ABSTRACT
In this study, we describe the detection of novel paramyxoviruses from the Eidolon helvum species of fruit bats. We extracted RNA from 312 spleen samples from bats captured in Zambia over a period of 4 years (2008–2011). Semi-nested RT-PCR detected a total of 25 (8%) positive samples for paramyxoviruses which were then directly sequenced and analyzed using phylogenetic analysis. Among the positive samples, seven novel paramyxoviruses were detected. Five viruses were closely related to the genus Henipavirus, while two viruses were related to the unclassified Bat paramyxoviruses from Ghana and Congo Brazzaville. Our study identified novel Henipavirus-related and unrelated viruses using RT-PCR in fruit bats from Kansaka National Park and indicated the presence of similar Bat paramyxoviruses originating from wide geographic areas, suggesting the ability of bats to harbor and transmit viruses. The presence of these viruses in fruit bats might pose a public health risk.
Keywords: bat viruses, epidemiology, paramyxoviruses, virus, Zambia
In the past 10 years, a lot of attention has been given to bats as reservoirs of emerging zoonotic viruses. This has been as a result of the high detection rate of previously unknown viral sequences in bats coupled with the emergence of pathogens, such as Hendra, Nipah, Severe acute respiratory syndrome (SARS)-Corona, Ebola and Marburg viruses, all of which are highly virulent and pose a great zoonotic risk [2, 3, 8, 9, 17]. Bats, being the only flying mammals with ancient evolutionary origins and long life span, are capable of covering great distances during migrations, rendering them suitable hosts and reservoirs for various viruses. Paramyxoviruses from the family Paramyxoviridae have been implicated in several human epidemics and mortalities [6, 10, 19]. Several studies have indicated bats as potential natural reservoirs of Paramyxoviruses, such as Henipavirus-, Respirovirus- and Morbillivirus-related viruses [1, 4]. This undoubtedly presents a threat to the health of the human population in areas where human beings live in close proximity to fruit bat species [7]. In Zambia, straw-colored fruit bats (Eidolon helvum) annually converge in Kasanka National Park (KNP).
In this study, we investigated the presence of paramyxoviruses in the Eidolon helvum bats captured over a period of 4 years (2008–2011) from KNP (S15:34.688 E28:16.513). During that period, a total of 312 spleen samples were collected from the same number of bats (Table 1). Appropriate research permits and hunting licenses were obtained from the Zambia Wildlife Authority (ZAWA).
Table 1. Paramyxoviruses in fruit bats from Kasanka National park.
| Year of collection | Total tested | Totalpositive | Positive sample Id | Accession number |
|---|---|---|---|---|
| 2008 | 104 | 1 | ZFB08-07 | AB853092 |
| 2009 | 60 | 7 | ZFB09-05 | AB853093 |
| ZFB09-07 | AB853094 | |||
| ZFB09-24 | AB853095 | |||
| ZFB09-32 | AB853096 | |||
| ZFB09-39 | AB853097 | |||
| ZFB 09-43 | AB853098 | |||
| ZFB 09-51 | AB853099 | |||
| 2010 | 51 | 10 | ZFB 10-03 | AB853100 |
| ZFB 10-07 | AB853101 | |||
| ZFB 10-14 | AB853102 | |||
| ZFB 10-22 | AB853103 | |||
| ZFB 10-23 | AB853104 | |||
| ZFB 10-24 | AB853105 | |||
| ZFB 10-26 | AB853106 | |||
| ZFB 10-27 | AB853107 | |||
| ZFB 10-39 | AB853108 | |||
| ZFB 10-42 | AB853109 | |||
| 2011 | 97 | 7 | ZFB 11-30 | AB853110 |
| ZFB 11-35 | AB853111 | |||
| ZFB 11-60 | AB853112 | |||
| ZFB 11-66 | AB853113 | |||
| ZFB 11-76 | AB853114 | |||
| ZFB 11-88 | AB853115 | |||
| ZFB 11-91 | AB853116 | |||
| Total | 312 | 25 | ||
The results presented are for the semi-nested RT-PCR-based Paramyxovirinae detection system. Samples shown in this table were collected from the spleens of Eidolon helvum fruit bats.
Total RNA was isolated from spleen tissues using TRIzol (Life Technologies, Carlsbad, CA, U.S.A.) according to the manufacturer’s instructions. A semi-nested broad spectrum RT-PCR targeting the paramyxovirus polymerase (L) gene was used to screen total RNA samples (n=312) for paramyxoviruses using PAR-F1, PAR-F2 and PAR-R primers and PCR conditions described by Tong et al. [16]. A total of 25 samples out of 312 bat spleens (8%) were positive for paramyxoviruses on semi-nested PCR. The positive products (584 bp) were then purified using the monofas purification kit (GL sciences, Tokyo, Japan), according to the manufacturer’s instructions. The purified PCR products were then subjected to Cycle sequencing reactions using the Big Dye Terminator v3.1 system (Life Technologies) and the PAR-F2 and PAR-R inner primers. Ethanol precipitation was used to remove the labeled dNTPs from cycle sequence products and subjected to electrophoresis in the ABI 3130 genetic analyzer (Life Technologies). Phylogenetic analysis was performed using reference sequences and positive samples by aligning all sequences using ClustalW1.6 followed by the creation of a MEGA file format created using MEGA ver.5.2 [14]. The neighbor joining method was used to generate the phylograms [12] with a 1,000 bootstrap replicate confidence level [5]. To compute the evolutionary distances, the Maximum Composite Likelihood method was used with the number of base substitutions per site as units [15].
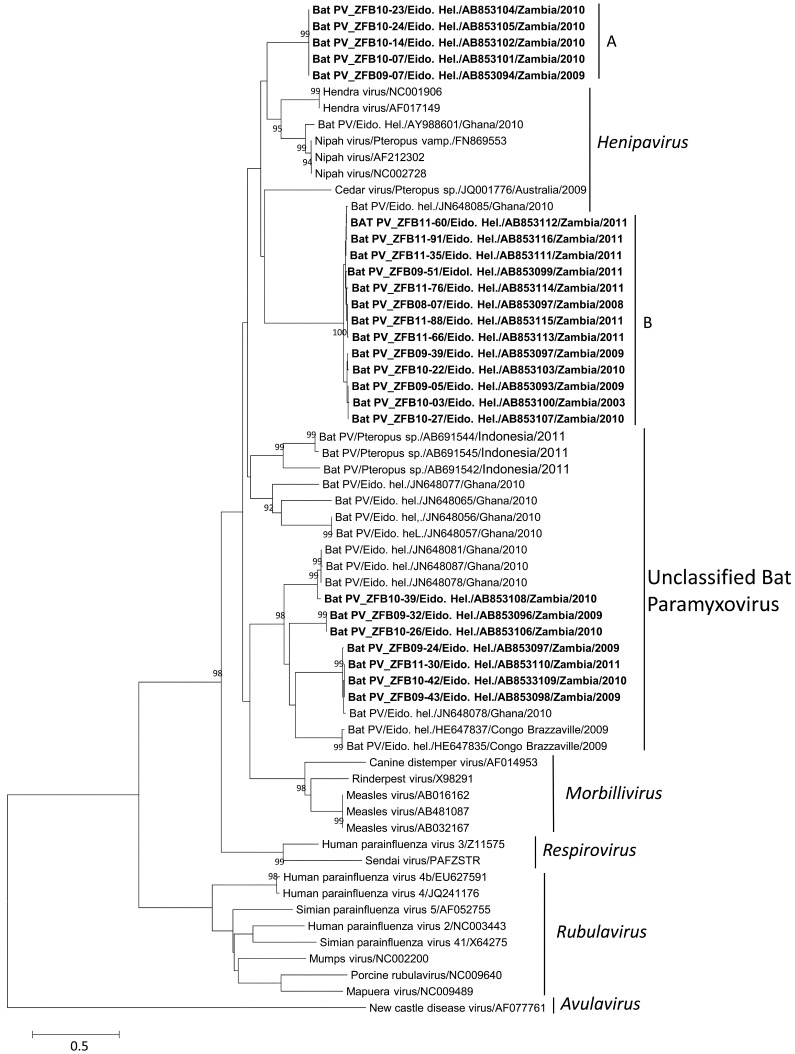
Samples AB853101, AB853102, AB853104, AB853105 and AB853094 showed a nucleotide homology of 73% with the Nipah virus (AF212302), while AB853106 and AB853096 had a nucleotide homology of 74% with the unclassified Bat paramyxovirus (Bat PV) (JN648087) from Ghana. The relatively low nucleotide homology might indicate that these sequences originate from novel paramyxoviruses. The samples from Zambia formed clusters with the Henipavirus-related viruses and with the unclassified Bat paramyxoviruses (Fig. 1). Within the Henipavirus-related virus cluster, two groups (A and B) were observed. Group A comprised novel Zambian strains closely related to the Nipah (NC002728, FN86955 and AF212302) and Hendra (AF017149 and NC001906) viral sequences, while Group B comprised a cluster of Zambian strains, in close relationship with an unclassified Bat PV from Ghana (JN648085) and Cedar virus (JQ001776) (Fig. 1). The remaining Zambian strains, including the novel AB853106 sequence, formed a cluster with the unclassified Bat PV sequences from Ghana (JN648078, JN648081, JN648087 and JN648089) and Congo Brazzaville (HE647835 and HE647837) (Fig. 1). The close relatedness of the viral sequence from Ghana and Congo Brazzaville strains with those from Zambia might imply the ability of bats to harbor and transmit similar viruses over long and diverse geographical distances. This is facilitated by their ability to migrate, covering thousands of kilometers to their hibernation and feeding sites [11]. Along their migratory path, they interact directly or indirectly with several terrestrial mammalian species in different geographical locations, thus enhancing the interspecies transmission of viruses [11]. Humans can become exposed to these viruses through environmental contamination with urine and feces from bats. Although paramyxovirus infections derived from bats have been reported in humans in Bangladesh [13], none have been reported in Africa. The absence of cases might be as a result of under-reporting. As such continued surveillance and assessment of the zoonotic risk posed by these viruses still remains important.
Fig. 1.
Phylogenetic analysis of paramyxovirus samples based on a 530 nucleotide sequence of the polymerase L gene from Zambia (in bold) and other areas of the world. The neighbor joining method was used to construct the phylogram using a confidence level of 1,000 bootstrap replicates. Species names and accession numbers are used to identify both the Zambian samples and the reference isolates. Bootstrap values for 1,000 replicates are indicated as percentage (>90%) and nucleotide substitutions per site as scale bars.
In order to isolate the detected viruses, spleens from PCR positive bats were homogenized in minimum essential media (MEM) followed by centrifugation at 1,000 × g for 3 min. The supernatant was then applied to Vero E6 cell with 70–80% growth confluence. The Vero E6 cells were cultured in MEM with 2% fetal bovine serum (FBS) and 2% antibiotic-antimycotic (Life Technologies). The inoculated Vero E6 cell cultures were then incubated at 37°C for 21 days, coupled with cell passage and microscopic examination. However, after several passages, cytopathic effects were not observed. We also performed semi-nested RT-PCR to detect paramyxovirus RNA in the supernatants of the inoculated cells. However, no positive signals were detected. Isolation of paramyxoviruses using Vero E6 cells has successfully been reported [18], implying that Vero E6 cell lines might be suitable for isolation of paramyxoviruses. In this study, the small amount of virus in the supernatant or the failure to successfully remove the virus from infected cells using the freeze and thaw technique might be responsible for the absence of cytopathic effects in the cell culture. In a study by Wilkinson [18], out of 8 positive samples, virus isolation was only successfully carried out in two samples. Furthermore, serological examination of bat sera may provide important information about their exposure to specific infections. Unfortunately, however, our study did not carry out any serological test.
In conclusion, we report the identification of novel Henipavirus-related (n=5) and unrelated (n=2) viruses in fruit bats from the KNP using RT-PCR. The viruses identified in this study were shown to originate from wide geographical areas, and their presence in fruit bat species might pose a public health risk and as such, continued surveillance of these viruses in fruit bats in essential.
ACKNOWLEDGMENTS
We thank Ms. Yamanouchi, Research Center for Zoonosis Control, Hokkaido University, for technical assistance. We also wish to thank the Zambia Wildlife Authority and the Kasanka Trust for providing permission and the enabling environment for sampling the fruit bats. This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT); the Ministry of Health, Labour and Welfare, Japan; the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID), MEXT Japan.
REFERENCES
- 1.Baker K. S., Todd S., Marsh G., Fernandez-Loras A., Suu-Ire R., Wood J. L., Wang L. F., Murcia P. R., Cunningham A. A.2012. Co-circulation of diverse paramyxoviruses in an urban African fruit bat population. J. Gen. Virol. 93: 850–856. doi: 10.1099/vir.0.039339-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermejo M., Rodriguez-Teijeiro J. D., Illera G., Barroso A., Vila C., Walsh P. D.2006. Ebola outbreak killed 5000 gorillas. Science 314: 1564. doi: 10.1126/science.1133105 [DOI] [PubMed] [Google Scholar]
- 3.Chua K. B., Bellini W. J., Rota P. A., Harcourt B. H., Tamin A., Lam S. K., Ksiazek T. G., Rollin P. E., Zaki S. R., Shieh W.2000. Nipah virus: a recently emergent deadly paramyxovirus. Science 288: 1432–1435. doi: 10.1126/science.288.5470.1432 [DOI] [PubMed] [Google Scholar]
- 4.Drexler J. F., Corman V. M., Muller M. A., Maganga G. D., Vallo P., Binger T., Gloza-Rausch F., Rasche A., Yordanov S., Seebens A., Oppong S., Adu Sarkodie Y., Pongombo C., Lukashev A. N., Schmidt-Chanasit J., Stocker A., Carneiro A. J., Erbar S., Maisner A., Fronhoffs F., Buettner R., Kalko E. K., Kruppa T., Franke C. R., Kallies R., Yandoko E. R., Herrler G., Reusken C., Hassanin A., Kruger D. H., Matthee S., Ulrich R. G., Leroy E. M., Drosten C.2012. Bats host major mammalian paramyxoviruses. Nat. Commun. 3: 796. doi: 10.1038/ncomms1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felsenstein J.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evol. 39: 783–791. doi: 10.2307/2408678 [DOI] [PubMed] [Google Scholar]
- 6.Field H. E., Mackenzie J. S., Daszak P.2007. Henipaviruses: emerging paramyxoviruses associated with fruit bats. Curr. Top. Microbiol. Immunol. 315: 133–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth A., Kohl C., Brinkmann A., Ebinger A., Harper J. A., Wang L. F., Muhldorfer K., Wibbelt G.2012. Novel paramyxoviruses in free-ranging European bats. PLoS ONE 7: e38688. doi: 10.1371/journal.pone.0038688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroy E. M., Kumulungui B., Pourrut X., Rouquet P., Hassanin A., Yaba P., Delicat A., Paweska J. T., Gonzalez J. P., Swanepoel R.2005. Fruit bats as reservoirs of Ebola virus. Nature 438: 575–576. doi: 10.1038/438575a [DOI] [PubMed] [Google Scholar]
- 9.Li W., Shi Z., Yu M., Ren W., Smith C., Epstein J. H., Wang H., Crameri G., Hu Z., Zhang H.2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310: 676–679. doi: 10.1126/science.1118391 [DOI] [PubMed] [Google Scholar]
- 10.Pallister J., Middleton D., Broder C. C., Wang L. F.2011. Henipavirus vaccine development. J. Bioterr. Biodef. 1:005. doi: 10.4172/2157-2526.S1-005 [DOI] [Google Scholar]
- 11.Richter H. V., Cumming G. S.2006. Food availability and annual migration of the straw-coloured fruit bat Eidolon helvum. J. Zool. 268: 35–44. doi: 10.1111/j.1469-7998.2005.00020.x [DOI] [Google Scholar]
- 12.Saitou N., Nei M.1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4: 406–425 [DOI] [PubMed] [Google Scholar]
- 13.Stone R.2011. Breaking the chain in Bangladesh. Science 331: 1128–1131. doi: 10.1126/science.331.6021.1128 [DOI] [PubMed] [Google Scholar]
- 14.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S.2011. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 28: 2731–2739. doi: 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura K., Nei M., Kumar S.2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. 101: 11030–11035. doi: 10.1073/pnas.0404206101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong S., Chern S. W., Li Y., Pallansch M. A., Anderson L. J.2008. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 46: 2652–2658. doi: 10.1128/JCM.00192-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L. F.2009. Bats and viruses: a brief review. Virol. Sin. 24: 93–99. doi: 10.1007/s12250-009-3032-5 [DOI] [Google Scholar]
- 18.Wilkinson D. A., Sarah T., Camille L., Erwan L., Julien C., Julia G., Beza R., Jean-Michel H., Xavier de Lamballerief, Steven M. G., Koussay D., Herve P.2012. Identification of novel paramyxoviruses in insectivorous bats of the Southwest Indian Ocean. Virus Res. 170: 159–163. doi: 10.1016/j.virusres.2012.08.022 [DOI] [PubMed] [Google Scholar]
- 19.Wong S., Lau S., Woo P., Yuen K. Y.2007. Bats as a continuing source of emerging infections in humans. Rev. Med. Virol. 17: 67–91. doi: 10.1002/rmv.520 [DOI] [PMC free article] [PubMed] [Google Scholar]