Abstract
Introduction
With advancing technology it has become possible to accurately record and assess fetal heart rate (FHR) patterns from gestations as early as 20 weeks. The aim of our study was to describe early patterns of FHR, as recorded by transabdominal fetal electrocardiogram according to the Dawes-Redman criteria. Accordingly, short-term variability, basal heart rate, accelerations and decelerations were quantified at 20-24 weeks gestation among women with uncomplicated pregnancies.
Methods
This study was conducted in a subset of participants enrolled in a large prospective pregnancy cohort study. Our final data set consisted of 281 recordings of women with good perinatal outcomes that had undergone fetal electrocardiographic assessment as part of the Safe Passage Study.
Results
The success rate of the recordings was 95.4%. The mean frequency of small and large accelerations was 0.5 and 0.1 per 10 minutes respectively and that of small and large decelerations 0.3 and 0.008 per 10 minutes respectively. The mean and basal heart rates were both equal to 148.0 bpm at a median gestation of 161 days. The mean short term variation was 6.2 (SD 1.4) milliseconds and mean minute range 35.1 (SD 7.1) milliseconds.
Conclusion
The 20 to 24 week fetus demonstrates FHR patterns with more accelerations and decelerations, as well as higher baseline variability than was anticipated. Information from this study provides an important foundation for further, more detailed, studies of early FHR patterns.
Introduction
Electronic fetal heart rate (FHR) monitoring is widely used to evaluate fetal well-being. Since the introduction of FHR monitoring, analytic strategies and software have been developed to improve the accuracy of interpretation of FHR patterns before and during labour (1,2). However, very few articles have been published on the quantification of FHR patterns before 26 weeks (3).
The maturation of sympathetic and parasympathetic influences on heart rate progresses throughout development and is not complete, even at term (4). However, the lack of developmental data detailing the effects of these cardio-regulatory mechanisms before 30 weeks makes the interpretations of heart rate dynamics much less precise earlier in gestation (5).
Fetal movement early in gestation often precludes collection of uninterrupted FHR tracings with ultrasound monitors. Another factor contributing to the relative lack of knowledge regarding early FHR patterns is that ultrasound techniques typically used for fetal heart rate monitoring cannot precisely determine the timing of each heart beat and employ heart rate averaging techniques (6).
Trans-abdominal acquisition of fetal ECG provides a non-invasive and inexpensive alternative to these methods. The technique is superior to Doppler ultrasound in evaluating fetal cardiac rhythms during epochs of fetal movement and it affords the temporal resolution required for true beat-to-beat variation (2, 7, 8). The equipment for recording fetal ECG is now commercially available. Using the AN24 (Monica Healthcare Ltd) device, more than 80% of antenatal recordings have been of high quality and more importantly, correlation with the scalp electrode derived recordings of the FHR and variation during labour is excellent (9).
In this current report, we have used trans-abdominal fetal electrocardiography (fECG) to describe the FHR patterns between 20-24 weeks’ gestation in 281 pregnancies with good perinatal outcomes.
Methods
The Monica AN24 recordings we analysed were obtained under the auspices of the Prenatal Alcohol in SIDS and Stillbirth (PASS) Network, which is conducting the Safe Passage Study (SPS), a multicentre, international collaboration, investigating the role of prenatal alcohol exposure in the risk for sudden infant death syndrome, stillbirth and fetal alcohol spectrum disorders (www.safepassagestudy.org). Part of the antenatal assessment is to record the FHR for at least 30 minutes at 20 to 24 weeks gestation. Early ultrasound examinations, to confirm the gestational age, are done in all participants. Written informed consent to record FHR is part of the consent for the main study. Ethical approval has been obtained from the Health Research Ethics Committee of Stellenbosch University.
Recordings of the FHR are carried out in quiet rooms between 08h00 and 16h00 on weekdays with participants lying in a 15° right or left lateral position. Four electrodes are placed in a diamond-shaped pattern on the maternal abdomen, one just below the umbilicus, one just above the pubic hairline, and the other two laterally, equal distances from the top and bottom ones. The fifth electrode, for reference, is placed just lateral to the one on the right side. Before application, the skin is lightly braised to remove superficial dry squamous cells (this is essential to reduce electrode impedance). The 5 electrodes are then connected to the Monica AN24 monitoring device which is attached to the abdominal wall with an elastic band to prevent it from falling down and to keep the devices in similar position across all studies. At the end of the recording, the device is removed and connected to a laptop for downloading of the raw data. During these study sessions, tracings of the FHR were not available to the person doing the recording and both participant and operator were kept blind from the results of all analyses.
The first 411 recordings (total duration 18,385 minutes) obtained at 20-24 week’s gestation (140 to 167 days), from 1 October 2008 to 31 December 2009, were analysed in this current study. All participants had only one recording at 20-24 weeks (Fig 1a); some participants also had recordings at 28-32 weeks and 34-38 weeks as part of the SPS but this information is not part of the present study. The Dawes and Redman criteria of the DK 1.4a programme were used for analyses of the raw data (5). Because the duration of the recordings differed, the number of accelerations and decelerations were expressed in rates, i.e., number per 10 minutes of recording. In order to limit the recordings to those where the outcome was normal, recordings of pregnancies that ended in stillbirths or neonatal deaths were excluded as well as cases where the fetus weighed less than 2 500 g, born before 259 days or weighed less than the 10th percentile on the Tygerberg population customised growth curves (10). Cases where the delivery data were not available were also excluded (some participants delivered in other hospitals or before arrival to hospital) as well as recordings with durations of less than 30 minutes or where the DK analysis indicated the success rate of the recording was less than 50% (the success rate depended on signal loss; the percentage of time, during at least 15 minutes of recording, where the FHR pattern could be computed accurately). The Dawes-Redman criteria of the Monica DK (version 1.4a) software were used to analyse the raw data. Small and large accelerations are defined as having duration of at least 15 seconds with amplitude of 10 or 15 beats per minute (bpm) respectively. Small decelerations are defined as a deviation from baseline of at least 10 bpm for a minimum of 60 seconds and for large decelerations the duration had to be at least 30 seconds with amplitude of 20 bpm or more.
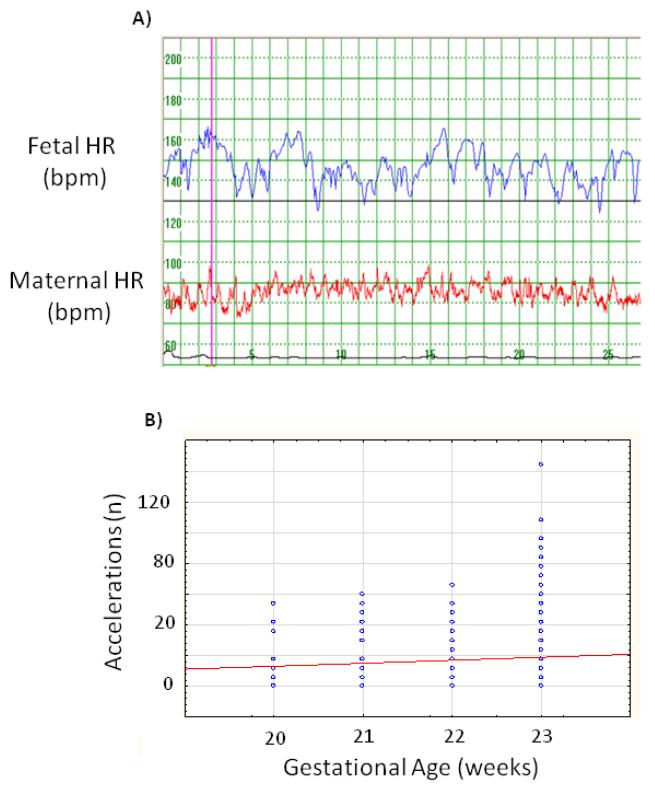
Figure 1.
a. The fetal heart rate patten as recorded by the Monica AN24 device
b. Number of fetal heart rate accelerations (large and small) as expressed for 60 minutes
All data from the Monica DK 1.4a program as well as information obtained from maternal records were entered into an Excel (Microsoft Inc.) spread sheet, specifically designed for this project. Frequency tables, categorized histograms and descriptive statistics (means, standard deviations, medians and interquartile ranges) were produced with the programme STATISTICA version 9 (StatSoft Inc. 2009). For the purpose of descriptive analysis a statistician from Stellenbosch University Department of Statistics and Actuarial Science was consulted.
Results
The initial study consisted of 411 participants. The percentage of signal loss per participant, from the initial 411 recordings, ranged from 0% to100% with a mean of 7.1% (SD 15.8 %; median 0.56%). Signal loss of more than 50% occurred in 19 [4.6%] of the recordings, giving a success rate of 95.4%. These 19 recordings were excluded as well as another 22 recordings (5.4%) which had a duration of less than 30 minutes. In addition, recordings from participants with the following characteristics were also excluded: delivery data not available, 11 (2.7%); preterm delivery, 56 (13.6%); term low birth weight, 21 (5.1%); intrauterine death, 11 (2.7 %); neonatal death, 2 (0.5%) and small-for-gestational age, 39 (9.5%). In some participants there was more than one reason for exclusion, and the recordings from the remaining 281 participants are the subject of this report.
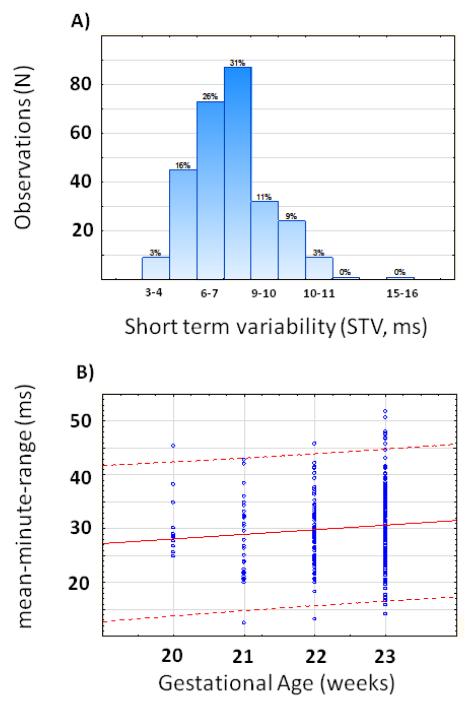
All but one participant belonged to the Cape Coloured population. Their ages ranged from 16 to 41 years with a median of 24 years. Prepregnancy BMIs were known in 137 participants; it ranged from 13.4 to 45.5 kg/m2 with a mean of 24 (SD 6.2) kg/m2. Information on the D.K. analyses of the recordings, gestational age at birth and birth weight was available in all participants but Apgar scores, only in 274 (Table I). The median gestational age when the recording was done was 161 days (23 weeks) with 55% of recordings done after 161 days gestation, 22% between 22 and 23 weeks and the remaining 23% before 22 weeks. Mean and basal heart rates were both equal to 148.0 bpm at this mean gestation of 161 days. The total duration of all the recordings was 13,028 minutes. Details of the analyses are given in Table I. We found that 71% of fetal heart rate recordings showed accelerations, and that decelerations were present in 67% of recordings. Only 29% of fetusses did not show any accelerations (Figure 2b). The short term variation ranged from 3.2 to 12.7 msec with a mean of 6.2 msec (Figure 2a). Mean minute range varied from 17.5 msec to 56.8 msec with a mean of 35.1 msec (Table I, Figure 2b).
Table I. Summary of fetal heart rate analyses.
| Variable | N | Mean | Median | Minimum | Maximum | Std. Dev. |
|---|---|---|---|---|---|---|
| Recording time (minutes) | 281 | 46.4 | 44.0 | 30.0 | 96.0 | 10.7 |
| Gestational age at recording (days) |
281 | 159.0 | 161.0 | 140.0 | 167.0 | 5.8 |
| Signal loss as % of recording time |
281 | 4.0 | 0.2 | 0.0 | 46.3 | 7.9 |
| Mean fetal heart rate (bpm) | 281 | 148.0 | 148.1 | 133.8 | 167.8 | 5.4 |
| Basal fetal heart rate (bpm) | 281 | 148.0 | 148.0 | 132.0 | 171.0 | 5.8 |
| SA in 10 minutes | 281 | 0.5 | 0.3 | 0.0 | 3.0 | 0.5 |
| LA in10 minutes | 281 | 0.1 | 0.0 | 0.0 | 1.5 | 0.2 |
| SD 10 minutes | 281 | 0.3 | 0.2 | 0.0 | 1.6 | 0.3 |
| LD in 10 minutes | 281 | 0.008 | 0.0 | 0.0 | 0.3 | 0.04 |
| STV (milliseconds) | 281 | 6.2 | 6.1 | 3.2 | 12.7 | 1.4 |
| MMR (milliseconds) | 281 | 35.1 | 34.6 | 17.5 | 56.8 | 7.1 |
| High variation (%) | 281 | 22.6 | 16.7 | 0.0 | 94.9 | 21.6 |
| High variation (milliseconds) | 281 | 32.6 | 42.9 | 0.0 | 109.8 | 22.1 |
| Low variation (%) | 281 | 22.6 | 15.0 | 0.0 | 100.0 | 24.7 |
| Low variation (milliseconds) | 281 | 15.7 | 21.4 | 0.0 | 39.0 | 11.7 |
| Delivery gestation (days) | 281 | 276.9 | 277.0 | 259 | 296.0 | 7.8 |
| Birth weight (gram) | 281 | 3168 | 3120 | 2520 | 4400 | 388.7 |
| Apgar score 1 minutes | 274 | 8.8 | 9.0 | 1.0 | 10.0 | 0.9 |
| Apgar score 5 minutes | 274 | 9.9 | 10.0 | 7.0 | 10.0 | 0.4 |
BPM = Beats per minute; SA = Small accelerations; LA = Large accelerations; SD = Small decelerations; LD = Large decelerations; STV = Short term variation; MMR = Mean minute range
Figure 2.
a. Short term variation - STV in milliseconds (ms)
b. Mean minute range – MMR in milliseconds (ms). The solid line represents the mean for the gestational age and the broken lines indicate plus or minus 2 standard deviations
Discussion
This study demonstrates that as early as 20 weeks gestations, heart rate can be accurately recorded using maternal trans-abdominal ECG. This study confirms the finding of Ribbert et al. (3) that accelerations of the FHR and baseline variation are present from as early as 20 weeks Our recording success rate of 95.4% compares very well with the findings of Graatsma et al (9) who collected signals overnight at different gestational ages and found 82% good quality recordings (where 60% or more fECG signals were present) in 150 women between 20 and 40 weeks gestation. Their success rate improved to 90.7% when only the night part of the recording (11 p.m. to 7 a.m.) was considered. For our analyses we were very strict in accepting only recordings with a high quality, excluding 4.6% of recordings where the heart rate could not be determined in more than 50% of the captured raw data. In this final data set of 281 participants, the mean loss of raw data was only 4% with a very low median of 0.2%. The mean basal heart rate of 148 bpm at a median gestation of 161 days (23 weeks) is within the 110-160 bpm normal range for viable fetusses (11).
The finding of more accelerations than decelerations is in contrast to previous reports based on ultrasound recordings at this gestational age. Sorokin et al described 97.1% (602 observations) of observed FHR changes in 20-22 week fetusses to be decelerations (defined as a negative deviation in baseline of 10 bpm) in comparison with 1.3% (a total of 8 observations) of FHR changes being accelerations (defined as a positive deviation in baseline of 10 bpm) (4). De Vries et al showed similar numbers with 99.4% of recordings (162 of 163 total recordings) between 20-22 weeks having small decelerations (deviation from baseline 10-15 bpm) and 90.2% (147 of 163) with a deviation of more than 15 bpm in comparison with 70.5% (115 of 163 recordings) showing small accelerations and only 28.8% (47 of 163) revealing large accelerations (baseline deviation more than 15bpm) (12). Unfortunately, no specifications as to the duration criteria per deviation required to meet definitions of either accelerations or decelerations are provided in these articles, therefore a direct comparison of our findings is not possible.
As reported, our mean and median basal heart rates were both 148 bpm. This compares well with previous ultrasound findings of a FHR of 148 bpm at 24 weeks and 149 bpm at 20 weeks which concurs with the described increase in parasympathetic activity leading to a steady decline in FHR as the fetus matures, overriding the sympathetic tone (3, 13-15).
If there were predominantly more accelerations than decelerations (considering both amplitude and duration) one would have expected a higher mean than median heart rate. Likewise, if decelerations were more prominent, one would have expected a lower mean than median basal heart rate. The fact that we did not find any difference between the mean and median heart rates at 20 - 24 weeks suggests that, at this age, there is a balance between accelerations and decelerations. An explanation for similar mean and median values in spite of more accelerations than decelerations is most probably that the changes in FHR during decelerations are greater than the changes during accelerations. The amplitude of accelerations and decelerations were not separately analysed by the DK 1.4a program; this information is therefore not available for a detailed amplitude comparison.. The number of accelerations increased from 20-23 weeks of gestation, as found by Libbert et al. (3)
We found a mean STV of 6.2 msec (SD 1.4 msec) and range from 3.2 to 12.7 msec. This compares well with the findings of Libbert et al. (3) who found mean STVs of 5.36 to 5.66 msec from 20 to 23 weeks’ gestation. In term fetusses, a reassuring STV is reported as > 3.0 msec (5). Our results suggest that abnormal STV values are not generally seen at 20-24 weeks gestation in healthy pregnancies. Low STV, even before 24 weeks may be abnormal and not caused by immaturity per se.
The mean minute range (MMR) in RR-intervals reflects the long term variation (LTV) of the fetal heart rate. We found a median value of 34.6 msec with a range between 17.5 msec and 58.2. Again, these findings compares favourably with that of Ribbert et al. (3) who found in a much smaller sample that the variation ranges from 21.2 msec at 20 weeks and 20.0 msec at 23 weeks to 36.3 msec at 20 weeks and 38,1 msec at 23 weeks. They found mean values of 28.9, 31.7, 33.4 and 32.0 msec at 20,21,22, and 23 weeks respectively while our mean values seem to be a little higher as demonstrated in Figure 2b. This similarity is interesting as two different techniques were used to obtain the raw FHR data. According to the Dawes Redman analyses, a period of low variation is identified when the mean minute range in at least 5 of 6 consecutive 1-minute intervals is less than or equal to 30 msec. A period of high variation is identified when the mean minute range in at least 5 of 6 consecutive 1-minute intervals is more than or equal to 32 msec (8). Although some cases were below 32 msec, it should be remembered that the 32 msec, as a normal lower limit, was derived from information obtained from more advanced gestations; lower levels for very early pregnancies is therefore less certain and needs to be established (5). The wide range of values, as we found in STV, could be explained in different ways.
Firstly, fetal state may play a role as active states and REM sleep are associated with a higher variation in contrast to periods of quiet sleep during which low FHR variation is usually observed (14, 19) However, consolidated sleep states are not thought to exist at 20-24 weeks (20). Secondly, the wide range in LTV could be explained by differences in gestational age when the recordings were done. However, the vast majority (55 %) were carried out at around 23 weeks with few cases around 20 week’s gestation. Thirdly, there is no doubt individual variation associated with different timelines of central nervous system maturation of the fetus. It is unlikely that the fetusses with LTV below 32 msec were compromised as no STV below 3.0 msec was found and they were all born alive after 37 weeks with no low birth weight or small for gestational age newborns in this sample.
The median proportion of a state of high FHR variation was 16.7%, ranging from 0% to 94.9%. A state of low variation was seen during 21.4% of the recordings, ranging from 0% to 100% of the recording time. De Vries at al. studied diurnal and other variations in fetal movement and heart rate patterns at 20-22 weeks (12). Significant diurnal changes were observed with the lowest values in the morning and the highest values during the evening. As we did all our recordings between the hours of 08h00 and 16h00, our findings were done when the fetus was generally expected to be less active (21-23).
The study could be criticized that the effects of smoking, alcohol and other drugs on early FHR patterns have not been investigated. However, this was also not done in leading articles on this subject (5, 11). It is the policy of the PASS Network to keep all preliminary analyses blind from exposures until the end of the study to ensure objective interpretation of findings.
With advances in neonatal intensive care the limits for fetal viability have been pushed forward to 24 weeks and earlier (24). This increases the importance of knowledge of FHR patterns in pregnancies with normal outcome to enable objective interpretation of suspicious patterns in high-risk pregnancies and alert clinicians to pending problems.
Acknowledgements
The Safe Passage Study was funded by the following grants from the National Institute on Alcohol Abuse and Alcoholism, the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute on Deafness and other Communication Disorders: U01 HD055154, U01 HD045935, U01 HD055155, U01 HD045991, and U01 AA016501.
Footnotes
Declaration of interest
The authors report no declaration of interest.
References
- 1.Reinhard J, Hayes-gill BR, Yi Q, Hatzmann H, Schiermeier S. Comparison of non-invasive fetal electrocardiogram to Doppler cardiotocogram during the 1st stage of labor. J Perinat Med. 2010;38:179–185. doi: 10.1515/jpm.2010.025. [DOI] [PubMed] [Google Scholar]
- 2.Sato N, Hoshiai T, Ito T, Owada K, Chisaka H, Aoyagi A, et al. Successful Detection of the Fetal Electrocardiogram Waveform Changes during Various States of Singletons. The Tohoku Journal of Experimental Medicine. 2011;225(2):89–94. doi: 10.1620/tjem.225.89. [DOI] [PubMed] [Google Scholar]
- 3.Ribbert LSM, Fidler V, Visser GHA. Computer-assisted analysis of normal second trimester fetal heart rate patterns. J Perinat Med. 1991;19:53–59. doi: 10.1515/jpme.1991.19.1-2.53. [DOI] [PubMed] [Google Scholar]
- 4.Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant Child Dev. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sorokin Y, Dierker LJ, Pillay SK, Zador IE, Schreiner ML, Rosen MG. The association between fetal heart rate patterns and fetal movements in pregnancies between 20 and 30 weeks’ gestation. Am J Obstet Gynecol. 1982;143(3):243–9. doi: 10.1016/0002-9378(82)90812-2. [DOI] [PubMed] [Google Scholar]
- 6.Pardey J, Moulden M, Redman CWG. A computer system for the numerical analysis of nonstress test. Am J Obstet Gynecol. 2002;186:1095–1103. doi: 10.1067/mob.2002.122447. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler T, Murrills A, Shelley T. Measurement of the fetal heart rate during pregnancy by a new electrocardiographic technique. Br J Obstet Gynaecol. 1978;85(1):12–17. doi: 10.1111/j.1471-0528.1978.tb15818.x. [DOI] [PubMed] [Google Scholar]
- 8.Dawes GS, Visser GHA, Goodman JDS, Redman CWG. Numerical analysis of the human fetal heart rate: The quality of ultrasound records. Am J Obstet Gynecol. 1981;141:43–52. doi: 10.1016/0002-9378(81)90673-6. [DOI] [PubMed] [Google Scholar]
- 9.Graatsma EM, Jacod BC, van Egmond LAJ, Mulder EJH, Visser GHA. Fetal electrocardiography: feasibility of long-term fetal heart rate recordings. Br J Obstet Gynaecol. 2009;116:334–338. doi: 10.1111/j.1471-0528.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 10.Theron GB, Thompson ML. A Centile chart for birth weight for an urban population of the Western Cape. S Afr Med J. 1995;85:1289–1292. [PubMed] [Google Scholar]
- 11.Macones GA, Hankins GDV, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretations and research guidelines. JOGNN. 2008;37(5):510–515. doi: 10.1111/j.1552-6909.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 12.De Vries JIP, Visser GHA, Mulder EJH, Prechtl HFR. Diurnal and other variations in fetal movement and heart rate patterns at 20 – 22 weeks. Early Human Development. 1987;15:333–348. doi: 10.1016/0378-3782(87)90029-6. [DOI] [PubMed] [Google Scholar]
- 13.Visser GH, Dawes GS, Redman CW. Numerical analysis of the normal human antenatal fetal heart rate. Br J Obstet Gynaecol. 1981;88(8):792–802. doi: 10.1111/j.1471-0528.1981.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 14.Swartjes JM, van Geijn HP, Mantel R, Schoemaker HC. Quantitated fetal heart rhythm at 20, 32 and 38 weeks of gestation and dependence on rest-activity patterns. Early Human Development. 1992;28:27–36. doi: 10.1016/0378-3782(92)90005-2. [DOI] [PubMed] [Google Scholar]
- 15.Van Leeuwen P, Lange S, Bettermann H, Gronemeyer D, Hatzmann W. Fetal heart rate variability and complexity in the course of pregnancy. Early Human Development. 1999;54:259–269. doi: 10.1016/s0378-3782(98)00102-9. [DOI] [PubMed] [Google Scholar]
- 16.Mantel R, van Geijn HP, Ververs IAP, Copray FJA. Automated analysis of near-term antepartum fetal heart rate in relation to fetal behavioural states: The Sonicaid System 8000. Am J Obstet Gynecol. 1991;165:57–65. doi: 10.1016/0002-9378(91)90223-e. [DOI] [PubMed] [Google Scholar]
- 17.Nijhuis JG, Prechtl HFR, Martin CB, Bots RSGM. Are there behaviour states in the human fetus? Early Human Development. 1982;6:177–195. doi: 10.1016/0378-3782(82)90106-2. [DOI] [PubMed] [Google Scholar]
- 18.Natale R, Nasello C, Turliuk R. The relationship between movements and accelerations in fetal heart rate at twenty-four to thirty-two weeks’ gestation. Am J Obstet Gynecol. 1984;148(5):591–5. doi: 10.1016/0002-9378(84)90754-3. [DOI] [PubMed] [Google Scholar]
- 19.Natale R, Nasello-Paterson C, Turliuk R. Longitudinal measurements of fetal breathing, body movements, heart rate, and heart rate accelerations and decelerations at 24 to 32 weeks of gestation. Am J Obstet Gynecol. 1985;151(2):256–63. doi: 10.1016/0002-9378(85)90022-5. [DOI] [PubMed] [Google Scholar]
- 20.DiPietro JA, Hodgson DM, Costigan KA, Hilton SC, Johnson TRB. Development of fetal movement – fetal heart rate coupling from 20 weeks through term. Early Human Development. 1996;44(2):139–51. doi: 10.1016/0378-3782(95)01704-6. [DOI] [PubMed] [Google Scholar]
- 21.Khan R, Burgoyne L, O’Donnell M, Dempsey EM. Antenatal management of expectant mother and extreme preterm infant at the limits of viability. Irish Medical Journal. 2010;103(9):266–9. [PubMed] [Google Scholar]