Abstract
Endoscopic ultrasound (EUS) has evolved to become a crucial tool for the evaluation of pancreatic diseases, among them solid pancreatic lesions. However, its ability to determine whether a lesion is malignant or not is difficult to establish based only in the endosonographic image. EUS-guided fine needle aspiration (EUS-FNA) allows obtaining a cytological and/or histological sample from pancreatic lesions, with a high overall accuracy and low complication rates. Although the clinical usefulness of EUS-FNA for pancreatic diseases is widely accepted, the indications for tissue diagnosis of pancreatic lesions suspected to be malignant is still controversial. This review highlights the diagnostic accuracy and complications of EUS-FNA, focusing on its current indications.
Keywords: Diagnostic accuracy, endoscopic ultrasound, fine needle aspiration, pancreatic mass
INTRODUCTION
Endoscopic ultrasound (EUS) has evolved to become a crucial tool for the evaluation of pancreatic diseases. Specifically, EUS plays a critical role in the evaluation of patients with a known or suspected pancreatic mass.[1,2] Published literature supports the superiority of EUS compared with cross-sectional imaging for tumor detection, mainly for the detection of tumors smaller than 2-3 cm.[2,3,4,5] Although the sensitivity for tumor detection is high it is also important to note that it has a very high negative predictive value (NPV).[6,7] This has important implications because it means that EUS can reliably exclude pancreatic cancer, especially in the setting of a low or indeterminate pretest probability. In fact, EUS is considered as the single best choice to detect a pancreatic neoplasm. However, whether a lesion is malignant or benign is difficult to assess only based on EUS imaging. In this setting, one of the big advantages of EUS is that allows performing a guided biopsy of several lesions, including pancreatic masses. In fact EUS-guided fine needle aspiration (EUS-FNA), by obtaining cytological and/or histological samples, is able to diagnose pancreatic lesions with high sensitivity and specificity.[8] This technique is becoming indispensible in the evaluation of patients with solid pancreatic tumors. EUS-FNA is considered as an accurate and safe technique to determine the specific diagnosis of solid pancreatic masses, being considered as the principal technique to establish the diagnosis of malignancy.[9,10]
This review attempts to highlights the diagnostic potential of EUS-FNA, its complications, for finally focusing on its current indications.
USEFULNESS OF EUS-FNA IN THE DIFFERENTIAL DIAGNOSIS OF SOLID PANCREATIC TUMORS
The role of EUS-FNA in the diagnosis of solid pancreatic tumors has been evaluated in several, well-designed studies. Reported sensitivity and accuracy for malignancy ranges from 75% to 92% and from 79% to 92%, respectively.[11,12,13,14,15,16,17,18,19,20,21] Two large reviews have been published evaluating the accuracy of EUS-FNA in solid pancreatic masses. One of them included 28 studies involving 4225 patients, evaluated the performance of EUS-FNA in differentiating between benign and malignant pancreatic masses. Sensitivity, specificity, NPV and diagnostic accuracy were 83% (range: 54-95%), 100% (range: 71-100%), 72% (range: 16-92%) and 88% (range: 65-96%), respectively.[22] The wide ranges reported may be related to the use of variable definitions to classify cytopathological results as benign or malignant and also to the exclusion of non-diagnostic specimens in some studies. The second one, a very recent meta-analysis, published by Hewitt et al.,[23] included 33 studies published between 1997 and 2009, with a total number of 4984 patients. Authors showed that the pooled sensitivity for malignant cytology was 85% (95% confidence interval [CI]: 84-86) and pooled specificity was 98% (95% CI: 0.97-0.99). If atypical and suspicious cytology results were included to determine true neoplasms, the sensitivity increased to 91% (95% CI: 90-92); however, the specificity was reduced to 94% (95% CI: 93-96). EUS-FNA also has a high positive predictive value (99%) and a reasonable NPV (64%). However, it is important to point out that the sensitivity of EUS-FNA for malignancy in parenchymal masses with features of chronic pancreatitis is inferior compared with when the surrounding parenchyma is normal.[24,25]
When trying to compare EUS-FNA with the classical percutaneous route for pancreatic masses, we found scarce data available.[26,27,28,29] In a single randomized control trial including 84 patients, 43 underwent computed tomography (CT) scan or trans-abdominal ultrasound-guided and 41 an EUS-FNA of a solid pancreatic mass.[27] EUS-FNA had higher sensitivity and diagnostic accuracy (84% vs. 62% and 89% vs. 72%, respectively; ns). Three other series retrospectively evaluated different FNA procedures.[26,28,29] The largest study showed a significant difference, with a higher accuracy for EUS-FNA when compared to CT scan or trans-abdominal ultrasound-guided FNA for masses <3 cm.[28] Importantly, a cost-minimization study demonstrated that EUS-FNA is the best initial test and the preferred secondary method after a failed alternative sampling procedure for the diagnosis of suspected pancreatic cancer.[30]
However EUS-FNA also present another important advantage, the ability to provide supplemental staging information by sampling lymph nodes, small liver lesions, both missed at other imaging techniques[31] and also small amounts of previously undetected ascites.[32] All these sites when positive for malignancy indicate a poor prognosis, with an impact on patient management.[33] In a prospective study, 12% of 99 operable patients were found by EUS-fine needle biopsy (EUS-FNB) to have metastasis in lymph nodes, liver, ascites and retroperitoneum; not detected nor suspected at trans-abdominal ultrasound not CT scan.[34]
In order to optimize tissue retrieval of EUS-FNA and thus, to improve its accuracy, various EUS-guided techniques have been explored, for instance by the use of Tru-Cut needles, with variable success and complication rates.[35,36,37] EUS-guided use of Quick-Core® needle has demonstrated that histological samples representative of the target organs can be obtained safely.[37,38] However, there are certain drawbacks with the Quick-Core® needle that restrict its use in clinical practice. Most importantly, its diagnostic yield is strongly limited for lesions located in the pancreatic head due to mechanical friction of the needle firing mechanism ensuing from the bended scope position.[39,40,41] In this setting, a novel needle have been designed (Procore™ histology needle) to overcome Tru-cut needle limitations (mainly in the second portion of the duodenum). A first study published with the 19-gauge caliber needle allowed a histological evaluation with overall accuracy of 85.9% (89.4% in pancreatic solid lesions),[42] with a very high interobserver agreement between Pathologist when evaluating the quality of the samples obtained.[43] A new study has been recently published using the 22-gauge Procore™ needle in pancreatic masses, being able to obtain a sample suitable for histological evaluation in 88.5% of the cases.[44]
EUS-FNA is also considered very useful for the diagnosis of other type of pancreatic tumors. For instances, it has a high sensitivity and diagnostic accuracy for the evaluation of neuroendocrine tumors.[45,46] Even more, EUS-FNA is very helpful in assessing the malignant behavior of this type of pancreatic tumors, being able also to predict 5-year survival.[47,48] Determination of Ki-67 expression, key in the evaluation of neuroendocrine tumors, can also be evaluated in EUS-FNA samples.[49,50] Metastatic lesions can also be demonstrated by EUS-FNA; in a series of 114 consecutive patients with focal pancreatic lesions identified on CT, EUS-FNA allowed demonstration of metastases of an extrapancreatic cancer in 11% of cases.[51] Finally, in cases suspicious for autoimmune pancreatitis or pancreatic lymphoma, where pancreas sampling is indicated, EUS-FNA has also shown to have a very important role.[52] EUS-FNB in these cases is essential, since surgical treatment is not indicated in these lesions.
However, certain drawbacks of EUS-FNA need to be emphasized. The procedure is difficult to perform in certain cases, owing to vessel interposition, duodenal stenosis and tumor hardness, particularly in chronic pancreatitis, which hampers the overall accuracy of the procedure. In some occasions, EUS-FNA samples cannot be interpreted due to bleeding or non-cellular samples. A systematic review of 53 studies estimated a NPV of EUS-FNA in the diagnosis of pancreatic adenocarcinoma of 60-70%.[22] In patients with indeterminate or negative findings at initial EUS-FNA and a high clinical suspicion for pancreatic cancer, repetition of EUS-FNA is strongly advised. Several studies have demonstrated that repeating EUS-FNA facilitated determination of the true status of disease in a high percentage of cases with inconclusive findings at initial EUS-FNA; in fact by repeating EUS-FNA up to 3 times sensitivity can increase up to 90%.[53,54,55] Hence, a new puncture seems mandatory in order to exclude malignancy in cases where the first EUS-FNA has been negative for malignancy. Given the high accuracy in the evaluation of pancreatic tumors, Eloubeidi and Tamhane recommend EUS-FNA for the differential diagnosis of solid pancreatic masses.[9]
COMPLICATIONS OF EUS-FNA
It has been recently published by the European Society of Gastrointestinal Endoscopy (ESGE) guidelines about EUS-FNA[56] referred to the technique and its complications. Regarding to pancreatic lesions they focus in the incidence of acute pancreatitis after EUS-FNA that ranged from 0.26% to 2% respectively. Certain factors have been associated to a predisposition to post-EUS-FNA pancreatitis as a recent history of acute pancreatitis and the puncture of a benign pancreatic lesion; however, a significant relationship was not demonstrated. This guidelines state that EUS-FNA is a safe procedure with a general complication rate of approximately 1% (for all kind of lesions), emphasizing that they are more frequent for EUS-FNA of cystic compared with solid lesions. One important point to notice is that the rate of complications does not seem to be superior depending on the needle size used. It also seems that the use of Tru-cut needles of new-histology needles (Procore™) in experienced hands do not increase the rate of complications.
The rate of severe complications, such as bleeding, perforation, or death, is extremely rare.[57,58] Malignant seeding attributed to EUS-FNA of a pancreatic adenocarcinoma has only been reported in isolated cases, one of them, for instances, in a FNA of tumor locates at the tail of the pancreas.[59] If the FNA involves a tumor in the head of the pancreas where the needle only traverses the duodenal wall, then any chance of malignant seeding would be localized to the duodenal wall, which would subsequently be resected along with the tumor. Overall, an important advantage of EUS-FNA over the percutaneous route is the presumed lower risk of peritoneal seeding.[60,61]
A recent study showed that pre-operative EUS-FNA is not associated with adverse perioperative or long-term outcomes in patients undergoing distal pancreatectomy for solid neoplasms of the pancreas. The potentially detrimental long-term impact of pre-operative EUS-FNA in patients with resectable pancreatic adenocarcinoma was not observed.[61]
INDICATIONS OF EUS-FNA IN SOLID PANCREATIC TUMORS, WHEN TO PUNCTURE
A fundamental principle in establishing indications for EUS-FNA is a determination as to whether or not the information obtained has the potential to change patient management. In addition, of course EUS-FNA must be technically feasible without intervening vascular structures and informed patient consent must be obtained before the procedure. If the information will not affect patient treatment, maybe the procedure should not be performed. It has been recently published the ESGE guidelines about EUS-guided sampling[62] including the accepted and suggested indications of this technique, trying to establish some recommendations.
As previously stated EUS-FNA presents a high diagnostic accuracy with a relatively low NPV for the diagnosis of pancreatic cancer and is also related to a low complication rate. Due to the universal drawback considered for all sampling techniques available for the pancreas, pre-operative sampling has been not generally advised (i.e., for potentially resectable pancreatic tumors in operable patients). However, differential diagnosis of solid pancreatic masses includes many different types of lesions. Although the predominant tissue type is adenocarcinoma, the differential diagnosis of a solid pancreatic tumor includes squamous-cell carcinoma, acinar-cell carcinoma, lymphoma, neuroendocrine tumor, solid pseudopapillary tumor, autoimmune pancreatitis and focal pancreatitis. In addition, other malignancies can metastasize to the pancreas and present as pancreatic tumors: Renal-cell carcinoma, melanoma, GI stromal tumors, as well as primary cancers of the breast, ovary, thyroid, lung, prostate and colon. Figure 1 shows some EUS imaging from solid pancreatic lesions, not corresponding to the classical pancreatic adenocarcinoma. Furthermore, tumors with a cystic component may represent other pathology, such as serous cystadenoma, mucinous cystadenoma, intrapapillary mucinous neoplasm, neuroendocrine tumor, simple cyst and pseudocyst. It has been estimated that approximately 6% of patients undergoing pancreaticoduodenoectomy have a benign process.[63] Another 6% of patient may have an unusual histology, including tumors that have metastasized to the pancreas.[57] Therefore, to minimize the number of patients undergoing non-therapeutic surgeries, a pre-treatment tissue diagnosis is strongly recommended in most cases.
Figure 1.
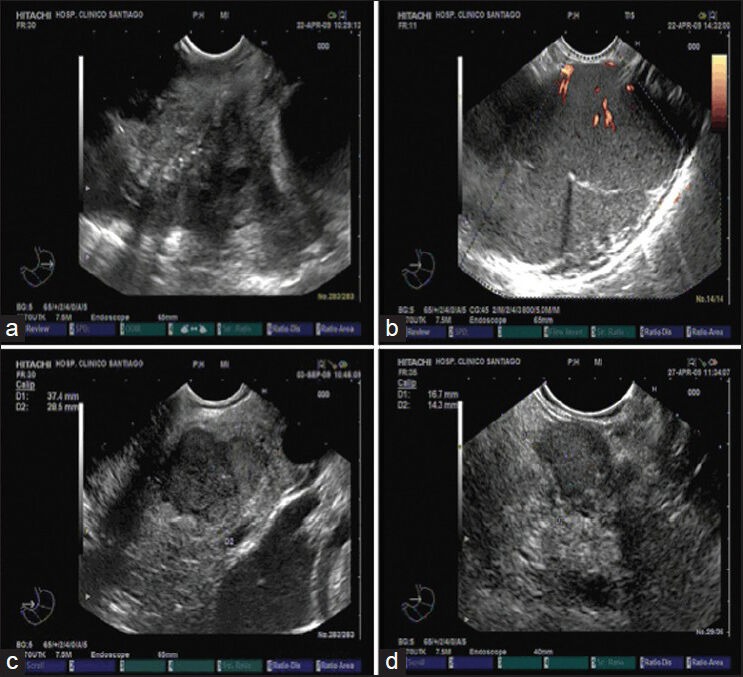
Endoscopic ultrasound image of different solid pancreatic lesion. (a) Solid lesion located at the pancreatic head, corresponding to a metastasis from an oat-cell lung cancer; (b) Pancreatic lymphoma located at pancreatic tail; (c) Endocrine pancreatic carcinoma located at pancreatic isthmus; (d) Insulinoma located in pancreatic body
When focusing in pancreatic solid masses suspicious for cancer, those may be classified into masses that will not be resected because they are locally advanced, associated with metastases, or they present in patients with a poor physical condition; and potentially resectable solid pancreatic tumors.
Unresectable pancreatic tumor
Sampling in order to obtain a definitive diagnosis is usually desirable to assist with counseling and planning palliation.[64] In fact, pathological confirmation is considered absolutely necessary in a patient with unresectable pancreatic cancer before chemotherapy and/or radiation therapy. Although some authors believe that histological evidence need not be obtained when imaging findings are typical of pancreatic cancer; however, non-ductal pancreatic cancer may have imaging features identical to the common ductal-adenocarcinoma. These patients may be refractory to chemotherapy. Even more, taking into account the latest advances in the field of oncology and in the knowledge about the biology of pancreatic cancer[65] we have now-a-days the possibility to perform different analysis from FNA and/or FNB samples (mainly biomarkers[66] which may provide with crucial information to guide the oncological treatment of pancreatic cancer patients.[67,68,69,70] This mean that we can direct the oncological treatment based on the evaluation of the sample obtained from the tumor. In this context, some patients with locally advanced pancreatic cancer have survived more than 2 years after chemotherapy/radiation therapy.
Resectable pancreatic tumor
However, in the second group, it is generally accepted that the procedure is not needed, since the results of any non-surgical sampling technique are unlikely to affect further management due to the relatively low NPV of sampling techniques for cancer diagnosis.[22] However, there are increasing arguments for performing biopsies in potentially resectable pancreatic tumors. An established protocol of pre-operative neoadjuvant therapy, a patient demand for a conclusive diagnosis of cancer before surgery and lastly, exclusion of unusual tumors (e.g., lymphoma, some pancreatic metastases, autoimmune pancreatitis) that would not benefit from surgery.[71] Although neoadjuvant therapy in resectable pancreatic cancer have been not generally advise, there are upcoming studies showing that the administration of oncological treatment in these patients may led to lower rates of local recurrence and increase rates of survival. Importantly, the effects of neoadjuvant chemoradiation clearly identified patients who were unlikely to benefit from surgery (patients with disease progression under treatment).[72,73] In this setting, for this strategy, a pre-treatment diagnosis is mandatory. Something similar occurs with those patients considered as borderline resectable pancreatic cancer.[74] There is an increasing consensus that these patients should be treated with induction chemotherapy, followed by chemoradiation and restaging, prior to surgery.[75,76] Again, obtaining a histological confirmation becomes essential. The differentiation of cancer from inflammation can be quite difficult but is mandatory when contemplating chemotherapy. In this case, positive cytologic confirmation is often needed and is a good indication for EUS-FNA. Another important point is that establishment of a histological diagnosis may influence the treatment and the operative procedure even when surgery is planned. Some patients, especially those at high risk for surgery, as well as many surgeons would like to know the histological diagnosis before a major surgery. There is also another important issue, regarding the pathological characteristics of the pancreatic tumor. A recent study has shown a different prognosis of resectable tumors depending on the degree of differentiation of pancreatic adenocarcinoma. Authors even preclude surgery in some cases with poorly differentiated carcinomas.[77] Moreover now-a-days, with latest advances is possible to obtain real cores from pancreatic tumors and establish the degree of differentiation of pancreatic tumors.[42,44]
Autoimmune pancreatitis
Autoimmune pancreatitis is a benign inflammatory disease of the pancreas that mimics pancreatic carcinoma both clinically and radiologically. It seems to be comparatively easy to differentiate between an inflammatory mass and pancreatic cancer by using EUS-FNA, but it is difficult to make a definite diagnosis by using FNA alone. Recently, EUS-FNA and EUS-guided Tru-cut biopsy in combination with immunohistochemical staining were reported to be useful for making a specific diagnosis of this disease.[1,78]
Neuroendocrine pancreatic solid tumors and other pancreatic tumors
EUS-FNA is very helpful in neuroendocrine pancreatic tumors.[79] We have already shown its ability to determine their malignant behavior and to predict 5-year survival. Acinar cell carcinomas, solid-pseudopapillary tumors and pancreatic metastasis can also be diagnosed by EUS-FNA and whenever each of these diagnoses is suspected, the procedure should be attempted.[80,81,82]
CONCLUSIONS
In summary, when a patient presents with a solid pancreatic lesion, a tissue diagnosis is strongly recommended in those cases in which oncological therapy is recommended (metastatic, locally advanced and unresectable pancreatic cancer). If pancreatic lesion is resectable or borderline resectable, a tissue diagnosis is, now-a-days, also recommended, as long as tissue acquisition has a high yield, is safe and does not delay management and may be necessary for neoadjuvant therapy. Certainly, if the patient is a good surgical candidate and the clinical presentation and imaging is typical for resectable adenocarcinoma, then one may proceed without a tissue diagnosis. Another circumstances mandatory for tissue diagnosis are patient demands for a conclusive diagnosis of cancer before surgery and exclusion of unusual tumors (e.g., lymphoma, some pancreatic metastases, autoimmune pancreatitis) if they are suspected, that would not benefit from surgery. As we have previously stated, EUS-FNA, due to its safety and accuracy, is the best modality for tumor detection and for obtaining a tissue diagnosis even if the tumor is poorly visualized by other imaging modalities.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Iglesias-García J, Lindkvist B, Lariño-Noia J, et al. The role of EUS in relation to other imaging modalities in the differential diagnosis between mass forming chronic pancreatitis, autoimmune pancreatitis and ductal pancreatic adenocarcinoma. Rev Esp Enferm Dig. 2012;104:315–21. doi: 10.4321/s1130-01082012000600006. [DOI] [PubMed] [Google Scholar]
- 2.Iglesias García J, Lariño Noia J, Domínguez Muñoz JE. Endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. Rev Esp Enferm Dig. 2009;101:631–8. doi: 10.4321/s1130-01082009000900006. [DOI] [PubMed] [Google Scholar]
- 3.Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: A systematic review. Clin Gastroenterol Hepatol. 2006;4:717–25. doi: 10.1016/j.cgh.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Sãftoiu A, Vilmann P. Role of endoscopic ultrasound in the diagnosis and staging of pancreatic cancer. J Clin Ultrasound. 2009;37:1–17. doi: 10.1002/jcu.20534. [DOI] [PubMed] [Google Scholar]
- 5.Varadarajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Gastrointest Endosc Clin N Am. 2005;15:497–511. doi: 10.1016/j.giec.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Klapman JB, Chang KJ, Lee JG, et al. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658–61. doi: 10.1111/j.1572-0241.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 7.Catanzaro A, Richardson S, Veloso H, et al. Long-term follow-up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointest Endosc. 2003;58:836–40. doi: 10.1016/s0016-5107(03)02301-0. [DOI] [PubMed] [Google Scholar]
- 8.Iglesias García J, Domínguez-Muñoz JE. Endoscopic ultrasound-guided biopsy for the evaluation of pancreatic tumors. Gastroenterol Hepatol. 2007;30:597–601. doi: 10.1157/13112588. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Tamhane A. Prospective assessment of diagnostic utility and complications of endoscopic ultrasound-guided fine needle aspiration. Results from a newly developed academic endoscopic ultrasound program. Dig Dis. 2008;26:356–63. doi: 10.1159/000177022. [DOI] [PubMed] [Google Scholar]
- 10.Puli SR, Bechtold ML, Buxbaum JL, et al. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20–6. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 11.Giovannini M, Seitz JF, Monges G, et al. Fine-needle aspiration cytology guided by endoscopic ultrasonography: Results in 141 patients. Endoscopy. 1995;27:171–7. doi: 10.1055/s-2007-1005657. [DOI] [PubMed] [Google Scholar]
- 12.Bhutani MS, Hawes RH, Baron PL, et al. Endoscopic ultrasound guided fine needle aspiration of malignant pancreatic lesions. Endoscopy. 1997;29:854–8. doi: 10.1055/s-2007-1004321. [DOI] [PubMed] [Google Scholar]
- 13.Gress FG, Hawes RH, Savides TJ, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy using linear array and radial scanning endosonography. Gastrointest Endosc. 1997;45:243–50. doi: 10.1016/s0016-5107(97)70266-9. [DOI] [PubMed] [Google Scholar]
- 14.Chang KJ, Nguyen P, Erickson RA, et al. The clinical utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of pancreatic carcinoma. Gastrointest Endosc. 1997;45:387–93. doi: 10.1016/s0016-5107(97)70149-4. [DOI] [PubMed] [Google Scholar]
- 15.Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: Diagnostic accuracy and complication assessment. Gastroenterology. 1997;112:1087–95. doi: 10.1016/s0016-5085(97)70164-1. [DOI] [PubMed] [Google Scholar]
- 16.Gress F, Gottlieb K, Sherman S, et al. Endoscopic ultrasonography-guided fine-needle aspiration biopsy of suspected pancreatic cancer. Ann Intern Med. 2001;134:459–64. doi: 10.7326/0003-4819-134-6-200103200-00010. [DOI] [PubMed] [Google Scholar]
- 17.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–91. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Chen VK, Eltoum IA, et al. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: Diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 19.Ardengh JC, Lopes CV, de Lima LF, et al. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–6. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iglesias-Garcia J, Dominguez-Munoz E, Lozano-Leon A, et al. Impact of endoscopic ultrasound-guided fine needle biopsy for diagnosis of pancreatic masses. World J Gastroenterol. 2007;13:289–93. doi: 10.3748/wjg.v13.i2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 22.Hartwig W, Schneider L, Diener MK, et al. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5–20. doi: 10.1002/bjs.6407. [DOI] [PubMed] [Google Scholar]
- 23.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 24.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–36. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 25.Ardengh JC, Lopes CV, Campos AD, et al. Endoscopic ultrasound and fine needle aspiration in chronic pancreatitis: Differential diagnosis between pseudotumoral masses and pancreatic cancer. JOP. 2007;8:413–21. [PubMed] [Google Scholar]
- 26.Erturk SM, Mortelé KJ, Tuncali K, et al. Fine-needle aspiration biopsy of solid pancreatic masses: Comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol. 2006;187:1531–5. doi: 10.2214/AJR.05.1657. [DOI] [PubMed] [Google Scholar]
- 27.Horwhat JD, Paulson EK, McGrath K, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–75. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 28.Volmar KE, Vollmer RT, Jowell PS, et al. Pancreatic FNA in 1000 cases: A comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–61. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 29.Mallery JS, Centeno BA, Hahn PF, et al. Pancreatic tissue sampling guided by EUS, CT/US, and surgery: A comparison of sensitivity and specificity. Gastrointest Endosc. 2002;56:218–24. doi: 10.1016/s0016-5107(02)70181-8. [DOI] [PubMed] [Google Scholar]
- 30.Chen VK, Arguedas MR, Kilgore ML, et al. A cost-minimization analysis of alternative strategies in diagnosing pancreatic cancer. Am J Gastroenterol. 2004;99:2223–34. doi: 10.1111/j.1572-0241.2004.40042.x. [DOI] [PubMed] [Google Scholar]
- 31.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am J Gastroenterol. 2003;98:1976–81. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 32.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609–15. doi: 10.1016/j.cgh.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 33.DeWitt J, Yu M, Al-Haddad MA, et al. Survival in patients with pancreatic cancer after the diagnosis of malignant ascites or liver metastases by EUS-FNA. Gastrointest Endosc. 2010;71:260–5. doi: 10.1016/j.gie.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 34.Mortensen MB, Pless T, Durup J, et al. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478–83. doi: 10.1055/s-2001-14966. [DOI] [PubMed] [Google Scholar]
- 35.Wiersema MJ, Levy MJ, Harewood GC, et al. Initial experience with EUS-guided trucut needle biopsies of perigastric organs. Gastrointest Endosc. 2002;56:275–8. doi: 10.1016/s0016-5107(02)70193-4. [DOI] [PubMed] [Google Scholar]
- 36.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–6. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 37.Levy MJ, Wiersema MJ. EUS-guided Trucut biopsy. Gastrointest Endosc. 2005;62:417–26. doi: 10.1016/j.gie.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 38.Jenssen C, Dietrich CF. Endoscopic ultrasound-guided fine-needle aspiration biopsy and trucut biopsy in gastroenterology – An overview. Best Pract Res Clin Gastroenterol. 2009;23:743–59. doi: 10.1016/j.bpg.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 39.Larghi A, Verna EC, Stavropoulos SN, et al. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: A prospective study. Gastrointest Endosc. 2004;59:185–90. doi: 10.1016/s0016-5107(03)02538-0. [DOI] [PubMed] [Google Scholar]
- 40.Wahnschaffe U, Ullrich R, Mayerle J, et al. EUS-guided Trucut needle biopsies as first-line diagnostic method for patients with intestinal or extraintestinal mass lesions. Surg Endosc. 2009;23:2351–5. doi: 10.1007/s00464-009-0345-2. [DOI] [PubMed] [Google Scholar]
- 41.Thomas T, Kaye PV, Ragunath K, et al. Efficacy, safety, and predictive factors for a positive yield of EUS-guided Trucut biopsy: A large tertiary referral center experience. Am J Gastroenterol. 2009;104:584–91. doi: 10.1038/ajg.2008.97. [DOI] [PubMed] [Google Scholar]
- 42.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 43.Petrone MC, Poley JW, Bonzini M, et al. Interobserver agreement among pathologists regarding core tissue specimens obtained with a new endoscopic ultrasound histology needle: A prospective multicentre study in 50 cases. Histopathology. 2013;62:602–8. doi: 10.1111/his.12041. [DOI] [PubMed] [Google Scholar]
- 44.Larghi A, Iglesias-Garcia J, Poley JW, et al. Feasibility and yield of a novel 22-gauge histology EUS needle in patients with pancreatic masses: A multicenter prospective cohort study. Surg Endosc. 2013;27:3733–8. doi: 10.1007/s00464-013-2957-9. [DOI] [PubMed] [Google Scholar]
- 45.Figueiredo FA, Giovannini M, Monges G, et al. Pancreatic endocrine tumors: A large single-center experience. Pancreas. 2009;38:936–40. doi: 10.1097/MPA.0b013e3181b365db. [DOI] [PubMed] [Google Scholar]
- 46.Pais SA, Al-Haddad M, Mohamadnejad M, et al. EUS for pancreatic neuroendocrine tumors: A single-center, 11-year experience. Gastrointest Endosc. 2010;71:1185–93. doi: 10.1016/j.gie.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Figueiredo FA, Giovannini M, Monges G, et al. EUS-FNA predicts 5-year survival in pancreatic endocrine tumors. Gastrointest Endosc. 2009;70:907–14. doi: 10.1016/j.gie.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Fasanella KE, McGrath KM, Sanders M, et al. Pancreatic endocrine tumor EUS-guided FNA DNA microsatellite loss and mortality. Gastrointest Endosc. 2009;69:1074–80. doi: 10.1016/j.gie.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Piani C, Franchi GM, Cappelletti C, et al. Cytological Ki-67 in pancreatic endocrine tumours: An opportunity for pre-operative grading. Endocr Relat Cancer. 2008;15:175–81. doi: 10.1677/ERC-07-0126. [DOI] [PubMed] [Google Scholar]
- 50.Chatzipantelis P, Konstantinou P, Kaklamanos M, et al. The role of cytomorphology and proliferative activity in predicting biologic behavior of pancreatic neuroendocrine tumors: A study by endoscopic ultrasound-guided fine-needle aspiration cytology. Cancer. 2009;117:211–6. doi: 10.1002/cncy.20025. [DOI] [PubMed] [Google Scholar]
- 51.Fritscher-Ravens A, Sriram PV, Krause C, et al. Detection of pancreatic metastases by EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:65–70. doi: 10.1067/mge.2001.111771. [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Pérez R, Iglesias-García J, Alvarez-del-Castillo M, et al. Usefulness of endoscopic ultrasound in the evaluation of a lymphoma with multiple gastric and pancreatic lesions. Rev Esp Enferm Dig. 2012;104:322–3. doi: 10.4321/s1130-01082012000600007. [DOI] [PubMed] [Google Scholar]
- 53.Eloubeidi MA, Varadarajulu S, Desai S, et al. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567–70. doi: 10.1111/j.1440-1746.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki R, Lee JH, Krishna SG, et al. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointestin Liver Dis. 2013;22:183–7. [PubMed] [Google Scholar]
- 55.DeWitt J, McGreevy K, Sherman S, et al. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610–9. doi: 10.1016/j.gie.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 56.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 57.Eloubeidi MA, Tamhane A, Varadarajulu S, et al. Frequency of major complications after EUS-guided FNA of solid pancreatic masses: A prospective evaluation. Gastrointest Endosc. 2006;63:622–9. doi: 10.1016/j.gie.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 58.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73:283–90. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 59.Chong A, Venugopal K, Segarajasingam D, et al. Tumor seeding after EUS-guided FNA of pancreatic tail neoplasia. Gastrointest Endosc. 2011;74:933–5. doi: 10.1016/j.gie.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 60.Micames C, Jowell PS, White R, et al. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–5. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 61.Beane JD, House MG, Coté GA, et al. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844–53. doi: 10.1016/j.surg.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 62.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 63.van Gulik TM, Reeders JW, Bosma A, et al. Incidence and clinical findings of benign, inflammatory disease in patients resected for presumed pancreatic head cancer. Gastrointest Endosc. 1997;46:417–23. doi: 10.1016/s0016-5107(97)70034-8. [DOI] [PubMed] [Google Scholar]
- 64.Paulson AS, Tran Cao HS, Tempero MA, et al. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–26. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 65.Matthaios D, Zarogoulidis P, Balgouranidou I, et al. Molecular pathogenesis of pancreatic cancer and clinical perspectives. Oncology. 2011;81:259–72. doi: 10.1159/000334449. [DOI] [PubMed] [Google Scholar]
- 66.Roberts AS, Campa MJ, Gottlin EB, et al. Identification of potential prognostic biomarkers in patients with untreated, advanced pancreatic cancer from a phase 3 trial (Cancer and Leukemia Group B 80303) Cancer. 2012;118:571–8. doi: 10.1002/cncr.26270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farrell JJ, Elsaleh H, Garcia M, et al. Human equilibrative nucleoside transporter 1 levels predict response to gemcitabine in patients with pancreatic cancer. Gastroenterology. 2009;136:187–95. doi: 10.1053/j.gastro.2008.09.067. [DOI] [PubMed] [Google Scholar]
- 68.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–7. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 69.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: A double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–62. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 70.Innocenti F, Owzar K, Cox NL, et al. A genome-wide association study of overall survival in pancreatic cancer patients treated with gemcitabine in CALGB 80303. Clin Cancer Res. 2012;18:577–84. doi: 10.1158/1078-0432.CCR-11-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mortenson MM, Katz MH, Tamm EP, et al. Current diagnosis and management of unusual pancreatic tumors. Am J Surg. 2008;196:100–13. doi: 10.1016/j.amjsurg.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 72.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 73.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–77. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 74.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–46. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 75.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: The importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–46. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–56. doi: 10.1002/cncr.27636. [DOI] [PubMed] [Google Scholar]
- 77.Wasif N, Ko CY, Farrell J, et al. Impact of tumor grade on prognosis in pancreatic cancer: Should we include grade in AJCC staging? Ann Surg Oncol. 2010;17:2312–20. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Levy MJ, Wiersema MJ, Chari ST. Chronic pancreatitis: Focal pancreatitis or cancer? Is there a role for FNA/biopsy. Autoimmune pancreatitis. Endoscopy. 2006;38(Suppl 1):S30–5. doi: 10.1055/s-2006-946648. [DOI] [PubMed] [Google Scholar]
- 79.Varas M, Gornals J, Prieto JL, et al. Diagnostic protocol for pancreatic neuroendocrine tumors (PNETs) Rev Esp Enferm Dig. 2012;104:29–32. doi: 10.4321/s1130-01082012000100006. [DOI] [PubMed] [Google Scholar]
- 80.Maimone A, Luigiano C, Baccarini P, et al. Preoperative diagnosis of a solid pseudopapillary tumour of the pancreas by Endoscopic Ultrasound Fine Needle Biopsy: A retrospective case series. Dig Liver Dis. 2013;45:957–60. doi: 10.1016/j.dld.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 81.Bertucci F, Araujo J, Giovannini M. Pancreatic metastasis from osteosarcoma and Ewing sarcoma: Literature review. Scand J Gastroenterol. 2013;48:4–8. doi: 10.3109/00365521.2012.711852. [DOI] [PubMed] [Google Scholar]
- 82.Singh D, Vaidya OU, Sadeddin E, et al. Role of endoscopic ultrasound and endoscopic retrograde cholangiopancreatography in isolated pancreatic metastasis from lung cancer. World J Gastrointest Endosc. 2012;4:328–30. doi: 10.4253/wjge.v4.i7.328. [DOI] [PMC free article] [PubMed] [Google Scholar]


