Abstract
Aims:
To evaluate the efficacy of repeated bevacizumab injection in rotational conjunctival flap surgery versus rotational conjunctival flap with adjunctive mitomycin C (MMC) or rotational conjunctival flap alone.
Materials and Methods:
Ninety eyes of 90 patients who underwent primary pterygium surgery with rotational flap were evaluated. Patients were randomly assigned to undergo conjunctival rotational flap alone (Group A) or conjunctival rotational flap with either 0.02% MMC application (Group B) or adjunctive subconjunctival 2.5 mg/0.1 ml bevacizumab injection (Group C). Each group consisted of 30 eyes. Recurrence rates at 9 months were evaluated.
Results:
There were no statistically significant differences in mean size of the pterygium across the limbus in terms of length (P > 0.5). The recurrence rates at 9 months were 26.6% (n = 8) in Group A, 13.3% (n = 4) in Group B, and 10% (n = 3) in Group C. The recurrence rates in Group B and C were significantly lower than in Group A (P =0.1806). The recurrence rates were similar in Group B and C (P > 0.05).
Conclusions:
Subconjunctival bevacizumab injection may decrease the recurrence rate of primary pterygium surgery with rotational conjunctival flap. Further studies with a larger population and longer follow-up period are needed to supplement this study.
Keywords: Mitomycin C, pterygium recurrence, pterygium surgery, subconjunctival bevacizumab injection
A pterygium is a wing-shaped growth of fibrovascular conjunctival tissue onto the cornea. Several hypotheses have been ascribed to its etiology. Prevalence rates range from 0.7-31% in various populations around the world and the condition is more common in warm, dry climates.[1] Ultraviolet radiation exposure is a major risk factor for its development.[2]
Treatment of pterygium is surgical and includes simple excision (the bare sclera technique) and excision with grafting (conjunctival or amniotic membrane grafts).[3] Simple excision carries a high recurrence rate, ranging from 24-89%.[4] Adjunctive treatments; including radiation, antimetabolites, conjunctival grafts, and limbal grafts; are used to reduce the rate of recurrence after surgical excision.[5,6]
Some findings suggest that an immunologic dysfunction plays a role in the pathogenesis of pterygium, and recent studies have shown that pterygia have increased levels of proangiogenic growth factors such as basic fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF).[7,8] However, the most prominent of these factors is VEGF, which is the main target of many current antiangiogenic therapies, including treatment with bevacizumab, a full-length humanized monoclonal antibody that binds VEGF and antagonizes its effects.[9,10] Topical or subconjunctival bevacizumab has been shown to be effective in treating corneal neovascularization in an in vivo animal study and in a recent case report.[11,12]
Another alternative adjunct is mitomycin C (MMC). Addition of MMC at various concentrations has been reported to be effective in preventing recurrence.[13,14] The mechanism of action seems to be inhibition of fibroblast proliferation at the level of the episclera. However, MMC may cause devastating complications such as scleral necrosis and microbial infections.[15,16]
In the present study, we compared the efficacy of rotational conjunctival flap alone versus rotational conjunctival flap combined with either MMC application on bare sclera or twice-repeated subconjunctival bevacizumab injections.
Materials and Methods
A prospective, comparative, blind, interventional clinical study was carried out from December 2009 until December 2011 Ninety eyes of 90 subjects with primary pterygia were included in the study. Informed consent was obtained from all patients before enrolment. The study was approved by the Bezmi Alem Vakif University Ethics Committee. All patients underwent full ophthalmologic examination before and after surgery, including visual acuity, slit-lamp examination, fundoscopy, and applanation tonometry. Exclusion criteria were collagen vascular disease or other autoimmune disease, pregnancy, ocular surface pathology or infection, and previous limbal surgery.
All 90 patients underwent rotational flap surgery performed by a single surgeon. The surgical technique was as follows: (i) subconjunctival anesthetic injection in the area to be excised; (ii) excision of the pterygium [Fig. 1]; (iii) light cautery for hemostasis; (iv) a U-shaped incision and preparation of the flap [Fig. 2]; and (v) suturation of the flap with 8-0 vicryl suture [Fig. 3]. Patients were randomized into three groups according to the last numerical digit of their medical records. Group A: Pterygium excision and rotational conjunctival flap on 30 eyes. Subconjunctival balanced salt solution was injected as a placebo. Group B: Pterygium excision and rotational conjunctival flap with adjunctive topical mitomycin C (0.02%) administered to the bare sclera on 30 eyes of 30 patients for 3 min. Group C: Pterygium excision and rotational conjunctival flap with adjunctive subconjunctival bevacizumab (2.5 mg/0.1 ml) injection performed on 30 eyes of 30 patients. Injections were given to the inferior fornix in order to prevent flap contraction. As per our protocol, all eyes received two subconjunctival bevacizumab injections, the first intraoperatively and the second at 1 week after the surgery. All patients were followed for 9 months by two independent examiners and recurrence rates were assessed at 3, 6, and 9 months. Recurrence was defined as any fibrovascular growth of conjunctival tissue extending more than 1.5 mm across the limbus.
Figure 1.

Excision of the pterygium tissue
Figure 2.
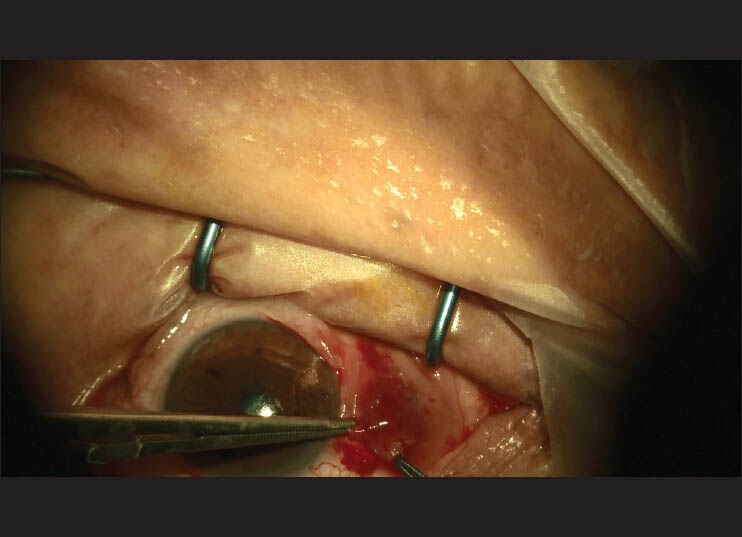
Preparation and testing the flap size
Figure 3.
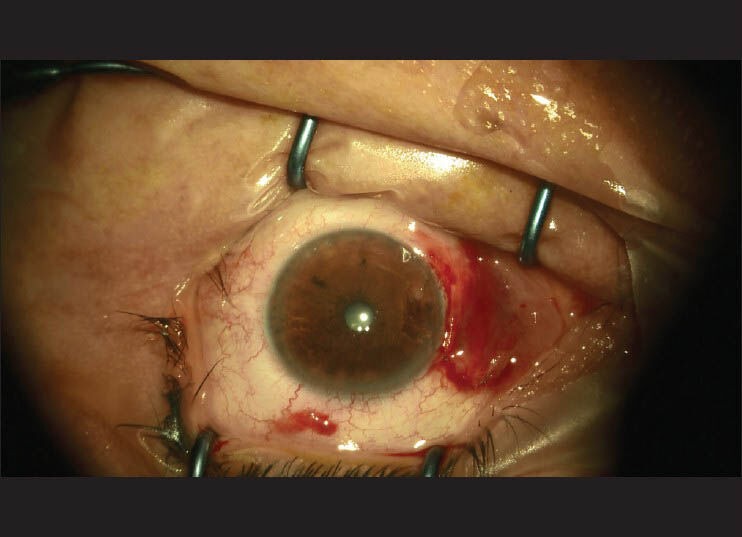
Appearance of the flap after suturation with 8-0 vicryl suture
Postoperatively, patients were treated with topical Tobradex (tobramycin and dexamethasone, Alcon Laboratories Ltd, Fort Worth, Texas, USA) eye drops four times daily for 1 week. Follow-up visits were at day 1; week 1; and months 1, 3, 6, and 9. Data were analyzed using SPSS version 10.0 software.
Results
This study was conducted on 90 patients. There were no statistically significant differences between the groups regarding sex (P =0.67) or age (P =0.68) [Table 1]. All patients had a pterygium on the medial side of the cornea and there were no statistically significant differences in mean size of the pterygium across the limbus (in length) (P =0.71). All patients completed the study. Patients were followed postoperatively for 9 months. The main outcome measure was postoperative recurrence. At 9 months postoperatively, the recurrence rate was 26.6% (n = 8) in Group A, 13.3% (n = 4) in Group B, and 10% (n = 3) in Group C [Table 2]. The recurrence rates in Group B and C were significantly lower than in Group A (P =0.1806). The recurrence rates were found to be similar in Group A and B (P > 0.05). No complications related to MMC application or bevacizumab injection were seen during the follow-up period.
Table 1.
Baseline demographics of patients
Table 2.
Number of recurrences

Discussıon
A pterygium is characterized by excessive fibrovascular proliferation on the exposed ocular surface. It is thought to be caused by increased ultraviolet light exposure due to climatic factors and aggravated by microtrauma and chronic inflammation from environmental factors.[4,17,18,19] Despite the multifactorial pathogenesis, surgery is the mainstay of treatment. The primary concern in pterygium surgery is recurrence, defined by regrowth of the fibrovascular tissue across the limbus and onto the cornea. In order to reduce the rate of recurrence, various modalities have been proposed. The majority of medical modalities involve measures to counter the fibrovascular activities that play key roles in pterygium recurrence.[20]
Generally, pterygium recurrences happen during the first 6 months after surgery. A number of factors such as the type of pterygium, age of the patient, environmental agents, and surgical technique may be responsible.[14]
Rotational conjunctival flaps to cover the pterygium excisional site have been employed since the 1940s.[21] Of the surgical interventions, these are associated with a recurrence rate of 2–39%. In our study, we followed 30 pterygium patients treated with the flap technique alone; the recurrence rate in this group was 26.6%. There were no serious complications in these cases. The most frequent symptom after this procedure was the formation of folds over the conjunctiva as a result of rotated tissues in the flap area. Although these folds can result in unsatisfactory cosmesis, including hyperemia at the beginning, after a time the conjunctiva improves and reaches an acceptable level cosmetically. Conjunctival flap tissue that is placed over bare sclera is adjacent to the excised pterygium tissue, and altered limbal cells that might be localized on the flap could contribute to the development of recurrence. We prefer this classical technique because it is easy to apply, but it also demonstrates that surgery alone cannot prevent recurrence.
The mechanism of action of MMC in the prevention of pterygium recurrence has been attributed to the inhibition of fibroblast proliferation of the episclera.[22,23] MMC has a prolonged, if not permanent, effect on suppressing human fibroblasts. This prevents the development of fibrosis and aggressive wound healing that is responsible for pterygium recurrence. Adjunctive MMC for pterygium surgery was first described by Kunitomo and Mori in Japan in 1963. In an attempt to decrease ocular morbidity, the intraoperative use of MMC applied directly to the scleral bed has gained increasing acceptance. In this technique, after bare sclera excision, 0.2-0.4 mg/ml MMC is applied directly to the scleral bed for 2-5 min.[24] The advantages of this technique include a lower MMC dosage, the use of MMC only in the operating room, and application of MMC directly to the area of pathology rather than to the entire ocular surface.
In Group B, where MMC was used intraoperatively at a concentration of 0.2 mg/ml over bare sclera for 3 min, the rate of recurrence was 20% in comparison with 38% reported by Chen et al.,[25] and 10.5% by Manning et al.,[26] with the application of 0.4 mg/ml for 3 min. This concurs with previous studies on intraoperative application of MMC with a rate of recurrence of 25%.[27] Various concentrations of MMC with different durations of application have been used, but the minimal safe and effective dosage and application time are still not certain.[13] Rubinfeld and colleagues[15] described scleral ulceration, necrotizing scleritis, perforation, iridocyclitis, cataract, infection, glaucoma, scleral calcification, and loss of an eye after pterygium excision with adjunctive MMC therapy. While the exact incidence of these complications is unknown, the safety of MMC therapy remains to be determined with future long-term trials.
The most common cause of recurrent pterygium is surgical trauma and the histopathological components include neovascularization and fibroblast proliferation. VEGF transcription and secretion are elevated in acute wounds, which mainly promotes the early events in angiogenesis, particularly endothelial cell migration and proliferation.[28] Because of this, many studies have focused on the efficacy and safety of avastin in ocular surface disorders. Kheirkhah et al.,[29] reported a significant role for inflammation in the induction of recurrence after pterygium surgery. They suggested that decreased angiogenic inhibitors together with increased stimulators might play a role in the formation and progression of pterygia. Thus, blocking VEGF, a crucial factor in wound healing, may result in a reduction in both fibrovascular tissue formation and the overall recurrence rate.
A case report demonstrated the efficacy of 2.5% topical bevacizumab administered four times daily for 3 weeks in inhibiting the recurrence in a patient with impending recurrent pterygium.[30] Bahar et al.,[31] reported on five patients with recurrent pterygium who received subconjunctival bevacizumab twice (2.5 mg/0.1 ml); at a 3-month follow-up no regression of corneal vessels in the recurrent pterygium was observed. Teng et al.,[32] reported that treatment of primary pterygium with subconjunctival bevacizumab (1.25 mg/0.05 ml) resulted in a short-term decrease in vascularization and irritation in one patient at 7 weeks of follow-up.[32] Fallah et al.,[33] evaluated the efficacy of intralesional bevacizumab injection (2.5 mg/0.1 ml), without rotational conjunctival flap, in reducing the size of pterygia and found it to be fairly effective and well tolerated. The mean percentage decrease of lesion size was 3.97 ± 3.84%. Razeghinejad et al.,[34] reported that a single intraoperative subconjunctival bevacizumab injection (1.25 mg/0.1 ml) had no effect on recurrence rate or early postoperative conjunctival erythema, lacrimation, photophobia, or healing of corneal epithelial defects following pterygium excision in 15 patients. In contrast to the finding of Razeghinejad et al.,[34] with respect to recurrence, we detected a decrease in recurrence rate after two subconjunctival bevacizumab injections (the first intraoperatively and the second at 1 week post-surgery, each 2.5 mg/0.1 ml). Because of this difference, it may be suggested to repeat the injection after the operation and apply a higher dose of bevacizumab.
In summary, in the present study we performed pterygium excision with a rotational flap technique in 90 eyes of 90 patients. Patients were divided into three groups, receiving rotational flap alone or with adjunctive 0.2 mg/ml MMC application or 2.5 mg/0.1 ml subconjunctival bevacizumab injection. The first bevacizumab injection was performed at the end of the surgery and the second 1 week after surgery to inhibit the acute phase of fibrovascular activity. Injections were performed in the inferior fornix in order to prevent conjuctival contraction around the wound site. When recurrence rates were compared at 9 months, both MMC application and subconjunctival bevacizumab injection had significantly less recurrence than rotational flap alone. No side effects related to bevacizumab injection were observed during the follow-up.
The limitations of the study were the short follow-up period and the moderate size of the study groups. With a longer follow-up period, recurrence rates and side effects related to the adjunctive drugs could be analyzed more accurately. Larger sample sizes could make the statistical analyses stronger. Nevertheless, this study showed that subconjunctival bevacizumab injection after primary pterygium surgery with the rotational flap technique had similar recurrence rates to MMC application but without the possible serious complications of MMC.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Tasman W, Jaeger EA. Vol. 6. Philadelphia: Lippincott Williams and Wilkins; 2002. Duane's Clinical Ophthalmology; p. 35. [Google Scholar]
- 2.Moran DJ, Hollows FC. Pterygium and ultraviolet radiation: A positive correlation. Br J Ophthalmol. 1984;68:343–6. doi: 10.1136/bjo.68.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prabhasawat P, Tesavibul N, Leelapatranura K, Phonjan T. Efficacy of subconjunctival 5-fluorouracil and triamcinolone injection in impending recurrent pterygium. Ophthalmology. 2006;113:1102–9. doi: 10.1016/j.ophtha.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Jaros PA, DeLuise VP. Pingueculae and pterygia. Surv Ophthalmol. 1988;33:41–9. doi: 10.1016/0039-6257(88)90071-9. [DOI] [PubMed] [Google Scholar]
- 5.Frucht-Pery J, Siganos CS, Ilsar M. Intraoperative application of topical mitomycin C for pterygium surgery. Ophthalmology. 1996;103:674–7. doi: 10.1016/s0161-6420(96)30635-0. [DOI] [PubMed] [Google Scholar]
- 6.Maldonado MJ, Cano-Parra J, Navea-Tejerina A, Cisneros AL, Vila E, Menezo JL. Inefficacy of low-dose intraoperative fluorouracil in the treatment of primary pterygium. Arch Ophthalmol. 1995;113:1356–7. doi: 10.1001/archopht.1995.01100110016008. [DOI] [PubMed] [Google Scholar]
- 7.Kria L, Ohira A, Amemiya T. Immunohistochemical localization of basic fibroblast growth factor, platelet derived growth factor, transforming growth factor-beta and tumor necrosis factor-alpha in pterygium. Acta Histochem. 1996;98:195–201. doi: 10.1016/s0065-1281(96)80038-9. [DOI] [PubMed] [Google Scholar]
- 8.Aspiotis M, Tsanou E, Gorezis S, Ioachim E, Skyrlas A, Stefaniotou M, et al. Angiogenesis in pterygium: Study of microvessel density, vascular endothelial growth factor, and thrombospondin-1. Eye (Lond) 2007;21:1095–101. doi: 10.1038/sj.eye.6702495. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini H, Nejabat M, Khalili MR. Bevacizumab (Avastin) as a potential novel adjunct in the management of pterygia. Med Hypotheses. 2007;69:925–7. doi: 10.1016/j.mehy.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–35. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 11.Barros LF, Belfort R., Jr The effects of the subconjunctival injection of bevacizumab (Avastin) on angiogenesis in the rat cornea. An Acad Bras Cienc. 2007;79:389–94. doi: 10.1590/s0001-37652007000300004. [DOI] [PubMed] [Google Scholar]
- 12.Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin) Br J Ophthalmol. 2007;91:804–7. doi: 10.1136/bjo.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lam DS, Wong AK, Fan DS, Chew S, Kwok PS, Tso MO. Intraoperative mitomycin C to prevent recurrence of pterygium after excision: A 30-month follow-up study. Ophthalmology. 1998;105:901–4. doi: 10.1016/S0161-6420(98)95034-5. [DOI] [PubMed] [Google Scholar]
- 14.Mutlu FM, Sobaci G, Tatar T, Yildirim E. A comparative study of recurrent pterygium surgery: Limbal conjunctival autograft transplantation versus mitomycin C with conjunctival flap. Ophthalmology. 1999;106:817–21. doi: 10.1016/S0161-6420(99)90172-0. [DOI] [PubMed] [Google Scholar]
- 15.Rubinfeld RS, Pfister RR, Stein RM, Foster CS, Martin NF, Stoleru S, et al. Serious complications of topical mitomycin-C after pterygium surgery. Ophthalmology. 1992;99:1647–54. doi: 10.1016/s0161-6420(92)31749-x. [DOI] [PubMed] [Google Scholar]
- 16.Dunn JP, Seamone CD, Ostler HB, Nickel BL, Beallo A. Development of scleral ulceration and calcification after pterygium excision and mitomycin therapy. Am J Ophthalmol. 1991;112:343–4. doi: 10.1016/s0002-9394(14)76738-8. [DOI] [PubMed] [Google Scholar]
- 17.Adamis AP, Starck T, Kenyon KR. The management of pterygium. Ophthalmol Clin North Am. 1990:3611–23. [Google Scholar]
- 18.Hilgers JH. Pterygium: Its incidence, heredity and etiology. Am J Ophthalmol. 1960;50:635–44. doi: 10.1016/0002-9394(60)90245-2. [DOI] [PubMed] [Google Scholar]
- 19.Mackenzie FD, Hirst LW, Battistutta D, Green A. Risk analysis in the development of pterygia. Ophthalmology. 1992;99:1056–61. doi: 10.1016/s0161-6420(92)31850-0. [DOI] [PubMed] [Google Scholar]
- 20.Ang LP, Chua JL, Tan DT. Current concepts and techniques in pterygium treatment. Curr Opin Ophthalmol. 2007;18:308–13. doi: 10.1097/ICU.0b013e3281a7ecbb. [DOI] [PubMed] [Google Scholar]
- 21.Krachmer JH, Mannis MJ, Holland EJ. Philadelphia: Mosby; 1998. Cornea; p. 1. [Google Scholar]
- 22.Wakaki S, Marumo H, Tomioka K. Isolation of new fractions of antitumor mitomycins. Antibiot Chemother. 1958;8:228–40. [PubMed] [Google Scholar]
- 23.Chen CW, Huang HT, Bair JS, Lee CC. Trabeculectomy with simultaneous topical application of mitomycin-C in refractory glaucoma. J Ocul Pharmacol. 1990;6:175–82. doi: 10.1089/jop.1990.6.175. [DOI] [PubMed] [Google Scholar]
- 24.Frucht-Pery J, Ilsar M. The use of low-dose mitomycin C for prevention of recurrent pterygium. Ophthalmology. 1994;101:759–62. doi: 10.1016/s0161-6420(94)31269-3. [DOI] [PubMed] [Google Scholar]
- 25.Chen PP, Ariyasu RG, Kaza V, LaBree LD, McDonnell PJ. A randomized trial comparing mitomycin C and conjunctival autograft after excision of primary pterygium. Am J Ophthalmol. 1995;120:151–60. doi: 10.1016/s0002-9394(14)72602-9. [DOI] [PubMed] [Google Scholar]
- 26.Manning CA, Kloess PM, Diaz MD, Yee RW. Intraoperative mitomycin in primary pterygium excision. A prospective, randomized trial. Ophthalmology. 1997;104:844–8. doi: 10.1016/s0161-6420(97)30224-3. [DOI] [PubMed] [Google Scholar]
- 27.Alpay A, Ugurbas SH, Erdogan B. Comparing techniques for pterygium surgery. Clin Ophthalmol. 2009;3:69–74. [PMC free article] [PubMed] [Google Scholar]
- 28.Gordon CR, Rojavin Y, Patel M, Zins JE, Grana G, Kann B, et al. A review on bevacizumab and surgical wound healing: An important warning to all surgeons. Ann Plast Surg. 2009;62:707–9. doi: 10.1097/SAP.0b013e3181828141. [DOI] [PubMed] [Google Scholar]
- 29.Kheirkhah A, Casas V, Sheha H, Raju VK, Tseng SC. Role of conjunctival inflammation in surgical outcome after amniotic membrane transplantation with or without fibrin glue for pterygium. Cornea. 2008;27:56–63. doi: 10.1097/ICO.0b013e31815873da. [DOI] [PubMed] [Google Scholar]
- 30.Wu PC, Kuo HK, Tai MH, Shin SJ. Topical bevacizumab eyedrops for limbal-conjunctival neovascularization in impending recurrent pterygium. Cornea. 2009;28:103–4. doi: 10.1097/ICO.0b013e3181822615. [DOI] [PubMed] [Google Scholar]
- 31.Bahar I, Kaiserman I, McAllum P, Rootman D, Slomovic A. Subconjunctival bevacizumab injection for corneal neovascularization in recurrent pterygium. Curr Eye Res. 2008;33:23–8. doi: 10.1080/02713680701799101. [DOI] [PubMed] [Google Scholar]
- 32.Teng CC, Patel NN, Jacobson L. Effect of subconjunctival bevacizumab on primary pterygium. Cornea. 2009;28:468–70. doi: 10.1097/ICO.0b013e31818d382d. [DOI] [PubMed] [Google Scholar]
- 33.Fallah Tafti MR, Khosravifard K, Mohammadpour M, Hashemian MN, Kiarudi MY. Efficacy of intralesional bevacizumab injection in decreasing pterygium size. Cornea. 2011;30:127–9. doi: 10.1097/ICO.0b013e3181e16d67. [DOI] [PubMed] [Google Scholar]
- 34.Razeghinejad MR, Hosseini H, Ahmadi F, Rahat F, Eghbal H. Preliminary results of subconjunctival bevacizumab in primary pterygium excision. Ophthalmic Res. 2010;43:134–8. doi: 10.1159/000252980. [DOI] [PubMed] [Google Scholar]


