Summary
Identification of key regulators of lipid metabolism and thermogenic functions has important therapeutic implications for the current obesity and diabetes epidemic. Here we show that Grb10, a newly identified direct substrate of mechanistic/mammalian target of rapamycin (mTOR), is expressed highly in brown adipose tissue, and its expression in white adipose tissue is markedly induced by cold exposure. In adipocytes, mTOR-mediated phosphorylation at Ser501/503 switches the binding preference of Grb10 from the insulin receptor to raptor, leading to the dissociation of raptor from mTOR and down-regulation of mTOR complex 1 (mTORC1) signaling. Fat-specific disruption of Grb10 increased mTORC1 signaling in adipose tissues, suppressed lipolysis, and reduced thermogenic function. The effects of Grb10 deficiency on lipolysis and thermogenesis were diminished by rapamycin administration in vivo. Our study has uncovered a novel feedback mechanism regulating mTORC1 signaling in adipose tissues and identified Grb10 as a key regulator of adiposity, thermogenesis, and energy expenditure.
Introduction
Adipose tissues, which include white adipose tissue (WAT) and brown adipose tissue (BAT), play an essential role in regulating whole body energy homeostasis. Excess expansion of WAT due to positive energy balance and defects in thermogenic gene expression in BAT are associated with obesity and various metabolic diseases. Recent studies also reveal the presence of a subset of cells in WAT that could be induced by environmental or hormonal factors to become “brown-like” cells and this “beigeing” process has been suggested to have strong anti-obesity and anti-diabetic benefits (Barbatelli et al., 2010; Bostrom et al., 2012; Petrovic et al., 2010). Over the last decade, great progress has been made in our understanding of the mechanisms regulating adipose tissue function and the “beigeing” process at the transcriptional level (Bostrom et al., 2012; Farmer, 2008; Jimenez-Preitner et al., 2011; Seale et al., 2007). However, the upstream signaling pathways regulating the transcriptional machinery involved in the regulation of adipogenesis, thermogenesis, and adipocyte function remain to be fully elucidated.
The mechanistic/mammalian target of rapamycin complex 1 (mTORC1) regulates many important metabolically processes including protein synthesis, lipogenesis, energy expenditure and autophagy (Laplante and Sabatini, 2012; Wullschleger et al., 2006). mTORC1 is highly active in tissues of obese and high fat diet (HFD)-fed rodents (Khamzina et al., 2005; Tremblay et al., 2005). In vitro, activation of the mTORC1 signaling pathway has been shown to suppress lipolysis, stimulate lipogenesis, and promote lipid accumulation in cells (Chakrabarti et al., 2010). On the other hand, inhibition of mTORC1 promotes triacylglycerol lipolysis and release of free fatty acids, blocks adipogenesis, and impairs the maintenance of fat cells (Chakrabarti et al., 2010; Polak et al., 2008; Soliman, 2011). However, the roles of mTORC1 in the regulation of lipid metabolism in vivo are much less clear. Although inhibition of mTOR by rapamycin or by knocking down raptor, a positive regulator of mTORC1, stimulates lipolysis through activation of adipose triglyceride lipase (ATGL) (Chakrabarti et al., 2010), adipose-tissue specific knockout of raptor had no effect on caloric intake, lipolysis, and absorption of lipids from the food, in spite of enhanced resistance to HFD-induced obesity, smaller and fewer adipocytes, and improved insulin sensitivity (Polak et al., 2008). On the other hand, fat-specific raptor knockout mice displayed enhanced expression levels of uncoupling protein 1 (UCP1), suggesting that mTORC1 regulates adipose metabolism and energy homeostasis mainly by negative regulation of mitochondrial uncoupling and energy expenditure (Polak et al., 2008).
Despite intensive studies, the precise mechanisms underlying the action of mTORC1 and its regulation, especially in vivo, remain elusive. One reason is that only a few direct substrates of mTOR have been identified so far. While phosphorylation of these substrates such as S6K and 4EBP1 by mTORC1 has been shown to play an important role in the regulation of translation, protein and lipid synthesis, cell size and growth (Laplante and Sabatini, 2012; Magnuson et al., 2012), it appears to be insufficient to explain the myriad functions of mTORC1. Very recently, the Src homology 2 (SH2)-domain-containing adaptor protein Grb10 (Growth factor receptor binding protein-10) has been identified as a direct substrate of mTOR (Hsu et al., 2011; Yu et al., 2011). Grb10 was originally identified by us and others as a negative regulator of insulin and IGF-1 signaling (Holt and Siddle, 2005; Lim et al., 2004a). The gene polymorphisms of Grb10 are reported to be related to the development of type 2 diabetes mellitus (Rampersaud et al., 2007; Vitai et al., 2009), yet the underlying mechanisms remain unknown. Grb10 is highly expressed in several mouse tissues including the pancreas, adipose tissues, and the brain (Wang et al., 2007; Zhang et al., 2012). Brain-specific ablation of Grb10 expression resulted in increased social dominance specifically among other aspects of social behavior (Garfield et al., 2011). Ablation of Grb10 in the pancreas improved diet-induced glucose intolerance and protected mice from streptozotocin-induced β-cell apoptosis (Zhang et al., 2012). However, the functional roles of Grb10 in adipose tissues remain unclear.
In the current study, we investigated the physiological function of Grb10 in adipose tissues. We show that, similar to UCP-1, Grb10 is highly expressed in BAT and its expression in both BAT and subcutaneous inguinal WAT (iWAT) is stimulated by cold stress. In addition, adipose tissue-specific ablation of Grb10 promotes adiposity and suppresses non-shivering thermogenesis and energy expenditure. We also demonstrate that Grb10 negatively regulates mTORC1 signaling in cells via a PI 3-kinase/Akt-independent novel mechanism and the mTOR-mediated phosphorylation switches the binding preference of Grb10 from the insulin receptor to raptor, thus providing a molecular mechanism to inhibit mTORC1.
Results
Grb10 expression is significantly induced during adipogenesis and by cold stress
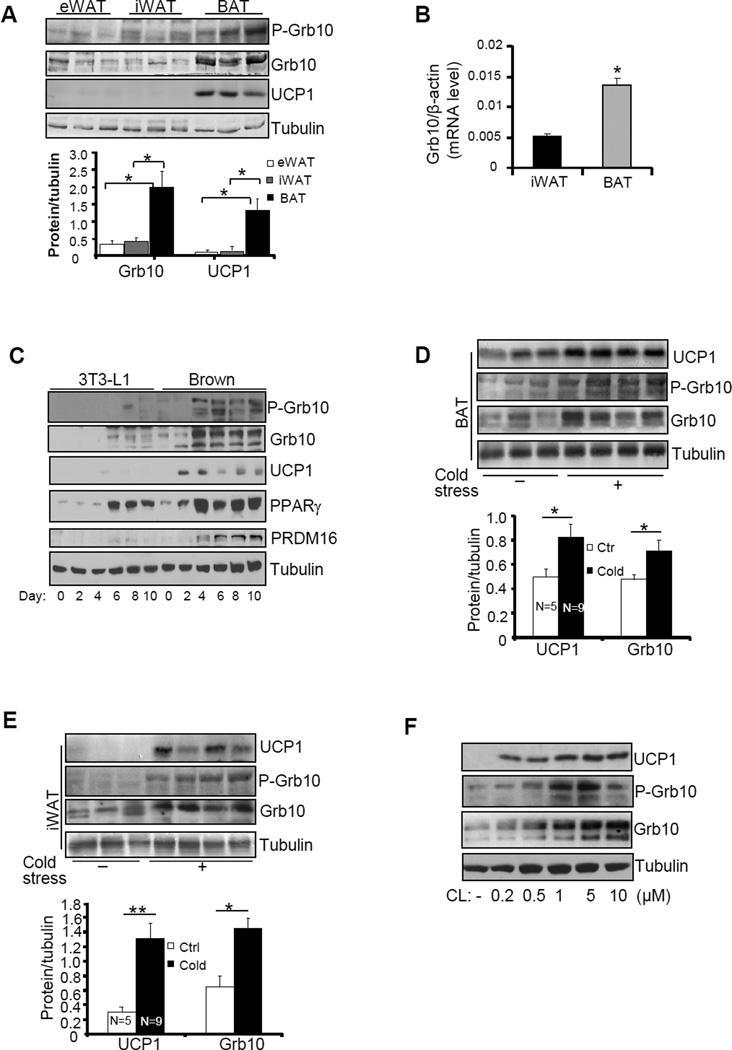
Grb10 was detected in epididymal WAT (eWAT), iWAT, and interscapular BAT, but the protein and phospho-protein levels of Grb10 were notably higher in BAT than in WAT (Fig. 1A). Consistent with this finding, the mRNA level of Grb10 was also significantly higher in BAT than in iWAT (Fig.1B). Western blot revealed multiple bands of Grb10 which may be due to alternative splicing events (Dong et al., 1997a; Lim et al., 2004a) and/or phosphorylation (Dong et al., 1997a; Langlais et al., 2005; Yu et al., 2011). Consistent with these in vivo findings, the cellular levels of Grb10 and phospho-Grb10 were much higher in brown adipocytes than that in 3T3-L1 adipocytes and Grb10 expression was greatly induced during differentiation of both cells (Fig. 1C). Cold exposure significantly stimulated the mRNA and protein levels of UCP1 and Grb10 in BAT and iWAT in mice (Figs. 1D, 1E, 4C and 4D), and protein levels of Grb10 in rat (Fig. S1A). The cellular levels of Grb10 and phospho-Grb10 were also stimulated by CL316243, a β3 adrenoceptor agonist that mimics cold stress, in brown adipocytes (Fig. 1F). Interestingly, high expression levels of Grb10 were detected not only in BAT but also iWAT of A/J mice (Jackson Laboratory) (Figs. S1B and S1C), which have been shown to possess higher thermogenic activities compared to C57BL/6 mice (Guerra et al., 1998). Taken together, these results suggest that Grb10 may play an important role in regulating thermogenic activities in BAT and the browning effect in WAT.
Fig. 1. Grb10 is highly enriched in brown adipose tissue and its expression level is positively correlated with the expression of UCP1 in adipose tissue.
(A) The expression levels of Grb10 and UCP1 and the phosphorylation of Grb10 at Ser501/503 in WAT and BAT of 3 month-old male mice. (B) The mRNA levels of Grb10 in BAT and iWAT of 3 month-old male mice. (C) Grb10 expression and phosphorylation at Ser501/503 during brown preadipocyte differentiation. Grb10 protein levels and phosphorylation at Ser501/503 in BAT (D) and iWAT (E) in 5 month-old Grb10fKO male mice (n=9) and their control littermates (n=5) individually housed in cages at room temperature or at 4 °C for 4 hrs everyday with free access to food and water continuously for 4 days. (F) Grb10 protein levels and phosphorylation at Ser501/503 in differentiated brown adipocytes treated with or without CL316243 at different doses as indicated for 20 hours. All data are presented as mean ± S.E.M. *<0.05 and **<0.01.
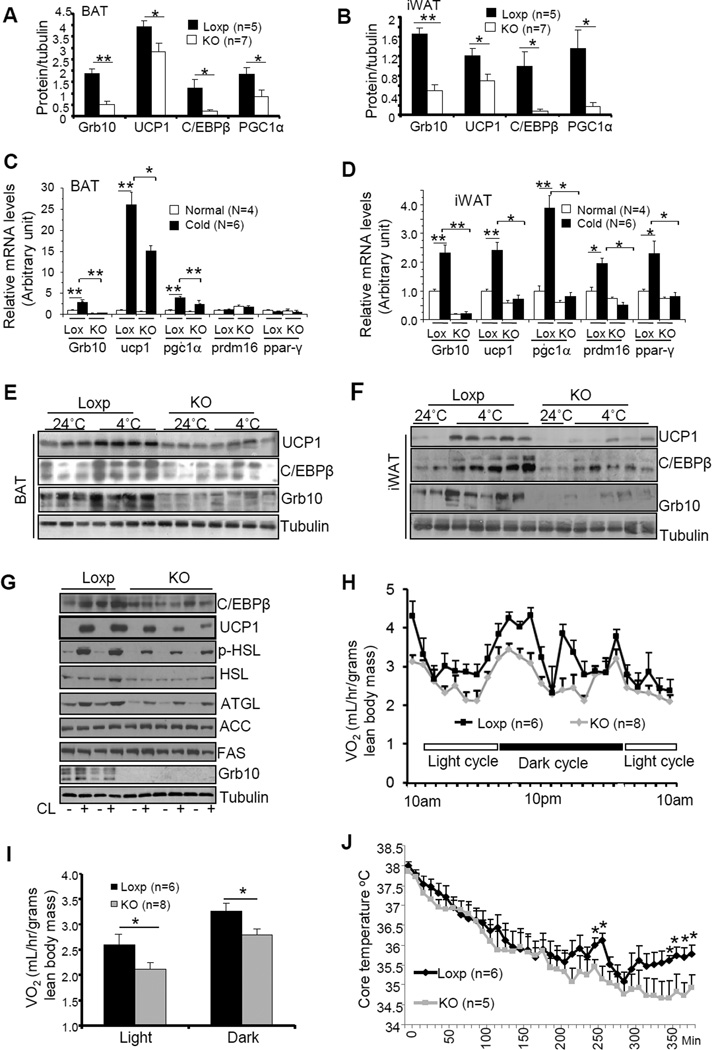
Fig. 4. Fat-specific disruption of Grb10 impaired thermogenic function in BAT and iWAT.
The protein levels of thermogenic proteins in BAT (A) and iWAT (B) of 5 month-old Grb10fKO and control mice. Cold exposure-induced mRNA expression in BAT (C) and iWAT (D) of Grb10fKO (KO) mice and Loxp control mice. The mRNA levels were determined by RT-PCR and normalized to β-actin. Cold exposure-induced protein expression in BAT (E) and iWAT (F) of Grb10fKO mice and control mice. (G) CL316243 (CL) (1μM, 22h)-induced protein expression in primary brown adipocytes isolated from Grb10fKO and Loxp control mice. (H) The oxygen consumption of Grb10fKO and control mice was measured during a 48 h period including two light cycles and dark cycles. (I) The average of oxygen consumption shown in (H) was normalized to whole body mass and analyzed by t-test. (J) Body temperature of mice exposed to cold (4°C) in the feeding condition. All data are presented as mean ± S.E.M. *<0.05 and **<0.01.
Adipose tissue-ablation of Grb10 promotes lipid accumulation in WAT and BAT
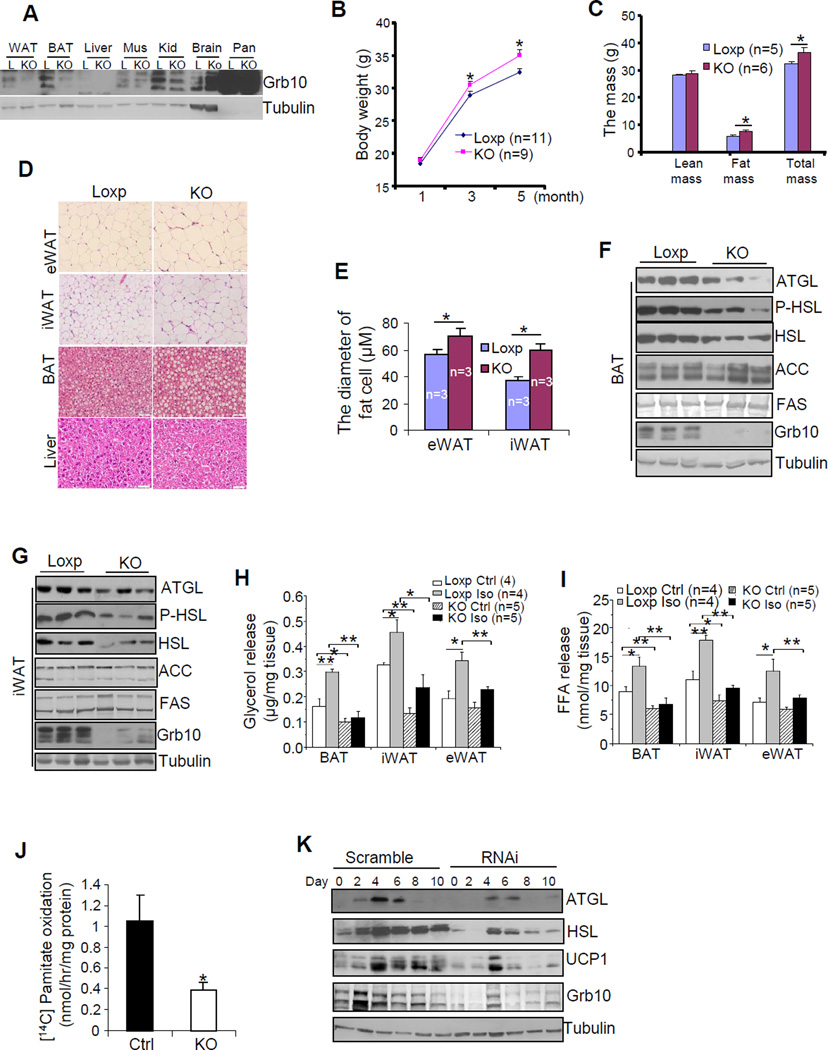
To determine the in vivo roles of Grb10, we generated fat-specific Grb10 knockout mice (Grb10fKO) by crossing the Grb10 floxed mice (Zhang et al., 2012) with the adiponectin-cre mice (Wang et al., 2010). Grb10 was specifically suppressed in WAT and BAT of the Grb10fKO mice, but not other tissues examined (Fig. 2A). The presence of residual Grb10-immunoreactive band in BAT and WAT may reflect the existence of cells other than adipocytes in these tissues. After 5 months, the wild-type control mice gained 55% of their initial weight at 1 month-old, but the Grb10fKO mice gained 70% of their initial body weight (Fig. 2B). At the age of 5 months, the Grb10fKO mice displayed increased fat mass and total body mass, but showed no significant difference in food intake, fasting glucose levels, bone mineral density (BMD), or lean mass compared with the loxp control littermates when maintained on the regular diet (Fig. 2C and data not shown). Fat-specific knockout of Grb10 also led to enlarged eWAT and increased lipid accumulation in interscapular BAT (Fig. S1E). Consistent with these results, H&E staining revealed that white fat cell sizes (Figs. 2D and 2E), but not cell number (Fig. S3F), were increased in eWAT and iWAT of the Grb10fKO mice compared to the control littermates. On the other hand, Grb10 deficiency had little effect on liver morphology under normal chow feeding condition (Fig. 2D). In addition, larger multilocular lipid droplets were observed in BAT of the Grb10fKO mice compared to the control mice (Fig. 2D). Furthermore, the expression levels of lipolytic enzymes such as ATGL and hormone-sensitive lipase (HSL) as well as the phosphorylation of HSL at Ser563 were decreased in BAT (Fig. 2F), iWAT (Fig. 2G), and eWAT (Fig. S1D) of Grb10fKO mice compared to control mice. Consistent with these findings, β-adrenoceptor agonist-stimulated glycerol and fatty acid release were significantly reduced in brown, inguinal, and epididymal fat isolated from overnight fasted Grb10fKO mice compared to their control mice (Figs. 2H and 2I). In addition, fatty acid oxidation was significantly reduced in primary brown adipocytes isolated from Grb10fKO mice compared with the control mice (Fig. 2J). However, fat-specific ablation of Grb10 had no significant effect on the expression levels of lipogenic enzymes such as acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) (Figs. 2F, 2G, and S1D). Consistently, the expression levels of ATGL and HSL, but not FAS and ACC, were markedly reduced in Grb10-suppressed brown adipocytes (Fig. 2K), suggesting that the effect of Grb10 on lipolysis in mouse fat tissues is cell autonomous. Similar phenotypes were also observed in fat-specific Grb10 knockout mice generated by using the aP2 promoter (Grb10fKO-aP2) (Fig. S2).
Fig. 2. Fat-specific disruption or suppression of Grb10 leads to impaired lipid metabolism and fat expansion.
(A) The expression of Grb10 in different tissue homogenates of 3 month-old male Grb10fKO (KO) and Loxp (L) control mice. Mus, muscle; Kid, kidney; Pan, Pancreas; (B) The body weight gain of male Grb10fKO and control mice. (C) Tissue composition of the Grb10fKO and Loxp control mice as determined by DEXA. (D) A hemotoxylin and eosin (H&E) staining of eWAT, interscapular BAT, and liver from 5 month-old Grb10fKO and control mice are shown. (E) The average diameters of fat cells in Grb10fKO and control mice were analyzed and quantified by the NIH Image J software. The protein levels of ACC, FAS, ATGL, and HSL and the phosphorylation of HSL at Ser563 in BAT (F) and iWAT (G) of Grb10fKO (KO) and control loxp mice. Fat tissues isolated from 5 month-old male Grb10fKO and control mice were treated with or without isoproterenol (10 μM) for 6 hrs. Glycerol release (H) and free fatty acid release (I) were determined as described in Experimental Procedures. (J) The oxidation of 14C-pamitate in primary brown adipocytes isolated from Grb10fKO and control mice. (K) The expression of ATGL, HSL, and UCP1 in Grb10-suppressed brown adipocytes and scramble control cells. All data are presented as mean ± S.E.M. *<0.05 and **<0.01.
Fat tissue-specific ablation of Grb10 predisposes mice to diet-induced obesity, hepatosteatosis, and insulin resistance
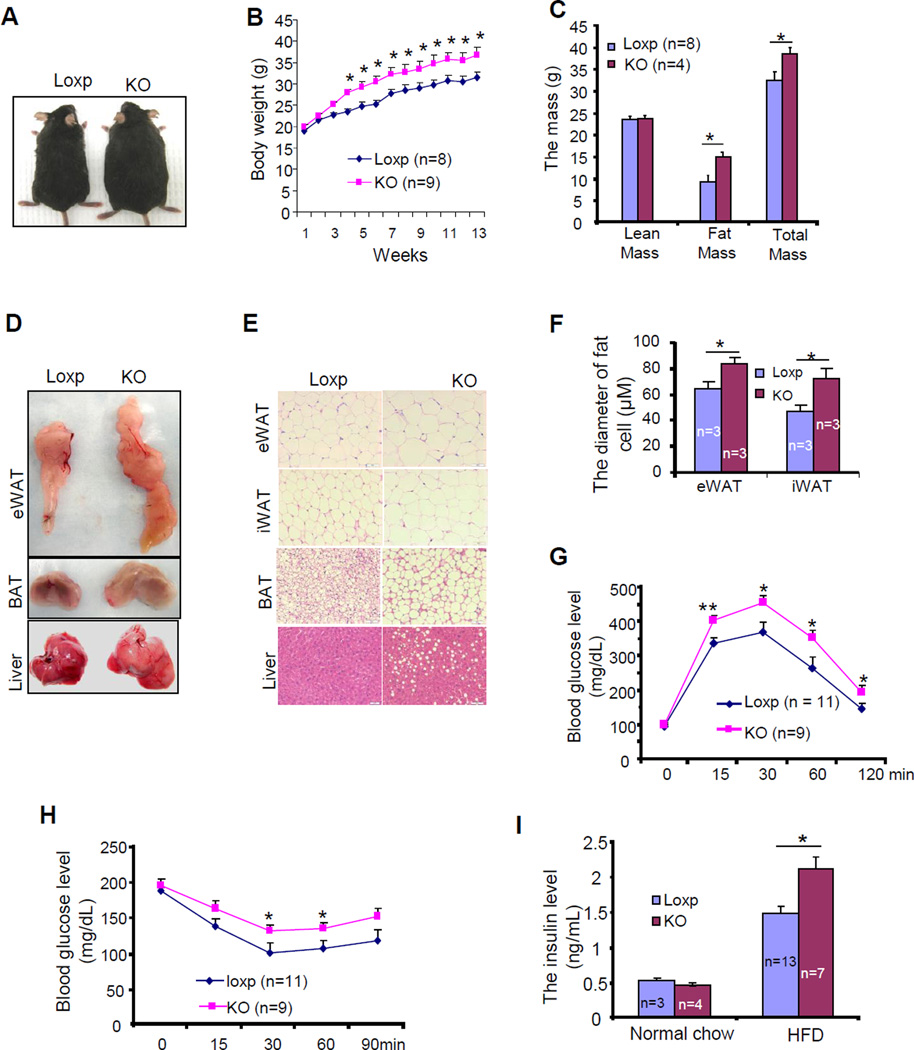
The finding that fat-specific knockout of Grb10 increased animal adiposity encouraged us to investigate the potential effect of Grb10 on diet-induced obesity and insulin resistance in vivo. On a HFD (45% kcal from fat) for 12 weeks, no significant difference in food intake was observed between Grb10fKO mice and their control mice (Fig. S3G). However, the difference in body size and weight persisted and was even more pronounced between Grb10fKO mice and the control littermates (Figs. 3A and 3B). The fat mass and total mass of the Grb10fKO mice were also significantly greater than that of the control littermates on HFD (Fig. 3C). Consistent with this finding, the Grb10fKO mice showed increased fat mass, larger fat cell size, enlarged fatty liver, and increased hepatosteatosis compared to wild-type littermates when fed with a HFD (Figs. 3D and 3E). However, HFD feeding had no significant effect on fat cell numbers between Grb10fKO mice and control littermates (Fig.S3F). Glucose and insulin tolerance tests revealed that the Grb10fKO mice were more glucose- and insulin-intolerant compared to control littermates (Figs. 3G and 3H). In addition, serum fasting insulin levels were significantly increased in Grb10fKO mice compared to control littermates under HFD condition (Fig. 3I). Similar metabolic phenotypes were also observed in HFD-fed Grb10fKO-aP2 mice (Fig. S3), further demonstrating that Grb10 in adipose tissues plays an important role in regulating adipocyte function and whole-body energy homeostasis.
Fig. 3. Fat tissue-specific disruption of Grb10 exacerbated diet-induced obesity and insulin resistance.
(A) A representative photograph of 5 month-old male Grb10fKO (KO) and Loxp control mice fed with HFD for 12 weeks. (B) Body weight gain of Grb10fKO and control mice during HFD feeding. (C) Tissue composition of Grb10fKO (KO; n=4) and control littermates (Loxp; n=8) fed with HFD for 12 weeks. A representative image (D) and H&E staining (E) of eWAT, BAT and liver tissue of Grb10fKO (KO) and Loxp control mice fed with HFD for 12 weeks. (F) Average diameters of fat cells in the HFD-fed Grb10fKO and control mice. Glucose tolerance test (G) and insulin tolerance test (H) were performed on Grb10fKO (KO) and Loxp control mice fed with HFD for 12 weeks. (I) Fasting serum insulin levels in Grb10fKO and Loxp control mice fed a normal chow or HFD for 12 weeks. All data are presented as mean ± S.E.M. *<0.05 and **<0.01.
Grb10 plays a critical role in regulating thermogenic gene expression and energy expenditure
To further elucidate the role of Grb10 in the regulation of thermogenesis, we examined the expression levels of several thermogenic genes in Grb10fKO mice. The protein levels of UCP1, PGC1α, and C/EBPβ were significantly reduced in BAT (Fig. 4A) and iWAT (Fig. 4B) of Grb10fKO mice compared to the wild-type littermates. Cold exposures significantly increased the mRNA levels of Grb10, UCP1, and PGC-1α in both BAT and iWAT of the control mice (Figs. 4C and 4D). The stimulatory effect of cold exposure, however, was significantly suppressed in adipose tissues of the Grb10fKO mice (Figs. 4C and 4D). Interestingly, cold exposure had little effect on the mRNA levels of PRDM16 and PPARγ in BAT of the control mice, but significantly stimulated the expression of these genes in iWAT of the mice (Figs. 4C and 4D). The stimulatory effects of cold exposure on the expression of PRDM16 and PPARγ were all significantly reduced in iWAT of Grb10fKO mice compared to the control littermates (Fig. 4D). Consistent with these findings, Western blot experiments revealed that cold exposure greatly increased the protein levels of UCP1, Grb10, and C/EBPβ in BAT and iWAT of control mice and the stimulatory effect of cold exposure on the expression of these proteins was markedly reduced in BAT and iWAT of Grb10fKO mice compared to the control littermates (Figs. 4E, and 4F). In addition, CL316243-stimulated thermogenic and lipolytic gene expression was impaired in primary brown adipocytes of the Grb10fKO mice compared to control cells (Fig.4G), suggesting that the effect of Grb10 deficiency on lipolysis and thermogenesis is cell autonomous. Furthermore, Grb10fKO mice displayed reduced resting metabolic rate (Fig. S1F) and oxygen consumption rate (Figs. 4H and 4I), but showed no significant difference in activities and respiratory quotient (Figs. S1G and S1H) compared to the control littermates. The core body temperature (Fig. S1I), and cold tolerance (Fig. 4J) were all significantly reduced in Grb10fKO mice compared to their respective control mice. Similar findings were also observed in Grb10 fKO-aP2 mice (Figs. S4A-S4E). Taken together, these results suggest that Grb10 plays an important role in regulating cold adaption and thermogenic function.
Grb10 is able to inhibit mTORC1 signaling via a PI3K/Akt-independent mechanism in brown adipocytes
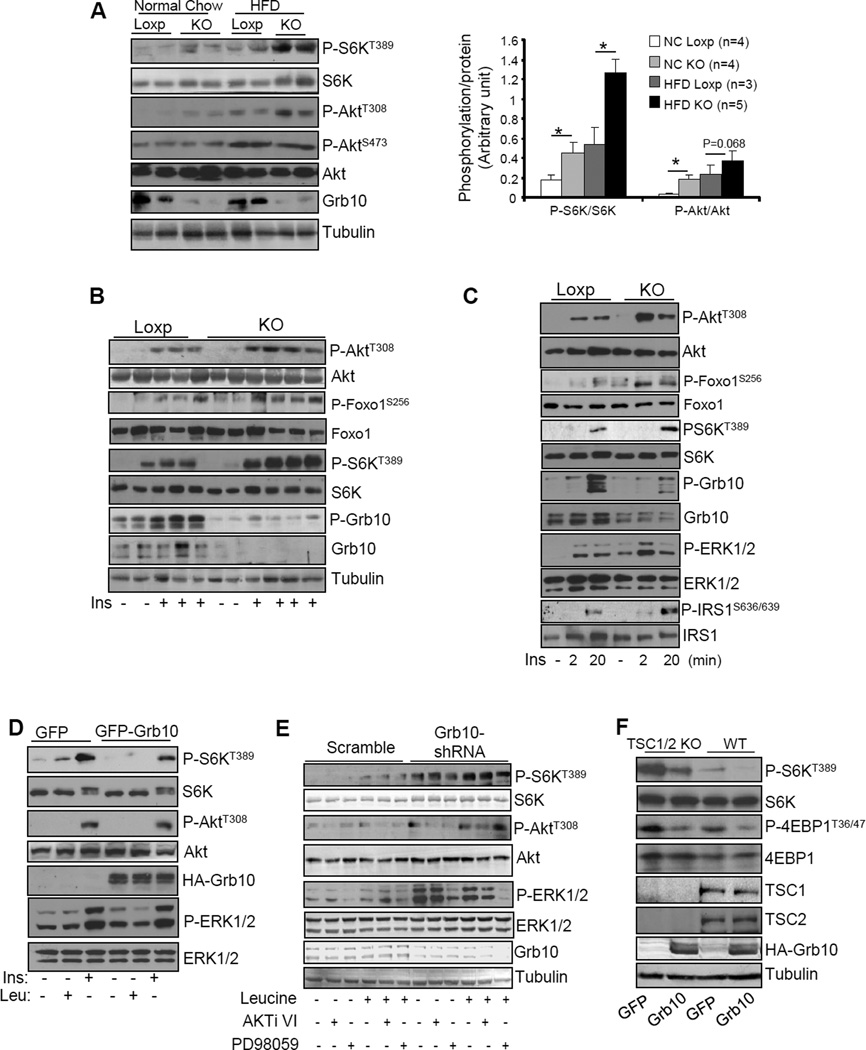
Grb10 was originally identified by us and others as a negative regulator of insulin-stimulated PI3K/Akt signaling (Dong et al., 1997b; Liu and Roth, 1995; O'Neill et al., 1996; Ooi et al., 1995). Consistent with this finding, fat-specific ablation of Grb10 increased Akt phosphorylation in BAT (Figs. 5A) and iWAT (Fig. S4I) of mice fed a regular diet. Fat-specific knockout of Grb10 in mice also led to a marked increase in S6 kinase phosphorylation at Thr389 in BAT, iWAT and eWAT (Figs. 5A, S4G and S1D), suggesting that Grb10 negatively regulates mTORC1 signaling in vivo. The phosphorylation of Akt and S6K was further increased when the mice were on HFD compared to the mice fed with normal chow (Figs. 5A and S4G), which is consistent with previous findings that basal Akt (Liu et al., 2009) and S6K (Khamzina et al., 2005; Um et al., 2006) phosphorylation was significantly elevated in tissues of obese mice. To further elucidate the roles of Grb10 in Akt/mTORC1 signaling, we examined insulin-stimulated Akt and S6K phosphorylation in various fat tissues of the Grb10fKO mice. Grb10 deficiency significantly potentiated insulin-stimulated phosphorylation of Akt and S6K in BAT (Fig. 5B) and iWAT (Fig. S4F). In primary brown adipocytes isolated from the Grb10fKO mice, insulin treatment led to a rapid increase in Akt phosphorylation, which peaked at 2 min post insulin treatment (Fig. 5C). Insulin treatment also led to a significant increase in S6K phosphorylation, but the phosphorylation peaked at 20 min (Fig. 5C). At this time point, phosphorylation of IRS1 at Ser636/639, an mTORC1-mediated phosphorylation site (Ozes et al., 2001), was markedly enhanced whereas insulin-stimulated phosphorylation of Akt and Foxo1 was greatly decreased (Fig. 5C). To determine whether Grb10 could negatively regulate mTORC1 signaling via a PI3K/Akt-independent mechanism, we examined the effect of Grb10 on S6K phosphorylation in brown adipocytes in response to insulin and leucine, the latter has been shown to activate the mTORC1 signaling pathway via an Akt-independent pathway (Hinault et al., 2004; Tokunaga et al., 2004). Under our experimental conditions, adenovirus-mediated overexpression of Grb10 greatly inhibited insulin- and leucine-stimulated S6K phosphorylation at Thr389, but had no significant effect on insulin-stimulated Akt and ERK1/2 phosphorylation (Fig. 5D), suggesting that Grb10 inhibits mTORC1 signaling by targeting at a site downstream of Akt. Consistent with this, inhibition of Akt or ERK1/2 by AKTi VI or PD98059 treatment, respectively, had little effect on leucine-stimulated S6K phosphorylation in Grb10 suppressed brown preadipocytes (5E). In addition, overexpression of Grb10 inhibited S6K phosphorylation in control and TSC1/2-knockout cells (Fig. 5F), further confirming that Grb10 is able to negatively regulate mTORC1 signaling via a PI3K/Akt-independent novel mechanism in brown adipocytes.
Fig. 5. Grb10 negatively regulates mTORC1 signaling in a PI3K/Akt-independent manner in brown adipocytes.
(A) Phosphorylation of S6K and Akt in BAT of Grb10fKO (KO) and Loxp control mice fed normal chow or HFD for 12 weeks. Data are presented as mean ± S.E.M. *<0.05. (B) Insulin and mTORC1 signaling in BAT of Grb10fKO and control mice. (C) The insulin-stimulated Akt and S6K phosphorylation in primary brown adipocytes isolated from Grb10fKO (KO) and Loxp control mice. (D) Brown adipocytes were infected with adenovirus encoding GFP or GFP plus Grb10 and then treated with or without 10 nM insulin (Ins) for 10 min or 20 mM leucine (Leu) for 30 min. (E) The phosphorylation and protein level of S6K, Akt and ERKp44/42 in Grb10-shRNA-suppressed and scramble control brown preadipocytes treated with or without 5μM AKTi VI or 4μM PD98059 for 1 hour, followed with 20 mM leucine for 30 minutes. (F) The phosphorylation and protein level of S6K and 4EBP1 in TSC1/2 knockout and wild-type MEFs infected with adenoviruses encoding GFP or GFP plus Grb10.
mTOR-mediated phosphorylation is essential for Grb10 to feedback regulate mTOR signaling in brown adipocytes
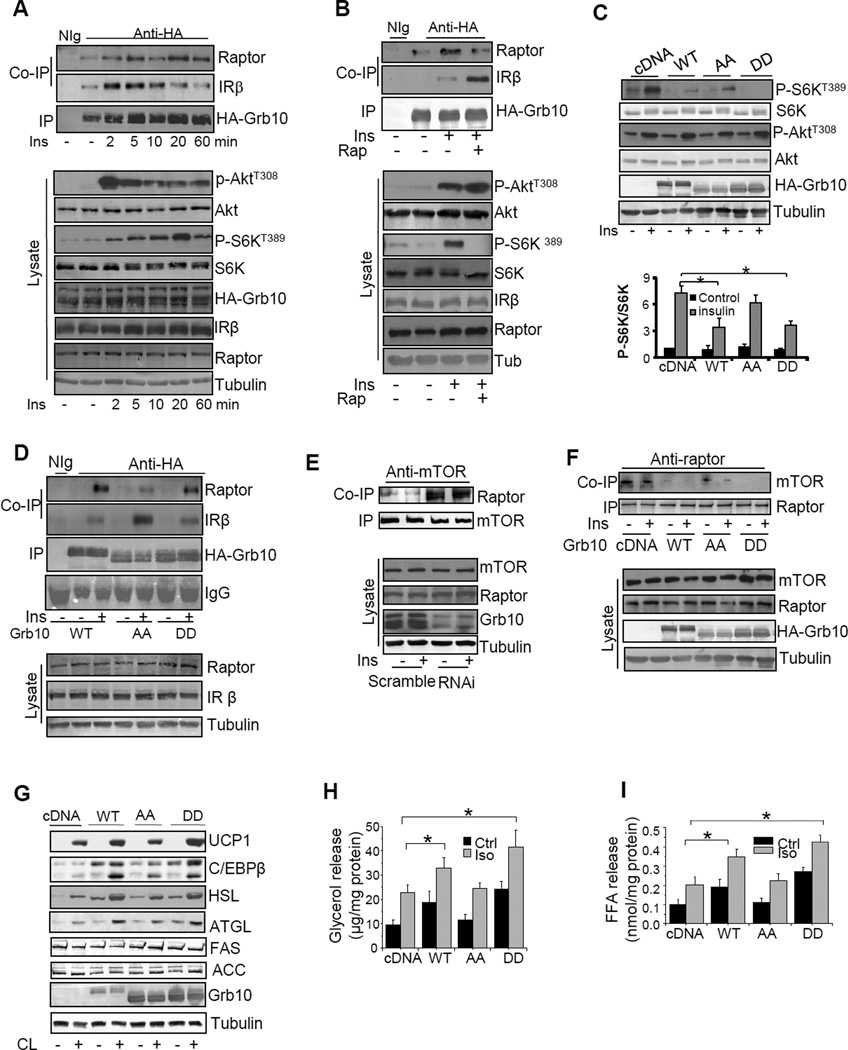
We previously showed that Grb10 interacts with the tyrosine phosphorylated insulin receptor (IR) in a number of cells including 3T3-L1 adipocytes (Dong et al., 1997a; Dong et al., 1997b; Liu and Roth, 1995). Consistent with this finding, Grb10 interacted with IR in brown adipocytes (Fig. 6A). The interaction between Grb10 and the IR was decreased after extended insulin treatment (Fig. 6A), suggesting that a negative regulation of the interaction might have occurred under this condition. In agreement with previous findings that Grb10 interacts with raptor in HEK293 cells (Hsu et al., 2011; Yu et al., 2011), we found that Grb10 interacted with raptor in brown adipocytes (Fig. 6A) and in HEK293 cells (data not shown). Interestingly, the interaction between Grb10 and raptor in brown adipocytes was stimulated by insulin treatment in a time-dependent manner (Fig. 6A), demonstrating a negative correlation between Grb10 interaction with the IR and raptor.
Fig. 6. Phosphorylation of Grb10 plays a critical role in its feedback inhibition of mTOR signaling in brown adipocytes.
(A) The immunoprecipitation (IP) of HA-Grb10 and Co-IP of raptor in starved brown adipocytes transiently expressing HA-tagged Grb10 treated with or without 10 nM insulin at different times as indicated. (B) The IP of HA-Grb10 and Co-IP of raptor and IRβ in starved brown adipocytes transiently expressing HA-tagged Grb10 treated with or without 5 nM rapamycin for 60 min, followed with or without 10 nM insulin for 10 min. (C) Insulin-stimulated phosphorylation of Akt and S6K and the expression of Grb10 in HEK293 cells transiently expressing wild-type Grb10 (WT), Grb10S501/503A (AA), or Grb10S501/503D (DD) treated with or without 10 nM insulin for 20 min. (D) The IP of HA-Grb10 and Co-IP of raptor and IRβ in starved HEK293 cells transiently expressing wild-type HA-tagged Grb10 (WT), Grb10S501/503A (AA), or Grb10S501/503D (DD) treated with or without 10 nM insulin. (E) The immunoprecipitation (IR)of mTOR and Co-IP of raptor in starved Grb10-suppressed brown preadipocytes (RNAi) and scramble control cells.(F) The IP of raptor and Co-IP of mTOR in starved HEK293 cells transiently expressing wild-type (WT) Grb10, Grb10S501/503A (AA), or Grb10S501/503D (DD) mutant treated with or without 10 nM insulin for 20 min. (G) The effect of overexpression of wild-type (WT), Grb10S501/503A (AA), or Grb10S501/503D (DD) on CL316343 (CL)-induced thermogenic and lipolytic gene expression in primary brown adipocytes isolated from Grb10fKO mice. Cells were treated with or without 1μM CL316243 for 22 hours. (H) The effect of overexpression of WT, AA, or DD mutants of Grb10 on isoproterenol (Iso)-induced glycerol released in primary brown adipocytes isolated from Grb10fKO mice. Data are presented as mean ± S.E.M. *<0.05. (I) Fatty acid released from Grb10-deficient primary brown adipocytes transfected with vector or vectors encoding WT or the phosphorylation site mutants of Grb10 (AA or DD). Data are presented as mean ± S.E.M. *<0.05.
To determine whether Grb10 phosphorylation affects the interaction between Grb10 and the IR or raptor, HEK293 cells overexpressing HA-tagged Grb10 were serum starved and treated with or without insulin in the presence or absence of rapamycin. Co-immunoprecipitation experiments showed that Grb10 interacted with both the IR and raptor and the interaction between Grb10 and raptor was stimulated by insulin (Fig. 6B). Treating the cells with rapamycin greatly stimulated the interaction between Grb10 and the IR, but suppressed the interaction between Grb10 and raptor (Fig. 6B), suggesting that the mTOR-mediated phosphorylation of Grb10 may provide a mechansim by which Grb10 specifically regulates distinct IR downstream signaling pathways. To futher test this, we transiently expressed wild-type Grb10 or the Grb10S501/503A (AA) and Grb10S501/503D (DD) mutants in HEK293 cells. Cells were serum starved and treated with or without insulin for 20 minites. Overexpression of wild-type Grb10 or Grb10S501/503D, but not Grb10S501/503A, greatly inhibited insulin-stimulated S6K phosphorylation in HEK293 cells (Fig. 6C). However, overexpression of wild-type or mutants of Grb10 had little effect on insulin-stimulated Akt phosphorylation under this condition (Fig. 6C), consistent with the finding that little Grb10 was found to be associated with the IR in brown adipocytes at this time point (Fig. 6B). Treating HEK293 cells with insulin for 10 min greatly stimulated the interaction between raptor and wild-type Grb10 or Grb10S501/503D (Fig. 6D). Under the same condition, much less raptor was co-immunoprecipitated by the AA mutant of Grb10 (Fig. 6D). On the other hand, the AA mutant immunoprecipitated more IR than the wild-type Grb10 and Grb10S501/503D (Fig. 6D). However, overexpression of Grb10S501/503D alone did not promote Grb10 interaction with raptor under serum-starved conditions (Fig. 6D), suggesting that phosphorylation at Ser501/503 may be necessary but not sufficient to trigger the binding of Grb10 to raptor. Raptor positively regulates mTOR downstream activation by binding to mTOR, and the stability of Raptor-mTOR complex has been shown to be nutrient-but not insulin-sensitive in cells (Kim et al., 2002). Consistent with these findings, insulin treatment had little effect on the association between mTOR and raptor in brown adipocytes (Figs. S4H and 6E) . However, suppressing Grb10 significantly increased the interaction between mTOR and raptor in brown adipocytes (Figs. S4H and 6E), suggesting that Grb10 negatively reguates the stability of raptor-mTOR complex. On the other hand, overexpression of wild-type but not the AA mutant of Grb10 inhibited the association between mTOR and raptor in HEK293 cells (Figs. 6F and S4I). Taken together, these results indicate that mTOR-mediated phosphorylation of Grb10 at Ser501/503 inhibits Grb10 interaction with the IR but contributes to Grb10 interaction with raptor, leading to the dissociation of raptor from mTOR and thus reduced mTORC1 signaling. Consistent with this, overexpression of wild-type and the DD, but not the AA mutant of Grb10, potentiated CL316243-stimulated lipolytic and thermogenic gene expression (Fig. 6G), increased basal and isoproterenol-stimulated glycerol and fatty acid release (Figs. 6H and 6I), and inhibited lipid accumulation (Fig. S4J) in primary brown adipocytes.
Grb10 inhibits lipogenesis and promotes thermogenesis in brown adipocytes by negative regulation of the mTORC1 signaling pathway
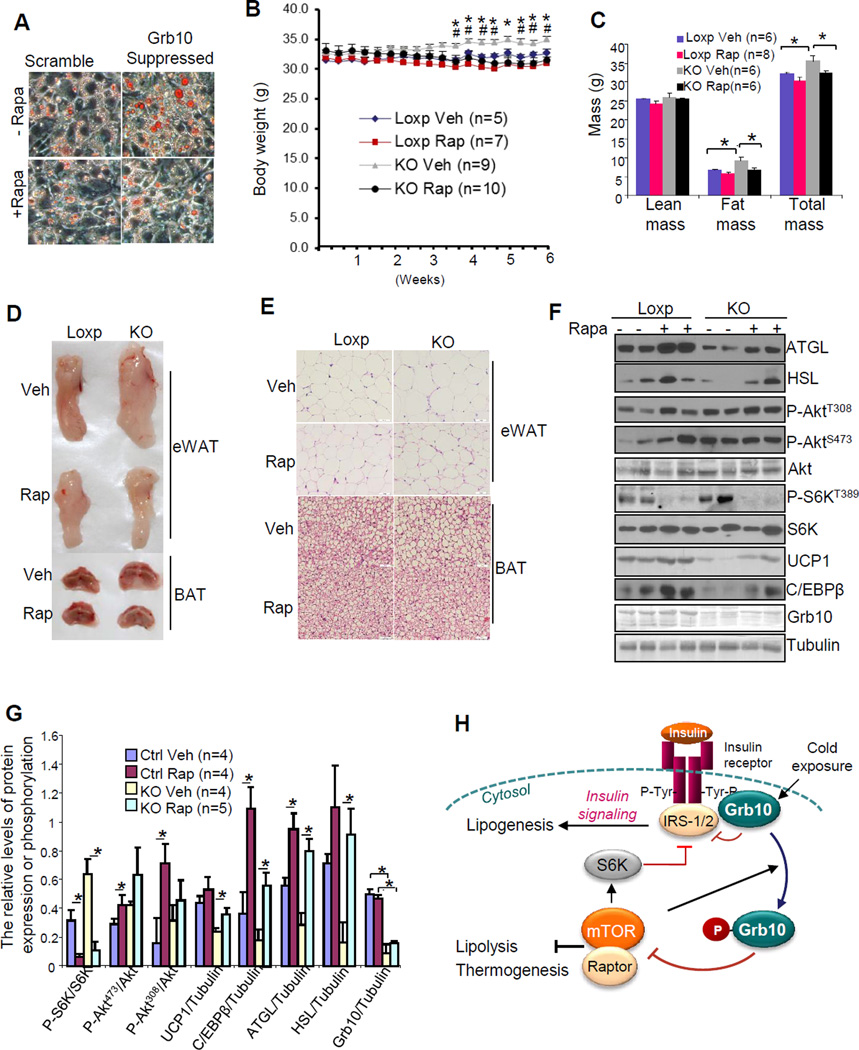
Suppressing Grb10 by siRNA increased lipid accumulation in brown adipocytes and the Grb10 deficiency-induced lipid accumulation was suppressed by treating the cells with rapamycin (Fig. 7A), suggesting that the effect of Grb10 is mediated by the mTORC1-signaling pathway. Grb10 deficiency had little effect on the mRNA and protein levels of differentiation markers such as PPARγ, C/EBPα and C/EBPγ in vivo and in brown adipocytes (Figs. 4C, 4D, S2H, S2I and S5A), and rapamycin treatment only slightly reduced the expression levels of PPARγ, C/EBPα and C/EBPγ in brown adipocytes (Fig. S5A), indicating that Grb10 deficiency-induced increase in lipid accumulation is mainly caused by reduced lipolysis rather than by increased adipocyte differentiation. To determine whether upregulation of mTORC1 signaling contributes to increased fat mass and decreased thermogenic function in vivo, we investigated whether rapamycin administration could inhibit fat mass gain and restore brown gene expression in BAT of 3 and a half-month-old male Grb10fKO mice fed with normal chow. Rapamycin treatment impaired glucose and insulin tolerance in both Grb10fKO and control mice (Fig. S5C and S5D), which has been suggested to be the result of increased gluconeogenesis in the liver (Houde et al., 2010; Lamming et al., 2012). On the other hand, Rapamycin treatment for 6-weeks markedly reduced body weight gains in 5 month-old Grb10fKO mice and diminished the difference in the body mass between Grb10fKO and control mice (Fig. 7B). Treating the Grb10fKO mice with rapamycin also greatly decreased fat mass and cell fat size in eWAT and BAT, reduced lipid accumulation in BAT, upregulated the expression of UCP1 and C/EBPβ, and partially restored the protein levels of lipolytic enzymes such as ATGL and HSL (Figs. 7C –7G). Taken together, these results further demonstrate that the effects of Grb10 on lipid metabolism and thermogenesis in fat are mediated by regulating the mTORC1-signaling pathway.
Fig. 7. mTORC1 signaling mediates the effect of Grb10 on lipid metabolism and thermogenesis in brown adipocytes.
(A) Oil red O staining of differentiated Grb10-shRNA-suppressed or scramble brown adipocytes. Post 48 hours induction of differentiation, cells were treated with or without 5 nM rapamycin for 72 hours. (B) The effect of rapamycin administration on body mass of Grb10fKO mice (KO) and Loxp littermates. 3 and half month-old mice were injected with rapamycin (4mg/Kg) or vehicle solution 3 times per week for 6 weeks. Data are presented as mean ± S.E.M. *P<0.05 (Grb10fKO mice treated with vehicle vs Grb10fKO mice treated with rapamycin) and #P<0.05 (Grb10fKO mice treated with vehicle compared with the Loxp control mice treated with vehicle). (C) The effect of rapamycin treatment on fat mass of the Grb10fKO mice (KO) and Loxp littermates described in (B). (D) The effect of rapamycin treatment on the size and morphology of distinct fat pad from Grb10fKO mice (KO) and Loxp littermates described in (B). (E) The effect of rapamycin treatment on fat cell size of Grb10fKO mice (KO) and control Loxp littermates described in (B). (F) The effect of rapamycin treatment on lipolytic and thermogenic gene expression in BAT of Grb10fKO mice and control littermates as described in (B). (G) Statistical analysis of the data presented in (F). Data are presented as mean ± S.E.M. *<0.05. (H) A proposed model on the mechanism by which Grb10 regulates insulin and mTORC1 signaling pathways in brown adipocytes. Insulin stimulates the tyrosine phosphorylation of the insulin receptor (IR), leading to the activation of downstream events such as promoting lipogenesis. The tyrosine phosphorylation of IR also creates a binding site for Grb10, providing a feedback mechanism to prevent insulin signaling from uncontrolled amplification. When mTOR is over-activated, it negatively regulates insulin signaling by S6K-mediated serine phosphorylation of IRS1/2. Activated mTOR also phosphorylates Grb10, which switches the binding affinity of Grb10 from the insulin receptor to raptor and inhibits mTORC1 signaling. Thus, mTOR-mediated phosphorylation of Grb10 provides a feedback mechanism to more efficiently and specifically control the mTORC1 signaling events.
Discussion
This study has discovered a novel negative feedback mechanism regulating mTORC1 signaling and identified Grb10 as a key regulator of lipid metabolism and thermogenic functions in adipose tissues. We demonstrated that fat-specific knockout of Grb10 led to an increase in total body mass and fat mass in mice regardless of being on a regular diet or HFD. In addition to regulating lipolysis, Grb10 appears to play an essential role in controlling brown gene expression and thermogenic function.
Adipose tissue expansion has recently been shown to have a buffering effect to store excess energy within adipose tissues, thus preventing excess lipids from being ectopically deposited in other metabolically important organs such as liver, muscle and pancreas, which are major causes of insulin resistance (Ailhaud and Reach, 2001). However, under excess nutrition, diet-induced obesity remains to be the leading cause of insulin resistance, unless the lipid storage capacity of adipose tissues is enhanced by other means, such as PPARγ stimulation (Chao et al., 2000). While we found that fat-specific disruption of Grb10 expression led to increased adiposity, we also found that the Grb10fKO mice were more insulin resistant compared to control mice when fed with HFD (Fig. 3). These findings suggest that disruption of Grb10 expression in adipose tissues promoted fat expansion but did not improve the lipid storage capacity of adipose tissues, thus leading to lipotoxicity. Our results are in agreement with the finding that lipolysis is the key step for fatty acid oxidation in adipose tissues, which is critical for lowering serum fatty acid levels and improving insulin action (Ashcroft et al., 1984).
What is the mechanism by which fat-specific ablation of Grb10 leads to increased fat mass and obesity? While we found that fat-specific knockout of Grb10 led to reduced CEBPβ, Grb10 deficiency in fat appeared to have no significant effect on the expression levels of other adipocyte differentiation markers such as PPARγ, C/EBPα and C/EBPγ in BAT and iWAT in vivo (Figs. 4C, 4D, S2H, and S2I). In addition, Grb10 deficiency had little effect on expression levels of these differentiation markers during brown adipogenesis in cells (Fig. S5A). In agreement with these findings, the expression level of Grb10 is very low at the earlier stage of adipocyte differentiation (Fig.1B), suggesting that the effect of Grb10 on lipid metabolism is mainly mediated by other mechanisms rather than inhibiting adipocyte differentiation. Consistent with this, Grb10 deficiency in adipose tissues significantly down-regulated lipolytic enzymes such as HSL and ATGL (Figs. 2F and 2G), In addition, the increased fat mass and reduced expression levels of lipolytic enzymes observed in Grb10fKO mice were rescued by rapamycin administration in vivo (Figs. 7C and 7F). Taken together, these results suggest that up-regulation of the mTORC1 signaling pathway may provide a mechanism by which Grb10 deficiency leads to reduced lipolysis and increased adiposity in cells and in vivo. While our previous studies showed that Grb10 negatively regulates insulin signaling in cells and in vivo (Lim et al., 2004b; Liu and Roth, 1995; Wang et al., 2007; Wick et al., 2003; Zhang et al., 2012), we found that ablation of Grb10 in adipose tissues had little effect on the expression level of lipogenic enzymes such as ACC and FAS (Figs. 2F – 2G). Thus, the promoting effect of Grb10 deficiency on lipid accumulation in cells is most likely via suppressing mTORC1-mediated lipolysis (Fig.7F and 7G), rather than by affecting the differentiation state of the cells (Fig. S5A).
We found that cold exposure increased total levels of phospho-Grb10, but had little effect on Grb10 phosphorylation per se since the ratio of phospho-Grb10 to total Grb10 levels was similar in adipose tissues of cold-stressed mice and control mice (Figs. 1C and 1D). These results indicate that cold stress does not stimulate mTOR activity, which is consistent with our finding that cold exposure did not stimulate S6K phosphorylation in mouse adipose tissues (data not shown). Thus, the stimulatory effect of cold exposure on thermogenic gene expression is most likely mediated by increasing the cellular levels of Grb10.
The precise mechanisms by which Grb10 regulates thermogenesis remain unknown. mTOR signaling has been shown to play a negative role in regulating the expression of UCP1 in fat tissue (Polak et al., 2008). In addition, reducing mTORC1 activity led to enhanced thermogenesis in fat tissues and improved obesity, inflammation and insulin resistance (Reilly et al., 2013). Furthermore, inhibition of mTORC1 by disruption of raptor increased the expression level of UCP1 and energy expenditure (Polak et al., 2008). Together, these results suggest that the mTORC1 signaling pathway may directly regulate thermogenic gene expression, thermogenesis, and energy expenditure. On the other hand, a recent study showed that reducing lipolysis by adipose tissue-specific ablation of Desnutrin/ATGL increased the expression of WAT-enriched genes and decreased BAT-associated genes, leading to the conversion of BAT to a white-like tissue (Ahmadian et al., 2011). In addition, inhibiting lipogenesis by fat-specific disruption of FAS increased brown-like cells in subcutaneous WAT, enhanced energy expenditure and protected from diet-induced obesity (Lodhi et al., 2012). Consistent with these results, inhibition of lipolysis has been shown to reduce thermogenic gene expression by suppressing the binding of PPARα to the UCP1 promoter (Ahmadian et al., 2011) while inhibition of de novo lipogenesis decreases PPARγ transcriptional activity and reprograms thermogenesis (Lodhi et al., 2012). Thus, it is possible that super activation of the mTORC1 signaling pathway may inhibit thermogenic gene expression through inhibiting lipolysis in Grb10fKO mice. Further studies will be needed to distinguish these possibilities.
How Grb10 mediates the signal event initiated by cold stress to regulate brown gene expression remains unknown. It is well established that cold stress increases UCP1 expression by activating the PKA signaling pathway (Cannon and Nedergaard, 2004). One possible mechanism may be that Grb10 deficiency-induced activation of the insulin and/or the mTORC1 signaling pathways inhibits the cAMP/PKA signaling pathway, leading to down-regulation of UCP-1 expression. Consistent with this, we found that basal and cold exposure-induced PKA substrate phosphorylation was reduced in adipose tissues of Grb10fKO mice compared to control mice (data not shown), which is in agreement with a recent finding that inhibition of mTORC1 via rapamycin augmented β-adrenergic agonist-stimulated and PKA-mediated lipolysis in 3T3-L1 adipocytes (Soliman, 2011). Nevertheless, it remains to be determined as to whether this crosstalk is the result of direct interactions between components of these two pathways or whether it is due to an indirect consequence of the activation of these pathways.
A novel finding made in this study is that Grb10 inhibits not only insulin but also leucine- and TSC1/2-deficiency-induced S6K phosphorylation, indicating that Grb10 inhibits mTORC1 signaling by targeting at the site downstream of TSC1/2. Since the binding of raptor to mTOR is critical for mTORC1 activation (Kim et al., 2002), the binding of Grb10 to raptor, which reduces the binding of raptor to mTOR, may thus provide a mechanism by which Grb10 negatively regulates mTORC1 activity. Our data showed that the mTOR-mediated phosphorylation switches the binding of Grb10 from the insulin receptor to raptor, uncovering a mechanism to specifically regulate signaling pathways downstream of the insulin receptor (Fig.7H). We anticipate that this feedback regulatory mechanism may be physiologically important because it may allow distinct signaling pathways downstream of the insulin receptor to be temporally and spatially regulated in order to meet the requirement of energy homeostasis in response to nutritional and environmental changes.
In summary, we have identified Grb10 as a critical regulator of lipid metabolism, the programming of the browning phenotype, and thermogenesis in adipose tissues. In addition, we have demonstrated for the first time a novel negative feedback mechanism regulating mTORC1 signaling and function. Identification of novel regulators of WAT and BAT function and understanding of the ability of adipose tissues to dissipate chemical energy may promote the development of new pharmacological tools to treat obesity and obesity-induced metabolic diseases.
Experimental Procedures
Generation of fat tissue-specific KO mice
Fat-specific Grb10 knockout mice (Grb10fKO) were generated by crossing female Grb10 floxed mice (Zhang et al., 2012) with male ap2-cre (Jackson Laboratory; Stock number: 005069) or the adiponectincre (Wang et al., 2010). The floxed littermates were used as a control.
Administration of rapamycin
Chronic rapamycin treatment was performed by injecting 4 and half month-old mice intraperitoneally with 4mg/Kg rapamycin or vehicle solution 3 times a week for 8 weeks. Rapamycin was first dissolved in 100% ethanol at 10 mg/ml, diluted in vehicle solution containing 5% Tween-80 and 5% PEG-400 in PBS to 0.5 mg/ml, and filtered (Chen et al., 2009).
Generation of Grb10-suppressed brown preadipocytes
The generation of Grb10-suppresed or scramble control brown preadipocytes is described in detail in the supplemental experimental procedures.
Primary cell culture
Primary stromal vascular fractions from interscapular brown fat depots of 8 week-old Grb10fKO or control mice were isolated and cultured as described previously (Fasshauer et al., 2001). Differentiation of brown adipocytes was performed according to the procedure as described previously (Fasshauer et al., 2001).
Fat cell size and number measurement
Fat cell size and number measurement is described in the supplemental experimental procedures.
Fatty acid oxidation
Fatty acid oxidation in primary brown adipocytes was determined by measuring 14CO2 produced from oxidation of 14C-pamitate. In brief, cells in 25 mm flasks were incubated with 1 ml medium containing 10 μM 14C-pamitate (53mCi/mmol) and 20 μM L-carnitine for 30 min at 37°C. The medium was acidified with 200 μl 2.6 N HClO4, and CO2 was trapped by hanging filter paper containing 30 μl 20% KOH in maintaining sealed flask at 37°C for 2 hours. The trapped radioactivity was quantified by scintillation counting.
Lipolysis
Lipolysis in fat tissue ex vivo and in primary brown adipocytes was performed by measuring isoproterenol-induced release of free fatty acid and glycerol as described in the supplemental experimental procedures.
Body weight, body composition, and energy expenditure measurement
Mouse body weight was measured on a weekly basis. To check body composition, mice were anesthetized by i.p. injection with avertin (120 mg/kg animal body weight). Bone mineral density, fat mass, lean mass and percentage of fat were determined using dual-energy X-ray absorptiometry (DEXA) (GE Medical Systems, Madison WI). For energy expenditure measurement, 3-month old male Grb10fKO and loxp control mice were placed on metabolic cages and energy expenditure was measured by indirect calorimetry as described previously (Liu et al., 2012).
H&E and Oil Red O staining
For H&E staining, adipose and liver tissues were fixed with a buffer containing 10% formalin for 24 hours and embedded in paraffin. Tissue sections (10 mm-thick) were stained with H&E. For IHC, tissue sections were deparaffinized, rehydrated, and followed with antigen retrieval using heat-induced epitope methods as suggested by the antibody company (Abcam). The sections were then incubated with 1:800 diluted UCP1 antibody, and IHC staining was performed using the HRP/DAB detection kit (Abcam). Oil Red O staining was performed as described in our previous study (Liu et al., 2008).
Cold stress experiments
The cold stress experiments were performed as described in the supplemental experimental procedures.
Real-time PCR
The Real-time PCR was performed as described in the supplemental experimental procedures.
Statistical analysis
For cell study, data are representative of at least three independent experiments with a similar result. The Western blot images were semi-quantified with the NIH-Scion Image program. Statistical analysis of the data was performed using analysis of variance (ANOVA) or Student’s t-test. All data are presented as mean ± S.E.M. Statistical significance was set at P values of *<0.05 and **<0.01.
Supplementary Material
Highlights.
Grb10 expression in WAT is markedly induced by cold exposure.
Knockout of Grb10 in fat increases adiposity due to suppression of lipolysis.
Grb10 is essential for cold-induced thermogenesis.
Grb10 directly inhibits mTORC1 via a phosphorylation-dependent feedback mechanism.
Acknowledgement
We are grateful to Drs. Nicole Nemetz (Laboratory of Animal Resource, UTHSCSA) and Holly Van Remmen (Department of Cellular & Structural Biology, UTHSCSA) for their kind help with the telemetry study. We also thank Dr. Milena Girotti and Ms. Jennifer Donegan from Dr. David Morilak’s laboratory (Department of Pharmacology, UTHSCSA) for providing fat tissues from cold stressed rats. This work was supported by an NIH RO1 grant (DK100697) and grants from the National Nature Science Foundation of China (81130015) and the National Basic Research Program of China (2014CB910500) (to F. L.), an ADA Junior Faculty Award 1-13-JF-37 and an AHA Beginning in Aid 11BGIA7620074 (To M. L.), NIH RO1 grants DK69930 (to L. Q. D.), GM51405 (to J. B.), DK55758 and DK099110 and P01-DK088761 (to P. E. S.), an ACRCF (to H. L.) and a CTRC P30 (to T. J. S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmadian M, Abbott MJ, Tang T, Hudak CS, Kim Y, Bruss M, Hellerstein MK, Lee HY, Samuel VT, Shulman GI, Wang Y, Duncan RE, Kang C, Sul HS. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell metabolism. 2011;13:739–748. doi: 10.1016/j.cmet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailhaud G, Reach G. Does obesity protect against diabetes? A new controversy. Annales d'endocrinologie. 2001;62:S43–S54. [PubMed] [Google Scholar]
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, Giacobino JP, De Matteis R, Cinti S. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. American journal of physiology. Endocrinology and metabolism. 2010;298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Shi J, Smas CM, Kandror KV. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes. 2010;59:775–781. doi: 10.2337/db09-1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao L, Marcus-Samuels B, Mason MM, Moitra J, Vinson C, Arioglu E, Gavrilova O, Reitman ML. Adipose tissue is required for the antidiabetic, but not for the hypolipidemic, effect of thiazolidinediones. The Journal of clinical investigation. 2000;106:1221–1228. doi: 10.1172/JCI11245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Science signaling. 2009;2:ra75. doi: 10.1126/scisignal.2000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong LQ, Du H, Porter SG, Kolakowski LF, Jr, Lee AV, Mandarino LJ, Fan J, Yee D, Liu F. Cloning, chromosome localization, expression, and characterization of an Src homology 2 and pleckstrin homology domain-containing insulin receptor binding protein hGrb10gamma. The Journal of biological chemistry. 1997a;272:29104–29112. doi: 10.1074/jbc.272.46.29104. [DOI] [PubMed] [Google Scholar]
- Dong LQ, Farris S, Christal J, Liu F. Site-directed mutagenesis and yeast two-hybrid studies of the insulin and insulin-like growth factor-1 receptors: The src-homology-2 domain-containing protein hGrb10 binds to the autophosphorylated tyrosine residues in the kinase domain of the insulin receptor. Mol. Endocrinol. 1997b;11:1757–1765. doi: 10.1210/mend.11.12.0014. [DOI] [PubMed] [Google Scholar]
- Farmer SR. Molecular determinants of brown adipocyte formation and function. Genes & development. 2008;22:1269–1275. doi: 10.1101/gad.1681308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer M, Klein J, Kriauciunas KM, Ueki K, Benito M, Kahn CR. Essential role of insulin receptor substrate 1 in differentiation of brown adipocytes. Molecular and cellular biology. 2001;21:319–329. doi: 10.1128/MCB.21.1.319-329.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield AS, Cowley M, Smith FM, Moorwood K, Stewart-Cox JE, Gilroy K, Baker S, Xia J, Dalley JW, Hurst LD, Wilkinson LS, Isles AR, Ward A. Distinct physiological and behavioural functions for parental alleles of imprinted Grb10. Nature. 2011;469:534–538. doi: 10.1038/nature09651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. The Journal of clinical investigation. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinault C, Mothe-Satney I, Gautier N, Lawrence JC, Jr, Van Obberghen E. Amino acids and leucine allow insulin activation of the PKB/mTOR pathway in normal adipocytes treated with wortmannin and in adipocytes from db/db mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18:1894–1896. doi: 10.1096/fj.03-1409fje. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Siddle K. Grb10 and Grb14: enigmatic regulators of insulin action--and more? The Biochemical journal. 2005;388:393–406. doi: 10.1042/BJ20050216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332:1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Preitner M, Berney X, Uldry M, Vitali A, Cinti S, Ledford JG, Thorens B. Plac8 Is an Inducer of C/EBPbeta Required for Brown Fat Differentiation, Thermoregulation, and Control of Body Weight. Cell metabolism. 2011;14:658–670. doi: 10.1016/j.cmet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Lamming D, Ye L, Katajisto P, Goncalves M, Saitoh M, Stevens D, Davis J, Salmon A, Richardson A, Ahima R, Guertin D, Sabatini D, Baur J. Rapamycin-Induced Insulin Resistance Is Mediated by mTORC2 Loss and Uncoupled from Longevity. Science. 2012;335 doi: 10.1126/science.1215135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlais P, Wang C, Dong LQ, Carroll CA, Weintraub ST, Liu F. Phosphorylation of Grb10 by mitogen-activated protein kinase: identification of ser(150) and ser(476) of human grb10zeta as major phosphorylation sites. Biochemistry. 2005;44:8890–8897. doi: 10.1021/bi050413i. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MA, Riedel H, Liu F. Grb10: more than a simple adaptor protein. Front Biosci. 2004a;9:387–403. doi: 10.2741/1226. [DOI] [PubMed] [Google Scholar]
- Lim MA, Yang L, Yi Z, Wu H, Dong LQ, Liu F. Roles of PDK-1 and PKN in regulating cell migration and cortical actin formation of PTEN-knockout cells. Oncogene. 2004b;23:9348–9358. doi: 10.1038/sj.onc.1208147. [DOI] [PubMed] [Google Scholar]
- Liu F, Roth RA. Grb-IR: A SH2-domain-containing protein that binds to the insulin receptor and inhibits its function. Proc. Natl. Acad. Sci., USA. 1995;92:10287–10291. doi: 10.1073/pnas.92.22.10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HY, Hong T, Wen GB, Han J, Zuo D, Liu Z, Cao W. Increased basal level of Akt-dependent insulin signaling may be responsible for the development of insulin resistance. American journal of physiology. Endocrinology and metabolism. 2009;297:E898–E906. doi: 10.1152/ajpendo.00374.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xiang R, Wilk SA, Zhang N, Sloane LB, Azarnoush K, Zhou L, Chen H, Xiang G, Walter CA, Austad SN, Musi N, DeFronzo RA, Asmis R, Scherer PE, Dong LQ, Liu F. Fat-specific DsbA-L overexpression promotes adiponectin multimerization and protects mice from diet-induced obesity and insulin resistance. Diabetes. 2012;61:2776–2786. doi: 10.2337/db12-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Zhou L, Xu A, Lam KS, Wetzel MD, Xiang R, Zhang J, Xin X, Dong LQ, Liu F. A disulfide-bond A oxidoreductase-like protein (DsbA-L) regulates adiponectin multimerization. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18302–18307. doi: 10.1073/pnas.0806341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodhi IJ, Yin L, Jensen-Urstad AP, Funai K, Coleman T, Baird JH, El Ramahi MK, Razani B, Song H, Fu-Hsu F, Turk J, Semenkovich CF. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARgamma activation to decrease diet-induced obesity. Cell metabolism. 2012;16:189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. The Biochemical journal. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- O'Neill TJ, Rose TW, Pillay TS, Hotta K, Olefsky JM, Gustafson TA. Interaction of a GRB-IR splice variant (a human GRB10 homolog) with the insulin and insulin-like growth factor I receptors. Evidence for a role in mitogenic signaling. J. Biol. Chem. 1996;271:22506–22513. doi: 10.1074/jbc.271.37.22506. [DOI] [PubMed] [Google Scholar]
- Ooi J, Yajnik V, Immanuel D, Gordon M, Moskow JJ, Buchberg AM, Margolis B. The cloning of Grb10 reveals a new family of SH2 domain proteins. Oncogene. 1995;10:1610–1630. [PubMed] [Google Scholar]
- Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. The Journal of biological chemistry. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell metabolism. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Rampersaud E, Damcott CM, Fu M, Shen H, McArdle P, Shi X, Shelton J, Yin J, Chang YP, Ott SH, Zhang L, Zhao Y, Mitchell BD, O'Connell J, Shuldiner AR. Identification of novel candidate genes for type 2 diabetes from a genome-wide association scan in the Old Order Amish: evidence for replication from diabetes-related quantitative traits and from independent populations. Diabetes. 2007;56:3053–3062. doi: 10.2337/db07-0457. [DOI] [PubMed] [Google Scholar]
- Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nature medicine. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell metabolism. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman GA. The integral role of mTOR in lipid metabolism. Cell cycle. 2011;10:861–862. doi: 10.4161/cc.10.6.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga C, Yoshino K, Yonezawa K. mTOR integrates amino acid- and energy-sensing pathways. Biochemical and biophysical research communications. 2004;313:443–446. doi: 10.1016/j.bbrc.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Tremblay F, Gagnon A, Veilleux A, Sorisky A, Marette A. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology. 2005;146:1328–1337. doi: 10.1210/en.2004-0777. [DOI] [PubMed] [Google Scholar]
- Um SH, D'Alessio D, Thomas G. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell metabolism. 2006;3:393–402. doi: 10.1016/j.cmet.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Vitai M, Buday B, Kulcsar E, Literati-Nagy B, Vecsei I, Bezzegh K, Peterfai E, Kurucz I, Koranyi L. Occurrence of GRB10 (+11275G >A) polymorphism in Hungarian population and its relationship to glucose metabolism. Orvosi hetilap. 2009;150:1845–1851. doi: 10.1556/OH.2009.28729. [DOI] [PubMed] [Google Scholar]
- Wang L, Balas B, Christ-Roberts CY, Kim RY, Ramos FJ, Kikani CK, Li C, Deng C, Reyna S, Musi N, Dong LQ, DeFronzo RA, Liu F. Peripheral disruption of the Grb10 gene enhances insulin signaling and sensitivity in vivo. Molecular and cellular biology. 2007;27:6497–6505. doi: 10.1128/MCB.00679-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZV, Deng Y, Wang QA, Sun K, Scherer PE. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151:2933–2939. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick KR, Werner ED, Langlais P, Ramos FJ, Dong LQ, Shoelson SE, Liu F. Grb10 inhibits insulin-stimulated IRS/PI 3-Kinase/Akt Signaling pathway by disrupting the association of IRS-1/IRS-2 with the insulin receptor. J. Biol. Chem. 2003;278:8460–8467. doi: 10.1074/jbc.M208518200. [DOI] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J. Phosphoproteomic analysis identifies Grb10 as an mTORC1 substrate that negatively regulates insulin signaling. Science. 2011;332:1322–1326. doi: 10.1126/science.1199484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang N, Liu M, Li X, Zhou L, Huang W, Xu Z, Liu J, Musi N, DeFronzo RA, Cunningham JM, Zhou Z, Lu XY, Liu F. Disruption of growth factor receptor-binding protein 10 in the pancreas enhances beta-cell proliferation and protects mice from streptozotocin-induced beta-cell apoptosis. Diabetes. 2012;61:3189–3198. doi: 10.2337/db12-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.