Abstract
Endoscopic ultrasound (EUS) is one of the best diagnostic methods for diseases of the digestive tract and surrounding organs. Whereas EUS-guided fine needle aspiration (FNA) has been very useful for providing histological confirmation for previously hard to reach lesions, elastography is aiming to obtain a “virtual biopsy” by assessing differences in elasticity between the normal and pathological – usually malignant – tissue. A question that arises is whether EUS-elastography has reached a stage where it might successfully supplant the use of EUS-FNA in some of its clinical indications. The main indications of EUS-guided FNA are listed in this article and published data on the usage of elastography in these settings is reviewed for each one. In some of the indications, a plethora of studies have been published, notably for the evaluation of solid pancreatic masses and lymph nodes, while in others there is little relevant data (submucosal masses, left liver lesions, left adrenal masses), or elastography simply is not suitable as a diagnostic means (cystic lesions). Our conclusion is that elastography is not yet ready to replace EUS-FNA in its indications, but should complement it in various settings, especially for the assessment of lymph nodes. It can only be considered an alternative on a case-by-case basis, in situations where FNA is regarded as a contraindication. Furthermore, it could be used in conjunction with other imaging techniques, such as contrast-enhanced EUS, in order to further improve the accuracy of non-invasive EUS assessment, possibly making the case for a more limited or targeted use of EUS-FNA in selected cases.
Keywords: Elastography, endoscopic ultrasound, fine needle aspiration
INTRODUCTION
Endoscopic ultrasound (EUS) offers high-resolution images of tissues and organs near the digestive tract as well as the digestive tract itself, thus becoming a landmark in the management of numerous mediastinal and abdominal diseases.[1,2,3] Its main focus are the benign and malignant tumors of the respective areas; the method has been proposed in various studies for both diagnosis and staging.[4,5,6,7,8,9,10]
Technological progress has led to the advent of fine needle aspiration (FNA) and/or biopsy, performed under ultrasonographic guidance. EUS-guided FNA has great accuracy, with 80-85% sensitivity and almost 100% specificity.[11,12,13,14,15,16,17,18] Even though, it is traditionally considered to be a gold standard.[19] There are drawbacks. Setting aside the associated morbidity,[20,21,22,23] the technique can be taxing to master and repeat punctures may be needed for a diagnosis.[24,25] Furthermore, false negatives tend to pile up in some circumstances, for example in patients with underlying chronic pancreatitis,[26,27,28] while in the case of multiple suspicious lymph nodes it is not always clear which one to puncture.[29]
Elastography is a newer method that seeks to improve the diagnostic yield of ultrasound. The underlying principle is that tissue consistency has a proportional effect on the strain produced by its compression.[30] The comparison between standard ultrasound images obtained before and after slight compression results in a transparent color overlay superimposed over the conventional gray-scale B-mode scans, representing the local index of tissue elasticity.[31,32,33,34] It has been shown that there is a good correlation between this index and histopathologic features, even more so for malignant masses.[30,35,36,37,38] It was thus only logical that EUS elastography was developed, seeking to complement conventional EUS in the assessment of previously hard to reach tumors in proximity of the digestive tract, such as pancreatic masses[39,40,41,42,43,44,45,46,47,48,49,50,51] and lymph nodes.[52,53,54] There have even been further enhancements of this technique, first using hue histogram analysis and artificial neural networks.[40,46] Afterwards, second-generation EUS elastography equipment introduced strain ratio (SR) and strain histogram (SH) as two reproducible, parametric measurements, that retrieve numerical values in real time, adding quantification possibilities to the technique and thus greatly reducing the human bias without the need for 3rd-party software.[55] SR computes the relative strain between two regions of interest (ROI), whereas SH measures the strain values of elemental areas inside a ROI and produces a graph as well as an average value. SR has already been used in vivo for pancreatic masses.[41]
As several studies have demonstrated, EUS-elastography is a promising technique with a high accuracy for the differential diagnosis of solid, otherwise hard to reach masses (i.e., pancreatic tumors and lymph nodes). This article will attempt to review current data on elastography, its technique and actual indications and assess whether this “virtual biopsy” might be able to supplant EUS-FNA in some of these instances.
QUALITATIVE AND QUANTITATIVE EUS-ELASTOGRAPHY
Qualitative elastography
The elastography module provided in the ultrasound device detects small structure deformations within the B-mode image caused by compression, which are smaller in hard tissue than in soft tissue.[56] The degree of deformation is then graded on a scale of 1-255 and each value is assigned a different shade from the red-green-blue (RGB) hue color spectrum for easier visual recognition. The two extremes are blue — hard and red — soft. The output is a real-time two-panel image, with the usual conventional gray-scale B-mode image on the right side and the elastographic image on the left side. The ROI for the elastographic evaluation is selected manually, with an emphasis on selecting the lesion in its entirety and also including normal surrounding tissue. Preliminary work with qualitative elastography was carried out on breast lesions, identifying several patterns that correctly correlated with the diagnosis of malignancy of breast masses.[36]
Other patterns have been described for EUS-elastography.[45,57] For example, in the case of pancreatic masses, studies have considered that mostly blue masses were malignant, while other color patterns were considered to show benignancy.[48] Needless to say, it has been suggested that there is a strong bias in this type of analysis, mostly observer-related, based on possible perception errors and the inability of the human eye to completely characterize all color hues. Even so, studies have shown a good interobserver correlation, with high sensitivity for pancreatic masses and somewhat lower for lymph nodes.[40,58]
Quantitative elastography
Quantitative EUS elastography can be performed in two different ways. One involves representing the color hues as a histogram; the other compares the average strain in two different areas of the ROI.
Hue/SH analysis
The histogram is a convenient way to quantify and represent a specific characteristic of a digital image through a graph. In this case, the histogram is used to represent the digitized color distribution. Using specific 3rd party software (ImageJ), such histograms can be obtained from standard qualitative elastography images.[59] The software analyzes the color of pixels inside the ROI and each pixel color is assigned a value from 0 to 255 (soft to hard). Elasticity values from 0 to 255 (soft to hard) are represented on the X-axis, while Y-axis values represent the number of pixels of each value. Side by side, the total numbers of pixels in each value create the graph. Newer ultrasound machines have included SH software, automatically calculating the graph in real time, with slight technical differences (for example, an inversed numeric scale, where 0 higher values denote softness while lower ones hardness). Both procedures return a mean value that gives a numeric representation to the overall elasticity of tissues.[40] The histogram analysis can even be taken one step further, by training artificial neural networks to make the distinction between benign and malignant histograms.[46,60,61]
SR
SR is based on a different principle than the histograms. Based on the assumption that some tissues (adipose, connective) have little to no inter-individual hardness variance, these are used as a control. Therefore, the elasticity of the target tissue is expressed not as an absolute value, but as a relative ratio to the reference provided by these tissues.[58] Using the standard qualitative EUS elastographic image, the operator selects two non-overlapping areas inside the ROI: The lesion (area A) and the reference zone (area B). The B/A quotient obtained represents the SR.[43]
CLINICAL APPLICATIONS OF FNA AND THE ROLE OF EUS-ELASTOGRAPHY IN THESE SETTINGS
In order to assess whether elastography is a viable alternative to FNA, one has to first take a look at the indications and contraindications of the latter. FNA fundamentals dictate that it should only be performed when the information obtained is likely to impact the management of the patient.[62] Its diagnostic accuracy, cost effectiveness and patient comfort and safety should also be taken into consideration.[11] Pooling the indications as stated by several authors from different countries,[11,63,64,65] the uses for EUS-FNA are presented in the following Table 1, together with the uses of EUS-elastography:
Table 1.
Indications of EUS-FNA and of EUS-elastography
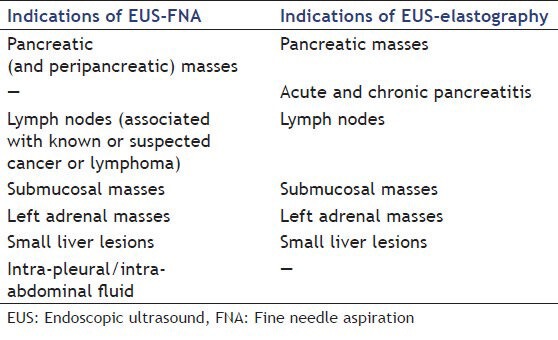
Contraindications to EUS-FNA include cases where patient management would not be affected, lesions which cannot be clearly visualized, interposition of other structures, such as vessels, between the needle and the target and a risk of bleeding.[11,62,65] Furthermore, as pointed out by some reviews and published case reports, in some settings there is a risk of seeding of malignant cells.[66,67,68]
Due to the principles behind it, EUS elastography can only assess solid lesions; therefore, we can say from the start that it cannot be used in some of the indications of FNA. As a general rule, fluids can greatly artifact the elasticity of tissue inside the ROI, so a lesion that has a cystic component, for example, cannot be evaluated by these means. In addition, including a large vessel in the elastography ROI has been, in our experience, a source of errors. After eliminating these indications, the following remain: Solid pancreatic masses, lymph nodes, submucosal masses, left liver lobe masses and left adrenal masses. Extensive studies have been performed for the first two instances, while consistent data is rather scarce for the others.
Pancreatic masses
EUS is considered one of the most accurate imaging techniques for the assessment of the pancreas.[4,19,69] In spite of efforts to implement criteria for the differential diagnosis between pancreatic cancer and mass-forming chronic pancreatitis, the accuracy has never been higher than 75%,[70] a percentage that elastography seeks to increase.
First qualitative EUS-elastography experiences were published by Giovannini et al. who implemented a qualitative scoring system based on the lesions color pattern; hard, mostly blue lesions were classified as malignant.[48] Sensitivity and specificity were, respectively, 100% and 67%. A refining of this system created a five-score classification: Score 1 (normal pancreatic tissue) was given to a homogeneous soft area (green); Score 2 (fibrosis) was given to images with soft heterogeneity (green, yellow and red); Score 3 (early pancreatic adenocarcinoma) was given to mostly blue images (hard) with minimal heterogeneity; Score 4 (neuroendocrine tumor, metastasis) was given to an image with a central green hypoechoic region and blue tissue outer layer; Score 5 (advanced pancreatic adenocarcinoma) was assigned to blue lesions with heterogeneity due to necrosis. Scores 1 and 2 were considered benign, while 3-5 were considered malignant. The scoring system achieved overall accuracy of 89.2% in a multicenter study,[44] with sensitivity and positive predictive value (PPV) being over 90%. Iglesias-Garcia has described four similar patterns: Homogeneous green for normal pancreas; heterogeneous, green-predominant for inflammatory pancreatic masses [Figure 1]; heterogeneous, blue-predominant for pancreatic malignant tumors; and homogeneous blue for pancreatic neuroendocrine malignant lesions [Figure 2]. This classification brought an almost 5% increase of accuracy, up to 94.0%.[45] However, other qualitative elastography studies have returned less optimistic figures. In one study, the patterns were found to be too similar and irregularly distributed between tumors and pancreatitis masses, perhaps with the exception of neuroendocrine tumors, while in another there were crippling rates of adequate lesion inclusion in the ROI, leading to disappointing results.[71,72]
Figure 1.
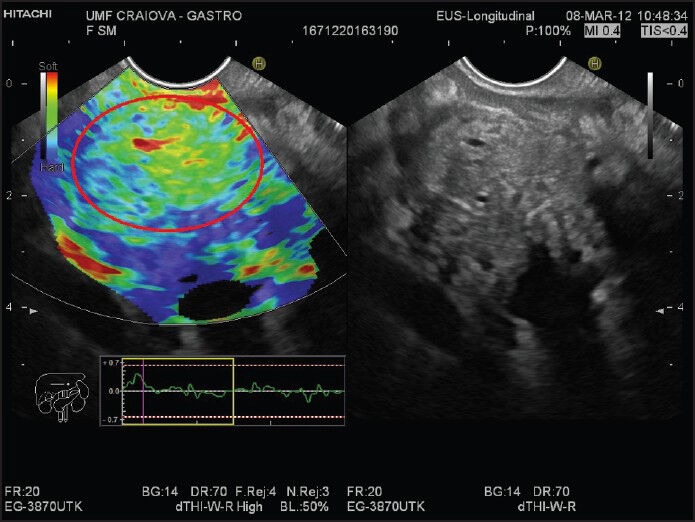
A patient with chronic pancreatitis. The elastography image in the left panel shows a heterogeneous green pancreatic mass (red circle). B-mode reference image is shown in the right panel
Figure 2.
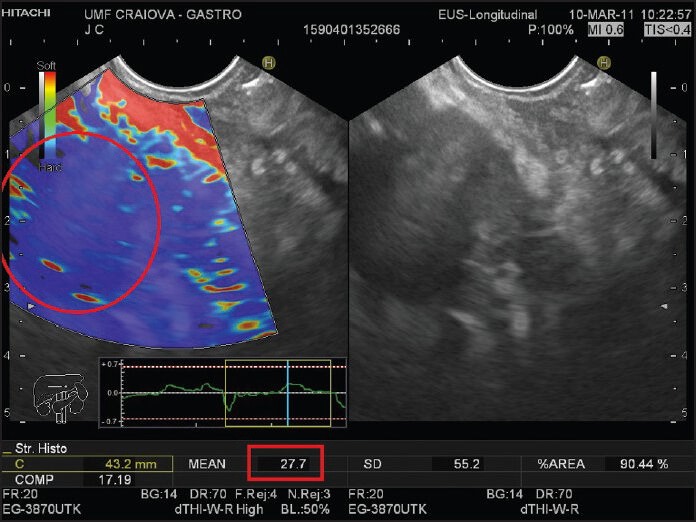
A patient with a malignant pancreatic tumor. The elastography image in the left panel shows a homogeneous blue pancreatic mass (red circle). The mean value of the strain histogram is also shown near the bottom of the image (red rectangle). B-mode reference image is shown in the right panel
More recently, studies have been published using quantitative EUS elastography for the differential diagnosis of pancreatic masses. A study by Săftoiu et al. used hue-histograms in two different studies,[40,46] obtaining good sensitivities (93.4% and 91.4%) but varying specificities (66.0% and 87.9%). Overall accuracies in these studies were only slightly lower than the ones obtained by Itokawa et al.[41] and Iglesias-Garcia[43] using a SR protocol. The same SR protocol was also used by Dawwas et al. in a large single-center prospective study.[73] He cited a sensitivity of 100.0%, poor specificity of 16.7%, a PPV of 86.1%, a negative predictive value of 100.0% and an overall accuracy of 86.5%.
The subject of elastographic differential diagnosis of solid pancreatic masses was also covered in some very recent meta-analyses.[74,75,76,77] They included between 6 and 13 studies, most of them dealing with both qualitative and quantitative elastography. Sensitivity was very high in all of them, between 95% and 99%, while specificity varied between 67% and 76% respectively.
Lymph nodes
Lymph nodes that are close to the digestive tract can be imaged with high accuracy via EUS. However, the differential diagnosis of pathologic ones remains a challenge. Since the presence of lymph node malignancy often changes the stage of the tumor to a more advanced one, which entails distinct treatment options, clinical decision-making is greatly influenced by this aspect. At the moment, there are established criteria that suggest lymph node malignancy: Round shape, hypoechogenicity and diameter >1 cm and distinct margins. Nevertheless, these features overlap with benign nodes and their specificity is rather low.[29] Therefore, it is essential to develop a minimally-invasive imaging procedure. In this setting, EUS-elastography might be useful to differentially diagnose lymph node malignancy or to single out the more suspicious nodes.
The first study using qualitative EUS-elastography[48] evaluated lymph nodes with different locations (cervical, mediastinal, celiac and aortocaval). Predominance of blue areas was considered to signify malignancy, while mostly green nodes, as well as indeterminate heterogeneous ones, were classified as benign. Switching to the previously described and more refined 5-point scoring system,[44] the same investigators obtained a great increase in specificity (from 50% to 82.5%), with only a slight decrease in sensitivity (from 100% to 91.8%) and an overall accuracy of 88.1%. A different team[57] described only the three elastographic patterns (mostly blue, mostly green and an indeterminate mixed pattern with no predominance). Their findings showed that green nodes were benign with a 100% probability [Figure 3], while blue ones had a 92.3% chance of being malignant. Indeterminate mixed-pattern nodes, however, were just as likely to be benign as malignant.
Figure 3.
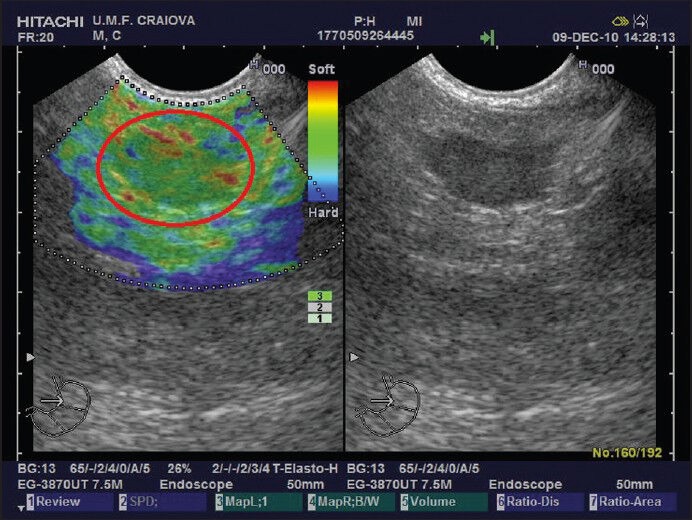
A patient with a benign lymph node. The elastography image in the left panel shows a mostly green mass (red circle). B-mode reference image is shown in the right panel
Săftoiu et al. also conducted a study that evaluated lymph nodes through qualitative elastography,[78] using the 5-score system, with better results (92.86% accuracy). More importantly, however, is the fact that their study is one of the few that also performed a quantitative analysis. Using separate RGB channel histograms, sensitivity, specificity and accuracy were all around the 95% threshold. Hue-histogram analysis performed by the same team[54] also yielded good figures, albeit slightly lower, but with specificity still over 90%. The most recent study,[79] which conducted both qualitative and quantitative evaluations of lymph nodes, complete with SR calculations, puts a damp on the enthusiasm of previous results. The investigators concluded that elastography and SR calculation were no better than standard EUS in differentiating between malignant and benign lymph nodes, at least for patients with resectable upper gastrointestinal cancer that were included in the study.
Submucosal masses
The most important prognostic question to answer is whether a submucosal mass is benign or malignant.[80] B-mode criteria for benignancy are: Small size (smaller than 3 cm), smooth margins, uniform echogenicity and the lack of signs of infiltration. Elastography typically shows an intermediate elasticity with a homogeneous pattern.[81,82] A follow-up is usually required, with little to no changes in regards to the aforementioned criteria. Some degenerative changes may develop, making a diagnosis of benignancy very difficult.[82] The most common submucosal lesions are lipomas which, in addition to these criteria, always originate between the mucosa and the muscularis propria and are usually soft.[82] Malignancy B-mode characteristics are as follows: Larger lesions (more than 3-4 cm), irregularities of the margins, heterogeneous structure (this includes anechoic internal structures) and infiltration. In contrast with benign lesions, elastography usually shows harder patterns.[82] A single criterion is not enough for the differential diagnosis and the combination of two criteria still shows low sensitivity, albeit specificity increases. In the study performed by Jenssen and Dietrich, almost a third of the tumors showed one or two criteria for malignancy, while 37.7% lacked any.[82] One type of submucosal tumor merits special attention. Gastrointestinal stromal tumors (GIST) are a difficult case because their malignancy is assessed based on size and the number of mitoses/50 high power fields (HPF). Benign GISTs must be under 50 mm and have a low mitotic count (<5/50 HPF). Further complicating this situation is the fact that one tumor may have several foci of highly different characteristics. In most cases, immunohistochemical analysis should be performed. Taking these factors into consideration, an elastography evaluation of malignancy proves difficult.[82]
Liver lesions
Scarcely no data is available regarding liver tumor detection through EUS-elastography. Mostly case studies have been reported, generally owing to the fact that the examiner can usually only image the left lobe and not the entire organ, while there are better imaging options in play (computed tomography, magnetic resonance imaging). One study has shown though that left liver lobe tumors can be differentiated via elastography.[83]
Left adrenal masses
In contrast to the right adrenal gland, which is technically demanding to visualize,[84] the left one is routinely seen with EUS due to its location near the posterior gastric body wall.[85,86] Studies regarding EUS-FNA have been published,[87] citing a very good accuracy, although the number of masses was very low. On the other hand, EUS elastography of the adrenal glands has only been described in reviews and case reports, not in prospective or retrospective studies.[30] As a general rule, malignant masses tend to be harder (blue), but no consistent elastography criteria have been proposed.
DISCUSSIONS
Solid pancreatic masses have been thoroughly examined via EUS-elastography, with several limitations being quite obvious. The method is observer-dependent, with a high selection bias of the images and in some cases showing even a lack of reproducibility. Excessive pressure applied to the tissue can artificially increase the strain. The presence of certain tissues in the ROI (vessels, cysts, bone) greatly impacts the elasticity and hence the reliability of the measurements. Furthermore, intra- and interobserver variability are still a factor in both qualitative and quantitative approaches. And even though some authors had very optimistic findings, others reported disappointingly low accuracy rates. Furthermore, we must keep in mind that, as far as pancreatic lesions are concerned, the golden standard is very often provided by EUS-FNA due to several factors, of which surgical inaccessibility of the organ and inoperability of the tumor stand out. Therefore, most authors have concluded that EUS-FNA should most likely be aided by elastography in negative or inconclusive cases with a strong suspicion of malignancy. The most recently published European guidelines[88] also suggest that elastography is more appropriate as a diagnostic aid to FNA than a first-line option.
Lymph node studies have shown accuracy of over 85% for EUS-FNA, thus delivering the most reproducible results in the diagnosis of lymph node metastatic infiltration. However, the accuracy depends on the proper selection of lymph nodes. Regarding elastography assessment, there have been conflicting results regarding its supremacy over B-mode evaluation of lymph nodes. Nevertheless, a good accuracy has been consistently reported in studies, especially when using quantitative evaluation. Lastly, there is a low risk of seeding that makes FNA a relative contraindication in some settings. Therefore, we should ask ourselves whether it is good practice to puncture a lymph node when the actual tumor is in the path of the needle [Figure 4]. The European guidelines[88] stress the idea that elastography adds information to the B-mode evaluation and can better guide a EUS-FNA procedure.
Figure 4.
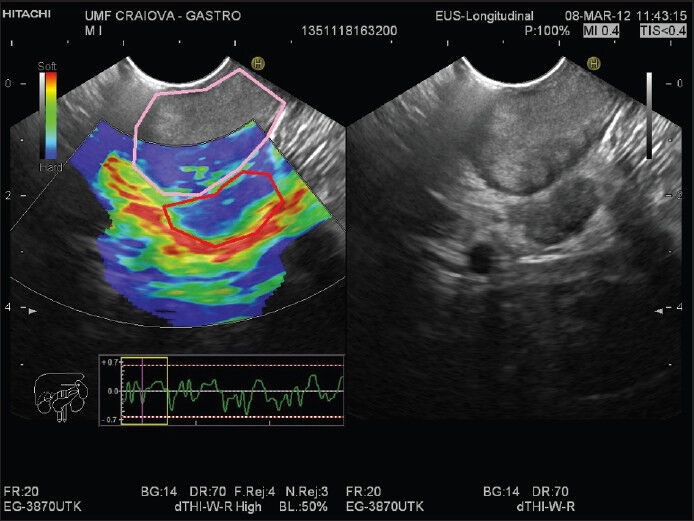
A patient with a malignant lymph node next to a primary tumor. The elastography image in the left panel shows the blue primary tumor partially encompassed in the regions of interest (pink outline) and the probably malignant satellite lymph node behind it (red outline). The node cannot be punctured without passing with the fine needle aspiration needle through the tumor. B-mode reference image is shown in the right panel
In the case of submucosal tumors, EUS elastography can add valuable information, helping to increase the accuracy of the staging. However, current knowledge has been based on individual experience and lacks prospective studies. Furthermore, GISTs warrant special attention when it comes to malignancy grading, which can sometimes only be achieved through immunohistochemical testing of each of the foci. Elastography simply does not currently provide a good enough resolution to properly asses the microfoci found in GISTs.
As for left liver lobe lesions and left adrenal masses, there is hardly any data available regarding elastography evaluation.
CONCLUSIONS
Reviewing published data and also based on our clinical experience, it is our conclusion that elastography is currently not ready to replace EUS-FNA in all or any of its indications. However, it should be a first-line means to complement FNA in various settings, as stated also by the most recent European guidelines. Negative or inconclusive cases of pancreatic mass FNA should be aided by elastography, especially when there is a strong suspicion of malignancy. Elastography can also be recommended to support discrimination of benign and malignant lymph nodes; this is especially true when used as a tool to select the nodes most likely to be malignant or malignant areas within nodes, for subsequent targeted EUS-FNA.
Both EUS elastography and subsequent EUS-FNA should be regarded as complementary methods that can give accurate information used for clinical decision making algorithms in individual cases. Due to its relatively ease of usage (at a touch of a button), low costs, lack of complications and valuable information regarding the strain characteristics of focal masses, EUS-elastography can be easily incorporated into the current clinical practice. Furthermore, EUS elastography might be the best option in settings where a high risk of tumor cells seeding makes FNA a relative contraindication, but on a case-by-case basis (for example, the FNA of a lymph node where the tumor is in the path of the needle).
Last but not least, elastography can be certainly used in conjunction with other imaging techniques, such as contrast-enhanced EUS, in order to further improve the accuracy of non-invasive EUS assessment, possibly making the case for a more limited or targeted use of EUS-FNA in selected cases.
ACKNOWLEDGMENTS
This paper was supported by the research grant “minimal invasive assessment of angiogenesis in pancreatic cancer based on imaging methods and molecular techniques (Angio-PAC)”, Ideas program, 164/2011, National Research Council-UEFISCDI, project number PN-II-ID-PCE-2011-3-0589.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Dye CE, Waxman I. Endoscopic ultrasound. Gastroenterol Clin North Am. 2002;31:863–79. doi: 10.1016/s0889-8553(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 2.Tamerisa R, Irisawa A, Bhutani MS. Endoscopic ultrasound in the diagnosis, staging, and management of gastrointestinal and adjacent malignancies. Med Clin North Am. 2005;89:139–58. doi: 10.1016/j.mcna.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 3.Byrne MF, Jowell PS. Gastrointestinal imaging: Endoscopic ultrasound. Gastroenterology. 2002;122:1631–48. doi: 10.1053/gast.2002.33576. [DOI] [PubMed] [Google Scholar]
- 4.Iglesias García J, Lariño Noia J, Domínguez Muñoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631–8. doi: 10.4321/s1130-01082009000900006. [DOI] [PubMed] [Google Scholar]
- 5.Giovannini M. The place of endoscopic ultrasound in bilio-pancreatic pathology. Gastroenterol Clin Biol. 2010;34:436–45. doi: 10.1016/j.gcb.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Gill KR, Wallace MB. Endoscopic ultrasound and staging of non-small cell lung cancer. Minerva Med. 2007;98:323–30. [PubMed] [Google Scholar]
- 7.De Luca L, Di Bella S, D’Amore E. Mediastinal and gastric EUS: Indications and technique of examination. Minerva Med. 2007;98:423–9. [PubMed] [Google Scholar]
- 8.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 9.Gheonea DI, Sãftoiu A. Beyond conventional endoscopic ultrasound: Elastography, contrast enhancement and hybrid techniques. Curr Opin Gastroenterol. 2011;27:423–9. doi: 10.1097/MOG.0b013e328349cfab. [DOI] [PubMed] [Google Scholar]
- 10.Săftoiu A. State-of-the-art imaging techniques in endoscopic ultrasound. World J Gastroenterol. 2011;17:691–6. doi: 10.3748/wjg.v17.i6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–79. doi: 10.1016/s0016-5107(04)01529-9. [DOI] [PubMed] [Google Scholar]
- 12.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 13.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 14.Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, et al. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289–93. doi: 10.3748/wjg.v13.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 16.Vilmann P, Annema J, Clementsen P. Endosonography in bronchopulmonary disease. Best Pract Res Clin Gastroenterol. 2009;23:711–28. doi: 10.1016/j.bpg.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TQ, Kalade A, Prasad S, et al. Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) of mediastinal lesions. ANZ J Surg. 2011;81:75–8. doi: 10.1111/j.1445-2197.2010.05266.x. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 19.Seicean A. Endoscopic ultrasound in chronic pancreatitis: Where are we now? World J Gastroenterol. 2010;16:4253–63. doi: 10.3748/wjg.v16.i34.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointest Endosc. 2006;63:622–9. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Voss M, Hammel P, Molas G, et al. Value of endoscopic ultrasound guided fine needle aspiration biopsy in the diagnosis of solid pancreatic masses. Gut. 2000;46:244–9. doi: 10.1136/gut.46.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Binmoeller KF, Thul R, Rathod V, et al. Endoscopic ultrasound-guided, 18-gauge, fine needle aspiration biopsy of the pancreas using a 2.8 mm channel convex array echoendoscope. Gastrointest Endosc. 1998;47:121–7. doi: 10.1016/s0016-5107(98)70343-8. [DOI] [PubMed] [Google Scholar]
- 23.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–5. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 24.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 25.Binmoeller KF, Rathod VD. Difficult pancreatic mass FNA: Tips for success. Gastrointest Endosc. 2002;56:S86–91. doi: 10.1016/s0016-5107(02)70093-x. [DOI] [PubMed] [Google Scholar]
- 26.Iglesias García J, Domínguez-Muñoz JE. Endoscopic ultrasound-guided biopsy for the evaluation of pancreatic tumors. Gastroenterol Hepatol. 2007;30:597–601. doi: 10.1157/13112588. [DOI] [PubMed] [Google Scholar]
- 27.DeWitt J, McGreevy K, Sherman S, et al. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610–9. doi: 10.1016/j.gie.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 28.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 29.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–9. doi: 10.1016/s0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 30.Saftoiu A, Vilman P. Endoscopic ultrasound elastography - A new imaging technique for the visualization of tissue elasticity distribution. J Gastrointestin Liver Dis. 2006;15:161–5. [PubMed] [Google Scholar]
- 31.Frey H. Realtime elastography. A new ultrasound procedure for the reconstruction of tissue elasticity. Radiologe. 2003;43:850–5. doi: 10.1007/s00117-003-0943-2. [DOI] [PubMed] [Google Scholar]
- 32.Ophir J, Alam SK, Garra B, et al. Elastography: Ultrasonic estimation and imaging of the elastic properties of tissues. Proc Inst Mech Eng H. 1999;213:203–33. doi: 10.1243/0954411991534933. [DOI] [PubMed] [Google Scholar]
- 33.Konofagou EE. Quo vadis elasticity imaging? Ultrasonics. 2004;42:331–6. doi: 10.1016/j.ultras.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Shiina T, Nitta N, Ueno E, et al. Real time tissue elasticity imaging using the combined autocorrelation method. Jpn J Med Ultrasonics. 2002;29:119–28. doi: 10.1007/BF02481234. [DOI] [PubMed] [Google Scholar]
- 35.Krouskop TA, Wheeler TM, Kallel F, et al. Elastic moduli of breast and prostate tissues under compression. Ultrason Imaging. 1998;20:260–74. doi: 10.1177/016173469802000403. [DOI] [PubMed] [Google Scholar]
- 36.Giovannini M. Contrast-enhanced endoscopic ultrasound and elastosonoendoscopy. Best Pract Res Clin Gastroenterol. 2009;23:767–79. doi: 10.1016/j.bpg.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 37.König K, Scheipers U, Pesavento A, et al. Initial experiences with real-time elastography guided biopsies of the prostate. J Urol. 2005;174:115–7. doi: 10.1097/01.ju.0000162043.72294.4a. [DOI] [PubMed] [Google Scholar]
- 38.Garra BS, Cespedes EI, Ophir J, et al. Elastography of breast lesions: Initial clinical results. Radiology. 1997;202:79–86. doi: 10.1148/radiology.202.1.8988195. [DOI] [PubMed] [Google Scholar]
- 39.Hocke M, Ignee A, Dietrich CF. Advanced endosonographic diagnostic tools for discrimination of focal chronic pancreatitis and pancreatic carcinoma – Elastography, contrast enhanced high mechanical index (CEHMI) and low mechanical index (CELMI) endosonography in direct comparison. Z Gastroenterol. 2012;50:199–203. doi: 10.1055/s-0031-1281824. [DOI] [PubMed] [Google Scholar]
- 40.Sãftoiu A, Vilmann P, Gorunescu F, et al. Accuracy of endoscopic ultrasound elastography used for differential diagnosis of focal pancreatic masses: A multicenter study. Endoscopy. 2011;43:596–603. doi: 10.1055/s-0030-1256314. [DOI] [PubMed] [Google Scholar]
- 41.Itokawa F, Itoi T, Sofuni A, et al. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843–53. doi: 10.1007/s00535-011-0399-5. [DOI] [PubMed] [Google Scholar]
- 42.Săftoiu A, Iordache SA, Gheonea DI, et al. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2010;72:739–47. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 43.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. Quantitative endoscopic ultrasound elastography: An accurate method for the differentiation of solid pancreatic masses. Gastroenterology. 2010;139:1172–80. doi: 10.1053/j.gastro.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 44.Giovannini M, Thomas B, Erwan B, et al. Endoscopic ultrasound elastography for evaluation of lymph nodes and pancreatic masses: A multicenter study. World J Gastroenterol. 2009;15:1587–93. doi: 10.3748/wjg.15.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iglesias-Garcia J, Larino-Noia J, Abdulkader I, et al. EUS elastography for the characterization of solid pancreatic masses. Gastrointest Endosc. 2009;70:1101–8. doi: 10.1016/j.gie.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 46.Săftoiu A, Vilmann P, Gorunescu F, et al. Neural network analysis of dynamic sequences of EUS elastography used for the differential diagnosis of chronic pancreatitis and pancreatic cancer. Gastrointest Endosc. 2008;68:1086–94. doi: 10.1016/j.gie.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 47.Deprez PH, Yeung CR, Weynand B. Contrast EUS versus EUS sonoelastography in the differentiation of atypical pancreatic masses. Gastrointest Endosc. 2007;65:AB103. [Google Scholar]
- 48.Giovannini M, Hookey LC, Bories E, et al. Endoscopic ultrasound elastography: The first step towards virtual biopsy? Preliminary results in 49 patients. Endoscopy. 2006;38:344–8. doi: 10.1055/s-2006-925158. [DOI] [PubMed] [Google Scholar]
- 49.Dawwas MF, Taha H, Leeds J, et al. The utility of quantitative endoscopic ultrasound elastography for the diagnosis of solid pancreatic masses. Gut. 2011;60:A78. [Google Scholar]
- 50.Badaoui A, Borbath I, Aouattah T, et al. Evaluation of pancreatic tumors with contrast enhanced-endoscopic ultrasonography and EUS-strain ratio elastography. Gastrointest Endosc. 2010;71:AB281. [Google Scholar]
- 51.Mayerle J, Simon P, Dickson EJ, et al. The role of EUS guided elastography to diagnose solid pancreatic mass lesions. Pancreas. 2010;39:1334. [Google Scholar]
- 52.Xu W, Shi J, Zeng X, et al. EUS elastography for the differentiation of benign and malignant lymph nodes;A meta-analysis. Gastrointest Endosc. 2011;74:1001–9. doi: 10.1016/j.gie.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 53.Janssen J, Dietrich CF, Will U, et al. Endosonographic elastography in the diagnosis of mediastinal lymph nodes. Endoscopy. 2007;39:952–7. doi: 10.1055/s-2007-966946. [DOI] [PubMed] [Google Scholar]
- 54.Sãftoiu A, Vilmann P, Ciurea T, et al. Dynamic analysis of EUS used for the differentiation of benign and malignant lymph nodes. Gastrointest Endosc. 2007;66:291–300. doi: 10.1016/j.gie.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 55.Havre RF, Waage JE, Gilja OH, et al. Euroson. Copenhagen: 2010. Aug 22-25th, Semi-quantification of elastic contrasts by strain ratio in elastography-possibilities and pitfalls. [Google Scholar]
- 56.Itoh A, Ueno E, Tohno E, et al. Breast disease: Clinical application of US elastography for diagnosis. Radiology. 2006;239:341–50. doi: 10.1148/radiol.2391041676. [DOI] [PubMed] [Google Scholar]
- 57.Larino-Noia J, Iglesias-Garcia J, Alvarez-Castro A, et al. Usefulness of endoscopic ultrasound (EUS) elastography for the detection of malignant infiltration of mediastinal and abdominal lymph nodes. Gastroenterology. 2009;136:A44. [Google Scholar]
- 58.Giovannini M. Endoscopic ultrasound elastography. Pancreatology. 2011;11:34–9. doi: 10.1159/000323496. [DOI] [PubMed] [Google Scholar]
- 59.Ferreira T, Rasband W. The Image J user guide – Version 1.44. [Last accessed on 2011 April 9]. Available from: http://www.imagej.nih.gov/ij/docs/user-guide.pdf .
- 60.Bishop C. New York: Oxford University Press; 1995. Neural Networks for Pattern Recognition. [Google Scholar]
- 61.Haykin S. 2nd ed. New Jersey: Prentice Hall; 1999. Neural Networks: A Comprehensive Foundation. [Google Scholar]
- 62.Hawes RH. Indications for EUS-directed FNA. Endoscopy. 1998;30(Suppl 1):A155–7. doi: 10.1055/s-2007-1001503. [DOI] [PubMed] [Google Scholar]
- 63.Yamao K, Ozawa S, Kida M. JSGE . Guidelines for Gastrointestinal Endoscopy. 2nd ed. Japan: Igaku-Shoin; 2002. Guideline for endoscopic ultrasound guided fine needle aspiration biopsy; pp. 327–36. [Google Scholar]
- 64.Kouzu T, Yamao K, Irisawa A. Guideline for endoscopic ultrasound guided fine needle aspiration biopsy. In: JSGE, editor. Guidelines for Gastrointestinal Endoscopy. 3rd ed. Japan: Igaku-Shoin; 2006. pp. 170–87. [Google Scholar]
- 65.Yamao K, Sawaki A, Mizuno N, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB): Past, present, and future. J Gastroenterol. 2005;40:1013–23. doi: 10.1007/s00535-005-1717-6. [DOI] [PubMed] [Google Scholar]
- 66.Yasuda K. Imaging alone is sufficient in most circumstances - Making the case for limited need for FNA. Gastrointest Endosc. 2009;69:S155–6. doi: 10.1016/j.gie.2008.12.039. [DOI] [PubMed] [Google Scholar]
- 67.Topazian M. Endoscopic ultrasonography in the evaluation of indeterminate biliary strictures. Clin Endosc. 2012;45:328–30. doi: 10.5946/ce.2012.45.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chong A, Venugopal K, Segarajasingam D, et al. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933–5. doi: 10.1016/j.gie.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Brugge WR. Evaluation of pancreatic cystic lesions with EUS. Gastrointest Endosc. 2004;59:698–707. doi: 10.1016/s0016-5107(04)00175-0. [DOI] [PubMed] [Google Scholar]
- 70.Galasso D, Carnuccio A, Larghi A. Pancreatic cancer: Diagnosis and endoscopic staging. Eur Rev Med Pharmacol Sci. 2010;14:375–85. [PubMed] [Google Scholar]
- 71.Janssen J, Schlörer E, Greiner L. EUS elastography of the pancreas: Feasibility and pattern description of the normal pancreas, chronic pancreatitis, and focal pancreatic lesions. Gastrointest Endosc. 2007;65:971–8. doi: 10.1016/j.gie.2006.12.057. [DOI] [PubMed] [Google Scholar]
- 72.Hirche TO, Ignee A, Barreiros AP, et al. Indications and limitations of endoscopic ultrasound elastography for evaluation of focal pancreatic lesions. Endoscopy. 2008;40:910–7. doi: 10.1055/s-2008-1077726. [DOI] [PubMed] [Google Scholar]
- 73.Dawwas MF, Taha H, Leeds JS, et al. Diagnostic accuracy of quantitative EUS elastography for discriminating malignant from benign solid pancreatic masses: A prospective, single-center study. Gastrointest Endosc. 2012;76:953–61. doi: 10.1016/j.gie.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 74.Pei Q, Zou X, Zhang X, et al. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: A meta-analysis. Pancreatology. 2012;12:402–8. doi: 10.1016/j.pan.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 75.Xu W, Shi J, Li X, et al. Endoscopic ultrasound elastography for differentiation of benign and malignant pancreatic masses: A systemic review and meta-analysis. Eur J Gastroenterol Hepatol. 2013;25:218–24. doi: 10.1097/MEG.0b013e32835a7f7c. [DOI] [PubMed] [Google Scholar]
- 76.Hu DM, Gong TT, Zhu Q. Endoscopic ultrasound elastography for differential diagnosis of pancreatic masses: A meta-analysis. Dig Dis Sci. 2013;58:1125–31. doi: 10.1007/s10620-012-2428-5. [DOI] [PubMed] [Google Scholar]
- 77.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: A meta-analysis. Gastrointest Endosc. 2013;77:578–89. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 78.Săftoiu A, Vilmann P, Hassan H, et al. Analysis of endoscopic ultrasound elastography used for characterisation and differentiation of benign and malignant lymph nodes. Ultraschall Med. 2006;27:535–42. doi: 10.1055/s-2006-927117. [DOI] [PubMed] [Google Scholar]
- 79.Larsen MH, Fristrup C, Hansen TP, et al. Endoscopic ultrasound, endoscopic sonoelastography, and strain ratio evaluation of lymph nodes with histology as gold standard. Endoscopy. 2012;44:759–66. doi: 10.1055/s-0032-1309817. [DOI] [PubMed] [Google Scholar]
- 80.Dietrich CF, Săftoiu A, Jenssen C. Real time elastography endoscopic ultrasound (RTE-EUS), a comprehensive review. Eur J Radiol. 2013 Apr 30; doi: 10.1016/j.ejrad.2013.03.023. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 81.Dietrich CF, Jenssen C, Hocke M, et al. Imaging of gastrointestinal stromal tumours with modern ultrasound techniques – A pictorial essay. Z Gastroenterol. 2012;50:457–67. doi: 10.1055/s-0031-1282076. [DOI] [PubMed] [Google Scholar]
- 82.Jenssen C, Dietrich CF. Endoscopic ultrasound of gastrointestinal subepithelial lesions. Ultraschall Med. 2008;29:236–56. doi: 10.1055/s-2008-1027388. [DOI] [PubMed] [Google Scholar]
- 83.Dietrich CF. Real-time tissue elastography. Multiple clinical applications. Multiple clinical solutions. Endoskopie Heute. 2011;24:177–212. [Google Scholar]
- 84.Eloubeidi MA, Beydoun M, Jurdi N, et al. Transduodenal EUS-guided FNA of the right adrenal gland to diagnose lung cancer where percutaneous approach was not possible. J Med Liban. 2011;59:173–5. [PubMed] [Google Scholar]
- 85.Jenssen C, Dietrich CF. Ultrasound and endoscopic ultrasound of the adrenal glands. Ultraschall Med. 2010;31:228–47. doi: 10.1055/s-0029-1245449. [DOI] [PubMed] [Google Scholar]
- 86.Dietrich CF, Wehrmann T, Hoffmann C, et al. Detection of the adrenal glands by endoscopic or transabdominal ultrasound. Endoscopy. 1997;29:859–64. doi: 10.1055/s-2007-1004322. [DOI] [PubMed] [Google Scholar]
- 87.Uemura S, Yasuda I, Kato T, et al. Preoperative routine evaluation of bilateral adrenal glands by endoscopic ultrasound and fine-needle aspiration in patients with potentially resectable lung cancer. Endoscopy. 2013;45:195–201. doi: 10.1055/s-0032-1325988. [DOI] [PubMed] [Google Scholar]
- 88.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–53. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]


