Abstract
In mammals, an array of MEF2C proteins is generated by alternative splicing (AS), yet specific functions have not been ascribed to each isoform. Teleost fish possess two MEF2C paralogues, mef2ca and mef2cb. In zebrafish, the Mef2cs function to promote cardiomyogenic differentiation and myofibrillogenesis in nascent skeletal myofibers. We found that zebrafish mef2ca and mef2cb are alternatively spliced in the coding exons 4–6 region and these splice variants differ in their biological activity. Of the two, mef2ca is more abundantly expressed in developing skeletal muscle, its activity is tuned through zebrafish development by AS. By 24 hpf, we found the prevalent expression of the highly active full length protein in differentiated muscle in the somites. The splicing isoform of mef2ca that lacks exon 5 (mef2ca 4–6), encodes a protein that has 50% lower transcriptional activity, and is found mainly earlier in development, before muscle differentiation. mef2ca transcripts including exon 5 (mef2ca 4–5–6) are present early in the embryo. Over-expression of this isoform alters the expression of genes involved in early dorso-ventral patterning of the embryo such as chordin, nodal related 1 and goosecoid, and induces severe developmental defects. AS of mef2cb generates a long splicing isoform in the exon 5 region (Mef2cbL) that predominates during somitogenesis. Mef2cbL contains an evolutionarily conserved domain derived from exonization of a fragment of intron 5, which confers the ability to induce ectopic muscle in mesoderm upon over-expression of the protein. Taken together, the data show that AS is a significant regulator of Mef2c activity.
Abbreviations: AS, Alternative Splicing; MEF2, Myocyte Enhancer Factor 2; BMP, Bone Morphogenetic Protein; MADS, Minichromosome maintenance, Agamous, Deficiens, Serum response factor; TAD, transcription activating domains; PKA, Protein Kinase A; qRTPCR, quantitative Real Time PCR; hpf, hours post fertilization; ss, somitic stage; WISH, Whole Mount In Situ Hybridization; CMV, Cytomegalovirus; LNA, Locked Nucleic Acid; myog, myogenin; actb2, beta-actin 2; chd, chordin; ndr1, nodal related 1; gsc, goosecoid; nog1, noggin1; ntl, no tail; smyhc1, slow myosin heavy chain 1; MyHC, Myosin Heavy Chain; I.M.A.G.E, Integrated Molecular Analysis of Genomes and their Expression; ascl1a, achaete-scute complex-like 1a; kdr1, kinase insert domain receptor; neurog 1, neurogenin 1; myl7, myosin, light polypeptide 7
Keywords: Mef2ca, Mef2cb, Zebrafish, Skeletal muscle, Alternative splicing, Development
Highlights
-
•
mef2ca and mef2cb gene products are alternatively spliced in zebrafish.
-
•
Inclusion of exon 5 in mef2ca transcripts is regulated during zebrafish development.
-
•
Exon 5 confers on Mef2ca the ability to activate early patterning genes.
-
•
Mef2cb includes an extra octapeptide encoded by a region of intron 5.
-
•
Inclusion of the extra-octapeptide confers on Mef2cb pro-myogenic activity.
1. Introduction
Alternative splicing (AS) creates diversity within proteins without the need for gene duplication. In addition, AS is also an important mechanism for modulating gene expression and has contributed substantially to the evolution of modern genomes (reviewed in [1], [2], [3]). Many transcription factors undergo AS that creates important functional differences in the encoded proteins: altered transcriptional regulation capacity, nuclear trafficking, sensitivity to signals or requirement for co-activators [4]. Splicing-sensitive microarrays and deep sequencing analysis of mRNA from various human tissues have revealed the prevalence of AS in skeletal muscle; dysregulation of AS is associated with human muscle diseases [5] (reviewed in [6], [7]).
Genes encoding the Myocyte Enhancer Factor 2 (MEF2) family of transcription factors undergo extensive AS, the function of which is generally unclear. All MEF2 proteins have an N-terminal DNA binding region composed of MADS (Minichromosome maintenance, Agamous, Deficiens, Serum response factor) and MEF2 domains, two central transcription activating domains (TAD1 and TAD2) and a C-terminal nuclear localization sequence (Fig. 1B). Invertebrates generally have a single MEF2 gene, whereas amniotes have four genes (MEF2A-D). The teleost-specific genome duplication has led to six mef2 genes in zebrafish, with two copies of mef2a and mef2c, designated mef2aa, mef2ab, mef2ca and mef2cb [8]. Most MEF2 proteins are highly expressed in muscle tissue, where they regulate the heart, skeletal and smooth muscle differentiation [9]. Like Drosophila D-Mef2, Mef2c is particularly important in early heart and skeletal muscle development in both mice and zebrafish [8], [10], [11], [12], [13], [14], [15], [16], [17]. MEF2s are also more broadly expressed and function to control the development and adaptation of the brain, immune system, blood vessel and many other tissues [18] (reviewed in [19]). In mammals, MEF2C is subjected to three different alternative splices. A mutually exclusive alternative splice occurs between exons α1 and α2, located in the region immediately adjacent to the MEF2 domain [20]. In the central TAD2 region, a skipping-type alternative splice can include exon β and a splice involving alternative 3′ splice site selection occurs in the γ region near the C-terminus [21], [22]. In the case of mouse Mef2d, AS of the α exon switches the protein from a transcriptional repressor regulated by protein kinase A (PKA), to an activator insensitive to PKA signaling [23]. This switch is thought to drive skeletal muscle terminal differentiation, but how AS in the Mef2d α exon relates functionally to AS at other alternate exons is unclear. Developmentally regulated switching of AS of MEF2 genes has been described during frog and mouse development [24], [25], [26]. Involvement of alternative splice variants of Mef2 in endomesoderm and neuron differentiation in the sea anemone Nematostella vectensis has been described recently [27]. However, it has not yet been determined whether functional differences among the splicing variants of the MEF2C genes are important in vertebrate development.
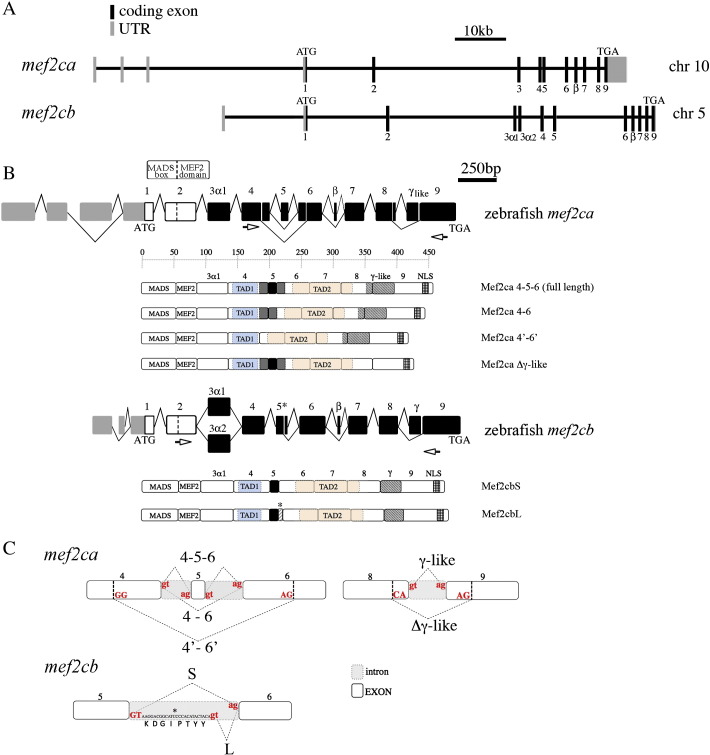
Fig. 1.
Genomic organization, transcripts and protein variants of zebrafish mef2ca and mef2cb genes. A) Schematics of zebrafish mef2ca and mef2cb genes. Exons are numbered and indicated by boxes. Black boxes indicate the mef2ca and mef2cb coding exons, whereas gray boxes represent the 5′- and 3′-untranslated regions. Introns are indicated by solid lines. The ATG translational start codons and the TGA stop codons of the two genes are also indicated. B) Schematic representation of zebrafish mef2ca and mef2cb transcript variants. Gray boxes represent UTR, white boxes represent the coding regions of the MADS and MEF2 domains in exons 1 and 2, black boxes represent the remaining translated sequence. Structures of zebrafish mef2ca and mef2cb genes transcripts are similar with the following exceptions: mef2ca lacks the 3α2, exon 5 alone or together with neighboring sequences from exons 4 and 6 may be excluded from the mature transcripts, the alternatively spliced γ region overlaps with the homologous γ regions of zebrafish mef2cb and of the other vertebrates mef2c genes, however it extends to neighboring sequences located in exons 8 and 9; mef2cb transcripts may include a short (24 nt) sequence of intron 5 (*). White arrows indicate the position of the primers used to amplify the cDNAs. The structures of the Mef2ca and Mef2cb protein isoforms deduced from the cloned cDNA sequences are schematized. The N-terminal region of the Mef2c proteins comprises the MADS-box and the MEF2 domain, involved in DNA binding and dimerization. By analogy with the mouse and human proteins, in the C-ter there are two putative transcriptional activation domains, TAD1 (blue) and TAD2 (orange), encoded respectively by exon 4 and by exons 6,7,8, downstream is localized the nuclear localization signal (NLS) (squared box). The position of exon 5 (black) and neighboring sequences that are excluded in the 4′–6′ isoform (gray) is indicated as well as the position of the γ-like and γ region of Mef2ca and Mef2cb respectively. Exon numbering is reported and the number of amino acids is indicated on the bar above. Mef2ca forms are named according to whether or not the exon 5 and neighboring regions or the γ-like region are present (Mef2ca 4–5–6, 4–6, 4′–6′, Δ γ-like). Mef2cb forms are named according to whether or not the octapeptide (*) in the exon 5 region is present or not (Mef2cbL and Mef2cbS). C) Details of the alternative splicing events that take place respectively: i. In the exon 5 region of mef2ca, showing the consensus and the non-canonical splice sites and the three species of mRNA generated; ii. In the γ region of mef2ca, splicing through a non-canonical CA alternative 5′ splice site in exon 8 and a canonical alternative 3′ splice site in exon 9 gives rise to the deletion of the γ-like region; iii. Exon 5 region of mef2cb transcript, the cartoon shows the sequence of the intron 5 that can be alternatively included in mef2cb transcripts, the competing donor splice sites (GT) and the two species of mRNA generated.
Here we describe the alternative splicing of the two zebrafish Mef2c genes, mef2ca and mef2cb. In addition to splicing events akin to the α, β, γ splices that were described in mice, we find novel splice forms varying in the region between the two TADs around the fifth coding exon. We provide the first evidence that the developmentally-regulated AS of mef2ca in this region affects Mef2c protein function. We report that mef2ca transcripts including exon 5 (mef2ca 4–5–6) are expressed early in development, and their over-expression causes severe defects in the embryos related to impaired gastrulation that are not created by variants lacking exon 5. Moreover, ectopic expression of Mef2ca 4–5–6 results in an increase of the transcript levels of genes such as chordin (chd), nodal related 1 (ndr1), no-tail a (ntla) and goosecoid (gsc), necessary during gastrulation for correct dorso-ventral patterning. Lastly, we describe a new evolutionarily conserved alternatively spliced isoform of mef2cb, here named Mef2cbL, containing an additional octapeptide in exon 5, that confers on Mef2cb the ability to induce ectopic skeletal myogenesis.
2. Materials and methods
2.1. Plasmids
The full-length coding regions of the zebrafish Mef2ca 4–5–6, Mef2ca 4–6, Mef2ca 4′–6′ and Mef2cbL variants were amplified from 24 hpf (hours post fertilization) zebrafish embryos cDNAs. The full length cDNA of Mef2cbS was obtained by a PCR reaction starting from a template made of three overlapping PCR products: the exon 5 region amplified from a Mef2cb I.M.A.G.E. clone (clone ID: 6519749, GenBank: CD282884.1), the upstream and downstream regions amplified from the Mef2cbL cDNA. The cDNAs were first inserted in the pCR2.1 vector (Invitrogen) or pGEM-T Easy vector (Promega), then sub-cloned into BamHI/NotI sites of the pcDNA 3.1(+) expression vector (Invitrogen). For RNA injections isoforms were sub-cloned into the XbaI/SalI sites of the βUT-3 vector [8]. Plasmids pGL3(desMEF2)3 and pRSVβ-gal were previously described [28].
2.2. Alternative splicing prediction and multiple alignments
TBLASTN (http://blast.ncbi.nlm.nih.gov) was used to predict alternative splicing isoforms of mef2ca or mef2cb and for multiple alignment to compare mef2cbL sequence to available sequences in database (GenBank and NCBI Reference sequence are listed in Fig. S3B). Sequence data were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) and then edited using GeneDoc software (http://www.psc.edu/biomed/genedoc).
2.3. Transcription reporter assays
Transactivation assays were performed by co-transfecting COS-1 cells with indicated expression vectors and cell lysates were analyzed as described previously [28].
2.4. RNA isolation, RT-PCR and Real Time PCR
RNA was isolated using TRIzol® Plus RNA Purification System (Ambion). For each developmental stage, 100 embryos were disrupted using Tissue Raptor (Qiagen). 500 ng of total RNA were reverse transcribed to cDNA using Superscript III reverse transcriptase (Invitrogen). Primers used to detect myogenin (myog), myod, beta-actin 2 (actb2), mef2ca and mef2cb are listed in Fig. S6A (other primer sequences are available upon request), quantitative Real Time PCR (qRTPCR) was performed on 2.5 ng of Poly A mRNA using SYBR Green method (SYBR® Green PCR Core Reagent, Applied Biosystems). Poly A mRNA has been purified using Ambion's protocol (MicroPoly(A) Purist Kit). To amplify the different mef2ca isoforms specific forward primers spanning exon-exon junctions were used with a common reverse primer (Fig. S6B). For each primer combination the optimal MgCl2 concentration was determined to obtain specific and high efficient amplification (slope values between − 2.95 and − 3.75). Absolute quantification of transcript copy number was achieved by generating calibration curve using plasmid DNA templates (listed above) as previously described [29], [30]. Analysis was performed using PCR ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems). Student's t-tests were performed for pairwise comparisons to determine significant differences between groups.
2.5. Zebrafish lines, maintenance and embryo manipulation
Wild-type zebrafish (Danio rerio) lines were maintained on King's College wild-type background, and staging and husbandry were as described [31].
2.6. Whole mount in situ hybridization (WISH)
In situ mRNA hybridization was performed as described [14]. Fluorescein- or digoxigenin-tagged probes used were mef2ca [32], mef2cb [8], myod [33], slow myosin heavy chain 1 (smyhc 1) [8], myosin, light polypeptide 7 (myl7) [34], kinase insert domain receptor (kdrl) [35], neurogenin 1 (neurog1) [36] or achaete-scute complex-like 1a (ascl1a) [37]. We have also used two non-overlapping dual digoxigenin-labelled custom mef2ca exon 5-specific locked nucleic acid (LNA) probes, LNA1 and LNA2 (Exiqon, sequence available upon request) to perform WISH as described [38], [39]. Embryos were photographed as wholemounts on Olympus DP70 or dissected and flatmounted in glycerol and photographed on a Zeiss Axiophot with AxioCam using Improvision Openlab.
2.7. mRNA injection and embryo manipulation
mRNA injection was performed as described previously [40]. βUT-3 vectors encoding Mef2ca and Mef2cb isoforms, were linearized using SfiI/PstI sites. mRNAs were made with mMESSAGE mMACHINE kit (Ambion). All RNAs were injected at 1–2 cell stage embryos at 10 pg, 25 pg or 50 pg/embryo. Tetramethyl-rhodamine Dextran (5% solution in 0.2 M KCl) was co-injected in order to sort phenotypes of injected embryos. At 20–28 hpf injected embryos were analyzed and sorted using a Zeiss Axiophot with AxioCam.
2.8. Western blot analysis and antibodies
Zebrafish embryos were dechorionated and lysed in RIPA buffer (50 mM Tris HCl pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Na Deoxycholate, 1% Igepal, 0.1% SDS, 1 mM DTT) containing 1 mM PMSF and Proteases Inhibitor Complete cocktail (ROCHE). Equal amounts of protein extracts were separated by SDS-PAGE and subsequently analyzed by Western blot as previously described [28]. The following antibodies were used: rabbit polyclonal anti-MEF2 (sc-313X; Santa Cruz Biotechnology, Inc.), mouse anti-αTubulin (T6074, Sigma Aldrich), mouse anti-Vinculin (V4505, Sigma Aldrich). Embryo staining was performed with a primary antibody against sarcomeric myosin heavy chain (MyHC; A4.1025 [41]) as previously described [8].
3. Results
To compare alternative splicing in MEF2C genes across species and paralogues, we use a standard nomenclature numbering exons 5′ to 3′ from the first coding exon, yet retaining the conventional α, β, γ designation for alternatively spliced exons. Each exon number thus corresponds to homologous sequences (Figs. 1A and S1).
3.1. Alternative splicing of zebrafish mef2ca and mef2cb
To predict splice variants of the zebrafish Mef2c proteins, we conducted in silico analysis of the zebrafish mef2ca and mef2cb genes on public databases (see Materials and methods). In addition to the known mef2ca transcript [32], [42], we detected two alternative 5′UTR sequences and several alternative splices (Fig. 1A and B). mef2ca lacks an alternative exon 3, the α exon, having a single exon most similar to the 3α1 form of amniote MEF2C, which has serine residues at positions 98 and 109 [43]. Mef2ca contains a putative β exon in intron 6 and, in addition, a γ-region flanked by a non-canonical 3′ splice site (GC) was found at the start of exon 9 (Fig. S2A). Of particular note in the in silico analysis, were three alternative splices in the region of exons 4, 5 and 6 (Fig. 1C), two of which correspond to the alternatively spliced δ exon (exon 5) of the mef2c gene product in Xenopus [24] (Fig. S1B,C). By sequence alignment we found that splicing of exon 5 is conserved among teleosts (Fig. S3). Zebrafish mef2cb splicing appeared more similar to amniote MEF2C genes than that of mef2ca. We predicted two 5′ UTR sequences, alternate exons 3, 3α1 and 3α2, a putative β exon, encoding the conserved octapeptide SEDVDLLL in intron 6 of mef2cb, and a putative γ region at the start of exon 9 (Fig. 1B). The sequences of alternative exons 3α1 and 3α2 are mostly similar to the corresponding alternate exons of amniote MEF2C, although neither of the two α exons contains a PKA target residue corresponding to serine 120 of MEF2D, which was found to direct binding of repressive or activating cofactors [23]. Additionally, we found a mef2cb variant with a long exon 5 resulting from a retained intron 5 sequence. We designate this Mef2cbL to distinguish it from the conventional exon 5 in Mef2cbS (Fig. 1B, C).
To characterize the major mef2ca and mef2cb spliced isoforms expressed in developing zebrafish skeletal muscle, we performed RT-PCR on RNA extracted from the dissected tail region of 24 hpf embryos using primer pairs that target conserved sequences (Fig. 1B). A series of mef2ca mRNA RT-PCR products were amplified, sub-cloned and their sequences compared to the nucleotide sequence of mef2ca genomic DNA, revealing the existence of at least four species of mef2ca mRNAs produced by AS in developing embryos (Fig. 1B). In addition to the transcript encoding the full length protein, here referred to as Mef2ca 4–5–6 (465 aa), two mef2ca isoforms, Mef2ca 4–6 (451 aa) and Mef2ca 4′–6′ (413 aa) derive, respectively, from skipping exon 5 or a larger region that also encompasses part of exons 4 and 6. Another variant, Mef2ca Δγ-like (411 aa) contains exon 5 but lacks the γ region and further sequences located in exons 8 and 9. The Mef2ca 4′–6′ and Mef2ca Δγ-like mRNAs are the results of splicing at the non-canonical 5′ donor splice sites GG and CA, respectively (Fig. 1C) (GenBank accession numbers: KF932282 and KF932281KF932282KF932281 respectively).
One mef2cb variant, named Mef2cbL, was obtained by RT-PCR; it includes exon 3α1 and γ but lacks exon β. Mef2cbL arises from the inclusion of an additional sequence from intron 5 (Fig. 1C). Indeed, two competing 5′ splice sites are present at the end of exon 5, these splice sites direct inclusion or exclusion of 24 nucleotides (nt) encoding the octapeptide KDGIPTYY (Fig. 1C). When aligned (Fig. S2B), the predicted amino acid sequences of the identified zebrafish mef2ca and mef2cb isoforms show that the major variation occurs in the exon 4–5–6 region of both genes, located between the two TADs described previously [44], [45].
The splicing pattern and the octapeptide sequence of Mef2cbL, appear to be conserved in other teleosts (Fig. S3). cDNA sequences from medaka (Oryzias latipes) and cavefish (Sinocyclocheilus anophtalmus and Sinocyclocheilus angustiporus) have a similar sequence at the end of intron 5 as in the mef2cbL homologue (Fig. S3). Such sequence conservation across the major teleost clades, combined with the location between TAD1 and TAD2, suggest that AS in the exon 5 region is functionally significant.
3.2. mef2ca is the main Mef2c orthologue expressed during skeletal muscle development
We sought to characterize the temporal and spatial expression patterns of mef2ca and mef2cb in developing zebrafish skeletal muscle. First, we quantified the expression levels of mef2ca and mef2cb transcripts by quantitative qRTPCR amplification using paralogue-specific primers, starting from equal amounts of RNA collected from zebrafish embryos at sequential developmental stages (from 12 to 72 hpf). mef2ca and mef2cb presented a similar profile of expression, with a higher abundance of the transcripts of mef2ca at all stages analyzed. Both genes were expressed at low levels at 12 hpf (mef2ca 26 copies/2.5 ng RNA and mef2cb 25 copies/2.5 ng RNA). The total number of mRNA copies increased by 24 hpf, when the first massive wave of muscle fibers differentiates (mef2ca 210/2.5 ng RNA, mef2cb 27/2.5 ng RNA) and stayed stable at later stages (Fig. 2A). These results were confirmed by a semi-quantitative PCR experiment (Fig. S4A).
Fig. 2.
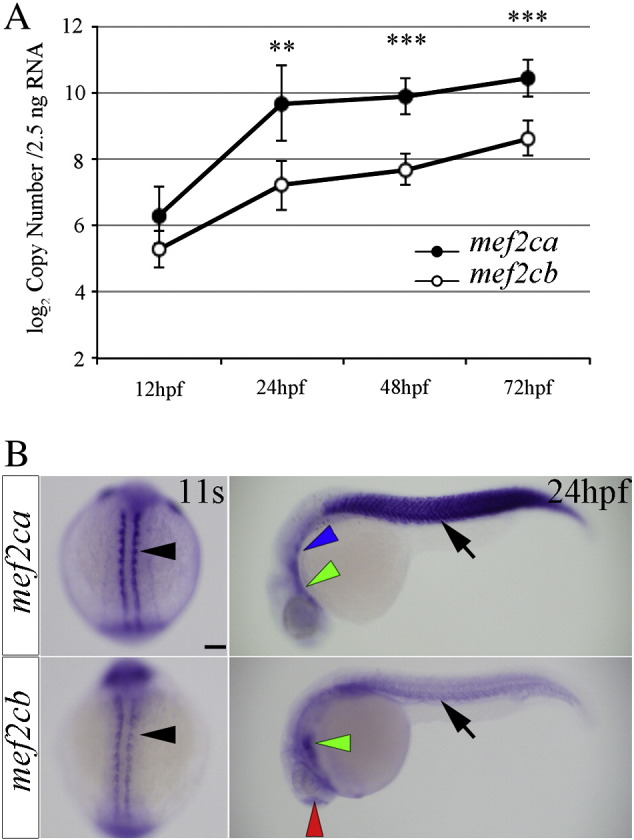
Expression of mef2ca and mef2cb genes in zebrafish embryos. A) Estimation of absolute mef2ca and mef2cb transcripts by qRTPCR during D. rerio development. The graph shows transcript-specific absolute quantification, reported as log2 copy number in equal amount of total RNA (2.5 ng) extracted from zebrafish embryos at 12, 24, 48 and 72 hpf. Graph showing mean ± SE from four independent experiments, ** and *** indicate P-values of ≤ 0.01 and ≤ 0.001 respectively. B) Wholemount in situ hybridization for mef2ca and mef2cb mRNA for embryos at 11 ss (dorsal view, anterior to top) and at 24 hpf (lateral view, anterior to left). At 11 ss, both genes are expressed in the adaxial cells (black arrowheads). By 24 hpf mef2ca is strongly expressed in the myotome (black arrows) and also in the heart (green arrowhead) and branchial arches (blue arrowhead). mef2cb transcripts are detected in the heart (green arrowhead), telencephalon (red arrowhead) and are weakly detected in the somites (black arrow). Scale bars = 100 μm.
These observations were confirmed by whole mount in situ mRNA hybridization on developing zebrafish embryos using probes specific for either mef2ca or mef2cb transcripts (Figs. 2B and S4B). At 11 somite stage (ss), mef2ca and mef2cb transcripts display an overlapping expression pattern in the adaxial cells next to the notochord and in the bilateral heart fields (Fig. 2B) [8]. At 24 hpf, most mef2ca mRNA is skeletal muscle-specific where it follows the expression of myod [14], [32] (Figs. 2B and S4B). In contrast, the transcripts of mef2cb are detected in the developing heart, blood vessels and telencephalon, as well as somitic muscle [8] (Figs. 2B and S4B). In summary, mef2ca is the more abundantly expressed in skeletal muscle of the two Mef2c paralogues.
3.3. Developmentally regulated expression of mef2ca and mef2cb splice variants
Levels of expression of alternatively spliced mef2ca and mef2cb during zebrafish development were determined by semi-quantitative RT-PCR and qRTPCR RNA quantification using SYBR and exon boundary spanning primers, that allow for selective PCR amplification of individual alternative transcripts [30]. At 12 hpf, the amount of mef2ca 4–6 transcript (lacking exon 5) represents about 30% of the total, whereas the amount of the full length 4–5–6 transcript the remaining 70%. At 24 hpf and beyond, mef2ca 4–6 expression increased slightly but remained less abundant than the mef2ca 4–5–6, whose predominance increases further (80% of the total mef2ca transcripts) (Fig. 3B–C). The shortest isoform, mef2ca 4′–6′ is present at low level (less than 1% of the total mef2ca transcripts) at every developmental stage and was therefore not considered further. mef2ca transcripts containing the β exon were barely detectable and were found exclusively at 72 hpf after five additional cycles of PCR amplification (data not shown). Transcripts without the γ-like region were expressed at early stages of development. However, they were less abundant, and were not detected beyond 24 hpf (Fig. 3A,B). Thus, almost all mef2ca transcripts contain the γ-like region and lack β exon, irrespective of their splicing at the 4–5–6 region.
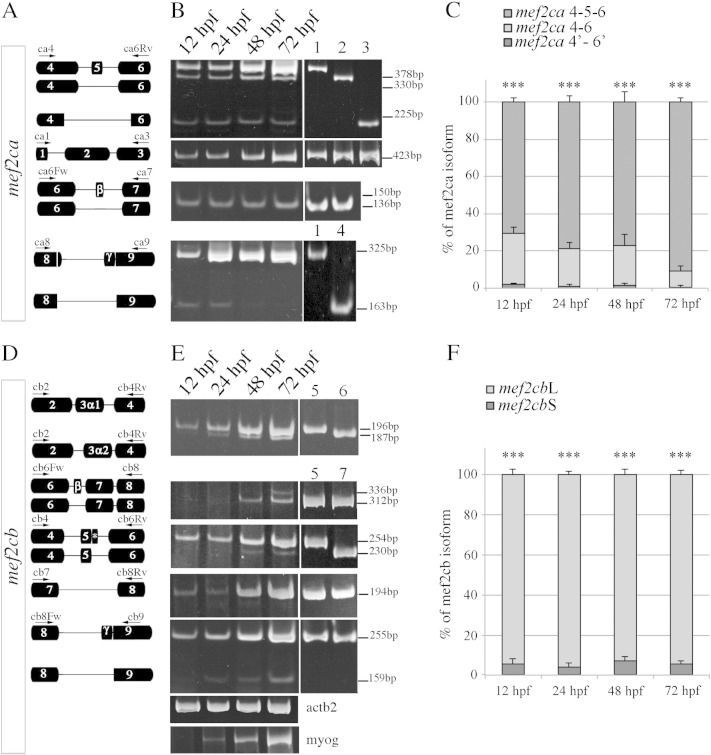
Fig. 3.
Developmental expression profile of mef2ca and mef2cb alternative splicing products.
A) Schematic representation of Mef2ca alternative exons. Arrows show primers annealing sites used in the RT-PCR analysis. B) Expression analysis of alternative splicing variants of mef2ca transcripts by RT-PCR. Total RNA was purified from staged embryos at 12, 24, 48 and 72 hpf. PCR was performed using primers that give amplification products of different sizes depending on the splice variant. The level of expression of total mef2ca transcripts was evaluated by using two primers (ca1 and ca3) that amplify a region not alternatively spliced between exons 1 and 3. Amplification of plasmid vectors containing the cDNAs of the various mef2ca splice variants cloned into the pcDNA 3.1 vector was used as controls of the correct size of expected amplicons: mef2ca 4–5–6 γ + (lane 1), mef2ca 4–6 γ + (lane 2), mef2ca 4′–6′ γ + (lane 3), and mef2ca 4–5–6 γ- (lane 4). PCR products were separated in 8% polyacrylamide gels. Length of PCR products in base pairs (bp) is indicated. C) Quantitative analysis of the mRNA levels of mef2ca exon 5 splice variants during D. rerio development. The amount of the transcripts of each splice variant was estimated by absolute qRTPCR. Original data (mRNA levels of each isoform) are reported as % of the total number of mef2ca transcripts (4–5–6 + 4–6 + 4′–6′ = 100%). Statistical analysis was performed on data obtained from three independent experiments, the means ± SE are represented. *** indicates a P-value ≤ 0.001. D) Schematic representations of Mef2cb alternative exons. Arrows show primers annealing sites. E) Developmental RT-PCR analysis of mef2cb mRNAs. To evaluate the amount of 3α1- and 3α2-containing mef2cb transcripts, we designed common PCR primers (cb2 and cb4Rv) annealing to flanking regions in exons 2 and 4 to generate two amplicons of different sizes: a 196-bp (3α1) and a 187-bp (3α2) RT-PCR products respectively. Flanking primers were also designed to investigate the expression of exon β, the extra sequence of intron 5 (*) and of the γ region. As control templates we used the pcDNA 3.1 expression vector containing the cDNAs of Mef2cbL3α1 β- γ + (lane 5), Mef2cbL3α2 β- γ + (lane 6) and Mef2cbS 3α1 β- γ + (lane 7). actb2 was used as a control, myog was used as a marker for skeletal muscle differentiation.
Expression of the mef2cb alternatively spliced exons was also determined by semi-quantitative RT-PCR and qRTPCR amplification. Transcripts containing exons 3α1 and 3α2 were detected throughout development using common primers that give two amplicons of different size and therefore electrophoretically distinguishable. The transcript that includes the 3α1 exon is the most abundant at all the developmental stages beyond 12 hpf (Fig. 3E). This result was also confirmed by using isoform-specific primers, given that, in identical experimental conditions, four additional PCR cycles are required to amplify an amount of exon 3α2-containing DNA similar to that containing exon 3α1 (Fig. S5A). Whereas the inclusion of exon 3α1 predominates in the developing embryo, RT-PCR analysis revealed that in adult skeletal and cardiac muscle the levels of the two isoforms are comparable (Fig. S5B). We did not detect the 3α2-containing transcript in the liver and brain, indicating a muscle-restricted pattern of expression of this splice variant, analogously to what has been reported for the mammalian counterpart (Fig. S5B) [20]. Inclusion of exon β was barely detected. In contrast, the γ region and the extra sequence from intron 5 (Mef2cbL) were readily detected at all developmental stages (Fig. 3D,E). qRTPCR quantification confirmed that more than 90% of mef2cb transcripts retain the extra intron 5 sequence, but less than 10% encode the Mef2cbS form (Fig. 3F). Thus, both mef2ca and mef2cb show striking variations in the exon 4–5–6 region.
3.4. Exon 5-containing mef2ca transcripts accumulate in skeletal muscle
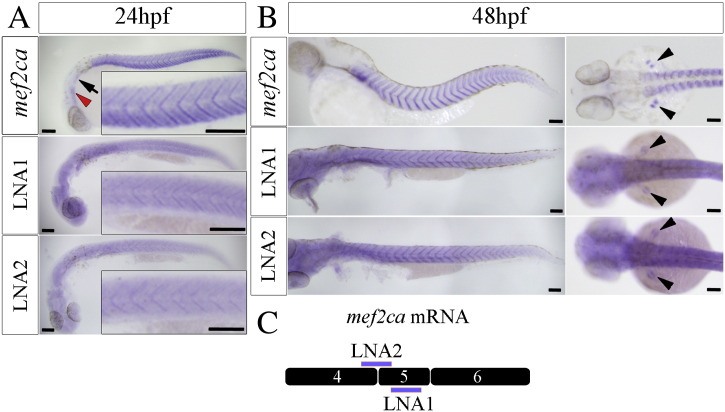
To examine where mef2ca mRNA(s) that include exon 5 are expressed in the developing zebrafish embryo, we performed in situ mRNA hybridization using a probe that recognizes all transcripts (mef2ca probe, [32]) and two non-overlapping dual digoxigenin-labelled LNA probes designed to recognize 21 base pair sequences located within exon 5 (LNA1) or within the exon 4/5 boundary (LNA2) (Fig. 4C). In 24 hpf embryos, mef2ca transcripts were detected throughout the somitic muscle and preferentially observed at somite borders (Fig. 4A upper panel), as well as in the heart and branchial arches (Fig. 4A upper panel, see also Fig. 2B). Similarly, both the exon 5-specific LNA probes gave signals above background only in skeletal muscle, preferentially observed at somite borders (Fig. 4A, middle and lower panels). By 48 hpf, the signals obtained with the generic and both exon 5-specific probes, are restricted almost entirely to the somite boundaries area (Fig. 4B, left and [14]). In addition, signals with all three probes show the typical separate dorsal and ventral muscle signal in the pectoral fin (Fig. 4B, right). Thus, even though we cannot exclude some levels of expression in other tissues, we conclude that the mef2ca 4–5–6 transcript is expressed primarily in skeletal muscle and is mainly localized to somite boundaries, suggesting it may have a distinct and specific function.
Fig. 4.
WISH analysis of zebrafish mef2ca transcripts in developing zebrafish embryos.
In situ hybridization using mef2ca and mef2ca-exon 5 specific probes as indicated. A) Lateral view of 24 hpf embryos. mef2ca mRNA localizes to both central and peripheral regions of the muscles in the somite, and also to the developing heart and branchial arches (red arrowhead and black arrow respectively). Exon 5 specific transcripts are detected by both LNA probes in a similar way in the muscle, with a slightly stronger expression at somite borders (see insets for magnified somatic muscle area). B, left panels.) Lateral view of 48 hpf embryos, anterior to left. mef2ca general and both LNA1 and LNA2 exon 5-specific probes show overlapping signals enriched at fiber ends. Right panels.) Dorsal view of the same embryos, anterior to left. mef2ca and both LNA probes detect expression in the pectoral fin dorsal and ventral muscle masses (black arrowheads). Scale bars = 100 μm. C) Drawing of the LNA1 and LNA2 probes annealing positions within the exon 4/5 region.
3.5. Mef2ca 4–5–6 is a potent transactivator
The transcriptional activities of mef2ca splice variants were tested in vitro by co-transfection into COS-1 cells of each Mef2c splice variant with a MEF2-responsive luciferase reporter containing three copies of the MEF2 binding site from the Desmin gene regulatory region (pGL3desMEF2) [46]. COS-1 cells have low endogenous MEF2 expression. Immunofluorescence analysis revealed that all Mef2ca and Mef2cb splice variants efficiently localized to the nucleus (data not shown), congruent with the observation that they all include the sequence corresponding to the nuclear localization signal described in the mouse [47].
Compared to other Mef2c isoforms tested, the Mef2ca 4–5–6 full length protein had the strongest transcriptional activity (Fig. 5A). Deletion of amino acids encoded by exon 5 and neighboring sequences results in a twofold reduction in transcriptional activity, even though the respective protein expression levels were comparable (Fig. 5B). Furthermore, we observed that a Mef2ca 4–5–6 isoform lacking the γ-like domain had 2-fold higher transcriptional activity than Mef2ca containing the γ-like domain, consistent with the finding that this region represses transcription (data not shown; [21]). Upon transfection, the Mef2cbL and Mef2cbS isoforms, each containing both exon 5 and γ, exhibited similar activity (about 70% of that of Mef2ca 4–5–6) (Fig. 5A and data not shown). However, Mef2cbL immunoreactivity was much lower than the Mef2ca isoforms (Fig. 5B). Given that we obtained similar results with other antibodies directed against different regions of MEF2 proteins (data not shown), it is unlikely that the low amount of Mef2cb protein detected is due to the low reactivity of our anti-Mef2 antiserum. Additional studies are required to characterize the stability and translational efficiency of Mef2c proteins, but our results suggest that Mef2cbL has higher activity per molecule than Mef2ca 4–5–6. Taken together, these data suggest that inclusion of exon 5 between TAD1 and TAD2 confers increased activity to Mef2ca.
Fig. 5.
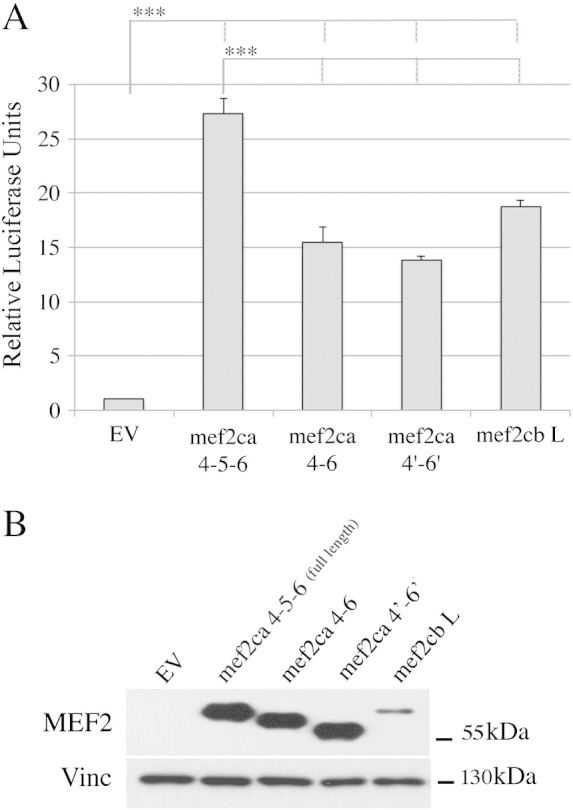
Transcriptional activity of zebrafish Mef2ca and Mef2cb splice variants.
A) COS-1 cells were co-transfected with pGL3(desMEF2)3 luciferase, the pRSVβ-gal reporter control and CMV (Cytomegalovirus)-driven expression plasmids encoding for the indicated Mef2c splicing isoforms. Firefly luciferase activities were normalized for transfection efficiency against the β galactosidase activity and expressed as relative luciferase units of the activity in cells transfected with the Empty Vector (EV) (= 1.0). Statistical analysis was performed on data obtained from three independent experiments, the means ± SE (error bars) are represented. *** indicates a P-value ≤ 0.001. B) Extracts from cells transfected in panel A were resolved by SDS PAGE, Mef2ca and Mef2cb expression was assessed by immunoblotting with anti-MEF2 antibody that recognizes all Mef2ca and Mef2cb splicing isoforms (upper panel). Sample loading was normalized using Vinculin immunoreactivity (lower panel).
3.6. Mef2cbL has unique myogenic potential in developing zebrafish
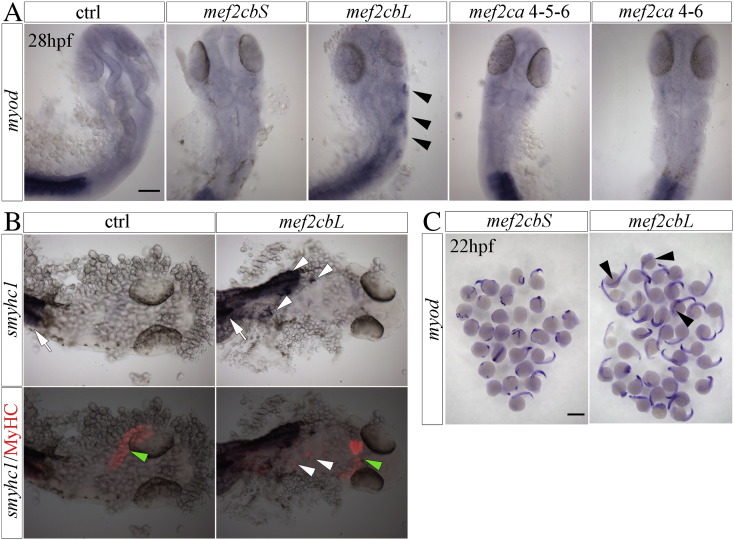
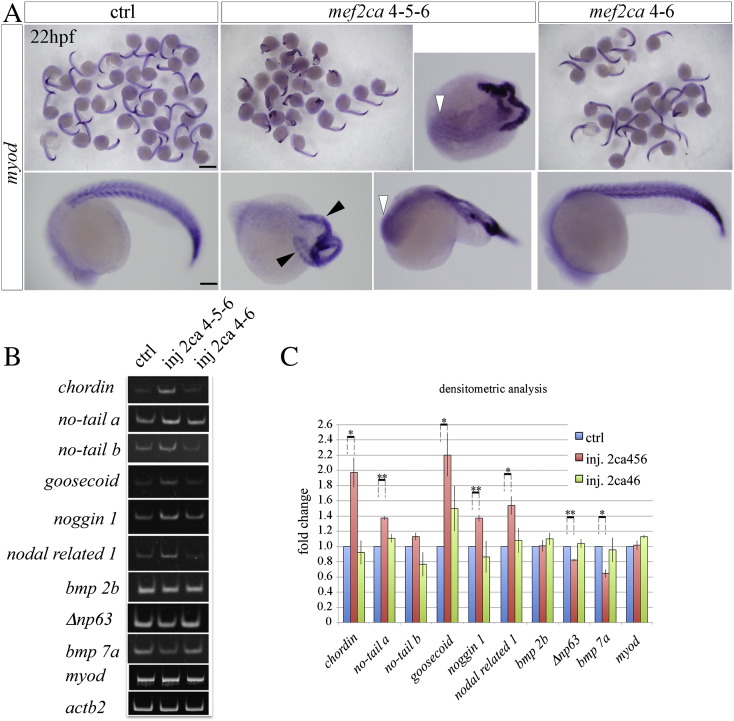
To investigate the biological significance of Mef2ca and Mef2cb splice variants in zebrafish embryonic development, we determined the effects of their ectopic expression by injecting embryos at the one-cell stage with synthetic Mef2c mRNAs and analyzing them at 24 hpf. We have shown previously that injection of mRNA of mef2cb induces ectopic skeletal muscle in embryos [8]. Here we report that injection of 10 pg/embryo of mRNAs of the Mef2cbL isoform induced ectopic skeletal muscle in the anterior mesoderm of 40% of the injected embryos, as revealed by wholemount in situ hybridization for myod mRNA in 28 hpf zebrafish embryo, a developmental stage where no endogenous muscle is normally observed in the head (Fig. 6A,B). In addition to myod transcripts we detected ectopic expression of smyhc1 transcripts and MyHC protein, further supporting the pro-myogenic activity of Mef2cbL (Fig. 6B). No induction of ectopic muscle was observed after ectopically expressing any Mef2ca isoform, even when higher quantities of mRNA were injected (Fig. 7). Interestingly, this effect depends on the inclusion of the KDGIPTYY octapeptide, because forced expression of the Mef2cbS isoform did not cause ectopic myogenesis (Fig. 6A, B). Thus, the form of Mef2cb that is normally present in developing zebrafish embryos during somitogenesis has unique myogenic potential that is not shared by Mef2ca 4–5–6, the predominant Mef2c isoform in skeletal muscle. Injection of higher amounts (25 pg/embryo) of both Mef2cbS and Mef2cbL mRNAs resulted in head and trunk developmental alterations (Fig. 6C).
Fig. 6.
Effects of Mef2cbL forced expression in zebrafish embryos.
Wholemount in situ mRNA hybridization of zebrafish embryos injected with in-vitro transcribed mRNA encoding Mef2c isoforms together with Rhodamine dextran at the 1-cell stage. Injected embryos or uninjected control embryos were analyzed during development.
A) Myod mRNA in 22 hpf embryos injected with 25 pg of mef2cb mRNAs. mef2cbL but not mef2cbS injected embryos have ectopic myod expression in head region (arrowheads). Both groups show an array of developmental defects in the head and trunk regions. B) Myod mRNA in head region at 28 hpf (dorsal view, anterior to top). Injection of 10 pg of Mef2cbL mRNA induces ectopic myod expression in head mesoderm (arrowheads). C) smyhc1 mRNA and immunofluorescence of MyHC protein in 28 hpf non-injected control embryos or embryos injected with 10 pg of Mef2cbL mRNA, ectopic muscle is clearly seen in the head region of injected embryos (white arrowheads). While arrow and green arrowhead indicate somitic muscle and heart respectively. Scale bars = 100 μm.
Fig. 7.
Effects of forced expression of mef2ca splice variants on development of zebrafish embryo.
A) Myod mRNA in 22 hpf embryos injected with the mRNAs of Mef2ca splice variants or not injected (control). Forced expression of Mef2ca 4–5–6 mRNA resulted in severe developmental defects: double axis (black arrowheads), trunk and brain defects (white arrowheads). Control embryos or embryos injected with 25 pg of Mef2ca 4–6 mRNA showed normal morphology. B) RT-PCR analysis of the total RNA extracted from 25 pg mef2ca mRNA injected or control uninjected embryos at 22 hpf. Mef2ca 4–5–6 injected embryos showed augmented expression of chordin, no-tail a, nodal related 1, noggin 1 and goosecoid, reduced expression of Δnp63 and bmp 7a, whereas bmp 2b, no-tail b and myod expression levels are unaffected. C) Densitometric analysis of the bands shown in B, normalized to actb2 signal. Expression levels of each gene were arbitrarily set to a value of 1 in the uninjected control embryos. Statistical analysis was performed on data obtained from three independent experiments, the means ± SE (error bars) are represented. * and ** indicate P-values of ≤ 0.05 and ≤ 0.01 respectively.
3.7. Mef2ca 4–5–6 over-expression causes defects in gastrulation
To investigate the functionality of the two main Mef2ca isoforms expressed during development (Mef2ca 4–5–6 and 4–6), high doses of Mef2ca mRNAs were employed. Injection of 25 pg of full length Mef2ca 4–5–6 RNA had dramatic effects on embryonic development, inducing lethality in approximately 30% of the embryos and marked developmental defects in 49% of the surviving embryos, classified as ‘severely defective’ (Fig. S6A,B). Such embryos already had defects evident at gastrulation stages (6–8 hpf, data not shown). Among the surviving embryos, a further 34% exhibited a milder phenotype classified as ‘defective’, with trunk convergent extension defects, occasional double axes, and some brain defects such as undeveloped eyes and absence of mid- and forebrain structures (Fig. 7A). Only 16% of embryos appeared unaffected by the Mef2ca 4–5–6 RNA. The percentage of severely defective embryos increased in a dose-dependent manner upon increasing the amount of injected RNA (Fig. S6B). In contrast to Mef2ca 4–5–6, forced expression of the Mef2ca 4–6 isoform was less active, having no detectable effect on the development of most (85%) of the injected embryos, even when expressed at comparable levels to Mef2ca 4–5–6 (Figs. 7A and S6B,C). These results indicate that ectopic Mef2ca activity in early stages disrupts normal development. The gross defects in gastrulation induced by over-expressed Mef2ca 4–5–6 suggested severe tissue patterning disruption, yet a survey of cell lineage markers revealed no indication of altered cell fates at lower doses of RNA (Fig. S6D).
To gain more insight into the mechanisms underlying the ability of Mef2ca 4–5–6 to disrupt development, the expression levels of genes encoding transcription factors and signaling molecules that are involved in early patterning of the embryo were screened by semi-quantitative RT-PCR. The chd gene, encoding a BMP (Bone Morphogenetic Protein) antagonist involved in dorsoventral patterning of early embryos [48] (reviewed in [49], [50]), was up-regulated (2-fold) in embryos injected with the mef2ca 4–5–6 mRNA, but not in those injected with the 4–6 spliced isoform (Fig. 7B,C). Mef2ca 4–5–6 also induced the expression of ndr1 (1.5-fold), gsc (2.2-fold) and other dorsally-expressed genes (no-tail a, noggin 1), and reduced the expression of ventralizing factors such as bmp7a (0.4-fold) and Δnp63 (0.2-fold), but did not alter the transcript level of myod or no-tail b and bmp2b (Fig. 7B,C), suggesting that the protein sequence encoded by exon 5 can modulate the expression level of a specific subset of early embryonic genes.
3.8. Mef2ca 4–5–6 mRNA is the prevalent Mef2c transcript present in the embryo before gastrulation
Our data indicate that forced expression of Mef2ca 4–5–6 protein induces the ectopic expression of genes involved in early dorso-ventral patterning of the embryo. In an attempt to get more insight into a putative role of Mef2ca in controlling endogenous patterning genes, we next determined the expression and alternative splicing patterns of mef2c genes during early stages of development and compared them to those of two of their putative target genes, i.e. chd and gsc. To this aim we performed RT-PCR analysis of the RNA from zebrafish embryos harvested at the 1K-cell (3 hpf), 50% epiboly (5.25 hpf) and bud (9–10 hpf) stages. Our analysis revealed that mef2ca transcripts are already detectable as early as at the 1K-cell stage, with predominant expression of the transcript including exon 5 (Fig. 8). We noticed a rapid loss of the 4–5–6 transcript that became undetectable by 10 hpf when the 4–6 mRNA is the only mef2ca transcript detected, inclusion of exon 5 is again detected later, by 12 hpf (Fig. 3) and the 4–5–6 full length transcript predominates upon muscle differentiation. The kinetics of expression of the mef2ca 4–5–6 transcript suggests that it might be of maternal origin. The presence of Mef2ca 4–5–6 transcripts early in development, which is temporally coincident with gsc expression and overlaps partially with that of chd (our data and [51]) is consistent with a role of this mef2ca splice variant in dorso-ventral patterning. No mef2cb expression is detected prior to 50% epiboly, in mid-gastrulation, where only the mef2cbS transcript is present. Nonetheless, at the onset of somitogenesis (9–10 hpf), we noticed that only the transcript encoding for Mef2cbL, the pro-myogenic variant, is expressed.
Fig. 8.
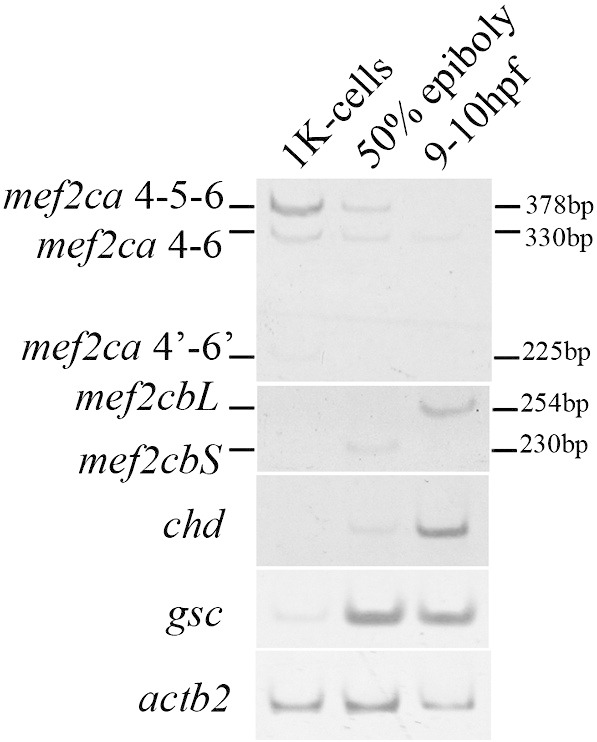
Expression of mef2ca and mef2cb splice variants during early zebrafish development.
Expression levels of the mef2ca and mef2cb splice variants in exon 5 region were evaluated by RT-PCR analysis of RNA harvested from zebrafish embryos at the indicated developmental stages. PCR was performed using primers that give amplification products of different sizes depending on the splice variant, as schematized in Fig. 3A. PCR products were separated in 8% polyacrylamide gels. Length of PCR products is indicated. Expression levels of gsc and chd were also determined. Expression levels of actb2 RNA are shown as loading control.
4. Discussion
Alternative splicing of transcription factors can have a wide impact on the regulation of transcriptional networks. However, the relevance of alternative splicing is often unclear as distinct roles of alternatively spliced isoforms are often not determined. In this study, we addressed the functions of alternatively spliced isoforms of zebrafish Mef2ca and Mef2cb, two transcription factors involved in the development of striated muscle and head skeletal patterning [8], [14], [16], [42]. Mef2c mRNA is alternatively spliced in several organisms [20], [21], [22], [24], [52], and a recent report suggests that aberrant splice variants of MEF2C are involved in myogenic disorders [53]. Nevertheless, the functional differences between alternatively spliced Mef2C variants remain elusive. Our findings make three major points regarding the function of alternative splicing in Mef2c proteins of teleost fish. Firstly, both mef2ca and mef2cb gene transcripts undergo specific alternative splicing and their splicing patterns change during development. Secondly, splicing of mef2ca transcripts to include the exon 5 enhances its positive transcriptional activity and ability to interfere with gastrulation when over-expressed. Thirdly, an evolutionarily conserved alternate splice of exon 5 in mef2cb transcripts creates a long form that has unique pro-myogenic capacity.
4.1. Regulation of Mef2ca activity by alternative splicing in zebrafish development
In addition to the well documented expression of mef2ca starting from 12 hpf [32], we found mef2ca transcripts in zebrafish embryo prior to gastrulation at the 1K-cell stage, likely from maternal contribution, with their level declining to a minimum at 9–10 hpf. Starting from 12 hpf we observe an overall increase in expression levels of mef2ca mRNA. Besides changes in the abundance of mef2ca transcripts, we found a dynamic regulation of the splicing in the exon 5 region: the mef2ca variant including exon 5 (mef2ca 4–5–6) is the major isoform detected very early in development (1K-cell stage), suggesting that it might play a role prior to gastrulation, by 10 hpf, the mef2ca transcripts lacking exon 5 (mef2ca 4–6) are predominant. Subsequently, mef2ca 4–5–6 again climbs as muscle precursors undergo terminal differentiation, becoming the predominant isoform at 24 hpf. Such splicing of exon 5 is evolutionary conserved between Xenopus and teleosts, suggesting it has biological significance [24] (Fig. S3). Moreover, muscle differentiation in zebrafish is associated with several other muscle-specific alternative splicing events involving changes in splicing efficiency [54]. Although no specific function was assigned to the exon 5 domain by mutational and deletion analysis of the mouse and human protein counterparts [44], [45], our cell culture data indicate that the peptide sequence encoded by exon 5 contributes to the transcriptional activity of Mef2ca. The early expression of mef2ca transcripts including exon 5 (mef2ca 4–5–6) may indicate their early function in embryo patterning. Later in development, the preferential accumulation of full length mef2ca 4–5–6 mRNA at skeletal muscle fiber ends, suggests that its normal function is in muscle, a view confirmed by the requirement for Mef2ca function for skeletal muscle fiber growth and heart myogenesis [8], [55]. In the current work the function of Mef2ca isoforms was probed by ectopic over-expression; mef2ca 4–5–6 RNA, but not mef2ca 4–6 RNA, causes gross defects during gastrulation. We suggest that these effects of Mef2ca 4–5–6 are attributable to its ability to activate, directly or indirectly, a specific subset of pivotal genes in gastrulation. We observed the induction in chd (2-fold) mRNA and a milder (1.4 fold) increase in noggin 1 (nog1) mRNAs that encode two inhibitors of the BMP signaling. chd is required to repress bmp2b function in formation of the organizer and dorsoventral patterning of mesoderm and neural tissue [56], [57], [58]. Over-expression of chd dorsalizes embryos [59], [60], a phenotype present in a fraction of embryos following Mef2ca 4–5–6 over-expression. Thus, up-regulation of these dorsalizing proteins may explain the effects of Mef2ca 4–5–6.
In Xenopus, MEF2D helps induce mesoderm by driving the expression of the Nodal-related 1 (ndr1) gene [61]. In zebrafish, Mef2ca 4–5–6 over-expression also increases in gsc and ndr1 mRNAs (2.2- and 1.5-folds, respectively), which regulate dorsoventral patterning in organisms ranging from Drosophila to mammals [49], [50], [62], [63], [64]. In line with our results it has been previously reported that expression of gsc, is reduced in mef2ca −/− (hoover) mutants [42]. Although we cannot exclude off-target effects, this specific ability of Mef2ca 4–5–6, but not of similar amounts of Mef2ca 4–6, suggests distinct transcriptional activity of the former. In silico analysis of promoter regions of chd and ndr1 genes revealed the presence of several putative MEF2 binding sites (YTA(A/T)4TAR) (data not shown), raising the possibility that Mef2ca 4–5–6 directly activates their expression during early development. Later in development chordin expression may be sustained by Mef2d, which constitutively includes the sequence encoded by exon 5, and which is expressed from mid-gastrulation in adaxial muscle cells that also express chordin [32], [60] or by Mef2cb proteins. Interestingly, injection of either mef2cbS or mef2cbL transcripts, both containing exon 5, has resulted in similar developmental defects to that of mef2ca 4–5–6 mRNA injection. Future studies will clarify whether these genes are indeed direct targets of a Mef2 protein containing exon 5.
After gastrulation, zebrafish mef2ca transcripts accumulate starting from 12 hpf [14], [32], and mef2ca 4–5–6 transcripts are particularly abundant by 24 hpf, We suggest that Mef2ca 4–5–6 function might modulate chordin and other target gene expression in the somites at later stages during myotome patterning, where later muscle differentiation is regulated by BMP signaling and where chordin expression has been observed [60], [65], [66], [67], [68].
The protein sequence encoded by exon 5 might represent a binding motif that mediates protein–protein interactions with specific co-factors, as one recognized function for alternatively spliced isoforms is to remodel the protein–protein interaction network [69]. Supporting this hypothesis is the recent demonstration that the domains encoded by the mutually exclusive α1/α2 exons of mouse MEF2D can mediate interactions with different sets of co-repressors or co-activators [23].
4.2. Gene duplication and evolutionary partitioning of alternative splicing
The importance of other splices in Mef2ca remains to be determined. The γ-like and 4′–6′ splices have low abundance and we were unable to display unique activities for these isoforms. On the other hand, unlike in mammals, exon 3 does not appear to show alternative splicing in Mef2ca, the gene only having an α1 version. As the α1 exon of mouse Mef2D mediates interactions with specific transcriptional co-regulators [23], Mef2ca may have a more restricted range of functions compared to Mef2cb, which retains alternative α exons in its genomic sequence. However, at the stages examined, transcripts of mef2cb containing the α2 exon had low abundance, suggesting that this splice may be significant in specific cell types or developmental stages. In the adult we found a high proportion of the mef2cb transcripts containing the 3α2 exon in striated muscle tissue where it might play a specific role in mediating muscle gene expression as shown for the analogous splice variant of Mef2d in mammals [23]. Conversely, mef2cb transcripts omitting exon 5 were not observed. Instead, teleost mef2cb has evolved a unique splice, possibly derived by exonisation [1] of a part of intron 5. The addition of this octapeptide and its conservation across teleosts appears to have conferred myogenic properties to Mef2cbL.
4.3. Alternative splicing of mef2cb gene generates a pro-myogenic transcription factor
We detected mef2cb transcripts in zebrafish embryo as early as 50% epiboly stage. Mef2cbL is the prevalent Mef2cb isoform starting from 9 to 10 hpf, concomitantly with the onset of somitogenesis and has a unique pro-myogenic capacity. mef2cb mRNA over-expression can convert cells to skeletal muscle (Fig. 6A; [8]). This result suggests a role for Mef2 as a skeletal muscle determination factor in zebrafish head, challenging the classical epistatic relationship between MyoD and MEF2 in which MyoD acts upstream of MEF2 to direct embryonic multipotent progenitors into the myogenic lineage. The myogenic activity of Mef2cbL relies on an octapeptide encoded by a short sequence of intron 5 retained in the transcript. This insert, being too short to form a domain, may act by changing the structural fold and leading to a new function of the protein [70]. Muscle conversion was not observed upon ectopic expression of Mef2cbL in mouse fibroblasts, congruent with previous observations made with the mouse MEF2 proteins [9], [71], [72]. Thus, we propose the existence of a specific co-factor expressed in zebrafish head mesoderm that confers myogenic capacity to Mef2cbL. Identifying Mef2cb's molecular partners recruited specifically in the presence of the octapeptide to activate the expression of myod and other muscle genes may help in deciphering the molecular mechanisms underlying the pro-myogenic activity of Mef2cbL.
5. Conclusions
Our data reveal novel alternative splicing events around exon 5 of zebrafish mef2ca and mef2cb transcripts. These various evolutionarily conserved transcripts expand the transcriptional range of activity of Mef2c proteins. We propose that by excluding or including sequences of the exon 5 region, Mef2cs can acquire distinct properties, which allow them to regulate different sets of target genes and execute unique developmental programs in vivo.
The following are the supplementary data related to this article.
Vertebrate MEF2 transcripts are alternatively spliced.
A) Schematic of the highly similar structures of three vertebrate MEF2C genes among coding exons (black boxes). To simplify the comparison, we assigned the number 1 to the exon containing the first translated ATG. Introns are indicated by solid lines. MEF2C genes from the three species share three alternative exons: the α1 and α2 mutually exclusive exons, the β skipping exon, and 3′ splice site selection at exon 9. B) Schematic of the vertebrate Mef2c gene exon numbering adopted in this paper. In the table the exon numbering of the mouse and frog MEF2C genes adopted in the indicated references is reported. C) Splicing patterns of frog, mouse and human MEF2C. The MADS box and MEF2 domain are encoded by exons 1 and 2.
Amino acid conservation of alternative spliced domains of vertebrate Mef2c proteins.
A) α2 alternative exon, β skipping exon and γ region in mef2cb and mef2ca genes predicted with the TBLASTN algorithm. The sequences of bona fide spliced out exons, the percentage of homology with the mouse sequence and the putative splice sites are indicated. B) Comparison of amino acid sequences for zebrafish Mef2ca and Mef2cb splice variants. Protein sequence encoded by different exons is indicated, and alternatively spliced out regions are marked in yellow and green. TADs are colored in blue and orange.
Amino acid conservation in the exon 5 encoded domain of teleosts Mef2 proteins.
A) Comparison of amino acid sequences encoded by exon 5 and surrounding regions for zebrafish Mef2ca and Mef2cb proteins and the predicted Mef2 proteins from cavefish (S. anophtalmus and S angustiporus), medaka (O. latipes), pufferfish (T. rubripes) and stickleback (G. aculeatus). B) GenBank and NCBI reference accession numbers of the sequences used for the sequence alignment in A.
Developmental expression profile of zebrafish mef2ca and mef2cb.
A) Developmental expression profile of mef2ca and mef2cb transcripts by semi-quantitative RT-PCR analysis of the RNA extracted from staged zebrafish embryos. To determine the concentration of the transcripts we constructed a standard curve by amplifying serial dilutions of plasmid DNA templates. As a control for the quantity of substrate RNA, we amplified the same samples for actb2. B) Double in situ hybridization for 22 hpf zebrafish embryos for myod, mef2ca and mef2cb transcripts. Wholemounts shown in lateral view, anterior to left.
Quantitative analysis of the mRNA levels of mef2ca and of mef2cb exon 3α splice variants during D. rerio development and in adult tissues.
A) Left panel. Schematic representation of mef2cb 3α1 or 3α2 alternative exons. Arrows show annealing sites of isoform-specific primers used in the RT-PCR analysis they were designed to give amplification products of the same size (190 bp). Right panel. Expression analysis of mef2cb transcripts including the mutually exclusive 3α1 or 3α2 exon by RT-PCR. Total RNA was purified from staged embryos. To amplify an amount of exon 3α2 containing DNA similar to that containing exon 3α2, four additional PCR cycles were required. (B) Left panel. Schematic representation of mef2ca 3α1 and of mef2cb 3α1 or 3α2 alternative exons. Arrows show annealing sites of the primers used in the RT-PCR analysis. They give amplification products of distinct sizes. Right panel. Expression analysis of mef2ca and mef2cb transcripts including the mutually exclusive 3α1 or 3α2 exon by RT-PCR in adult tissues. Total RNA was purified from the brain, liver, skeletal and cardiac muscle of adult zebrafish. The level of expression of the transcripts was evaluated by using primers that anneal to exons 2 and 4 for both mef2c genes, in the case of mef2cb, they give two amplification products of distinct sizes: 196 and 187 bp, depending on the incorporation of 3α1 or 3α2 alternative exons in the transcripts. PCR products were separated in 8% polyacrylamide gels. Length of PCR products (bp) is indicated.
Effects of Mef2ca splice variants overexpression in zebrafish embryos.
A) Zebrafish embryos were injected with 25 pg of in vitro-transcribed mef2ca 4–5–6 RNA together with rhodamine dextran at the 1–2 cells stage and analyzed at 20 hpf. Successfully injected embryos were distinguished on the basis of the red fluorescence (insets) and classified on the basis of morphology into ‘severely defective’ (blocked development), ‘defective’ (altered development) or ‘normal’. B) Dose-dependent effects of in vitro-transcribed mef2ca mRNAs on embryos development. The graph reports the quantification of defective embryos upon injection of increasing doses (25 pg and 50 pg) of RNA encoding Mef2ca 4–5–6 and 4–6 splice variants. Controls were uninjected embryos (Ctrl). The number of embryos tested in each experiments is indicated by (n) on top of each column. C) Western blot analysis showing over-expression of Mef2ca 4–5–6 and Mef2ca 4–6 following RNA injection (25 pg) into embryos. COS-1 cell extracts over-expressing Mef2ca 4–5–6 or 4–6 were used as electrophoretic mobility controls (a and b, respectively). α-Tubulin was used as loading control. D) To assess whether injection of 10 pg mef2ca 4–5–6 RNA leads to aberrant maturation of vascular, neuronal or cardiac tissues, injected embryos (right panels) or controls (left panels) were subjected to in situ hybridization for myl7, kdrl, neurog1 and ascl1a mRNAs, respectively.
Primers used in semi-quantitative RT-PCR and qRTPCR.
A) In the table is reported a restricted list of PCR primer pairs used in the semi-quantitative PCR reaction, missing primers are available on request. B) Schematic drawing of mef2ca isoform specific and isoform common primers used in qRTPCR. Sequences are available on request.
Acknowledgements
We thank Veronica Mantovani for her help in performing qRTPCR and transactivation assays, Isabella Della Noce and Filippo Schepis for their help in RNA and protein extraction from zebrafish embryos, Cristina Valensisi for her help in designing splice variants-specific primers. We are grateful to Sharon Amacher, Tom Gallagher and Tod Gulick for sharing their results before publication. We are grateful to Andrea Martello, Tommaso Selmi and Tommaso Zanocco-Marani for helpful discussion. We greatly thank Carol Imbriano and Alessandro Magli for reading the manuscript and helpful suggestions.
Research support in the laboratory of S. Molinari was provided by: Fondazione Cassa di Risparmio di Modena, progetto internazionale bando 2010 (grant n° E91J10000170003), AFM (Association Francaise contre les Myopathies) (grant n°16252) and Optistem (European collaborative project HEALTH-2007-1.4-6). M.G. was a recipient of short term fellowships from EMBO (ref. ASTF 414.00-2009), EufishBioMed (COST action BM0804, ref code 141111-012508), University of Modena and Reggio Emilia (Bando di Mobilità), Myores (Exchange Fund) and CIB (Consorzio Italiano Biotecnologie) that allowed him to visit and perform experiments in the laboratory of S. M. Hughes. A.P. was a recipient of a short-term fellowship from CIB (Consorzio Italiano Biotecnologie) that supported his visit to the laboratory of S. M. Hughes. S. M. H. is a Medical Research Council Scientist with Programme Grant G1001029 support that funded Y.H. and studies in the Hughes laboratory. Y. H. was also supported by a collaborative grant from the UK British Heart Foundation Centres of Excellence at King's College London, Imperial College London and the Universities of Edinburgh and Oxford.
Contributor Information
Y. Hinits, Email: yaniv.hinits@kcl.ac.uk.
S. Molinari, Email: susanna.molinari@unimore.it.
References
- 1.Xing Y., Lee C. Alternative splicing and RNA selection pressure—evolutionary consequences for eukaryotic genomes. Nat. Rev. Genet. 2006;7:499–509. doi: 10.1038/nrg1896. [DOI] [PubMed] [Google Scholar]
- 2.Keren H., Lev-Maor G., Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nat. Rev. Genet. 2010;11:345–355. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 3.Roy S.W., Irimia M. Splicing in the eukaryotic ancestor: form, function and dysfunction. Trends Ecol. Evol. 2009;24:447–455. doi: 10.1016/j.tree.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Kornblihtt A.R., Schor I.E., Allo M., Dujardin G., Petrillo E., Munoz M.J. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nat. Rev. Mol. Cell Biol. 2013;14:153–165. doi: 10.1038/nrm3525. [DOI] [PubMed] [Google Scholar]
- 5.Trapnell C., Williams B.A., Pertea G., Mortazavi A., Kwan G., van Baren M.J., Salzberg S.L., Wold B.J., Pachter L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llorian M., Smith C.W. Decoding muscle alternative splicing. Curr. Opin. Genet. Dev. 2011;21:380–387. doi: 10.1016/j.gde.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Pistoni M., Ghigna C., Gabellini D. Alternative splicing and muscular dystrophy. RNA Biol. 2010;7:441–452. doi: 10.4161/rna.7.4.12258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinits Y., Pan L., Walker C., Dowd J., Moens C.B., Hughes S.M. Zebrafish Mef2ca and Mef2cb are essential for both first and second heart field cardiomyocyte differentiation. Dev. Biol. 2012;369:199–210. doi: 10.1016/j.ydbio.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black B.L., Olson E.N. Transcriptional control of muscle development by myocyte enhancer factor-2 (MEF2) proteins. Annu. Rev. Cell Dev. Biol. 1998;14:167–196. doi: 10.1146/annurev.cellbio.14.1.167. [DOI] [PubMed] [Google Scholar]
- 10.Bour B.A., O'Brien M.A., Lockwood W.L., Goldstein E.S., Bodmer R., Taghert P.H., Abmayr S.M., Nguyen H.T. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- 11.Lilly B., Zhao B., Ranganayakulu G., Paterson B.M., Schulz R.A., Olson E.N. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 12.Ranganayakulu G., Zhao B., Dokidis A., Molkentin J.D., Olson E.N., Schulz R.A. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev. Biol. 1995;171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- 13.Lin Q., Schwarz J., Bucana C., Olson E.N. Control of mouse cardiac morphogenesis and myogenesis by transcription factor MEF2C. Science. 1997;276:1404–1407. doi: 10.1126/science.276.5317.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinits Y., Hughes S.M. Mef2s are required for thick filament formation in nascent muscle fibres. Development. 2007;134:2511–2519. doi: 10.1242/dev.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh T.K., Song F.F., Packham E.A., Buxton S., Robinson T.E., Ronksley J., Self T., Bonser A.J., Brook J.D. Physical interaction between TBX5 and MEF2C is required for early heart development. Mol. Cell. Biol. 2009;29:2205–2218. doi: 10.1128/MCB.01923-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lazic S., Scott I.C. Mef2cb regulates late myocardial cell addition from a second heart field-like population of progenitors in zebrafish. Dev. Biol. 2011;354:123–133. doi: 10.1016/j.ydbio.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 17.Potthoff M.J., Arnold M.A., McAnally J., Richardson J.A., Bassel-Duby R., Olson E.N. Regulation of skeletal muscle sarcomere integrity and postnatal muscle function by Mef2c. Mol. Cell. Biol. 2007;27:8143–8151. doi: 10.1128/MCB.01187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodou E., Sparrow D.B., Mohun T., Treisman R. MEF2 proteins, including MEF2A, are expressed in both muscle and non-muscle cells. Nucleic Acids Res. 1995;23:4267–4274. doi: 10.1093/nar/23.21.4267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potthoff M.J., Olson E.N. MEF2: a central regulator of diverse developmental programs. Development. 2007;134:4131–4140. doi: 10.1242/dev.008367. [DOI] [PubMed] [Google Scholar]
- 20.McDermott J.C., Cardoso M.C., Yu Y.T., Andres V., Leifer D., Krainc D., Lipton S.A., Nadal-Ginard B. hMEF2C gene encodes skeletal muscle- and brain-specific transcription factors. Mol. Cell. Biol. 1993;13:2564–2577. doi: 10.1128/mcb.13.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu B., Gulick T. Phosphorylation and alternative pre-mRNA splicing converge to regulate myocyte enhancer factor 2C activity. Mol. Cell. Biol. 2004;24:8264–8275. doi: 10.1128/MCB.24.18.8264-8275.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu B., Ramachandran B., Gulick T. Alternative pre-mRNA splicing governs expression of a conserved acidic transactivation domain in myocyte enhancer factor 2 factors of striated muscle and brain. J. Biol. Chem. 2005;280:28749–28760. doi: 10.1074/jbc.M502491200. [DOI] [PubMed] [Google Scholar]
- 23.Sebastian S., Faralli H., Yao Z., Rakopoulos P., Palii C., Cao Y., Singh K., Liu Q.C., Chu A., Aziz A., Brand M., Tapscott S.J., Dilworth F.J. Tissue-specific splicing of a ubiquitously expressed transcription factor is essential for muscle differentiation. Genes Dev. 2013;27:1247–1259. doi: 10.1101/gad.215400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.della Gaspera B., Armand A.S., Sequeira I., Lecolle S., Gallien C.L., Charbonnier F., Chanoine C. The Xenopus MEF2 gene family: evidence of a role for XMEF2C in larval tendon development. Dev. Biol. 2009:392–402. doi: 10.1016/j.ydbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari S., Molinari S., Melchionna R., Cusella-De Angelis M.G., Battini R., De Angelis L., Kelly R., Cossu G. Absence of MEF2 binding to the A/T-rich element in the muscle creatine kinase (MCK) enhancer correlates with lack of early expression of the MCK gene in embryonic mammalian muscle. Cell Growth Differ. 1997;8:23–34. [PubMed] [Google Scholar]
- 26.Guo Y., Kuhl S.J., Pfister A.S., Cizelsky W., Denk S., Beer-Molz L., Kuhl M. Comparative analysis reveals distinct and overlapping functions of Mef2c and Mef2d during cardiogenesis in Xenopus laevis. PLoS One. 2014;9:e87294. doi: 10.1371/journal.pone.0087294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genikhovich G., Technau U. Complex functions of Mef2 splice variants in the differentiation of endoderm and of a neuronal cell type in a sea anemone. Development. 2011;138:4911–4919. doi: 10.1242/dev.068122. [DOI] [PubMed] [Google Scholar]
- 28.Angelelli C., Magli A., Ferrari D., Ganassi M., Matafora V., Parise F., Razzini G., Bachi A., Ferrari S., Molinari S. Differentiation-dependent lysine 4 acetylation enhances MEF2C binding to DNA in skeletal muscle cells. Nucleic Acids Res. 2008;36:915–928. doi: 10.1093/nar/gkm1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walton H.S., Gebhardt F.M., Innes D.J., Dodd P.R. Analysis of multiple exon-skipping mRNA splice variants using SYBR Green real-time RT-PCR. J. Neurosci. Methods. 2007;160:294–301. doi: 10.1016/j.jneumeth.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 30.Vandenbroucke I.I., Vandesompele J., Paepe A.D., Messiaen L. Quantification of splice variants using real-time PCR. Nucleic Acids Res. 2001;29:E68–68. doi: 10.1093/nar/29.13.e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Westerfield M. Institute of Neuroscience, University of Oregon; Eugene, OR: 1993. The Zebrafish Book a Guide for the Laboratory Use of Zebrafish Danio (Brachydanio) rerio. [Google Scholar]
- 32.Ticho B.S., Stainier D.Y., Fishman M.C., Breitbart R.E. Three zebrafish MEF2 genes delineate somitic and cardiac muscle development in wild-type and mutant embryos. Mech. Dev. 1996;59:205–218. doi: 10.1016/0925-4773(96)00601-6. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg E.S., Allende M.L., Kelly C.S., Abdelhamid A., Murakami T., Andermann P., Doerre O.G., Grunwald D.J., Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- 34.Yelon D., Horne S.A., Stainier D.Y. Restricted expression of cardiac myosin genes reveals regulated aspects of heart tube assembly in zebrafish. Dev. Biol. 1999;214:23–37. doi: 10.1006/dbio.1999.9406. [DOI] [PubMed] [Google Scholar]
- 35.Thompson M.A., Ransom D.G., Pratt S.J., MacLennan H., Kieran M.W., Detrich H.W., 3rd, Vail B., Huber T.L., Paw B., Brownlie A.J., Oates A.C., Fritz A., Gates M.A., Amores A., Bahary N., Talbot W.S., Her H., Beier D.R., Postlethwait J.H., Zon L.I. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev. Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- 36.Blader P., Fischer N., Gradwohl G., Guillemot F., Strahle U. The activity of neurogenin1 is controlled by local cues in the zebrafish embryo. Development. 1997;124:4557–4569. doi: 10.1242/dev.124.22.4557. [DOI] [PubMed] [Google Scholar]
- 37.Allende M.L., Weinberg E.S. The expression pattern of two zebrafish achaete-scute homolog (ash) genes is altered in the embryonic brain of the cyclops mutant. Dev. Biol. 1994;166:509–530. doi: 10.1006/dbio.1994.1334. [DOI] [PubMed] [Google Scholar]
- 38.Hinits Y., Williams V.C., Sweetman D., Donn T.M., Ma T.P., Moens C.B., Hughes S.M. Defective cranial skeletal development, larval lethality and haploinsufficiency in Myod mutant zebrafish. Dev. Biol. 2011;358:102–112. doi: 10.1016/j.ydbio.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagendijk A.K., Moulton J.D., Bakkers J. Revealing details: whole mount microRNA in situ hybridization protocol for zebrafish embryos and adult tissues. Biol. Open. 2012;1:566–569. doi: 10.1242/bio.2012810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborn D.P., Li K., Hinits Y., Hughes S.M. Cdkn1c drives muscle differentiation through a positive feedback loop with Myod. Dev. Biol. 2011;350:464–475. doi: 10.1016/j.ydbio.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blagden C.S., Currie P.D., Ingham P.W., Hughes S.M. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–2175. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller C.T., Swartz M.E., Khuu P.A., Walker M.B., Eberhart J.K., Kimmel C.B. mef2ca is required in cranial neural crest to effect Endothelin1 signaling in zebrafish. Dev. Biol. 2007;308:144–157. doi: 10.1016/j.ydbio.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magli A., Angelelli C., Ganassi M., Baruffaldi F., Matafora V., Battini R., Bachi A., Messina G., Rustighi A., Del Sal G., Ferrari S., Molinari S. Proline isomerase Pin1 represses terminal differentiation and myocyte enhancer factor 2C function in skeletal muscle cells. J. Biol. Chem. 2010;285:34518–34527. doi: 10.1074/jbc.M110.104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molkentin J.D., Black B.L., Martin J.F., Olson E.N. Mutational analysis of the DNA binding, dimerization, and transcriptional activation domains of MEF2C. Mol. Cell. Biol. 1996;16:2627–2636. doi: 10.1128/mcb.16.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janson C.G., Chen Y., Li Y., Leifer D. Functional regulatory regions of human transcription factor MEF2C. Brain Res. Mol. Brain Res. 2001;97:70–82. doi: 10.1016/s0169-328x(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 46.Naya F.J., Olson E. MEF2: a transcriptional target for signaling pathways controlling skeletal muscle growth and differentiation. Curr. Opin. Cell Biol. 1999;11:683–688. doi: 10.1016/s0955-0674(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 47.Borghi S., Molinari S., Razzini G., Parise F., Battini R., Ferrari S. The nuclear localization domain of the MEF2 family of transcription factors shows member-specific features and mediates the nuclear import of histone deacetylase 4. J. Cell Sci. 2001;114:4477–4483. doi: 10.1242/jcs.114.24.4477. [DOI] [PubMed] [Google Scholar]
- 48.Xu P.F., Houssin N., Ferri-Lagneau K.F., Thisse B., Thisse C. Construction of a vertebrate embryo from two opposing morphogen gradients. Science. 2014;344:87–89. doi: 10.1126/science.1248252. [DOI] [PubMed] [Google Scholar]
- 49.De Robertis E.M. Spemann's organizer and self-regulation in amphibian embryos. Nat. Rev. Mol. Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langdon Y.G., Mullins M.C. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu. Rev. Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- 51.Branam A.M., Hoffman G.G., Pelegri F., Greenspan D.S. Zebrafish chordin-like and chordin are functionally redundant in regulating patterning of the dorsoventral axis. Dev. Biol. 2010;341:444–458. doi: 10.1016/j.ydbio.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Infantino V., Convertini P., Menga A., Iacobazzi V. MEF2C exon alpha: role in gene activation and differentiation. Gene. 2013;531:355–362. doi: 10.1016/j.gene.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 53.Bachinski L.L., Sirito M., Bohme M., Baggerly K.A., Udd B., Krahe R. Altered MEF2 isoforms in myotonic dystrophy and other neuromuscular disorders. Muscle Nerve. 2010;42:856–863. doi: 10.1002/mus.21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallagher T.L., Arribere J.A., Geurts P.A., Exner C.R., McDonald K.L., Dill K.K., Marr H.L., Adkar S.S., Garnett A.T., Amacher S.L., Conboy J.G. Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev. Biol. 2011;359:251–261. doi: 10.1016/j.ydbio.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yogev O., Williams V.C., Hinits Y., Hughes S.M. eIF4EBP3L acts as a gatekeeper of TORC1 in activity-dependent muscle growth by specifically regulating mef2ca translational initiation. PLoS Biol. 2013;11:e1001679. doi: 10.1371/journal.pbio.1001679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hammerschmidt M., Serbedzija G.N., McMahon A.P. Genetic analysis of dorsoventral pattern formation in the zebrafish: requirement of a BMP-like ventralizing activity and its dorsal repressor. Genes Dev. 1996;10:2452–2461. doi: 10.1101/gad.10.19.2452. [DOI] [PubMed] [Google Scholar]
- 57.Oelgeschlager M., Kuroda H., Reversade B., De Robertis E.M. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev. Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- 58.Schulte-Merker S., Lee K.J., McMahon A.P., Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- 59.Sasai Y., Lu B., Steinbeisser H., Geissert D., Gont L.K., De Robertis E.M. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miller-Bertoglio V.E., Fisher S., Sanchez A., Mullins M.C., Halpern M.E. Differential regulation of chordin expression domains in mutant zebrafish. Dev. Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- 61.Kolpakova A., Katz S., Keren A., Rojtblat A., Bengal E. Transcriptional regulation of mesoderm genes by MEF2D during early Xenopus development. PLoS One. 2013;8:e69693. doi: 10.1371/journal.pone.0069693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dal-Pra S., Furthauer M., Van-Celst J., Thisse B., Thisse C. Noggin1 and Follistatin-like2 function redundantly to Chordin to antagonize BMP activity. Dev. Biol. 2006;298:514–526. doi: 10.1016/j.ydbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Imai Y., Gates M.A., Melby A.E., Kimelman D., Schier A.F., Talbot W.S. The homeobox genes vox and vent are redundant repressors of dorsal fates in zebrafish. Development. 2001;128:2407–2420. doi: 10.1242/dev.128.12.2407. [DOI] [PubMed] [Google Scholar]
- 64.Tian T., Meng A.M. Nodal signals pattern vertebrate embryos. Cell. Mol. Life Sci. 2006;63:672–685. doi: 10.1007/s00018-005-5503-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Du S.J., Devoto S.H., Westerfield M., Moon R.T. Positive and negative regulation of muscle cell identity by members of the hedgehog and TGF-beta gene families. J. Cell Biol. 1997;139:145–156. doi: 10.1083/jcb.139.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meng A., Moore B., Tang H., Yuan B., Lin S. A Drosophila doublesex-related gene, terra, is involved in somitogenesis in vertebrates. Development. 1999;126:1259–1268. doi: 10.1242/dev.126.6.1259. [DOI] [PubMed] [Google Scholar]
- 67.Yin C., Kikuchi K., Hochgreb T., Poss K.D., Stainier D.Y. Hand2 regulates extracellular matrix remodeling essential for gut-looping morphogenesis in zebrafish. Dev. Cell. 2010;18:973–984. doi: 10.1016/j.devcel.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patterson S.E., Bird N.C., Devoto S.H. BMP regulation of myogenesis in zebrafish. Dev. Dyn. 2010;239:806–817. doi: 10.1002/dvdy.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellis J.D., Barrios-Rodiles M., Colak R., Irimia M., Kim T., Calarco J.A., Wang X., Pan Q., O'Hanlon D., Kim P.M., Wrana J.L., Blencowe B.J. Tissue-specific alternative splicing remodels protein–protein interaction networks. Mol. Cell. 2012;46:884–892. doi: 10.1016/j.molcel.2012.05.037. [DOI] [PubMed] [Google Scholar]
- 70.Garcia J., Gerber S.H., Sugita S., Sudhof T.C., Rizo J. A conformational switch in the Piccolo C2A domain regulated by alternative splicing. Nat. Struct. Mol. Biol. 2004;11:45–53. doi: 10.1038/nsmb707. [DOI] [PubMed] [Google Scholar]
- 71.Black B.L., Molkentin J.D., Olson E.N. Multiple roles for the MyoD basic region in transmission of transcriptional activation signals and interaction with MEF2. Mol. Cell. Biol. 1998;18:69–77. doi: 10.1128/mcb.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Molkentin J.D., Black B.L., Martin J.F., Olson E.N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Vertebrate MEF2 transcripts are alternatively spliced.
A) Schematic of the highly similar structures of three vertebrate MEF2C genes among coding exons (black boxes). To simplify the comparison, we assigned the number 1 to the exon containing the first translated ATG. Introns are indicated by solid lines. MEF2C genes from the three species share three alternative exons: the α1 and α2 mutually exclusive exons, the β skipping exon, and 3′ splice site selection at exon 9. B) Schematic of the vertebrate Mef2c gene exon numbering adopted in this paper. In the table the exon numbering of the mouse and frog MEF2C genes adopted in the indicated references is reported. C) Splicing patterns of frog, mouse and human MEF2C. The MADS box and MEF2 domain are encoded by exons 1 and 2.
Amino acid conservation of alternative spliced domains of vertebrate Mef2c proteins.
A) α2 alternative exon, β skipping exon and γ region in mef2cb and mef2ca genes predicted with the TBLASTN algorithm. The sequences of bona fide spliced out exons, the percentage of homology with the mouse sequence and the putative splice sites are indicated. B) Comparison of amino acid sequences for zebrafish Mef2ca and Mef2cb splice variants. Protein sequence encoded by different exons is indicated, and alternatively spliced out regions are marked in yellow and green. TADs are colored in blue and orange.
Amino acid conservation in the exon 5 encoded domain of teleosts Mef2 proteins.
A) Comparison of amino acid sequences encoded by exon 5 and surrounding regions for zebrafish Mef2ca and Mef2cb proteins and the predicted Mef2 proteins from cavefish (S. anophtalmus and S angustiporus), medaka (O. latipes), pufferfish (T. rubripes) and stickleback (G. aculeatus). B) GenBank and NCBI reference accession numbers of the sequences used for the sequence alignment in A.
Developmental expression profile of zebrafish mef2ca and mef2cb.
A) Developmental expression profile of mef2ca and mef2cb transcripts by semi-quantitative RT-PCR analysis of the RNA extracted from staged zebrafish embryos. To determine the concentration of the transcripts we constructed a standard curve by amplifying serial dilutions of plasmid DNA templates. As a control for the quantity of substrate RNA, we amplified the same samples for actb2. B) Double in situ hybridization for 22 hpf zebrafish embryos for myod, mef2ca and mef2cb transcripts. Wholemounts shown in lateral view, anterior to left.
Quantitative analysis of the mRNA levels of mef2ca and of mef2cb exon 3α splice variants during D. rerio development and in adult tissues.
A) Left panel. Schematic representation of mef2cb 3α1 or 3α2 alternative exons. Arrows show annealing sites of isoform-specific primers used in the RT-PCR analysis they were designed to give amplification products of the same size (190 bp). Right panel. Expression analysis of mef2cb transcripts including the mutually exclusive 3α1 or 3α2 exon by RT-PCR. Total RNA was purified from staged embryos. To amplify an amount of exon 3α2 containing DNA similar to that containing exon 3α2, four additional PCR cycles were required. (B) Left panel. Schematic representation of mef2ca 3α1 and of mef2cb 3α1 or 3α2 alternative exons. Arrows show annealing sites of the primers used in the RT-PCR analysis. They give amplification products of distinct sizes. Right panel. Expression analysis of mef2ca and mef2cb transcripts including the mutually exclusive 3α1 or 3α2 exon by RT-PCR in adult tissues. Total RNA was purified from the brain, liver, skeletal and cardiac muscle of adult zebrafish. The level of expression of the transcripts was evaluated by using primers that anneal to exons 2 and 4 for both mef2c genes, in the case of mef2cb, they give two amplification products of distinct sizes: 196 and 187 bp, depending on the incorporation of 3α1 or 3α2 alternative exons in the transcripts. PCR products were separated in 8% polyacrylamide gels. Length of PCR products (bp) is indicated.
Effects of Mef2ca splice variants overexpression in zebrafish embryos.
A) Zebrafish embryos were injected with 25 pg of in vitro-transcribed mef2ca 4–5–6 RNA together with rhodamine dextran at the 1–2 cells stage and analyzed at 20 hpf. Successfully injected embryos were distinguished on the basis of the red fluorescence (insets) and classified on the basis of morphology into ‘severely defective’ (blocked development), ‘defective’ (altered development) or ‘normal’. B) Dose-dependent effects of in vitro-transcribed mef2ca mRNAs on embryos development. The graph reports the quantification of defective embryos upon injection of increasing doses (25 pg and 50 pg) of RNA encoding Mef2ca 4–5–6 and 4–6 splice variants. Controls were uninjected embryos (Ctrl). The number of embryos tested in each experiments is indicated by (n) on top of each column. C) Western blot analysis showing over-expression of Mef2ca 4–5–6 and Mef2ca 4–6 following RNA injection (25 pg) into embryos. COS-1 cell extracts over-expressing Mef2ca 4–5–6 or 4–6 were used as electrophoretic mobility controls (a and b, respectively). α-Tubulin was used as loading control. D) To assess whether injection of 10 pg mef2ca 4–5–6 RNA leads to aberrant maturation of vascular, neuronal or cardiac tissues, injected embryos (right panels) or controls (left panels) were subjected to in situ hybridization for myl7, kdrl, neurog1 and ascl1a mRNAs, respectively.
Primers used in semi-quantitative RT-PCR and qRTPCR.
A) In the table is reported a restricted list of PCR primer pairs used in the semi-quantitative PCR reaction, missing primers are available on request. B) Schematic drawing of mef2ca isoform specific and isoform common primers used in qRTPCR. Sequences are available on request.