Abstract
Background and Objective:
Endoscopic transmural drainage is an effective, but technically demanding treatment modality for walled off pancreatic necrosis (WOPN). The factors that determine the outcome of endoscopic treatment for WOPN have been infrequently studied. We aim to retrospectively correlate the morphological features of WOPN on endoscopic ultrasound (EUS) with the outcome of endoscopic transmural drainage.
Patients and Methods:
Over the last 3 years, 43 patients (36 males; mean age 36.04 ± 10.06 years) with symptomatic WOPN were treated by an attempted endoscopic drainage. The correlation between the morphological features of WOPN and the type of treatment offered as well as the number of endoscopic procedures undergone by the patient was assessed.
Results:
The mean size of WOPN was 9.95 ± 2.75 cm with <10%, 10-40% and >40% solid debris being present in 6, 33, and 4 patients, respectively. Patients with <10% necrotic debris needed only single session of endoscopic drainage, whereas patients with 10-40% solid debris needed two or more sessions. Patients with >40% solid debris either needed direct endoscopic debridement or surgical necrosectomy. The extent of necrosis correlated significantly (r = 0.703, P < 0.001) with the type of treatment received by the patient. With increasing size of the collection (r = 0.320, P = 0.047) and the amount of the solid debris (r = 0.800, P < 0.001), there was a significant increase in the number of endoscopic procedures required for successful outcome by the patient.
Conclusions:
The morphological features of WOPN on EUS have important therapeutic implications with collections having large size and more solid debris needing more aggressive therapeutic method for the successful outcome.
Keywords: Acute pancreatitis, computed tomography, endoscopic ultrasound, walled off pancreatic necrosis
INTRODUCTION
The results of endoscopic therapy for walled off pancreatic necrosis (WOPN) have considerably improved after adoption of more aggressive endoscopic techniques such as larger tract dilation, placement of multiple stents, aggressive irrigation, and direct debridement of necrotic tissue by endoscopic necrosectomy.[1,2,3,4,5] A recent systematic review on the results of endoscopic necrosectomy reported 76% success rates with a mortality of 5% and morbidity of 27%.[6]
Although many patients with WOPN can be successfully treated with aggressive endoscopic transmural drainage involving active irrigation and placement of multiple transmural stents, still a subset of patients do not improve with these techniques and these patients either require direct endoscopic necrosectomy (DEN) or surgical necrosectomy. Furthermore, studies have shown the DEN has better resolution rates when compared to insertion of multiple stents and one comparative study comparing DEN with transmural endoscopic drainage for the treatment of WOPN has demonstrated that successful resolution in 88% of patients who underwent DEN versus only 45% in patients who received standard drainage.[5]
The factors that determine the outcome of standard endoscopic drainage in patients with WOPN have been infrequently studied. One study by Bang et al. have reported that using multiple transluminal gateway technique (MTGT), whereby several openings are created in the stomach or duodenum to facilitate drainage of necrosis improved the treatment outcomes.[7] They also found that on multivariate logistic regression analysis, only MTGT and the need for fewer endoscopic sessions predicted the treatment success. Since the WOPN contains a mixture of both fluid and solid necrotic debris and these are usually best delineated on endoscopic ultrasound (EUS), we hypothesized that the morphological appearances of WOPN on EUS may also determine the outcome of endoscopic drainage of WOPN. In the current study, we retrospectively evaluated the morphological features of WOPN on EUS that could impact the outcome of endoscopic transmural drainage.
PATIENTS AND METHODS
Over last 3 years, 43 patients (36 males; mean age 36.04 ± 10.06 years) with symptomatic WOPN were treated by an attempted endoscopic drainage. All the enrolled patients had been earlier diagnosed with acute necrotizing pancreatitis (ANP) based on the Atlanta classification.[8] An informed consent was obtained from all the patients before the enrollment in the study and the study protocol was approved by the Institute Ethics Committee. Patients with pregnancy, age less than 18 years, presence of congestive cardiac failure, compromised pulmonary status or any contraindication to EUS were excluded. All patients provided procedural informed consent at the time of EUS guided drainage.
Indications for endoscopic drainage were computed tomography and EUS confirmed WOPN located adjacent to the stomach or duodenum, with ongoing infection as evident by abdominal pain and fever despite administration of intravenous antibiotics, or gastric outlet or biliary obstruction by WOPN. Patients with coagulopathy, thrombocytopenia and distance of WOPN being more than 1 cm from the gastrointestinal lumen were excluded.
The EUS examination was done with a linear scanning echoendoscope (EG-3870 UTK Linear Echoendoscope, Pentax Inc., Tokyo, Japan) at 7.5 MHz. On EUS, the size as well as the detailed morphology of the pancreatic fluid collection (PFC) was studied with special emphasis on the presence as well as the amount of the solid necrotic debris. The echogenic material present in the PFC was suggestive of solid debris. An attempt to quantify the amount of solid debris present in the PFC as a percentage of the total size of the collection was done. The quantification of the solid debris was an approximate visual judgment of the endoscopist. Two experienced Endosonologists (SSR, DKB) separately reviewed the EUS images to quantify the solid debris in the PFC and mean of their findings was taken as the final figure of solid debris in each PFC.
We have been performing endoscopic drainage of WOPN under EUS guidance with placement of multiple 7 or 10 Fr stents along with a 7 Fr nasocystic catheter with the details of the procedure described in our earlier published study.[1] The initial puncture was done under EUS guidance only and the subsequent dilatations of the transmural tract were performed under fluoroscopic guidance. Ours is a step up approach where we initially do aggressive transmural drainage with multiple stents and irrigation of the cavity with a nasocystic catheter and in case of nonresponse or partial response the tract is further dilated with placement of additional stents. If even after two sessions of drainage the symptoms persist a decision for additional transmural drainage by stents, DEN or surgery is taken after interdisciplinary consultation with pancreatic surgeons.
The morphological features of WOPN on initial EUS were correlated with the number of endoscopic sessions required for the successful outcome, need for DEN or surgery and the complications of endoscopic procedure.
Statistical analysis
The descriptive analysis was performed and the results were presented as percentages for categorical variables and mean ± standard deviation for continuous variables. The correlation between the morphological features of the PFC and the type of treatment offered to the patient and the number of endoscopic procedures undergone by the patient was assessed by using Pearson correlation coefficient and significance was interpreted at 0.05 level.
RESULTS
Over a period of 3 years, 43 patients (36 males; mean age 36.04 ± 10.06 years) with symptomatic WOPN were treated endoscopically. The etiology of ANP was: Alcohol (24 patients), gall stones (14 patients) and idiopathic (5 patients). The mean size of the WOPN was 9.95 ± 2.75 cm with the extent of solid necrotic debris ranging from 5% to 60% with an average of 28.72%. The proportion of solid necrotic component was <10%, 10-40% and >40% solid debris in 6, 33 and 4 of these patients respectively. In 41 patients, the WOPN was accessed through the stomach, whereas the duodenum was used for entry into the WOPN in two patients. The entry tract was dilated from 8 to 15 mm and multiple 7-10 Fr double pigtail stents (maximum: three 10 Fr stents) were placed.
Of these 43 patients, 39 (91%) patients could be successfully drained with aggressive standard endoscopic transluminal drainage (1-7 endoscopic sessions) and four patients needed either DEN or surgical necrosectomy following failed endoscopic transluminal drainage. No significant complications of the procedure were observed.
Morphological features of walled off pancreatic necrosis and its impact on treatment outcome
The morphological features of WOPN on EUS in patients successfully treated with standard endoscopic transluminal drainage and the patients needing DEN or surgical necrosectomy is given in Table 1. Patients with <10% necrotic debris needed only single session of endoscopic drainage whereas patients with 10-40% solid debris needed two or more sessions. Patients with >40% solid debris either needed direct endoscopic debridement or surgical necrosectomy. The extent of necrosis as evident by echogenic solid debris in the PFC correlated significantly (r = 0.703, P < 0.001) with the type of treatment received by the patient. Furthermore, with increasing size of the collection (r = 0.320, P = 0.047) and the amount of the solid debris (r = 0.800, P < 0.001), there was a significant increase in the number of endoscopic procedures required for the successful outcome by the patient [Table 2 and Figures 1–4].
Table 1.
Morphological characteristics of the WOPN in patients who underwent endoscopic or surgical drainage
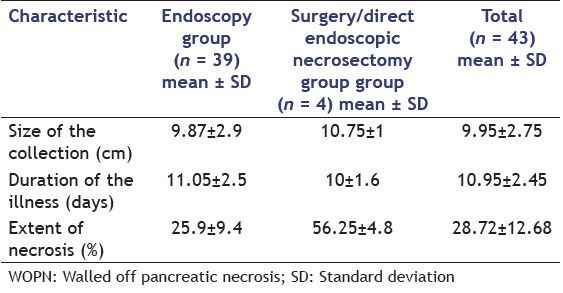
Table 2.
Correlation of disease parameters with the number of endoscopic procedures
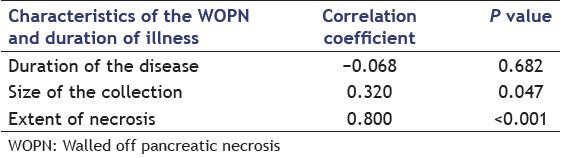
Figure 1.
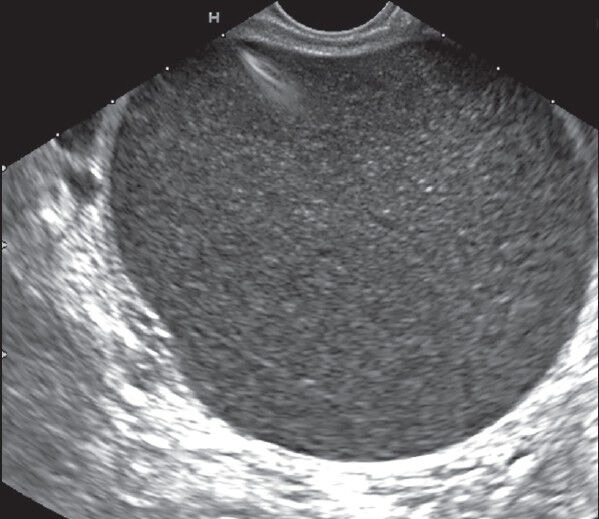
Walled off pancreatic necrosis with predominantly liquid content and was successfully treated with a single session of endoscopic transmural drainage
Figure 4.
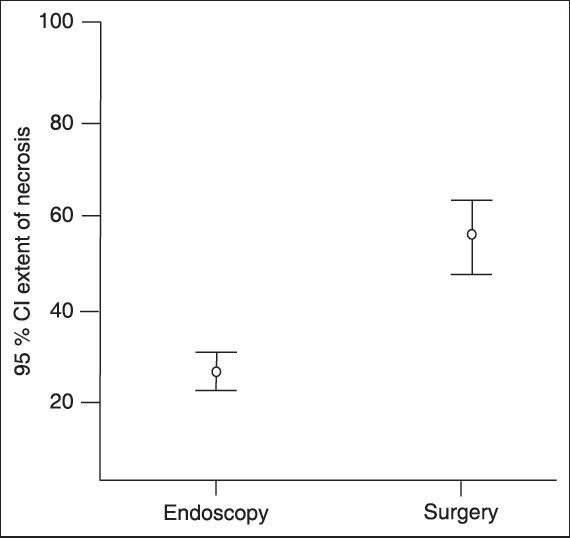
% solid necrotic debris in patients treated by endoscopic transluminal drainage or surgical drainage
Figure 2.
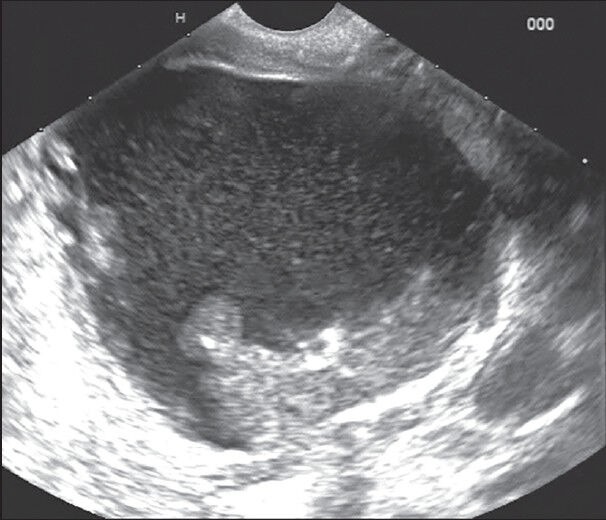
Walled off pancreatic necrosis with <40% solid content and needed three sessions of endoscopic transmural drainage with multiple stents and nasocystic catheter
Figure 3.
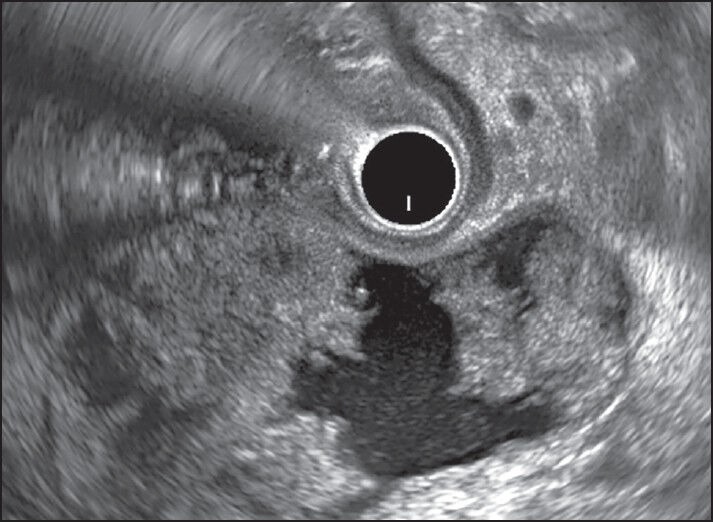
Walled off pancreatic necrosis >40% solid necrotic debris and needed direct endoscopic necrosectomy
DISCUSSION
The factors that determine the outcome of endoscopic treatment of WOPN have been infrequently studied and it has been suggested that aggressive drainage with direct removal of debris from the cavity under endoscopic guidance is the crucial step for improving the results of endoscopic necrosectomy. However, we have earlier reported that the majority of patients with WOPN can be successfully treated with standard endoscopic transmural drainage with only a few patients needing DEN or surgical necrosectomy.[1] Furthermore, similar results have been reported by Bang et al. as well as Varadarajulu et al.[7,9] They reported that using MTGT, irrigation of the cavity can be performed with an 18-gauge nasogastric tube through one channel while the other channel could act as a conduit for rapid drainage of the necrotic debris and thus by facilitating better drainage decreased the need for a rescue endoscopic necrosectomy or surgical necrosectomy.
Our results have shown that those patients with <10% necrotic debris needed only a single session of endoscopic drainage whereas patients with 10-40% solid debris needed multiple sessions of endoscopic drainage. Moreover, patients with >40% solid debris either needed direct endoscopic debridement or surgical necrosectomy, thereby suggesting that increasing solid debris needed more aggressive therapeutic method for the successful outcome. We also found that the size of WOPN also has an impact on the outcome of endoscopic drainage with increasing size leading on to an increased number of endoscopic sessions being required for the successful outcome.
Hence, how do our results compare with the results obtained by Bang et al.? We have shown that increasing solid debris in the WOPN adversely affects the results of standard endoscopic drainage and Bang et al. have shown that by doing aggressive transluminal drainage using MTGT which provides a better drainage route for necrotic debris, DEN or surgical necrosectomy can be avoided. Thus, by carefully delineating the morphology of WOPN by EUS appropriate endoscopic treatment method can be planned and MTGT by providing better egress channel for the necrotic debris can decrease the number of endoscopic sessions as well as obviate the need of DEN or surgery. However, it would be interesting to know how the morphology of WOPN determines the outcome of MTGT.
Our study has several limitations. The small sample size especially the number of patients undergoing DEN or surgical necrosectomy as well as retrospective nature of the study is important limitations. Furthermore, all these procedures were done at a highly specialized tertiary care center with extensive experience in pancreatic endotherapy, which may not be available in smaller institutions.
CONCLUSION
The morphological features of WOPN on EUS have important therapeutic implications with collections having large size and more solid debris needing more aggressive therapeutic method for the successful outcome.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Rana SS, Bhasin DK, Rao C, et al. Non-fluoroscopic endoscopic ultrasound-guided transmural drainage of symptomatic non-bulging walled-off pancreatic necrosis. Dig Endosc. 2013;25:47–52. doi: 10.1111/j.1443-1661.2012.01318.x. [DOI] [PubMed] [Google Scholar]
- 2.Seewald S, Groth S, Omar S, et al. Aggressive endoscopic therapy for pancreatic necrosis and pancreatic abscess: A new safe and effective treatment algorithm (videos) Gastrointest Endosc. 2005;62:92–100. doi: 10.1016/s0016-5107(05)00541-9. [DOI] [PubMed] [Google Scholar]
- 3.Charnley RM, Lochan R, Gray H, et al. Endoscopic necrosectomy as primary therapy in the management of infected pancreatic necrosis. Endoscopy. 2006;38:925–8. doi: 10.1055/s-2006-944731. [DOI] [PubMed] [Google Scholar]
- 4.Seifert H, Biermer M, Schmitt W, et al. Transluminal endoscopic necrosectomy after acute pancreatitis: A multicentre study with long-term follow-up (the GEPARD Study) Gut. 2009;58:1260–6. doi: 10.1136/gut.2008.163733. [DOI] [PubMed] [Google Scholar]
- 5.Gardner TB, Chahal P, Papachristou GI, et al. A comparison of direct endoscopic necrosectomy with transmural endoscopic drainage for the treatment of walled-off pancreatic necrosis. Gastrointest Endosc. 2009;69:1085–94. doi: 10.1016/j.gie.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Haghshenasskashani A, Laurence JM, Kwan V, et al. Endoscopic necrosectomy of pancreatic necrosis: A systematic review. Surg Endosc. 2011;25:3724–30. doi: 10.1007/s00464-011-1795-x. [DOI] [PubMed] [Google Scholar]
- 7.Bang JY, Wilcox CM, Trevino J, et al. Factors impacting treatment outcomes in the endoscopic management of walled-off pancreatic necrosis. J Gastroenterol Hepatol. 2013;28:1725–32. doi: 10.1111/jgh.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–90. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 9.Varadarajulu S, Phadnis MA, Christein JD, et al. Multiple transluminal gateway technique for EUS-guided drainage of symptomatic walled-off pancreatic necrosis. Gastrointest Endosc. 2011;74:74–80. doi: 10.1016/j.gie.2011.03.1122. [DOI] [PubMed] [Google Scholar]


