichrome (MT) staining, the tissue that was fixed in formalin was incubated for 30 min with Bouin’s fixatives at 56 °C, stained with Weigert’s hematoxyline for 10 min for nuclear staining, and washed with running water for 10 min. Biebrich scarlet-acid fuchsin was used to initiate the reaction or 10 min, after which time each sample was incubated in phosphomolybdic-phosphotungstic acid for 10 min for mordant treatment and discoloration. The tissue was incubated with aniline blue for 10 min and, then treated with acetic acid for 3 min to allow time for the discoloration of the uncombined aniline blue. Finally, the tissue was washed in running water for 2 min.
Immunohistochemistry
α-SMA was used for the staining of the monoclonal mouse anti-human smooth muscle actin (DAKO Co., Denmark). First, paraffin-removed tissue sections were treated with a 0.1 mol/L citric acid solution (pH 6.0) for antigen retrieval. The antibody was treated with dilutions in multiples of 1:40-1:80.
CK 19 also involved the use of paraffin-removed tissue sections for antigen retrieval with a PT link (DAKO Co., Denmark). This antibody (DAKO Co.) was diluted in multiples of 1:250 and then stained using the DAKO stain.
RNA extraction, cDNA generation and PCR
To extract the total RNA from the control and BDL rat livers, 1 mL of Trizol (Invitrogen, CA, United States) was added to the tissues, and the resulting samples were homogenized. The homogenates were mixed with 200 μL of chloroform. After incubation for 5 min at room temperature, the homogenates were centrifuged at room temperature for 10 min at 13200 g. The supernatants were transferred to clean tubes containing 1000 μL of isopropyl alcohol (Sigma-Aldrich, St. Louis, MO, United States) followed by centrifugation at 13200 rpm for 30 min. The resulting supernatant was mixed with 500 μL of DEPC-treated water and centrifuged at room temperature for 10 min at 13200 g. The resulting supernatant was discarded, and the pellet was dried at room temperature, dissolved in DEPC-treated water (Sigma-Aldrich, St. Louis, MO, United States), and stored at -75 °C. The quality and integrity of the RNA were confirmed by agarose gel electrophoresis. A total of 1 μg of the RNA was used to prepare the cDNA by random priming using a First-Strand cDNA Synthesis Kit (Enzynomics, Daejeon, Korea). The PCR conditions were as follows: initial denaturation at 95 °C for 5 min followed by 35 cycles at 95 °C for 25 s, 54.5 °C for 25 s, and 72 °C for 25 s, followed by a final extension step of 72 °C for 5 min. The PCR products were analyzed by electrophoresis on 1.5% agarose gels. GAPDH was used as a housekeeping gene control. The primer sequences used are listed in Table 1.
Table 1.
Primer sequences for the polymerase chain reaction
| Gene | Primer sequence | Product size (bp) | |
| TGF-β1 | Forward | 5'-TACAGGGCTTTCGCTTCAGT-3' | 394 |
| Reverse | 5'-TGGTTGTAGAGGGCAAGGAC-3' | ||
| PDGF | Forward | 5'-GTCGAGTCGGAAAGCTCATC-3' | 416 |
| Reverse | 5'-GTCACCCGAGTTTGAGGTGT-3' |
TGF-β1: Transforming growth factor-β1; PDGF: Platelet derived growth factor.
Western blotting
The frozen tissue was pulverized with a precooled mortar and pestle and then homogenized at 4 °C in 1 × radio-immunoprecipitation assay buffer (Sigma-Aldrich). Following centrifugation of the homogenates at 13000 g for 20 min at 4 °C, the pellets were discarded and the supernatants were either used immediately or stored at -70 °C. The TP was measured using a Bradford dye-binding protein assay kit (Thermo Scientific, MA, United States). An aliquot of the supernatant was kept for protein determination and Laemmli sample buffer (Bio-Rad, CA, United States) containing β-mercaptoethanol was added to the remainder of the supernatant. The samples were boiled for 5 min, and 50 μg of the TP samples were loaded onto a 10% polyacrylamide gel after cooling. The samples were electrophoresed in a Mini-Protean TetraCell electrophoresis assembly (Bio-Rad) under constant voltage. The proteins were then transferred to polyvininylidene fluoride membranes using a Mini-Protean trans-blot semidry transfer cell for 2 h at 4 °C. Non-specific binding sites were blocked by incubation in 1 × phosphate buffered saline (PBS) containing 5% skim milk and 0.1% Tween 20 (PBSTM) for 1 h. The membranes were then incubated overnight at 4 °C with an antibody targeting p70s6k (1:250) or, p-p70s6k (1:250). After washing with 1 × PBSTM, the membrane was incubated for 1 h at room temperature with goat anti-rabbit IgG-horseradish peroxidase (1:2000) and then washed for 5 min in 1 × PBSTM a total of four times. The membranes were also probed with an anti-actin monoclonal antibody (1:1000) as an internal control. Immunoreactive proteins were detected and visualized with a chemiluminescence reagent (Daeillab service, Seoul, Korea) and the scanned films were quantified using a gel documentation system (Dongjinsa, Seoul, Korea). All antibodies were purchased from Santa Cruz Biotechnology (CA, United States) or Cell Signaling Technology (MA, United States).
Statistical analysis
After measuring the total weight and liver weights, calculating the ratio of the total weights to the liver weights, and averaging the blood test results, a one-way analysis of variance (ANOVA) was performed to examine the between-group significance. PCR and Western blot bands were examined using the IMT i-solution program to calculate the multiples of the densities and areas. In addition, we measured the ratio of GAPDH (control protein) to actin twice. The results are reported as the mean ± SE. SPSS 20.0 was used for the one-way ANOVA. We compared the P-values to assess the statistical significance. A P value of less than 0.05 was considered significant.
RESULTS
The baseline characteristics and blood chemistry of the experimental groups
One rat in each of the rapamycin-treated groups (the BDL+/Rapa+ and BDL+/Rapa++) died on the second and third days after the 28 d of drug administration. Thus, 4 rats in BDL+/Rapa-, 7 rats in the BDL+/Rapa+, 7 rats in the BDL+/Rapa++, and 4 rats in the BDL-/Rapa- group completed the experiment. A total of 22 rats underwent anesthesia for blood collection, and their total weights and liver weights were measured before performing the autopsies. The experimental animals were killed 6 wk after BDL, and the liver tissues were collected. According to the one-way ANOVA, there was no difference in the total weights between groups (P = 0.136), but a significant difference was found in the ratio of the liver weights to the total weights (liver wt/TB wt) between the groups (P = 0.010). Similarly, the AST levels were significantly different between the groups (P = 0.023), as were the TB and Alb levels (P < 0.0001 and, P = 0.008, respectively) (Table 2).
Table 2.
Basal characteristics and results of blood chemistry
| Treatment group | BDL+/Rapa- (n = 4) | BDL+/Rapa+ (n = 7) | BDL+/Rapa++ (n = 7) | BDL-/Rapa- (n = 4) | P value1 |
| TBWt (g) | 323.25 ± 85.84 | 351.28 ± 55.42 | 338.57 ± 21.16 | 276.25 ± 15.9 | 0.136 |
| Liver Wt (g) | 20.49 ± 2.68a | 23.55 ± 4.90a | 21.53 ± 3.10a | 10.85 ± 0.38 | < 0.0001 |
| Liver Wt/TBWt | 0.065 ± 0.011ac | 0.069 ± 0.017a | 0.064 ± 0.010ac | 0.040 ± 0.003c | 0.010 |
| AST (U/L) | 254.50 ± 143.79 | 244.43 ± 97.56 | 293.57 ± 89.67 | 86.75 ± 10.69 | 0.023 |
| ALT (U/L) | 47.00 ± 17.87 | 50.86 ± 21.97 | 52.00 ± 17.58 | 34.25 ± 2.63 | 0.429 |
| TB (mg/dL) | 9.15 ± 2.16a | 8.74 ± 0.92a | 11.17 ± 2.11a | 0.50 ± 0.14 | < 0.0001 |
| TP (g/dL) | 5.48 ± 1.28 | 5.37 ± 0.33 | 5.74 ± 0.61 | 5.48 ± 0.38 | 0.773 |
| Alb (g/dL) | 2.65 ± 0.85a | 2.81 ± 0.41a | 3.43 ± 0.43a | 3.83 ± 0.33 | 0.008 |
Statistical significances were tested by one-way analysis of variances among groups and statistically significant results are indicated by P values.
P < 0.05 vs BDL-/Rapa-;
P < 0.05 vs BDL+/Rapa+ based on Scheffe multiple comparison test. Basal characteristics and results of blood chemistry in bile duct ligation (BDL+/Rapa-), bile duct ligation with rapamycin (BDL+/Rapa+, BDL+/Rapa++) and sham-operated (BDL-/Rapa-) groups. Each values indicate mean ± SD. TBWt: Total body weight; Wt: Weight; TP: Total protein; Alb: Abumin; AST: Aspartate transaminase; ALT: Alanine transaminase; TB: Total bilirubin; Rapa: Rapamycin; Rapa+: Rat administered rapamycin for 28 d from the 14th day post-BDL; Rapa++: Rat administered rapamycin for 28 d beginning the first day post-BDL.
HE and MT staining
HE staining demonstrated that the portal areas of the BDL+/Rapa- rats were markedly expanded with evidence of ductular proliferation and a severe inflammatory reaction (moderate to severe periportal activity). The BDL+/Rapa+ rats, which received drug treatment 14 d after BDL, exhibited a moderate expansion in their portal area, with marked ductular proliferation and a mild to moderate inflammatory reaction (mild to moderate periportal activity). The BDL+/Rapa++ rats, which received drug treatment immediately after BDL, exhibited a mild in their portal areas, with mild ductular proliferation and a mild inflammatory reaction (mild periportal activity) (Figure 1A). The MT staining results revealed showed marked portal-portal fibrotic septa formation and collagen deposition (blue) in the BDL+/Rapa- group. Reduced fibrotic septa formation was observed in the BDL+/Rapa+ and BDL+/Rapa++ groups, and the BDL+/Rapa++ group exhibited the lowest levels of fibrotic septa formation and collagen deposition (Figure 1B).
Figure 1.
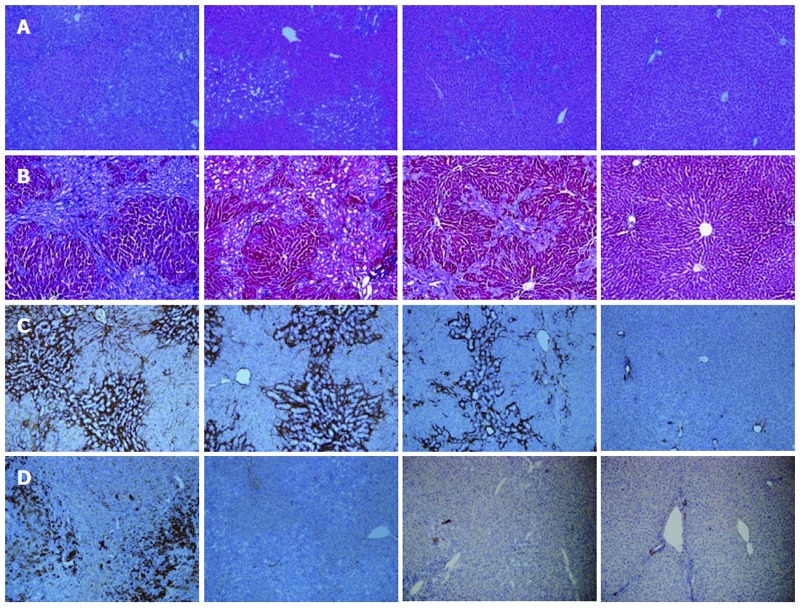
Comparison of histological findings between the groups that underwent bile duct ligation (BDL) with/without rapamycin treatment and the control. A: Hematoxylin and eosin (HE) staining. The bile duct ligation (BDL)+/Rapa++ group showed the smallest expansion of portal areas, with the least amount of ductular proliferation and the most mild inflammatory reaction among the BDL groups; B: Masson-Trichrome staining. The BDL+/Rapa++ group showed the lowest degree of fibrotic septa formation and deposition of collagen among BDL groups (blue); C: α-smooth muscle actin (α-SMA) staining (dark brown). The BDL+/Rapa++ group showed the lowest amount of staining among BDL groups; D: Cytokeratin 19 (CK 19) protein expression (dark brown). The BDL+/Rapa++ group showed the lowest amount of staining among BDL groups. Rapa: Rapamycin; Rapa+: Rat administered rapamycin for 28 d from the 14th day post-BDL; Rapa++: Rat administered rapamycin for 28 d beginning the first day post-BDL. Original magnification: × 100(a-d).
Immunohistochemical staining of α-SMA and CK 19
The results of the α-SMA staining in the BDL+/Rapa- rat livers revealed strong staining (dark brown) in the portal-portal area compared with the BDL-/Rapa- rat livers. The treated BDL+/Rapa+ and BDL+/Rapa++ livers showed relatively decreased expression, especially in the BDL+/Rapa++ group, in which the rapamycin was administered immediately following BDL (Figure 1C). CK 19 protein staining confirmed the presence of greater bile duct proliferation in the BDL+/Rapa- group (dark brown) compared with the BDL-/Rapa- group, as well as a decreased ductular reaction in the BDL+/Rapa+ and BDL+/Rapa++ groups (Figure 1D).
TGFβ1 mRNA expression
The inhibitory effect of rapamycin on fibrosis was confirmed by the expression levels of TGFβ1 mRNA, which increased with fibrosis. According to the results of the one-way ANOVA, the TGFβ1/GAPDH ratios exhibited significant differences between the groups, as shown in the figures and by the densitometry results (P < 0.0001). According to Scheffe’s multiple comparison test, the BDL+/Rapa- rats exhibited significantly increased expression compared with the controls (P < 0.0001), and the difference in expression between the BDL+/Rapa+ and BDL+/Rapa++ rats was also significant (P < 0.001 and P < 0.0001). Decreased expression was found in the BDL+/Rapa+ and BDL+/Rapa++ groups compared with the BDL+/Rapa- group, further, suggesting that rapamycin has an inhibitory effects on fibrosis (Figure 2A).
Figure 2.
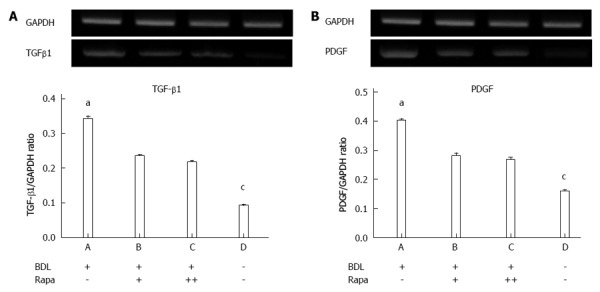
Transforming growth factor β1 and platelet-derived growth factor mRNA expression was assessed by polymerase chain reaction analysis. A: Transforming growth factor β1 (TGFβ1); B: Platelet-derived growth factor (PDGF). BDL: Bile duct ligation; Rapa: Rapamycin; Rapa+: Rat administered rapamycin for 28 d starting the 14th day after BDL; Rapa++: Rat administered rapamycin treatment for 28 d starting the 1st day after BDL; GAPDH: Glyceraldehyde 3-phosphate dehydrogenase. aP < 0.05 vs other group; cP < 0.05 vs BDL+/Rapa++ group.
PDGF mRNA expression
In the PDGF band and density one-way ANOVA, the PDGF/GAPDH values showed statistically significance differences between the groups (P < 0.0001). The BDL+/Rapa+ and BDL+/Rapa++ group showed significantly decreased level of PDGF expression compared with the BDL+/Rapa- group (P = 0.001, P = 0.001) (Figure 2B).
mTOR down-stream molecules p70s6k and p-p70s6k
The protein p-p70s6k is the active form of p70s6k and is involved in the mTOR signaling pathway, the action site of rapamycin, cell cycle control, and HSCs proliferation. Its expression was therefore compared with that of a control protein (actin).
The BDL+/Rapa+ and BDL+/Rapa++ groups showed a significant decrease in the expression of p70s6k compared with the BDL+/Rapa- group (P < 0.013, P < 0.0001). The BDL+/Rapa++ group was found to have a significantly decreased p70s6k expression level compared with the BDL+/Rapa- group ( with no drug administration) and the BDL+/Rapa+ group (with drug administration at a different time ) (P < 0.0001, P < 0.0001).
Based on the analyses of p-p70s6k protein expression, the treated BDL+/Rapa++ group showed a significantly decreased expressions compared with the BDL+/Rapa+ (P = 0.005) and BDL+/Rapa- (P = 0.001) groups. These results demonstrate that p-p70s6k has different expression levels according to the time of drug administration, making the effects of drug administration at each time predictable (Figure 3).
Figure 3.
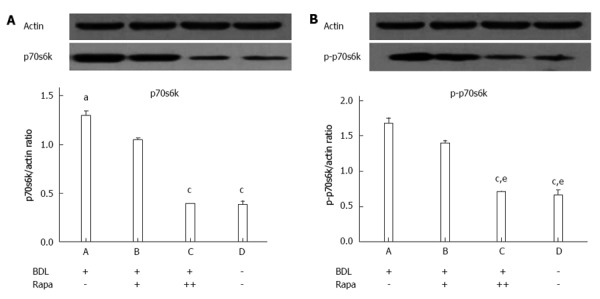
Western blot analysies showed the p70s6k and p-p70s6k expression levels in the advanced liver fibrosis samples. A: 70-kDa ribosomal S6 kinase (p70s6k); B: p-p70s6k. BDL: Bile duct ligation; Rapa: Rapamycin; Rapa+: Rat administered rapamycin for 28 d beginning the 14th day post-BDL; Rapa++: Rat administered rapamycin for 28 d beginning the first day post-BDL. aP < 0.05 vs other group; cP < 0.05 vs BDL+/Rapa+ group; eP < 0.05 vs BDL+/Rapa- group.
DISCUSSION
In our study, we aimed to determine whether rapamycin has an inhibitory effects on liver fibrosis. We examined changes in relevant cytokines when this drug was administered to early and more progressed cases of liver fibrosis in animal models of liver fibrosis that was induced by BDL. We administered rapamycin for 28 d during the different stages of fibrosis.
Liver fibrosis develops from the excessive deposition of extracellular matrix as proliferation-promoting cytokines and growth factors lose their regulatory control. This occurs after chronic liver damage as in the cases of viral hepatitis, alcoholic fatty liver, and non-alcoholic steatohepatitis[23,24]. Liver cirrhosis is the most advanced stage of liver fibrosis and can yield complications such as esophageal varices, hepatic encephalopathy, and peritonitis. Cirrhosis patients eventually die because of these complications. Rapamycin is mainly used to prevent rejection after transplantation; it inhibits cell proliferation and is used in coronary artery stenting to treat cardiovascular diseases[25,26]. Following additional studies on the inhibitory effects of rapamycin on the liver, the lung, and renal fibrosis, it is expected that rapamycin will be deemed suitable as next anti-fibrosis treatment[27]. Several human studies have evaluated the antifibrotic effects of rapamycin on fibrosis and cirrhosis in humans. McKenna et al[28] described the impact of sirolimus in reducing the extent and progression of fibrosis in liver transplant recipients with recurrent HCV[29]. Kelly et al[30] suggested that sirolimus-based immunosuppression is associated with a lower risk of significant graft fibrosis following liver transplantation in HCV-infected recipients.
In this study, we confirmed the effects of rapamycin on the inhibition of fibrosis in a BDL rat model. In the previous studies of rapamycin, the drug was administered immediately after BDL through either intraperitoneal or subcutaneous injections. Non-oral administration has different absorption routes compared with the current route for rapamycin in humans. Our study used the same drug delivery mechanism as that used in humans to compensate for this limitations. The rapamycin used in this study was ground into a powder, mixed with water, and provided through a small-diameter tube (sonde) at the same time every day directly into the stomach of the rats. Drug administration was initiated at a different time points to enable partial progression of fibrosis.
Liver tissues stained with HE and MT were used for the analysis, and depositions of the extracellular matrix in the treated BDL+/Rapa+ and BDL+/Rapa++ groups were found to be decreased compared with those in the BDL+/Rapa- group. In particular, the BDL+/Rapa++ group exhibited less collagen deposition than the BDL+/Rapa+ group. This result demonstrates the potential of rapamycin to inhibit liver fibrosis. In addition, the effects were greater in the animals in earlier stages of fibrosis.
HSCs, portal fibroblasts, and myofibroblasts are involved in liver fibrosis. α-SMA is a marker of HSCs and myofibroblasts, which were compared in this study to confirm the difference in their expression levels in the portal veins and interface zone. The BDL+/Rapa- group was found to exhibit strong staining, and the treated BDL+/Rapa+ and BDL+/Rapa++ groups showed significantly decreased staining compared with the BDL+/Rapa- group (Figure 1C). This finding is interpreted as a demonstration of the anti-fibrosis capacity of rapamycin.
CK 19 is expressed in normal epithelial cells, and its expression is increased during ductular reactions in biliary sclerosis[31]. Decreased CK 19 staining was found in the BDL+/Rapa+ and BDL+/Rapa++ groups compared with the BDL+/Rapa- groups (Figure 1D).
TGF-β1 is a marker of the active form of HSCs and functions as a key cytokine in the progression liver fibrosis. Once tissue healing after damage is complete, its production is terminated for an unknown reason; however, in the case of chronic and repeated damage, this self regulation is lost[32]. The results of this study also showed increased expression in the BDL+/Rapa- group compared with the control. However, the BDL+/Rapa+ and BDL+/Rapa++ groups were found to have decreased expression compared with the BDL+/Rapa- group, thus confirming the inhibitory effects of rapamycin (Figure 2A). The P values of the BDL+/Rapa+ and BDL+/Rapa++ groups compared to the BDL+/Rapa- group were statistically significant, at 0.001 and 0.0001, respectively. These results correspond to those of Bierker et al[18], suggesting that rapamycin has inhibitory effects on cell proliferation following liver damage.
PDGF-β is the strongest known mitogen and is auto-secreted in HSCs. In our comparison of the density of PCR bands between the experimental groups, the BDL+/Rapa- group showed the greatest increase in PDGF mRNA expression (P < 0.0001), and the treated groups (BDL+/Rapa+ and BDL+/Rapa++ ) showed a statistically significant decrease in the expression of PDGF mRNA compared to the BDL+/Rapa- group (P = 0.001). These results demonstrate that rapamycin stops the production of cytokines and decreases their expression in chronic liver damage.
p70s6k is a downstream protein of the PI3K-Akt signaling system, and its secretion is stimulated by various hormones and growth factors. p70s6k controls protein synthesis, proliferation, and the cell cycle. Activation of p70s6k occurs when serine or threonine is phosphorylated, and this active form is inhibited by rapamycin[33-35]. In our experiment, p70s6k/Actin showed significantly decreased expression levels in the BDL+/Rapa+ and BDL+/Rapa++ groups compared with the BDL+/Rapa- group (P = 0.013, P < 0.0001). The BDL+/Rapa++ group exhibited significantly decreased expression compared with the BDL+/Rapa+ group (P < 0.0001). With regard to the p-p70s6k expression, the BDL+/Rapa+ group showed slightly decreased expression levels compared with the BDL+/Rapa- group, although this finding was not statistically significant (P = 0.117). However, compared with the BDL+/Rapa++ group, there was a statistically significant decrease in the expression (P = 0.005). The BDL+/Rapa++ group was observed to have decreased expression level compared with the BDL+/Rapa- group (P = 0.001) the BDL+/Rapa+ group (P = 0.005), (Figure 3).
The observed differences in p70s6k and p-p70s6k protein expression are consistent with the results of previous studies investigating the effects of rapamycin inhibition on p70s6k phosphorylation. However, the differences in p70s6k expression between the BDL+/Rapa+ and BDL+/Rapa++ groups are not consistent with the results of Biecker et al[18] and Zhu et al[36]. These authors predicted that different mechanism was involved in the mTOR pathway. The inhibition of these two proteins is believed to inhibit cell proliferation. The BDL+/Rapa++ and BDL+/Rapa+ groups showed significant differences in their expression levels of p70s6k and p-p70s6k, suggesting that the administration of rapamycin is more effective in the early stages of fibrosis.
We confirmed the inhibitory effects of rapamycin on fibrosis, including partially progressed fibrosis, and determined that these effects were greater during the early stages of fibrosis. Therefore, the results of this study should be useful in developing drugs to inhibit or reduce fibrosis in patients with chronic liver diseases. One limitation of this study is the fact that rapamycin is insoluble in water. We therefore ground it into a powder mixed with water to enable administation directly into the stomach of the rats through small-diameter tubes. Given this experimental setup, it is possible that residual drug remained inside the tube, potentially decreasing the dosage to less than 2 mg/kg per day. However, this possible error was minimized by injecting air into the tube after drug administration.
In conclusion, this study confirmed that rapamycin, which is an immunosuppressant used in the treatment of transplant patients, has inhibitory effects on liver fibrosis. Importantly, these effects are more pronounced when the drug is administered immediately after the start of liver fibrosis or before progression of fibrosis. Our results showed that better treatment effects can be expected when rapamycin is administered during the early stages of fibrosis. However, administration of this drug after the progression of fibrosis is also effective. No severe side effects or adverse events due to the administration of rapamycin were noted in this study.
Therefore, the treatment of chronic liver disease patients with rapamycin is likely to inhibit the progression of liver cirrhosis while improving fibrosis is more progressed cases.
ACKNOWLEDGMENTS
The authors are grateful for the expert technical assistance of Lee EJ in performing the Western blot and PCR experiments.
COMMENTS
Background
Liver cirrhosis is the endpoint of the fibrogenic process that accompanies chronic liver disease. Complications arising from cirrhosis frequently result in death. Thus, the inhibition of fibrosis progression is associated with improvements in the survival rate. Rapamycin is used as an immunosuppressant agent but is also believed to be an anti-fibrotic drug. Indeed, several studies have reported that rapamycin inhibits fibrosis.
Research frontiers
Rapamycin is a bacterial macrolide with immunosuppressive properties. Many studies have reported that rapamycin had an inhibitory effect on fibrogenesis in the lung, skin, and liver. In the field of of liver disease, one research area of interest in the development of a drug that can ameliorate or prevent fibrosis.
Innovations and breakthroughs
In previous studies of the ability of rapamycin to inhibit liver fibrosis, rapamycin was administered by peritoneal injection or by mixing it with drinking water in animal models. However, these are not the modes of delivery in humans. Authors therefore designed our study to be more consistent with actual treatment, such that the rapamycin was ground into a powder and mixed with water for direct injection into the rat stomach through small-diameter tubes. They also divided their treatment groups into a group treated 14 d after bile duct ligation (BDL) and a group treated 1 d after BDL. They offer a comparison of the inhibitory effects of rapamycin in the different stages of liver fibrosis.
Applications
The results suggest that rapamycin is a potential therapeutic drug that could be used to inhibit liver fibrosis.
Terminology
Fibrosis represents is the endpoint of a fibrogenic process that accompanies chronic liver injury in cases of viral hepatitis and other diseases. The complications of fibrosis are the cause of death in many cirrhotic patients. Rapamycin is a bacterial macrolide antibiotics that blocks cell proliferation. It is a clinically important immunosuppressive drugs used to prevent rejection after organ transplantation.
Peer review
The authors examined the immunohistochemical staining of α-smooth muscle actin in liver tissues, transforming growth factor and platelet-derived growth factor mRNA expression levels, and p70s6k and p-p70s6k protein levels in the experimental groups. The treated groups (BDL+/Rapa++ and BDL+/Rapa+) showed decreased staining and expression levels compared with the un-treated BDL group (BDL+/Rapa-). These results suggested that rapamycin has an inhibitory effect on liver fibrosis and is therefore a potential treatment drug.
Footnotes
Supported by Grants from YUHAN Cooperation
P- Reviewers: He JY, Kouroumalis E, Tsuchiya A S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–S53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Wiegand J, Berg T. The etiology, diagnosis and prevention of liver cirrhosis: part 1 of a series on liver cirrhosis. Dtsch Arztebl Int. 2013;110:85–91. doi: 10.3238/arztebl.2013.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Aziz G, Lebeau G, Rescan PY, Clément B, Rissel M, Deugnier Y, Campion JP, Guillouzo A. Reversibility of hepatic fibrosis in experimentally induced cholestasis in rat. Am J Pathol. 1990;137:1333–1342. [PMC free article] [PubMed] [Google Scholar]
- 4.Aronson DC, Chamuleau RA, Frederiks WM, Gooszen HG, Heijmans HS, James J. Reversibility of cholestatic changes following experimental common bile duct obstruction: fact or fantasy? J Hepatol. 1993;18:85–95. doi: 10.1016/s0168-8278(05)80014-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee BS, Kim NJ, Jeong HY, Lee HY, Kang DY, Noh SM. Changes in serum cytokine concentration: a morphological study of liver cirrhosis induced by common bile duct ligation in rats. Korean J Intern Med. 2003;18:6–12. doi: 10.3904/kjim.2003.18.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isaka Y, Fujiwara Y, Ueda N, Kaneda Y, Kamada T, Imai E. Glomerulosclerosis induced by in vivo transfection of transforming growth factor-beta or platelet-derived growth factor gene into the rat kidney. J Clin Invest. 1993;92:2597–2601. doi: 10.1172/JCI116874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 8.Saile B, Matthes N, Knittel T, Ramadori G. Transforming growth factor beta and tumor necrosis factor alpha inhibit both apoptosis and proliferation of activated rat hepatic stellate cells. Hepatology. 1999;30:196–202. doi: 10.1002/hep.510300144. [DOI] [PubMed] [Google Scholar]
- 9.Pinzani M, Knauss TC, Pierce GF, Hsieh P, Kenney W, Dubyak GR, Abboud HE. Mitogenic signals for platelet-derived growth factor isoforms in liver fat-storing cells. Am J Physiol. 1991;260:C485–C491. doi: 10.1152/ajpcell.1991.260.3.C485. [DOI] [PubMed] [Google Scholar]
- 10.Borkham-Kamphorst E, Kovalenko E, van Roeyen CR, Gassler N, Bomble M, Ostendorf T, Floege J, Gressner AM, Weiskirchen R. Platelet-derived growth factor isoform expression in carbon tetrachloride-induced chronic liver injury. Lab Invest. 2008;88:1090–1100. doi: 10.1038/labinvest.2008.71. [DOI] [PubMed] [Google Scholar]
- 11.Bridle KR, Li L, O’Neill R, Britton RS, Bacon BR. Coordinate activation of intracellular signaling pathways by insulin-like growth factor-1 and platelet-derived growth factor in rat hepatic stellate cells. J Lab Clin Med. 2006;147:234–241. doi: 10.1016/j.lab.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Wong L, Yamasaki G, Johnson RJ, Friedman SL. Induction of beta-platelet-derived growth factor receptor in rat hepatic lipocytes during cellular activation in vivo and in culture. J Clin Invest. 1994;94:1563–1569. doi: 10.1172/JCI117497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-q. [DOI] [PubMed] [Google Scholar]
- 14.Yang X, Yang C, Farberman A, Rideout TC, de Lange CF, France J, Fan MZ. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. J Anim Sci. 2008;86:E36–E50. doi: 10.2527/jas.2007-0567. [DOI] [PubMed] [Google Scholar]
- 15.Gaben AM, Saucier C, Bedin M, Barbu V, Mester J. Rapamycin inhibits cdk4 activation, p 21(WAF1/CIP1) expression and G1-phase progression in transformed mouse fibroblasts. Int J Cancer. 2004;108:200–206. doi: 10.1002/ijc.11521. [DOI] [PubMed] [Google Scholar]
- 16.Hashemolhosseini S, Nagamine Y, Morley SJ, Desrivières S, Mercep L, Ferrari S. Rapamycin inhibition of the G1 to S transition is mediated by effects on cyclin D1 mRNA and protein stability. J Biol Chem. 1998;273:14424–14429. doi: 10.1074/jbc.273.23.14424. [DOI] [PubMed] [Google Scholar]
- 17.Bridle KR, Popa C, Morgan ML, Sobbe AL, Clouston AD, Fletcher LM, Crawford DH. Rapamycin inhibits hepatic fibrosis in rats by attenuating multiple profibrogenic pathways. Liver Transpl. 2009;15:1315–1324. doi: 10.1002/lt.21804. [DOI] [PubMed] [Google Scholar]
- 18.Biecker E, De Gottardi A, Neef M, Unternährer M, Schneider V, Ledermann M, Sägesser H, Shaw S, Reichen J. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313:952–961. doi: 10.1124/jpet.104.079616. [DOI] [PubMed] [Google Scholar]
- 19.Carpino G, Morini S, Ginanni Corradini S, Franchitto A, Merli M, Siciliano M, Gentili F, Onetti Muda A, Berloco P, Rossi M, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37:349–356. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Ballardini G, Fallani M, Biagini G, Bianchi FB, Pisi E. Desmin and actin in the identification of Ito cells and in monitoring their evolution to myofibroblasts in experimental liver fibrosis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;56:45–49. doi: 10.1007/BF02890000. [DOI] [PubMed] [Google Scholar]
- 21.Gibelli NE, Tannuri U, Mello ES. Immunohistochemical studies of stellate cells in experimental cholestasis in newborn and adult rats. Clinics (Sao Paulo) 2008;63:689–694. doi: 10.1590/S1807-59322008000500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho SN, Lira DC, Oliveira GP, Thole AA, Stumbo AC, Caetano CE, Marques RG, Carvalho L. Decreased collagen types I and IV, laminin, CK-19 and α-SMA expression after bone marrow cell transplantation in rats with liver fibrosis. Histochem Cell Biol. 2010;134:493–502. doi: 10.1007/s00418-010-0746-2. [DOI] [PubMed] [Google Scholar]
- 23.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234–1248. doi: 10.1097/01.tp.0000219703.39149.85. [DOI] [PubMed] [Google Scholar]
- 26.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 27.Gao Y, Xu X, Ding K, Liang Y, Jiang D, Dai H. Rapamycin inhibits transforming growth factor β1-induced fibrogenesis in primary human lung fibroblasts. Yonsei Med J. 2013;54:437–444. doi: 10.3349/ymj.2013.54.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF, et al. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379–2387. doi: 10.1111/j.1600-6143.2011.03767.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim M, Curry MP, Rogers CC. Impact of sirolimus duration on hepatitis C related fibrosis progression in liver transplant recipients. Am J Transplant. 2012;12:1356–1357. doi: 10.1111/j.1600-6143.2011.03942.x. [DOI] [PubMed] [Google Scholar]
- 30.Kelly MA, Kaplan M, Nydam T, Wachs M, Bak T, Kam I, Zimmerman MA. Sirolimus reduces the risk of significant hepatic fibrosis after liver transplantation for hepatitis C virus: a single-center experience. Transplant Proc. 2013;45:3325–3328. doi: 10.1016/j.transproceed.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Chatzipantelis P, Lazaris AC, Kafiri G, Papadimitriou K, Papathomas TG, Nonni A, Patsouris ES. Cytokeratin-7, cytokeratin-19, and c-Kit: Immunoreaction during the evolution stages of primary biliary cirrhosis. Hepatol Res. 2006;36:182–187. doi: 10.1016/j.hepres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Border WA, Ruoslahti E. Transforming growth factor-beta in disease: the dark side of tissue repair. J Clin Invest. 1992;90:1–7. doi: 10.1172/JCI115821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gäbele E, Reif S, Tsukada S, Bataller R, Yata Y, Morris T, Schrum LW, Brenner DA, Rippe RA. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374–13382. doi: 10.1074/jbc.M409444200. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A. Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer. 2009;125:1710–1720. doi: 10.1002/ijc.24472. [DOI] [PubMed] [Google Scholar]
- 35.Fenton TR, Gout IT. Functions and regulation of the 70kDa ribosomal S6 kinases. Int J Biochem Cell Biol. 2011;43:47–59. doi: 10.1016/j.biocel.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 36.Zhu J, Wu J, Frizell E, Liu SL, Bashey R, Rubin R, Norton P, Zern MA. Rapamycin inhibits hepatic stellate cell proliferation in vitro and limits fibrogenesis in an in vivo model of liver fibrosis. Gastroenterology. 1999;117:1198–1204. doi: 10.1016/s0016-5085(99)70406-3. [DOI] [PubMed] [Google Scholar]


