Abstract
Background:
Neurosurgery and ophthalmology residents need many years to improve microsurgical skills. Laboratory training models are very important for developing surgical skills before clinical application of microsurgery. A simple simulation model is needed for residents to learn how to handle microsurgical instruments and to perform safe dissection of intracranial or intraorbital nerves, vessels, and other structures.
Materials and Methods:
The simulation material consists of a one-year-old fresh cadaveric sheep cranium. Two parts (Part 1 and Part 2) were designed to approach structures of the orbit. Part 1 consisted of a 2-step approach to dissect intraorbital structures, and Part 2 consisted of a 3-step approach to dissect the optic nerve intracranially.
Results:
The model simulates standard microsurgical techniques using a variety of approaches to structures in and around the orbit and the optic nerve.
Conclusions:
This laboratory training model enables trainees to gain experience with an operating microscope, microsurgical instruments and orbital structures.
Keywords: Microneurosurgery, microsurgery, microsurgical training, optic nerve, orbita surgery
Introduction
As surgical specialties, neurosurgery and ophthalmology require the development of dexterity and skills for a variety of surgical procedures. In delicate organs such as the central nervous system and eye, the surgeon's individual skills play a crucial role in determining patients’ outcome. Hence, the emphasis has been placed on laboratory training, preparing surgical trainees for the operating room experience.[1] Laboratory training models are indispensable for improving and purifying surgical skills before clinical application of microsurgery.[2] Education and training in microsurgical techniques have historically relied on the use of live animal models. However, increasing sensitivity toward the ethical aspects of scientific research demands a significant reduction in the numbers of live animals used in surgical and academic education.[2,3,4,5,6,7,8] Many articles are reporting on the use of alternatives to live animals in microsurgical training.[2,3,4,5,6,7,8,9,10,11,12,13]
We propose a simple fresh sheep cranium model for orbita and optic nerve microsurgical training. The fresh sheep cranium material has been previously described as a useful model to learn microneurosurgical skills[2,3,4,11,14] and we have applied the same model in the current study.
Materials and Methods
The model presented in this report is one step of a basic microneurosurgery training program that was used in the Microneurosurgery Laboratory at Trakya University's Department of Neurosurgery. Second- to fifth-year residents regularly have to complete this basic laboratory training for microsurgery.
The material for the model was obtained from a local butcher under official veterinary control and consisted of a one-year-old fresh sheep cranium with the scalp removed. The material was kept in the refrigerator at 4°C for the next 6 hours.
The cranium was positioned in the neutral vertical plane. The frontal and periorbital periosteum were removed bilaterally. Superior and lateral orbital border with supraorbital notch and supraorbital nerve were exposed [Figure 1]. The microneurosurgical training was started under magnifications (×6 to ×10) of the operating microscope (OpMi 99 Zeiss Inc., Oberkocken, Germany). Bipolar forceps, an arachnoid knife, microscissors, and a suction tube were used for intraorbital and intracranial dissection. All surgical procedures, with the exception of craniotomies, were performed under operating microscope. Photographs were taken with a digital camera (Nikon Coolpix 4500, Japan) from the microscope eyepiece. Two microsurgical parts were designed to approach structures of the orbit. Part 1 consisted of a 2-step approach to dissect intraorbital structures, and Part 2 consisted of a 3-step approach to dissect the optic nerve intracranially.
Figure 1.
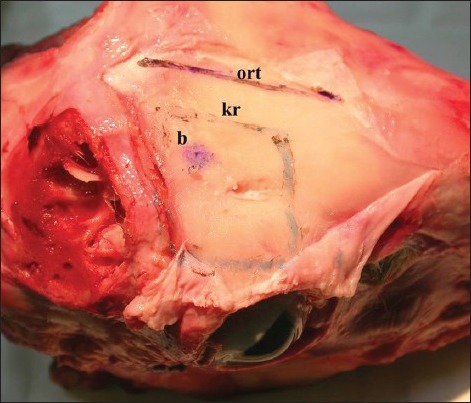
Exposed superior and lateral orbital border with supraorbital notch and supraorbital nerve
Results
The model simulates standard microsurgical techniques using a variety of approaches to neural structures in and around the orbit.
Part 1 simulates the superior orbitotomy approach and microdissection of the intraorbital anatomic structures. The first step consists of subperiostal dissection of the periorbita and bony removal of the superior orbital border and roof by rongeur [Figure 2a]. The second step consists of microsurgical identification and gentle dissection of the retroocular anatomic structures in the orbital fatty tissue and approaches of the orbital apex [Figure 2b].
Figure 2.
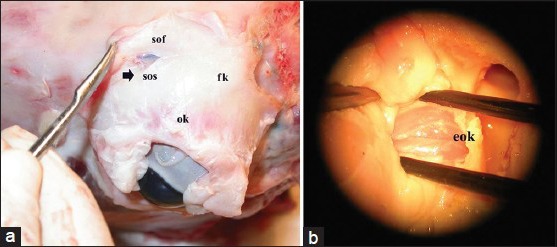
(a) First step of the dissection consisting of subperiostal dissection of the periorbita and bony removal of the superior orbital border and roof by rongeur (b) Microsurgical identification and dissection of the retroocular anatomic structures in the orbital fatty tissue in order to approach the orbital apex
The simulation of the superior orbitotomy approach was performed using the same microsurgical maneuvers and manipulations, alike fatty tissue dissection of the orbit and differentiation of the normal anatomic structures.
Part 2 simulates the frontal intracranial approach to the optic nerve and optic canal. One burr hole is placed 3 cm to the midline and 3 cm to the superior orbital border after identifying the supraorbital nerve and removing the periosteum. The frontal bone was removed using Rongeurs to make a 3 × 3 cm craniectomy to simulate the standard frontal craniotomy. The dura overlying the frontal (rostral) lobe is opened in a semicircular fashion, simulating the standard frontal approach in the human brain (first step) [Figure 3a].
Figure 3.
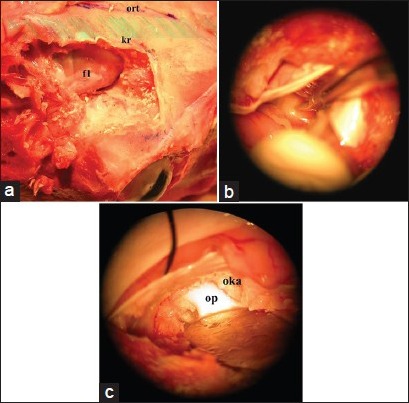
(a) Following the craniectomy to simulate the standard frontal craniotomy and dura was opened in a semicircular fashion. This way it is possible to simulate the standard frontal approach in the human brain (b) The next step is identification and microsurgical dissection of the optic nerve. The filled arteries in Sylvian dissection step allows to feel a real surgery experience for inexperienced neurosurgery residents (c) At the end of the dissection, the last step is the opening of optic canal and exposure of the optic nerve within the canal
The second step consists of identification and microsurgical dissection of the optic nerve [Figure 3b]. The exposed brain was human-like: The arteries were light red, the veins were dark red and filled, and a clear fluid simulated the release of cerebrospinal fluid when the arachnoid was opened. The sylvian fissure was splitted, followed to the carotid and basal cisterns, and dissected the optic nerve in the anterior skull base.
The third step consists of opening the optic canal and exposing the optic nerve [Figure 3c]. Kerrison rongeur or high-speed drill was used for opening and unroofing the optic canal.
Discussion
Historically, learning, invention, and development in microsurgery started with the human subject, moved toward the animal subject, then synthetic models. In today's world, surgical residents need to start in the laboratory and work their way up to human subjects, focusing on exercises that closely mimic the real-life situation in order to facilitate skills transfer.[13]
Practicing surgical skills and gaining experience with microsurgical procedures on several models such as laboratory animals, human and animal cadavers, and synthetic materials before exposure to patients is useful.[2,3,4] These training methods help surgeons learn basic microneurosurgery skills.[1,3,6,7,8,11,13,15]
The best way to maintain surgical skills and consistent ability is to routinely perform procedures. Surgeons who are not routinely presented with such clinical experiences must regularly repeat correct rehearsals in order to maintain or improve their skills.[1,13] Books, lectures, videotapes, and the assistance of experienced surgeons facilitate the development of cognitive and perceptual skills, but motor skills can be developed and maintained only with regular practice.[7,8,15]
The authors present a training model in sheep cranium that allows residents in neurosurgery and ophthalmology to practice the superior orbitotomy and frontal craniotomy procedure used in the surgical approach of various orbit pathology. The model comprises a two-part, five-step approach: Intraorbital (Part 1) and intracranial dissection (Part 2); superior orbitotomy approach; subperiostal dissection of the periorbita and bony removal of the superior orbital border and roof; dissection of the retroocular anatomic structures; approaches of the orbital apex, frontal craniotomy, dissection of the optic nerve and opening the optic canal. We recommend that trainees attempt this model before working with live animals.[2,3,4,11,14] This model will surely facilitate the development and maintenance of surgical skills. The major advantage of this new training system is the possibility of learning to manipulate biological material that is very easily procured at a low cost. Sheep cranium is an ideal material because it is inexpensive, convenient to manage and easy to obtain; furthermore, it is a biologic human-like material, and neither specific facilities to maintain live animals nor anesthesia is needed.[2,3,4,7,11] However, sheep may not be found very often in some parts of the world, as a result the general costs of this model may be increased. In that case, other animal models can be used in that particular areas. Generally, the abovementioned advantages remain true for fresh cadaveric animal models. The rationale behind the use of animal models is not because they mimick the human anatomy exactly, but they create a real-life surgical training opportunity for inexperienced residents.
The main disadvantage related to the presented model is the absence of bleeding, as it is in any of the cadaveric animal models.[4] One should note that real-life surgery contains a risk of bleeding and the ability to deal with hemostasis is essential for any kind of surgery. Lack of bleeding results in limitations in the practice of hemostasis. Also the surgical material is different than in real-life surgery, although it is a fresh cadaveric sheep cranium. The surgical “feeling” through the microinstruments, as well as tissue handling may be different. Live animal models may be a good complementary training alongside our suggested model, which resemble more to the real-life surgery by the means of bleeding, tissue handling and tissue preservation. Although the medical risk of contracting animal diseases, such as transmissible spongiform encephalopathie (scrapie), is low in younger sheep, precautions need to be taken.[3,16] The specimen should be provided from a known source and from animals under veterinary control. We also recommend that surgical instruments used in the study should not be used in human subjects and all sterilization measures should be absolutely strict.[16]
The sheep brain and orbit are similar to that of the human with some differences. For instance, the volume of a sheep orbit is slightly more than half that of a human orbit, and the topographic anatomy of the orbit of the sheep cranium is different from that of the human orbit.[17] The cadaveric sheep cranium model presented here is intended only for laboratory training. Ours is not an anatomic study of the sheep brain or orbit in the context of veterinary medicine; therefore, except for the microsurgical similarities to corresponding structures of the human orbit and brain, other definitions, locations and variations of anatomic structures mentioned in this article are not the subject of the training model and are beyond the scope of this study.
Acquiring microsurgical skills is essential for neurosurgeons and physicians from several other disciplines like ophthalmology, otorhinolaryngology, plastic surgery etc., Recent studies have shown that programs focusing on early microsurgical training of physicians might result in early and better acquisition of the proper microsurgical technique.[18,19] Studies by Scholz et al.[18] and Mücke et al.[19] are focused on the effects of the participants age, who have completed a standard microsurgical training program. Participants of younger age or medical students showed better results compared to older individuals or surgeons, by the means of microinstrument handling, tissue handling, time management and theoretical knowledge. However our microsurgical model is planned for the training of second to fifth year residents. As indicated in several other publications microsurgical courses or training programs aiming to train younger individuals, for example during medical school education or before the acceptance to the residency program may be more useful.
This model can also be used for training in eye microsurgery. However, our aim is to familiarize junior residents of neurosurgery and ophthalmology with the basic techniques of microsurgery in dissecting major anatomic structures in and around the orbit under an operating microscope. For this reason, this training model should not be exchanged for other training, especially on live animal models, but should serve only as a supplementary training model in microsurgery for beginners.[2,3,4,11,14,15]
Conclusions
Advantages of using this model over traditional training techniques are ease of use, reproducibility, minimal cost, easy access to the materials; most importantly, this model allows surgeons to practice microsurgery outside of the operating room.[2,11,14] We would argue that use of this model is helpful to improve microsurgical techniques and reduce time taken to perform basic microsurgical maneuvers, factors that will lead to greater success when performing microsurgery on patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Aboud E, Al-Mefty O, Yaşargil MG. New laboratory model for neurosurgical training that simulates live surgery. J Neurosurg. 2002;97:1367–72. doi: 10.3171/jns.2002.97.6.1367. [DOI] [PubMed] [Google Scholar]
- 2.Hicdonmez T, Hamamcioglu MK, Parsak T, Cukur Z, Cobanoglu S. A laboratory training model for interhemispheric-transcallosal approach to the lateral ventricle. Neurosurg Rev. 2006;29:159–62. doi: 10.1007/s10143-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 3.Hicdonmez T, Hamamcioglu MK, Tiryaki M, Cukur Z, Cobanoglu S. Microneurosurgical training model in fresh cadaveric cow brain: A laboratory study simulating the approach to the circle of Willis. Surg Neurol. 2006;66:100–4. doi: 10.1016/j.surneu.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Hicdönmez T, Birgili B, Tiryaki M, Parsak T, Çobanoğlu S. Posterior fossa approach: Microneurosurgical training model in cadaveric sheep. Turk Neurosurg. 2006;16:111–4. [Google Scholar]
- 5.Bao JY. Rat tail: A useful model for microvascular training. Microsurgery. 1995;16:122–5. doi: 10.1002/micr.1920160216. [DOI] [PubMed] [Google Scholar]
- 6.Galeano M, Zarabini AG. The usefulness of a fresh chicken leg as an experimental model during the intermediate stages of microsurgical training. Ann Plast Surg. 2001;47:96–7. doi: 10.1097/00000637-200107000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Hino A. Training in microvascular surgery using a chicken wing artery. Neurosurgery. 2003;52:1495–8. doi: 10.1227/01.neu.0000065174.83840.62. [DOI] [PubMed] [Google Scholar]
- 8.Steffens K, Koob E, Hong G. Training in basic microsurgical techniques without experiments involving animals. Arch Orthop Trauma Surg. 1992;111:198–203. doi: 10.1007/BF00571477. [DOI] [PubMed] [Google Scholar]
- 9.Lausada NR, Escudero E, Lamonega R, Dreizzen E, Raimondi JC. Use of cryopreserved rat arteries for microsurgical training. Microsurgery. 2005;25:500–1. doi: 10.1002/micr.20153. [DOI] [PubMed] [Google Scholar]
- 10.Menovsky T. A human skull cast model for training of intracranial microneurosurgical skills. Microsurgery. 2000;20:311–3. doi: 10.1002/1098-2752(2000)20:7<311::aid-micr1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Hamamcioglu MK, Hicdonmez T, Tiryaki M, Cobanoglu S. A laboratory training model in fresh cadaveric sheep brain for microneurosurgical dissection of cranial nerves in posterior fossa. Br J Neurosurg. 2008;22:769–71. doi: 10.1080/02688690802477573. [DOI] [PubMed] [Google Scholar]
- 12.Ayoubi S, Ward P, Naik S, Sankaran M. The use of placenta in a microvascular exercise. Neurosurgery. 1992;30:252–4. doi: 10.1227/00006123-199202000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Ilie VG, Ilie VI, Dobreanu C, Ghetu N, Luchian S, Pieptu D. Training of microsurgical skills on nonliving models. Microsurgery. 2008;28:571–7. doi: 10.1002/micr.20541. [DOI] [PubMed] [Google Scholar]
- 14.Hicdonmez T, Parsak T, Cobanoglu S. Simulation of surgery for craniosynostosis: A training model in a fresh cadaveric sheep cranium. J Neurosurg. 2006;105(Suppl 2):150–2. doi: 10.3171/ped.2006.105.2.150. [DOI] [PubMed] [Google Scholar]
- 15.Taylor JB, Binenbaum G, Tapino P, Volpe NJ. Microsurgical lab testing is a reliable method for assessing ophthalmology residents’ surgical skills. Br J Ophthalmol. 2007;91:1691–4. doi: 10.1136/bjo.2007.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grist EP. Transmissible spongiform encephalopathy risk assessment: The UK experience. Risk Anal. 2005;25:519–32. doi: 10.1111/j.1539-6924.2005.00619.x. [DOI] [PubMed] [Google Scholar]
- 17.Popesko P. I. Head and Neck (article in german); 1989. Atlas of the topographic anatomy of the pet animals; pp. 44–5. [Google Scholar]
- 18.Scholz M, Mücke T, Hölzle F, Schmieder K, Engelhardt M, Pechlivanis I, et al. A program of microsurgical training for young medical students: Are younger students better? Microsurgery. 2006;26:450–5. doi: 10.1002/micr.20269. [DOI] [PubMed] [Google Scholar]
- 19.Mücke T, Borgmann A, Ritschl LM, Kesting MR, Loeffelbein DJ. Microvascular training of medical students and surgeons-a comparative prospective study. J Craniomaxillofac Surg. 2013;41:e187–90. doi: 10.1016/j.jcms.2013.01.017. [DOI] [PubMed] [Google Scholar]


