ABSTRACT
The cystine transport activity of a lens epithelial cell line originated from a canine mature cataract was investigated. The distinct cystine transport activity was observed, which was inhibited to 28% by extracellular 1 mM glutamate. The cDNA sequences of canine cysteine/glutamate exchanger (xCT) and 4F2hc were determined. The predicted amino acid sequences were 527 and 533 amino acid polypeptides, respectively. The amino acid sequences of canine xCT and 4F2hc showed high similarities (>80%) to those of humans. The expression of xCT in lens epithelial cell line was confirmed by western blot analysis. RT-PCR analysis revealed high level expression only in the brain, and it was below the detectable level in other tissues.
Keywords: cysteine/glutamate exchanger, glutathione, lens epithelial cells, SLC7A11, system xc–
Among many amino acid transporters, system xc− is a heterodimeric transporter comprised of a light chain, xCT (xc− transporter) and heavy chain (4F2hc) which mediates the exchange of extracelluar cystine and intracellular glutamate at the plasma membrane [4, 32]. As a heterodimeric transporter, system xc− requires both xCT and 4F2hc for its activity as a functional transport-unit of this carrier protein, which belongs to the SLC7 gene family [35].
Glutathione (GSH) is a tripeptide consisting of glutamate, glycine and cysteine, which plays an important role in several physiologic processes, including protection of cells against oxidative damage. Glutamate and glycine occur at relatively high intracellular concentrations, so that cysteine availability largely determines GSH synthesis. Therefore, system xc− is critically important for GSH production as a cysteine supplier into the cells. As a potent antioxidant, GSH maintains enzymes and protein thiols in their reduced state and scavenges free radicals and other reactive oxygen species [9, 22, 23].
The lens epithelial cells (LEC) are the progenitors of the lens fibers in vivo and undergo a developmental transition into fiber cells of the lens cortex, a process characterized by distinct biochemical and morphologic changes, such as the synthesis of crystallins proteins, cell elongation, loss of cellular organelles and disintegration of the nucleus [1]. Notably, LEC possesses a milli-molar order of high GSH. While glutamate and glycine transport system in lens epithelial cells are well documented [18, 19], the cystine transport system is poorly understood, especially LEC from mature cataract. Previously, we developed a lens epithelial cell line originated from a mature cataract of dog and reported several characteristics of this cell line [16]. In this study, we investigated functional analysis of cystine transport of the lens epithelial cell line, clarified the cDNA sequence of canine xCT and 4F2hc and examined distribution of xCT in various canine tissues.
MATERIALS AND METHODS
Animals: All experiments were performed according to the guidelines of The Laboratory Animal Care Committee of Azabu University and were in compliance with the Fundamental Guideline for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions. All canine tissues were obtained from a healthy male Shiba dog.
Determination of cDNA sequence of xCT and 4F2hc: Total RNA was isolated from canine tissues using an RNA extraction solution (Isogen, Nippon Gene, Tokyo, Japan) as described previously [16]. The primers were selected from the conserved region of cDNA sequence between humans and rodents (DDBJ accession Nos. AF252872 and AY766236, respectively). The primers used in this study are shown in Table 1. Previously, we reported the partial DNA sequence of canine 4F2hc [26]. In order to determine the 3′ and 5′ regions of cDNA, RACE methods were carried out using a SMARTer RACE cDNA amplification kit (Takara Bio, Kyoto, Japan) and a set of canine xCT or 4F2hc gene-specific primers, respectively. RT-PCR products were purified from the agarose gel using a Wizard SV gel clean-up system (Promega, Madison, WI, U.S.A.). The extracted and purified DNA was cloned into a pCR II-TOPO cloning vector (Invitrogen, Carlsbad, CA, U.S.A.) and sequenced with a BigDye terminator kit ver. 3 (Applied Biosystems, Carlsbad, CA, U.S.A.).
Table 1. Sequences of oligonucleotides used in this study.
| Primer | Sequence (5’–3’) | Product (bp) | |
|---|---|---|---|
| Oligonucleotide for cloning of canine xCT1 and 4F2hc | |||
| xCT-gsp1 (for 3’RACE) | sense | TGTCCGCAAGCACACTCCTCTGCCAGC | |
| xCT-gsp2 (for 5’RACE) | anti | CCGGTGTTCTGGAGCACGCCCTTAGGAG | |
| 4F2hc-gsp2 (for 3’RACE) | sense | TGCGGGCTGGTGTGGATGGGTTCCAGGT | |
| 4F2hc-gsp1 (for 5’RACE) | anti | GGGAGTGAGGACCAGAATGACCCGGATG | |
| Oligonucleotide for RT-PCR | |||
| transcript | |||
| xCT | sense | CGGGCTCAGCITACCTCTACAGCT | 365 |
| (AB847157) | anti | AGTGCCAATGGACATGAGGTCCACCA | |
| GAPDH | sense | ATC ACC ATC TTC CAG GAG CGA GA | 192 |
| (AB038240) | anti | GTC TTC TGG GTG GCA GTG ATG G | |
(accession number) is indicated.
Measurement of cystine transport activity in LEC: The canine lens epithelial cell line originated from mature cataract was maintained as described previously [16]. Radioactive (14C-) cystine was purchased from Perkin Elmer (Waltham, MA, U.S.A.). The transport activity was measured as follows. The cells were plated in a 5 × 105 cell/ 6-well plate 24 hr before the experiment. The cells were washed 3 times with 130 mM NaCl, 5 mM KCl, 2 mM MgCl2, 10 mM glucose, 15 mM Tris/MOPS pH 7.4 and 0.1% BSA. Then, a medium containing 10 µM radiolabeled cystine was added and incubated at 37°C for 10 min. Uptake was terminated by washing with ice-cold phosphate-buffered saline. After solubilizing the cells with 1% SDS, the radioactivity was measured with a liquid scintillation counter, and protein content was determined by the Micro BCA method. The transport activity was expressed as nmol/min/mg protein. To evaluate the inhibitory effect of extracellular homocysteic acid (HCA) and other amino acids, they were added to the incubation medium to a final concentration of 100 µM and 1 mM, respectively.
RT-PCR analysis of xCT mRNA in canine tissues: All RNA samples from various canine tissues were treated with DNase 1 (Invitrogen). cDNA synthesis was carried out using the Superscript III first-strand synthesis system for RT-PCR (Invitrogen), according to the manufacturer’s instructions. We performed RT-PCR using newly designed primers specific to canine xCT (Table 1). RT-PCR conditions were as follows: 94°C 2 min and 25 cycles of three steps; 94°C 15 sec 60°C 10 sec and 72°C 30 sec. Integrity of RNA was tested by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as reported previously [26].
Western blot analysis of xCT protein in various canine tissues: The cell membrane of brain tissue for Western blot analysis was prepared as reported by Denker et al. [6]. In brief, LEC or brain was homogenized at 4°C in the buffer containing 0.1 M KCl, 5 mM Na2HPO4 pH 7.5, 0.75 mM Na-EGTA pH 7.5, 1 mM DDT, 5 mM MgCl2, 200 µg/ml phenylmethylsulfonyl fluoride and 4 µg/ml leupeptin. Homogenates were centrifuged for 10 min to remove debris. The 1-vol. supernatant was layed over a 5-vol. sucrose solution containing 0.8 mM sucrose and 2 mM Na-EGTA and was centrifuged at 32,000 × g for 40 min. The membranes protein of the pellet was solubilized, and protein concentrations were determined by the BCA method [26]. The membranes protein samples were mixed with Laemmli sample buffer (Bio-Rad, Hercules, CA, U.S.A.), heated at 94°C for 5 min and electrophoresed into 12% polyacrylamide gels (190V, 40 min). After the transferring proteins to PVDF membrane (90V, 150 min), it was then treated with the primary antibody (rabbit anti-human xCT, x 500, Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.), followed by the secondary antibody (anti-rabbit IgG (H+L) goat IgG Fab’ HRP, x20,000, Seikagaku Corp., Tokyo, Japan). The xCT protein was detected with an ECL plus a chemiluminescence detection system (GE Healthcare Bioscience, Piscataway, NJ, U.S.A.) and exposed to an x-ray film.
RESULTS
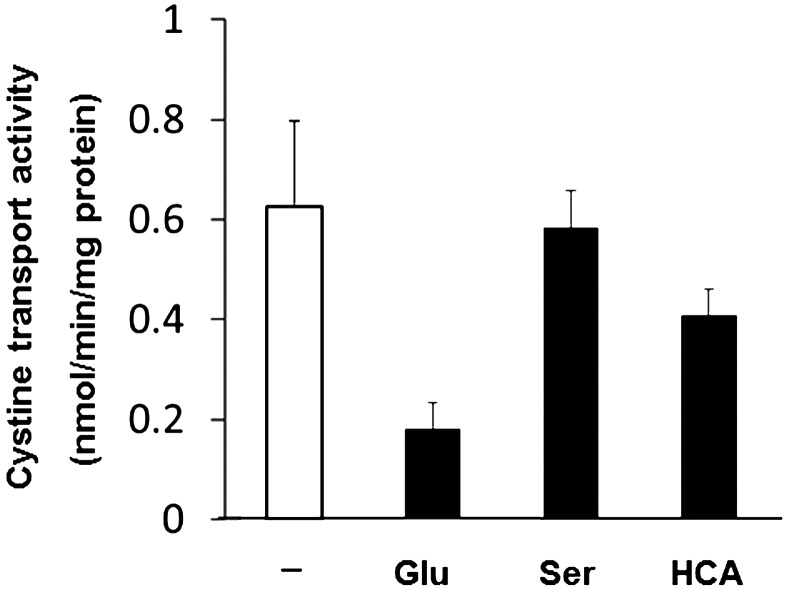
Figure 1 shows the cysteine transport activity of lens epithelial cells with or without extracellular 1 mM glutamate. The cystine transport activity at this experimental condition was 0.626 ± 0.169 nmol/min/mg protein, and it was reduced to 28% by existence of 1 mM glutamate in the medium which hindered the intracellular Glu efflux. On the other hand, neutral amino acid serine did not affect the cystine transport activity in LECs. Homocysteic, the inhibitor of cystine/glutamate transporter, reduced 35% of cystine transport activity. The cDNA sequence of canine xCT was 1,727 bp which encodes 527 amino acids (Fig. 2). The amino acid sequence of canine xCT indicated 84% identity to that of humans. The cDNA sequence of canine xCT was identical to the accession No. EF143580 which was deposited as putative canine xCT in DDBJ, but lacked N-terminus 21 amino acids. The deduced amino acid sequence obtained in this study was 26 amino acids longer than human in the C-terminus (Fig. 3). Figure 4 shows the cDNA sequence of canine 4F2hc. Previously, we reported the partial cDNA sequence of canine 4F2h [26] and the cDNA sequence of canine xCT and 4F2hc were deposited in the DDBJ (Accession Nos.AB847157 and AB786709, respectively). An obtained nucleotide sequence corresponding to canine 4F2hc cDNA was 2,228 bp in length and contained an entire open reading frame of 1,599 bp, encoding 533 amino acids (Fig. 4). The amino acid sequence of canine 4F2hc showed 80% similarities to that of human (Fig. 5). Canine 4F2hc possesses 3 amino acid insertions between amino acid No. 31 and 32 of rat 4F2hc. Humans and dogs have 2 amino acid insertions between amino acid No. 49 and 50 of the rat. Conversely, rats have three amino acids between 501 and 502 amino acids of canines. To determine the genomic distribution of each cDNA, the UCSC genome browser site (http://genome.ucsc.edu/) was used to align canine genomic sequences and each cDNA. It was revealed that the cDNA sequence of xCT and 4F2hc consisted of 11 exons of 97–406 base pairs and 9 exons of 59–587 base pairs, respectively (Figs. 2B and 4B).
Fig. 1.
Cystine transport activity of LEC with (closed column) or without (open column) 1 mM Glu, serine or 100µM homocysteic acid (HCA). The values are means and SD of more than 4 individual experiments.
Fig. 2.
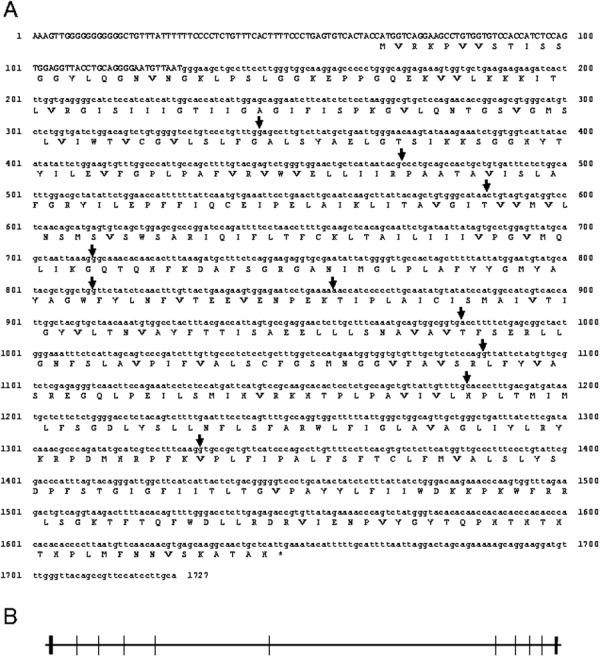
Nucleotide and deduced amino acid sequences of canine xCT are shown. Canine xCT cDNA was 1,727 bp and contained an entire open reading frame of 1,581 bp, encoding canine xCT of 485 amino acids. (DDBJ accession number is AB847157) (A). The termination codon is asterisked. Arrows indicate the positions of introns. Alignment of the cDNA sequence with the canine genome chromosome 19 predicts 10 exons of 97– 406 base pairs. Coding regions ■; untranslated regions. (B).
Fig. 3.

Amino acid sequences of canine xCT were compared with those of humans and mice. Multiple sequence alignments were performed using the Genetyx Programme (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively. The conserved cysteine (#) was predicted to be the disulfide bond site to 4F2hc.
Fig. 4.

Nucleotide and deduced amino acid sequence of canine 4F2hc are shown. Full-length canine 4F2hc cDNA was 2,230 bp and contained an entire open reading frame of 1,599 bp, encoding canine 4F2hc of 533 amino acids (DDBJ accession number is AB786709). The termination codon is asterisked. The polyadenylation signal is boxed (A). Arrows indicate the positions of introns. Alignment of the cDNA sequence with the canine genome chromosome 18 predicts 9 exons of 59–587 base pairs. Coding regions ■; untranslated regions. (B).
Fig. 5.

Amino acid sequences of canine 4F2hc were compared with those of humans and mice. Multiple sequence alignments were performed using the Genetyx Programme (ver. 10). Asterisks and dots indicate identical residues and conservative substitutions, respectively. The conserved cysteine (#) was predicted to be the disulfide bond site to xCT.
Figure 6A shows the detection of canine xCT mRNA in various tissues from healthy dog (A). The discrete bands of 181 bp in length, which were derived from canine RNA, were observed only in the brain tissue of healthy dog. Western blot analysis using anti-human xCT detected at ca 58 kDa in the membrane of both LEC and brain (Fig. 6B).
Fig. 6.
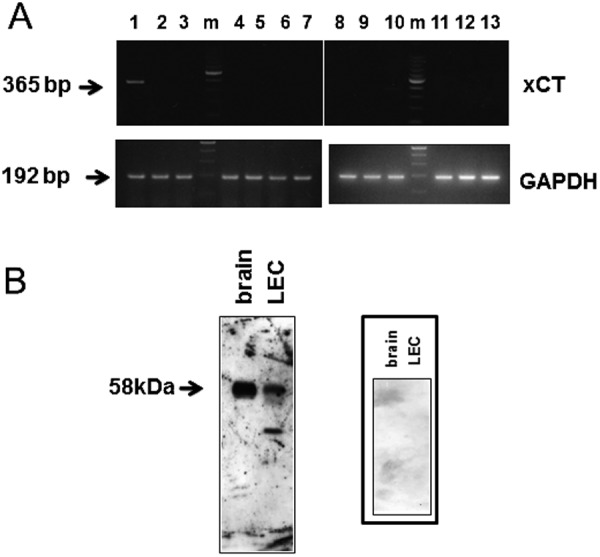
RT-PCR analysis of canine xCT in various healthy dog tissues (A) and Western blot analysis of the membrane of canine LEC were examined using anti-human xCT antibody (B). (A) Lane 1: cerebellum, lane 2; trachea, lane 3; kidney, lane 4; lung, lane 5; liver, lane 6; spleen, lane 7; salivary gland, lane 8; pancreas, lane 9; testis, lane 10; heart, lane 11; intestine, lane 12; bladder, lane 13; skeleton muscle. Integrity of mRNA was examined by glyceraldehyde-3-phosphate dehydrogenase (GAPDH). m: 100 bp ladder. (B) The membrane protein samples of LEC and brain were 30 µg and 5 µg, respectively. The right panel represents negative control using normal rabbit serum (× 10).
DISCUSSION
The GSH is ubiquitous and essential to many physiological processes. It maintains enzyme and protein thiols in their reduced state and scavenges free radicals and other reactive oxygen species [12, 30, 37]. This reducing potential is particularly important in the lens, which is continually exposed to free radicals and reactive oxygen species generated from peroxisome, inflammatory cytokines, exposure to ultraviolet light, ionizing radiation and chemotherapeutic agents. Cataract is associated with protein modifications brought about by oxidative damage to the lens. The young lens is protected from such damage through a robust oxygen radical scavenger system that is essential in the detoxification of reactive species and vital for maintaining lens transparency. With advancing age, the ability of this system to protect the lens from oxidative damage is reduced, ultimately resulting in opacification of the lens and formation of old-age cataract.
In this study, we investigated the cystine transport activity of a lens epithelial cell line originated from mature cataract of a canine and confirmed the distinct cystine transport activity, which was inhibited to 28% and 65% by extracellular glutamate and homosteaic acid, respectively (Fig. 1). It was reported that the transport activity of the cystine/glutamate exchanger was hindered by extracellular glutamate [32]. Therefore, the cystine transport of LEC was due to system xc−. The cDNA sequence of xCT and 4F2hc which consisted of system xc− was determined (Figs. 2 and 4). The deduced amino acid sequences of canine xCT and 4F2hc showed high similarities to those of humans and mice (Figs. 3 and 5). The distribution of xCT was investigated using various healthy canine tissues. RT-PCR analysis revealed high level expression only in the brain (Fig. 5A). It was reported that xCT mRNA is most prominently expressed in the brain in human, but was not at detectable levels in other tissues [32], whereas 4F2hc was ubiquitously expressed [26, 29]. In this study, it was confirmed that distribution of canine xCT was conserved among mammalians [3]. The expression of xCT protein in the lens epithelial cell line was examined by western blot analysis using anti-human xCT. It was detected at 58 kDa in the membrane of LEC as well as brain, which was identical to the calculated molecular mass of canine xCT polypeptide (58 kDa) (Fig. 3).
Amino acid transport system xc− mediates the entry of cystine into cells coupled to the efflux of glutamate. The xc− is a heterodimer, consisting of 4F2hc as the heavy chain and xCT as the light chain. 4F2hc is a subunit common to 6 amino acid transport systems, including LAT1, LAT2, y+LAT1, y+LAT2, asn1 and xCT as light chain, whereas xCT is unique to system xc− [28]. It has been demonstrated that the 4F2hc is essential for the functional expression of xCT in the plasma membrane, while xCT is not capable of amino acid transport on its own [15, 24]. 4F2hc is reportedly necessary for trafficking of the light chain to the plasma membrane, whereas the light chain is thought to determine the transport characteristics [36].
Under normal physiological conditions, system xc− is expressed ubiquitously at low levels in mammalian cells [11]. However, under a condition of oxidative stress, the transport activity of this carrier appears to be up-regulated [17, 25, 34], possibly via an amino acid response-element [31]. As a result of this up-regulation, an increased number of cystine molecules can be transported into the cells, which in turn would provide an increased number of molecules of cysteine (rate limiting substrate) for GSH synthesis. Interesingly, the xCT is reportedly up-regulated in cancer in humans and plays a critical role in the growth of cancer cells [13, 21, 27]. In cancer cells, the GSH levels maintain DNA synthesis, growth and multidrug resistance, and sustenance of GSH levels through GSH biosynthesis is vital for growth and survival of tumors. Therefore, GSH is regarded as an important target in cancer therapy, and various therapeutic approaches based on GSH depletion of cancer cells have been suggested [5, 7 , 8, 10, 14, 20, 21, 33]. In summary, we investigated cystine transport activity in a canine lens epithelial cell line originated from a mature cataract, clarified the cDNA sequence of canine xCT and 4F2hc and examined the distribution of xCT of canine tissues. We will next investigate xCT expression in various canine cancer samples as a first step to clarify the relationship between xCT level and cancer development in canines.
ACKNOWLEDGMENTS
This study was in part supported by a Grant-in-Aid to HO from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 19580376 and No. 23580451) and a research project grant awarded by the Azabu University Research Services Division.
REFERENCES
- 1.Babizhayev M. A., Costa E. B.1994. Lipid peroxide and reactive oxygen species generating systems of the crystallin lens. Biochim. Biochem. Acta 1225: 326–337. doi: 10.1016/0925-4439(94)90014-0 [DOI] [PubMed] [Google Scholar]
- 2.Berthoud V. M., Beyer E. C.2009. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 11: 339–353. doi: 10.1089/ars.2008.2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burdo J., Dargusch R., Schubert D.2006. Distribution of the cystine/glutamate antiporter system xc- in the brain, kidney, and duodenum. J. Histochem. Cytochem. 54: 549–557. doi: 10.1369/jhc.5A6840.2006 [DOI] [PubMed] [Google Scholar]
- 4.Christensen H. N.1990. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 70: 43–77 [DOI] [PubMed] [Google Scholar]
- 5.Chung W. J., Lyons S. A., Nelson G. M., Hamada H., Gladson C. L., Gillespie G. Y., Sontheimer H.2005. Inhibition of cysteine uptake disrupts the growth of primary brain tumour. J. Neurosci. 25: 7101–7110. doi: 10.1523/JNEUROSCI.5258-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denker B. M., Kwon E. D., Kuhajda F. P., Agre P.1988. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 263: 15634–15642 [PubMed] [Google Scholar]
- 7.Doxsee D. W., Gout P. W., Kurita T., Lo M., Buckley A. R., Wang Y., Xue H., Karp C. M., Cutz J. C., Cunha G. R., Wang Y. Z.2007. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 67: 162–171. doi: 10.1002/pros.20508 [DOI] [PubMed] [Google Scholar]
- 8.Estrela J. M., Ortega A., Obrador E.2006. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 43: 143–181. doi: 10.1080/10408360500523878 [DOI] [PubMed] [Google Scholar]
- 9.Ganea E., Harding J. J.2006. Glutathione-related enzymes and the eye. Curr. Eye Res. 31: 1–11. doi: 10.1080/02713680500477347 [DOI] [PubMed] [Google Scholar]
- 10.Gout P. W., Kang Y. J., Buckley D. J., Bruchovsky N., Buckley A. R.1997. Increased cysteine uptake capability associated with malignant progression of Nb2 lymphoma. Leukemia Cells 11: 1329–1337. doi: 10.1038/sj.leu.2400739 [DOI] [PubMed] [Google Scholar]
- 11.Guidotti G. G., Gazzola G. C.1992. Amino acid transporters: systematic approach and principles of control. pp3–30. In: Mammalian Amino Acid Transport. Mechanisms and Control. Plenum, New York. [Google Scholar]
- 12.Gukasyan H. J., Kannan R., Lee V. H., Kim K. J.2003. Regulation of L-cystine transport and intracellular GSH level by a nitric oxide donor in primary cultured rabbit conjunctival epithelial cell layers. Invest. Ophthalmol. Vis. Sci. 44: 1202–1210. doi: 10.1167/iovs.02-0409 [DOI] [PubMed] [Google Scholar]
- 13.Ishimoto T., Nagano O., Yae T., Tamada M., Motohara T., Oshima H., Oshima M., Ikeda T., Asaba R., Yagi H., Masuko T., Shimizu T., Ishikawa T., Kai K., Takahashi E., Imamura Y., Baba Y., Ohmura M., Suematsu M., Baba H., Saya H.2011. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(−) and thereby promotes tumor growth. Cancer Cell 19: 387–400. doi: 10.1016/j.ccr.2011.01.038 [DOI] [PubMed] [Google Scholar]
- 14.Iwata S., Hori T., Sato N., Hirota K., Sasada T., Mitsui A., Hirakawa T., Yodoi J.1997. Adult T lymphoma (ATL)-derived factor/human thioredoxin prevents apoptosis of lymphoid cells induced by lcystine and glutathione depletion: possible involvement of thiol-mediated redox reglation in apoptosis caused by pro-oxidant states. J. Immunol. 158: 3108–3117 [PubMed] [Google Scholar]
- 15.Kanai Y., Segawa H., Miyamoto K., Uchino H., Takeda E., Endou H.1998. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). J. Biol. Chem. 273: 23629–23632. doi: 10.1074/jbc.273.37.23629 [DOI] [PubMed] [Google Scholar]
- 16.Kanemaki N., Saito M., Onda K., Maruo T., Ogihara K., Naya Y., Morishita T., Ochiai H.2012. Establishment of a lens epithelial cell line from a canine mature cataract. Exp. Anim. 61: 41–47. doi: 10.1538/expanim.61.41 [DOI] [PubMed] [Google Scholar]
- 17.Kim J. Y., Kanai Y., Chairoungdua A., Cha S. H., Matsuo H., Kim D. K., Inatomi J., Sawa H., Ida Y., Endou H.2001. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim. Biophys. Acta 1512: 335–344. doi: 10.1016/S0005-2736(01)00338-8 [DOI] [PubMed] [Google Scholar]
- 18.Lim J., Lorentzen K. A., Kistler J., Donaldson P. J.2006. Molecular identification and characterisation of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Exp. Eye Res. 83: 447–455. doi: 10.1016/j.exer.2006.01.028 [DOI] [PubMed] [Google Scholar]
- 19.Lim J., Li L., Jacobs M. D., Kistler J., Donaldson P. J.2007. Mapping of glutathione and its precursor amino acids reveals a role for GLYT2 in glycine uptake in the lens core. Invest. Ophthalmol. Vis. Sci. 48: 5142–5151. doi: 10.1167/iovs.07-0649 [DOI] [PubMed] [Google Scholar]
- 20.Lo M., Ling V., Wang Y. Z., Gout P. W.2008. The xc− cystine/glutamate antiporter: a mediator of pancreatic cancer growth with a role in drug resistance. Br. J. Cancer 99: 464–472. doi: 10.1038/sj.bjc.6604485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo M., Wang Y. Z., Gout P. W.2008. The X(c)(−) cysteine/glutamate antiporter: a potential target for therapy of cancer and other disease. J. Cell Physiol. 215: 593–602. doi: 10.1002/jcp.21366 [DOI] [PubMed] [Google Scholar]
- 22.Lou M. F.2003. Redox regulation in the lens. Prog. Retin. Eye Res. 22: 657–682. doi: 10.1016/S1350-9462(03)00050-8 [DOI] [PubMed] [Google Scholar]
- 23.Lou M. F.2000. Thiol regulation in the lens. J. Ocul. Pharmacol. Ther. 16: 137–148. doi: 10.1089/jop.2000.16.137 [DOI] [PubMed] [Google Scholar]
- 24.Mastroberardino L., Spindler B., Pfeiffer R., Skelly P. J., Loffing J., Shoemaker C. B., Verrey F.1998. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature 395: 288–291. doi: 10.1038/26246 [DOI] [PubMed] [Google Scholar]
- 25.Miura K., Ishii T., Sugita Y., Bannai S.1992. Cystine uptake and glutathione level in endothelial cells exposed to oxidative stress. Am. J. Physiol. 262: C50–C58 [DOI] [PubMed] [Google Scholar]
- 26.Ochiai H., Morishita T., Onda K., Sugiyama H., Maruo T.2012. Canine Lat1: molecular structure, distribution and its expression in cancer samples. J. Vet. Med. Sci. 74: 917–922. doi: 10.1292/jvms.11-0353 [DOI] [PubMed] [Google Scholar]
- 27.Okuno S., Sato H., Kuriyama-Matsumura K., Tamba M., Wang H., Sohda S., Hamada H., Yoshikawa H., Kondo T., Bannai S.2003. Role of cystine transport in intracellular glutathione level and cisplatin resistance in human ovarian cancer cell lines. Br. J. Cancer 88: 951–956. doi: 10.1038/sj.bjc.6600786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palacín M., Nunes V., Font-Llitjós M., Jiménez-Vidal M., Fort J., Gasol E., Pineda M., Feliubadaló L., Chillarón J., Zorzano A.2005. The genetics of heteromeric amino acid transporters. Physiology 20: 112–124. doi: 10.1152/physiol.00051.2004 [DOI] [PubMed] [Google Scholar]
- 29.Parmacek M. S., Karpinski B. A., Gottesdiener K. M., Thompson C. B., Leiden J. M.1989. Structure, expression and regulation of the murine 4F2 heavy chain. Nucleic Acids Res. 17: 1915–1931. doi: 10.1093/nar/17.5.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reddy V. N.1990. Glutathione and its function in the lens–an overview. Exp. Eye Res. 50: 771–778. doi: 10.1016/0014-4835(90)90127-G [DOI] [PubMed] [Google Scholar]
- 31.Sato H., Nomura S., Maebara K., Sato K., Tamba M., Bannai S.2004. Transcriptional control of cystine/glutamate transporter gene by amino acid deprivation. Biochem. Biophys. Res. Commun. 325: 109–116. doi: 10.1016/j.bbrc.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Sato H., Tamba M., Ishii T., Bannai S.1999. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J. Biol. Chem. 274: 11455–11458. doi: 10.1074/jbc.274.17.11455 [DOI] [PubMed] [Google Scholar]
- 33.Schnelldorfer T., Gansauge S., Gansauge F., Schlosser S., Beger H. G., Nussler A. K.2000. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 89: 1440–1447. doi: [DOI] [PubMed] [Google Scholar]
- 34.Tomi M., Hosoya K., Takanaga H., Ohtsuki S., Terasaki T.2002. Induction of xCT gene expression and L-cystine transport activity by diethyl maleate at the inner blood-retinal barrier. Invest. Ophthalmol. Vis. Sci. 43: 774–779 [PubMed] [Google Scholar]
- 35.Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y.2004. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 447: 532–542. doi: 10.1007/s00424-003-1086-z [DOI] [PubMed] [Google Scholar]
- 36.Wagner C. A., Lang F., Broer S.2001. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 281: C1077–C1093 [DOI] [PubMed] [Google Scholar]
- 37.Wu G., Fang Y. Z., Yang S., Lupton J. R., Turner N. D.2004. Glutathione metabolism and its implications for health. J. Nutr. 134: 489–492 [DOI] [PubMed] [Google Scholar]