ABSTRACT
Rapid turnover proteins, such as transferrin (Tf), are used as dynamic nutritional assessment proteins in human medicine. However, nutritional status in veterinary medicine is mostly assessed on the basis of classical static factors, such as body weight, body condition score and plasma albumin level. This study evaluated the clinical usefulness of Tf as a nutritional assessment marker by measuring plasma Tf concentrations in malnourished dogs before and after nutritional treatment. Posttreatment plasma Tf concentrations were significantly higher than the pretreatment concentrations, although the albumin concentration did not change significantly. The numbers of dogs that exhibited increases in plasma Tf concentrations were significantly related to weight gain. Furthermore, the survival rates at day 60 after treatment initiation were significantly higher in dogs with plasma Tf concentrations above the reference value (180 mg/dl) after the nutritional treatment than in those with a plasma Tf concentration<180 mg/dl. In conclusion, the plasma Tf concentration is related to nutritional condition and would be a candidate for a novel nutritional assessment marker in dogs.
Keywords: nutritional assessment, rapid turnover protein, transferrin
Malnutrition negatively affects the recovery process in various diseases. Compromised immune function and delayed wound healing are reported in humans with malnutrition [3, 9, 26, 27, 30, 32, 35]. Effective nutritional therapy improves mortality in malnourished surgical patients or seriously ill patients [11, 16, 19, 29]. Nutritional assessment should be the first step to provide appropriate nutritional treatment [22].
In dogs, malnutrition during hospitalization is reported to impair clinical signs and prognosis [4, 25]. The nutritional status of diseased dogs is currently estimated on the basis of body weight [2, 17, 18], body condition score (BCS) [17, 18] and several serum biochemical values [17]. Body weight change is the most reliable factor for assessing nutritional status. However, it is a static factor and does not reflect short-term changes. In addition, fluid accumulation in the third space directly affects body weight, making assessment difficult. Although BCS is a convenient and noninvasive method for estimating the nutritional status of dogs, it is a subjective marker and is influenced by body size and shape among different breeds. The plasma albumin concentration is a biochemical parameter commonly used to assess nutritional status in dogs [17]. As the half-life of albumin is 20 days in humans [33] and 8.2 days in dogs [6], it is expected to be a short- to medium-term nutritional assessment marker. However, the plasma albumin concentration is readily influenced by fluid accumulation, hepatic function and protein loss from the gut and kidneys. In addition, it rarely decreases unless malnutrition is in the advanced stage, possibly in part because of the large capacity of the liver to synthesize albumin [28].
Tf is mainly synthesized in the liver and plays an important role in iron transport [14]. Decreased intestinal protein uptake promptly reduces Tf production, which directly depletes the plasma Tf concentration [5, 10]. We previously reported that plasma Tf concentrations are decreased in experimentally induced undernourished dogs, suggesting that Tf may be a dynamic nutritional marker in dogs [21]. Therefore, this study evaluated the relationships between plasma Tf concentration and the nutritional condition and prognosis in diseased dogs receiving nutritional treatment.
MATERIALS AND METHODS
Dogs and nutritional treatment: Thirty-three dogs referred to the Veterinary Medical Center of the University of Tokyo with clinical signs of anorexia (i.e., taking<50% of the resting energy requirement [RER] per day over 3 days) were included in this study. Caloric intake was estimated on the basis of interviews with the dogs’ owners before nutritional treatment. RER was calculated by the following formula: RER=70 × (body weight in kg)0.75 [4]. All dogs received nutritional treatments that met their respective RER through oral-assisted feeding (OF), enteral-assisted feeding (EF) including esophagostomy, gastrostomy or jejunostomy tube feeding, or parenteral-assisted feeding (PF). The OF and EF dogs, except for jejunostomy tube feeding, were fed mainly semi-digestion liquid diet (CliniCare, Abbott Laboratories, Abbott Park, IL, U.S.A.) or high density diet (a/d, Hill’s Pet Nutrition, Topeka, KS, U.S.A.). For jejunostomy tube feeding, a highly digested nutrition agent (Convalescence Support, Royal Canin, Aimargues, France) was used. The PF dogs received continuous intravenous fluids prepared by mixing dextrose, lipid and amino acid products to meet the RER for each patient. Body weight, BCS and plasma Tf and albumin concentrations were monitored in all dogs before and after the nutritional treatment. Dogs with incomplete medical records or liver failure were excluded from this study.
Subject characteristics: For the purpose of evaluating the relationships between the plasma Tf concentration and the effect of nutritional treatment, dogs with pleural effusion and ascites were excluded in order to accurately measure body weight changes. Twenty-one of the 33 anorexic dogs were included for this purpose; 9 were female (3 intact), and 12 were male (6 intact). The median age was 113 months (range: 7–173 months), and the median BCS was 2/5 (range: 1–3). The primary disease causing malnutrition was chronic inflammatory gastrointestinal disease in 6 dogs; pancreatitis in 3 dogs; pyloric stenosis in 2 dogs; and lymphangiosarcoma, gastric adenocarcinoma, gastric delayed emptying, oral melanoma, holoprosencephaly, gastrointestinal lymphoma, esophageal diverticulum, megaesophagus, myelodysplastic syndrome and encephalomyelitis in 1 dog each, respectively. The nutritional treatments performed included OF only (n=11), EF (n=5), PF (n=1) and both EF and PF (n=4).
For the purpose of evaluating the association between Tf and prognosis, neoplastic diseases were excluded, and diseases were restricted to the chronic inflammatory gastrointestinal diseases. Twenty dogs histopathologically diagnosed with chronic inflammatory gastrointestinal diseases were recruited. Nine dogs were female (2 intact), and 11 were male (10 intact). The median age was 105.5 months (range: 58–174 months), and the median BCS was 2/5 (range: 1–3). Fifteen dogs were diagnosed with chronic enteritis, 3 were diagnosed with chronic gastritis accompanying pyloric stenosis, and 1 each was diagnosed with chronic colitis and functional ileus. Six of the 20 dogs had apparent ascites due to hypoalbuminemia as a result of protein-losing enteropathy (PLE). The nutritional treatments included OF only (n=9), EF (n=3), PF (n=6) and both EF and PF (n=2).
Measurement of plasma Tf and albumin concentrations: Fasting plasma was separated from heparinized blood and immediately stored at −20°C. Plasma Tf concentrations were determined using a commercial kit (Canine Transferrin ELISA kit, GenWay Biotech, San Diego, CA, U.S.A.) according to the manufacturer’s protocol; all samples were measured in duplicate. Plasma albumin concentrations were measured using a biochemical analyzer (DRI-CHEM 7000V: Fujifilm Corporation, Tokyo, Japan). Plasma Tf and albumin concentrations were measured at the onset of nutritional treatment (pretreatment) and after treatment (posttreatment). The interval between both time points ranged from 8–30 days. The reference value for the plasma Tf concentration was 180 mg/dl according to the lower limit of the reference range (i.e., mean − 2SD) for healthy dogs [21]. The reference value for the plasma albumin concentration was 2.6 g/dl according to the lower limit of our reference range. The survival rate at day 60 after the initial treatment was assessed in each group.
Statistical analysis: Statistical analyses were performed using JMP version 9 (SAS Institute, Cary, NC, U.S.A.). The Wilcoxon Signed–Rank Test was used to compare the pre- and posttreatment plasma Tf and albumin concentrations. The Fisher’s exact test was used to compare the proportion dogs categorized by plasma Tf and albumin concentrations and body weight change. The survival rates at 60 days after the initiation of nutritional treatment were analyzed using the log-rank test. The level of statistical significance was set at P<0.05.
RESULTS
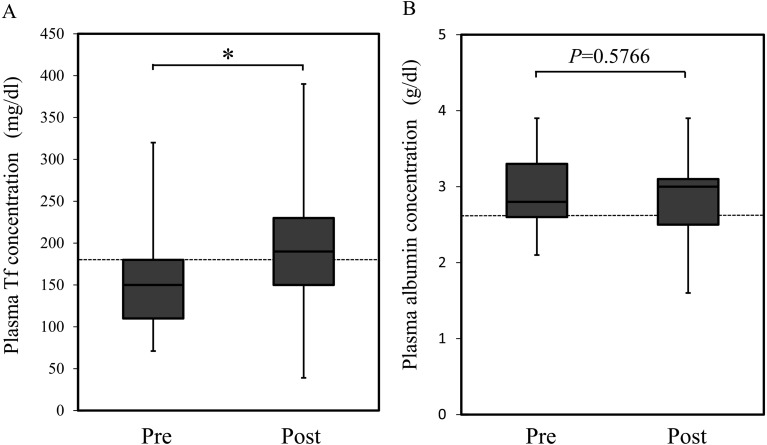
Changes in plasma Tf and albumin concentrations before and after nutritional treatment: Plasma Tf and albumin concentrations were compared before and after nutritional treatment. The median plasma Tf concentrations before and after treatment were 150 mg/dl (range: 71–320 mg/dl) and 190 mg/dl (range: 39–390 mg/dl), respectively. The median plasma albumin concentrations before and after treatment were 2.8 g/dl (range: 2.1–3.9 g/dl) and 3 g/dl (range: 1.6–3.9 g/dl). Plasma Tf concentrations were significantly higher after treatment than before treatment (P<0.05) (Fig. 1A). In contrast, plasma albumin concentrations did not differ between before and after treatment (P=0.5766) (Fig. 1B).
Fig. 1.
Plasma transferrin (Tf) (A) and albumin (B) concentrations before and after nutritional treatment. Data are presented as the median with the 25th-75th percentile range in each box plot. Whiskers indicate the highest and lowest data points. Dotted lines show the lower limit of the reference ranges of plasma (A) Tf (i.e., 180 mg/dl) and (B) albumin (i.e., 2.6 g/dl) concentrations. Pre, pretreatment; Post, posttreatment; Tf, transferrin. A significant difference was observed between the pre- and posttreatment Tf concentrations (P<0.05), but not for the albumin concentrations (P=0.5766).
The changes in plasma Tf and albumin concentrations and body weight in each case were subsequently analyzed. Among 15 dogs showing increased plasma Tf concentrations, 10 dogs showed increases in body weight. By contrast, only 1 of the 6 dogs that did not show a change or decrease in plasma Tf concentration showed an increase in body weight. The proportion of dogs that exhibited increases in plasma Tf concentrations was significantly related to weight gain (P<0.05) (Table 1). Meanwhile, the numbers of dogs that showed increased plasma albumin concentrations were not significantly related to weight gain (P=0.4663).
Table 1. Plasma transferrin, albumin concentration and body weight change in dogs with various primary diseases, but without pleural effusion and ascites.
| Body weight |
P values | |||
|---|---|---|---|---|
| Increased a) (n=11) | Not changed or decreased b) (n=10) | |||
| Transferrin | Increased (n=15) | 10 | 5 | 0.0382* |
| Not changed or decreased (n=6) | 1 | 5 | ||
| Albumin | Increased (n=8) | 5 | 3 | 0.4663 |
| Not changed or decreased (n=13) | 6 | 7 | ||
* Statistically significant by Fisher’s exact test. a) Number of dogs showing increased body weight after the nutritional therapy. b) Number of dogs in which body weight did not change or decreased after the nutritional therapy.
Next, the number of dogs with plasma Tf concentrations above the reference range (i.e.,≥180 mg/dl) was examined [21]. Nine of 11 dogs with increased body weight and 5 of 10 dogs without increase of body weight had ≥180 mg/dl plasma Tf concentrations after treatment, respectively. The number of dogs with plasma Tf concentrations ≥180 mg/dl was not significantly related to body weight gain (P=0.1223) (data not shown).
Survival rates 60 days after nutritional treatment initiation: The dogs were divided into 2 groups according to the Tf and albumin concentrations: plasma Tf concentrations before or after treatment ≥180 and <180 mg/dl and plasma albumin concentrations before or after treatment ≥2.6 and <2.6 g/dl. The survival rates 60 days after the initiation of treatment for each group are summarized in Table 2. There was a significant difference in the survival rate 60 days after treatment between dogs with posttreatment plasma Tf concentrations ≥180 mg/dl and those with posttreatment plasma Tf concentrations <180 mg/dl (P<0.05). The survival rates 60 days after treatment were also significantly different between dogs with pretreatment plasma albumin concentrations ≥2.6 g/dl and those with pretreatment plasma albumin concentrations <2.6 g/dl (P<0.05).
Table 2. Plasma transferrin, albumin concentration and survival rate on day 60 after the nutritional treatment in dogs histopathologically diagnosed with chronic inflammatory gastrointestinal diseases.
| Evaluation criteria | Survival rate (%) | P value | ||
|---|---|---|---|---|
| Before treatment | Transferrin (mg/dl) | ≥ 180 (n=5) | 60 | 0.4655 |
| < 180 (n=15) | 40 | |||
| Albumin (g/dl) | ≥ 2.6 (n=9) | 78 | 0.0234* | |
| < 2.6 (n=11) | 18 | |||
| After treatment | Transferrin (mg/dl) | ≥ 180 (n=9) | 78 | 0.0147* |
| < 180 (n=11) | 18 | |||
| Albumin (g/dl) | ≥ 2.6 (n=7) | 71 | 0.1176 | |
| < 2.6 (n=13) | 31 | |||
* Statistically significant by the log-rank test.
DISCUSSION
We previously reported that plasma Tf concentrations decrease in both anorexic dogs with various diseases and experimentally induced undernourished dogs [21]. The present results show that plasma Tf concentrations changed depending on nutritional state after nutritional treatment. Furthermore, the results suggest that Tf concentrations after nutritional treatment would be indicative of the prognosis of malnourished dogs with gastrointestinal diseases.
The plasma Tf concentrations increased significantly in many of the malnourished dogs that gained weight after nutritional treatment. Although the rate of change varied among cases, the variation in the plasma Tf concentrations during nutritional treatment could be a useful marker in dogs as well as humans [12, 20, 34]. By contrast, the plasma albumin concentrations did not change significantly before and after treatment. This is considered to be due to the relatively large body pool of albumin in dogs. Therefore, nutritional condition is not reflected by an increased plasma albumin concentration.
The survival rate at day 60 after nutritional treatment was significantly higher in dogs with a plasma Tf concentration ≥180 mg/dl. Therefore, the plasma Tf concentration level after nutritional treatment is a candidate prognostic factor for malnourished dogs. In human medicine, patients with higher serum Tf concentrations have longer survival times than those with lower Tf concentrations [13, 24]. The present results are concordant with these human studies. Another study showed that an increase in plasma Tf concentration within 4–7 days after the initiation of treatment is associated with better prognosis [23]. As the interval between the 2 measurement points (i.e., before and after treatment) varied among cases (range: 12–29 days) in this study, it is necessary to evaluate the appropriate monitoring time for plasma Tf concentrations during nutritional treatment. The reference value used in the present study, 180 mg/dl, was defined on the basis of the data of healthy dogs in our previous study [21]. According to the present data, this reference value may be appropriate when using the plasma Tf concentration as a clinical marker of nutritional condition.
Lower plasma albumin concentrations before treatment also influenced the survival rates. Considering that 6 dogs with low plasma albumin concentrations at the onset of treatment were diagnosed with PLE and that hypoalbuminemia is reported to be a prognostic factor of canine chronic enteropathy [1], the significance of the association between survival rate and albumin concentration before treatment is indicative of the severity of PLE itself. As rapid turnover proteins, such as Tf, exhibit preserved blood levels even in human patients with PLE [31], Tf could be especially useful for assessment of the nutritional conditions in dogs with PLE.
This study has several limitations that should be addressed. First, various diseases including malignant tumors were included to evaluate the association between plasma Tf concentrations before and after nutritional treatment. The type of disease and its severity could affect the plasma Tf concentrations. Tf is reported to decrease with infection or inflammation; moreover, the plasma Tf and C-reactive protein (CRP) concentrations are reported to be negatively correlated [7, 8, 15]. However, CRP was not measured in many dogs in the present study. Second, several nutritional therapies including force feeding, enteral and parenteral feeding and surgery were administered in this study. For example, parenteral intravenous feeding may affect Tf concentrations by altering protein synthesis in the liver. Therefore, additional studies using greater numbers of dogs with a single disease and the same nutritional treatment are required.
In conclusion, to the best of our knowledge, this is the first study demonstrating the clinical usefulness of the plasma Tf concentration as a nutrition assessment marker in dogs. Monitoring the plasma Tf concentration together with the conventional static markers albumin, body weight and BCS could be useful for evaluating nutritional status and prognosis during nutritional treatment in dogs.
REFERENCES
- 1.Allenspach K., Wieland B., Gröne A., Gaschen F.2007. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21: 700–708. doi: 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 2.Armstrong P. J., Lippert A. C.1988. Selected aspects of enteral and parenteral nutritional support. Semin. Vet. Med. Surg. (Small Anim.) 3: 216–226 [PubMed] [Google Scholar]
- 3.Bistrian B. R., Blackburn G. L., Scrimshaw N. S., Flatt J. P.1975. Cellular immunity in semistarved states in hospitalized adults. Am. J. Clin. Nutr. 28: 1148–1155 [DOI] [PubMed] [Google Scholar]
- 4.Chan D. L.2004. Nutritional requirements of the critically ill patient. Clin. Tech. Small Anim. Pract. 19: 1–5. doi: 10.1053/S1096-2867(03)00079-3 [DOI] [PubMed] [Google Scholar]
- 5.de Jong F. A., Howlett G. J., Schreiber G.1988. Messenger RNA levels of plasma proteins following fasting. Br. J. Nutr. 59: 81–86. doi: 10.1079/BJN19880012 [DOI] [PubMed] [Google Scholar]
- 6.Dixon F. J., Maurer P. H., Deichmiller M. P.1953. Half-lives of homologous serum albumins in several species. Proc. Soc. Exp. Biol. Med. 83: 287–288. doi: 10.3181/00379727-83-20336 [DOI] [PubMed] [Google Scholar]
- 7.Fleck A.1989. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc. Nutr. Soc. 48: 347–354. doi: 10.1079/PNS19890050 [DOI] [PubMed] [Google Scholar]
- 8.Fuhrman M. P., Charney P., Mueller C. M.2004. Hepatic proteins and nutrition assessment. J. Am. Diet. Assoc. 104: 1258–1264. doi: 10.1016/j.jada.2004.05.213 [DOI] [PubMed] [Google Scholar]
- 9.Haider M., Haider S. Q.1984. Assessment of protein-calorie malnutrition. Clin. Chem. 30: 1286–1299 [PubMed] [Google Scholar]
- 10.Hassanein el- S. A., Assem H. M., Rezk M. M., el-Maghraby R. M.1998. Study of plasma albumin, transferrin, and fibronectin in children with mild to moderate protein-energy malnutrition. J. Trop. Pediatr. 44: 362–365. doi: 10.1093/tropej/44.6.362 [DOI] [PubMed] [Google Scholar]
- 11.Heyland D. K.2000. Enteral and parenteral nutrition in the seriously ill, hospitalized patient: a critical review of the evidence. J. Nutr. Health Aging 4: 31–41 [PubMed] [Google Scholar]
- 12.Ingenbleek Y., Van Den Schrieck H. G., De Nayer P., De Visscher M.1975. Albumin, transferrin and the thyroxine-binding prealbumin/retinol-binding protein (TBPA-RBP) complex in assessment of malnutrition. Clin. Chim. Acta 63: 61–67. doi: 10.1016/0009-8981(75)90379-4 [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y., Nezu R., Matsuda H., Takagi Y., Okada A.1995. Rapid turnover proteins as a prognostic indicator in cancer patients. Surg. Today 25: 498–506. doi: 10.1007/BF00311305 [DOI] [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K., Kleiner M., Dunne E., Ahern K., Nelson M., Koslowe R., Luft F. C.1998. Total iron-binding capacity-estimated transferrin correlates with the nutritional subjective global assessment in hemodialysis patients. Am. J. Kidney Dis. 31: 263–272. doi: 10.1053/ajkd.1998.v31.pm9469497 [DOI] [PubMed] [Google Scholar]
- 15.Krzystek-Korpacka M., Matusiewicz M., Diakowska D., Grabowski K., Blachut K., Kustrzeba-Wojcicka I., Terlecki G., Gamian A.2008. Acute-phase response proteins are related to cachexia and accelerated angiogenesis in gastroesophageal cancers. Clin. Chem. Lab. Med. 46: 359–364. doi: 10.1515/CCLM.2008.089 [DOI] [PubMed] [Google Scholar]
- 16.Kuvshinoff B. W., Brodish R. J., McFadden D. W., Fischer J. E.1993. Serum transferrin as a prognostic indicator of spontaneous closure and mortality in gastrointestinal cutaneous fistulas. Ann. Surg. 217: 615–622. doi: 10.1097/00000658-199306000-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Michel K. E.1993. Prognostic value of clinical nutritional assessment in canine patients. J. Vet. Emerg. Crit. Care 3: 96–104. doi: 10.1111/j.1476-4431.1993.tb00107.x [DOI] [Google Scholar]
- 18.Michel K. E., Sorenmo K., Shofer F. S.2004. Evaluation of body condition and weight loss in dogs presented to a veterinary oncology service. J. Vet. Intern. Med. 18: 692–695. doi: 10.1111/j.1939-1676.2004.tb02607.x [DOI] [PubMed] [Google Scholar]
- 19.Mullen J. L., Buzby G. P., Matthews D. C., Smale B. F., Rosato E. F.1980. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann. Surg. 192: 604–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakajima A., Shibazaki M., Shimetani N., Yamaguchi M., Mori M.2005. Clinical Significance of Measurement of Transfferin, Transthyretin, and Retinol-Binding Protein as Nutrition Assessment Proteins. Dokko. J. Med. Sci. 21–28 (in Japanease). [Google Scholar]
- 21.Nakajima M., Ohno K., Takeuchi Y., Takeuchi A., Nakashima K., Fujino Y., Tsujimoto H.2012. Usefulness of plasma transferrin levels as dynamic assessment of protein nutrition in dog. J. Pet. Anim. Nutr. 15: 65–71(in Japanease) [Google Scholar]
- 22.Oka R., Nakagawa Y., Shoji T., Matsuda Y., Hamamoto Y., Takeshita M.2006. Usefulness of a nutrition assessment system for parenteral/enteral nutrition therapy. Yakugaku Zasshi 126: 1351–1356. doi: 10.1248/yakushi.126.1351 [DOI] [PubMed] [Google Scholar]
- 23.Reddy S., Adcock K. J., Adeshina H., Cooke A. R., Akene J., McFAarlane H.1970. Immunity, transferrin, and survival in kwashiorkor. Br. Med. J. 4: 268–270. doi: 10.1136/bmj.4.5730.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeds P. J., Laditan A. A.1976. Serum albumin and transferrin protein-energy malnutrition. Their use in the assessment of marginal undernutrition and the prognosis of severe undernutrition. Br. J. Nutr. 36: 255–263. doi: 10.1079/BJN19760077 [DOI] [PubMed] [Google Scholar]
- 25.Remillard R. L., Darden D. E., Michel K. E., Marks S. L., Buffington C. A., Bunnell P. R.2001. An investigation of the relationship between caloric intake and outcome in hospitalized dogs. Vet. Ther. 2: 301–310 [PubMed] [Google Scholar]
- 26.Roubenoff R., Kehayias J. J.1991. The meaning and measurement of lean body mass. Nutr. Rev. 49: 163–175. doi: 10.1111/j.1753-4887.1991.tb03013.x [DOI] [PubMed] [Google Scholar]
- 27.Seltzer M. H., Bastidas J. A., Cooper D. M., Engler P., Slocum B., Fletcher H. S.1979. Instant nutritional assessment. JPEN J. Parenter. Enteral. Nutr. 3: 157–159. doi: 10.1177/0148607179003003157 [DOI] [PubMed] [Google Scholar]
- 28.Shetty P. S., Watrasiewicz K. E., Jung R. T., James W. P.1979. Rapid-turnover transport proteins: an index of subclinical protein-energy malnutrition. Lancet 2: 230–232. doi: 10.1016/S0140-6736(79)90241-1 [DOI] [PubMed] [Google Scholar]
- 29.Smale B. F., Mullen J. L., Buzby G. P., Rosato E. F.1981. The efficacy of nutritional assessment and support in cancer surgery. Cancer 47: 2375–2381. doi: [DOI] [PubMed] [Google Scholar]
- 30.Steffee W. P.1980. Malnutrition in hospitalized patients. JAMA 244: 2630–2635. doi: 10.1001/jama.1980.03310230032019 [DOI] [PubMed] [Google Scholar]
- 31.Takeda H., Ishihama K., Fukui T., Fujishima S., Orii T., Nakazawa Y., Shu H. J., Kawata S.2003. Significance of rapid turnover proteins in protein-losing gastroenteropathy. Hepatogastroenterology 50: 1963–1965 [PubMed] [Google Scholar]
- 32.Thibault R., Pichard C.2010. Nutrition and clinical outcome in intensive care patients. Curr. Opin. Clin. Nutr. Metab. Care 13: 177–183. doi: 10.1097/MCO.0b013e32833574b9 [DOI] [PubMed] [Google Scholar]
- 33.Winkler M. F., Gerrior S. A., Pomp A., Albina J. E.1989. Use of retinol-binding protein and prealbumin as indicators of the response to nutrition therapy. J. Am. Diet. Assoc. 89: 684–687 [PubMed] [Google Scholar]
- 34.Winkler M. F., Pomp A., Caldwell M. D., Albina J. E.1989. Transitional feeding: the relationship between nutritional intake and plasma protein concentrations. J. Am. Diet. Assoc. 89: 969–970 [PubMed] [Google Scholar]
- 35.Young M. E.1988. Malnutrition and wound healing. Heart Lung 17: 60–67 [PubMed] [Google Scholar]