Abstract
Aim:
To study the effects of triamcinolone acetonide (TA) on cultured human trabecular meshwork (HTM) cells.
Materials and Methods:
HTM cells were cultured and treated with 125, 250, 500 and 1000 μg/mL concentration of TA for 24 h. The cells were treated with both crystalline TA (TA-C) (commercial preparation) and solubilized TA (TA-S). Cell viability was measured by a trypan blue dye exclusion test. The activity of caspse-3/7 was measured by a fluorescence caspase kit and DNA laddering was evaluated by electrophoresis on 3% agarose gel. Levels of lactate dehydrogenase (LDH) were assessed with LDH cytotoxicity assay kit-II.
Results:
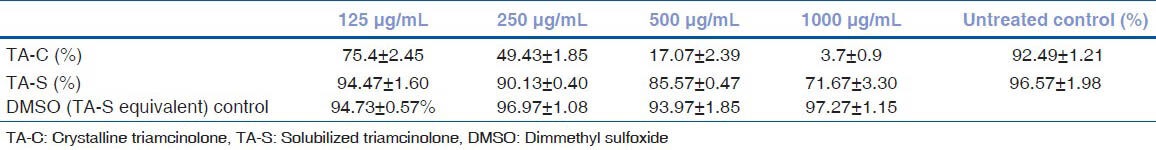
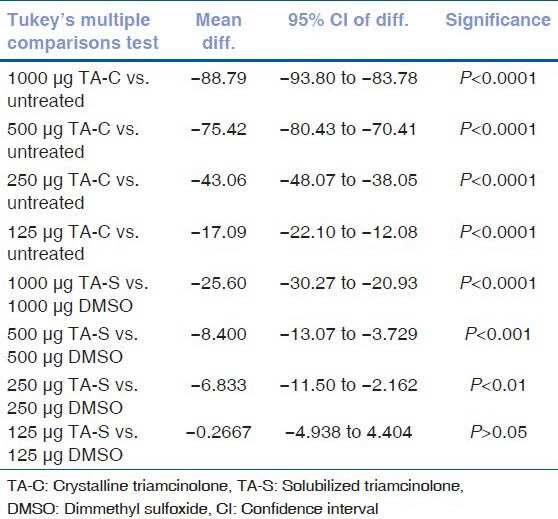
Mean cell viabilities of HTM cells after 24 h exposure to TA-C 125, 250, 500, and 1000 μg/mL were 75.4 ±2.45% (P < 0.0001), 49.43 ± 1.85% (P < 0.0001), 17.07 ± 2.39% (P < 0.0001), and 3.7 ± 0.9% (P < 0.0001), respectively, compared with the untreated HTM cells 92.49 ± 1.21%. The mean cell viabilities with 125, 250, 500, and 1000 μg/mL of TA-S were 94.47 ± 1.60% (P > 0.05), 90.13 ± 0.40% (P < 0.01), 85.57 ± 0.47% (P < 0.001), and 71.67 ± 3.30% (P < 0.0001), respectively, compared to DMSO-equivalent cultures. Untreated HTM control had a cell viability of 96.57 ± 1.98%. DMSO-treated controls of 125, 250, 500, and 1000 μg/mL had a cell viability of 94.73 ± 0.57%, 96.97 ± 1.08%, 93.97 ± 1.85%, and 97.27 ± 1.15%, respectively. There was no increase of caspase-3/7 activity in cultures treated with either TA-C or TA-S. DNA laddering showed no bands in the TA-C or TA-S treated cultures. There were significantly higher LDH release rates at all concentrations of TA-C compared to TA-S.
Conclusions:
Results show that the effect of TA-C and TA-S on HTM cells is due to cell death by necrosis at all concentrations except 125 μg/mL of TA-S. Elevated levels of LDH confirmed necrotic cell death. Our study also infers the relative safety of TA-S over TA-C.
Keywords: Triamcinolone acetonide, human trabecular meshwork cells, in vitro
Triamcinolone acetonide (TA) is extensively used in intravitreal injections to treat macular diseases such as macular edema due to diabetic retinopathy,[1] venous occlusive diseases,[1,2] ocular inflammation,[3,4] and also in the cases of choroidal neovascularization from AMD.[5] With recent clinical and experimental studies confirming the pivotal role of intravitreal triamcinolone acetonide (IVTA) in the successful management of routine and refractory cases of inflammatory, neovascular and macular edematous conditions, the use of IVTA has increased in the past decade.[6] The indications have widened to treat other anterior ocular diseases such as neovascularization of the iris. With its expanded use, the complications related to the injection of TA are being reported more frequently.
Complications of IVTA, include increased intraocular pressure (IOP),[7,8] cataract formation,[9] retinal detachment, vitreous hemorrhage, and pseudoendophthalmitis.[10,11] Bioanalytical and pharmacokinetic studies have shown TA in the aqueous following IVTA.[12] Alternative review shows that the most common complications reported are the rise in IOP causing secondary chronic open angle glaucoma that requires medications or antiglaucoma surgeries,[13] progression of cataracts,[14] and accumulation of triamcinolone crystals, resulting in pseudohypopyon. The passage of emulsified TA into the anterior chamber occurs commonly in many clinical scenarios. It can be seen in patients with zonular dehiscence and in those with rupture of the posterior capsule. Presumably, the TA crystals are carried into the anterior chamber by currents generated by saccadic eye movements in these conditions.
Moshfeghi et al. reported a rate of 0.8% of pseudohypopyon after IVTA.[11] Although pseudohypopyon does not cause any ocular morbidity, persistence of even a trace amount of TA may lead to prolonged ocular hypertension occasionally seen in some patients. TA crystals induce cytotoxic and stress response in trabecular meshwork (TM) cells.[15] Although several mechanisms have been put forth to explain the elevated IOP with IVTA, it is still not clear as to the role of TA on the human trabecular meshwork (HTM) cells.
A dose of 25 mg of IVTA led to ocular hypertension in about 50% of treated eyes that lasted for approximately 6 months.[1] However, in another study using 4 mg of IVTA, there was no effect on IOP at 7 days after injection.[16] Although, the intravitreal TA lasts for 3-6 months, persistence of even a trace amount of the drug can lead to a rise in the IOP. HTM cells constantly bathe in the aqueous and an altered aqueous humor outflow resistance plays an important role in the regulation of IOP. Previous works in our laboratory have shown the toxicity of TA on the retinal pigment epithelial (ARPE-19), neurosensory retinal (R28), and human lens epithelial cell lines.[19,20] TA might affect the trabecular meshwork structural framework by increasing the protein expression and inhibiting the proteases or may have a direct effect on the trabecular meshwork cells. This might disrupt the aqueous outflow and prove glaucomagenic.[17]
The aim of this study was to investigate the effects of TA on the HTM cell line. We tested the effects of two forms of TA on the trabecular cells, one being the commercially available crystalline TA suspension (TA-C) and other being the TA without the supernatant, but solubilized in dimethyl sulfoxide (DMSO). Solubilized TA (TA-S) is a solution without any particulate matter. This is in contrast with the TA-C that has a suspension of varying sized particles/crystals that may cause a mechanical effect on the cells. Comparing the TA-S and TA-C formulations, will help distinguish the possible toxicity of the TA crystals on the cells.
Materials and Methods
HTM cells were obtained from Dr. Vincent Raymond, MD, PhD, at the Laboratoire de Genetique et Geonmique Oculaires, CREMO (Quebec, QC). Cells were grown in Dulbecco's modified Eagle's medium (DMEM, Low glucose; Invitrogen-Gibco, Carlsbad, CA), 1000 mg/L D-glucose, L-glutamine, 25 mM HEPES buffer, and 110 mg L-sodium pyruvate, 25 μg/mL, 2.5 μg/mL, amphotericin B, 10% fetal bovine serum.
The cells were plated in 6 and 24-well plates (Fisher Scientific, Park Lane, Pittsburgh, PA) for cell viability (6 × 105 cells/well) and caspase assays (1.2 × 105 cells/well), respectively, and were incubated at 37°C until confluent. The cells were then exposed to TA-C or TA-S at four different concentrations. As controls for the TA-S cultures, some cells were treated with dimethyl sulfoxide (DMSO) at all equivalent concentrations. Before the treatment, the cells were incubated for 2 h in 2% fetal bovine serum containing medium, to make them relatively nonproliferating. This simulates the HTM cells, which remain in a nonproliferating phase.
HTM cells were treated for 24 h with TA-C (Kenalog, Bristol-Meyers Squibb, Princeton, NJ) in the concentration of 125, 250, 500, and 1000 μg/mL. For the TA-S solution, the TA-C was centrifuged at 5000 rpm for 1 min and the supernatant containing the vehicle was discarded. The pellet of TA was resuspended in an equivalent amount of DMSO to achieve the same concentrations of TA as in TA-C. The new TA solution was resuspended in an equivalent amount of culture medium to achieve the desired concentrations of solubilized Triamcinolone (TA-S) (125, 250, 500, and 1000 mg/mL) for 24 h. HTM cells were also treated with equivalent amounts of DMSO as control. We maintained untreated controls in all our experiments.
Cell viability assay was performed as described by Luthra et al. and Narayanan et al.[18,19,20] Cell passages used were between 10 and 15. Briefly, cells were harvested from the 35-mm dishes by treatment with 0.2% trypsin-EDTA at 37°C for 5 min. The cells were centrifuged at 1000 rpm for 1 min. The supernatant was removed and the cell pellet resuspended in 1 mL of culture medium. Automated cell viability analysis was performed using ViCell analyzer (Beckman Coulter Inc., Fullerton, CA). The analyzer performs an automated trypan blue dye-exclusion assay and gives the percentage viability of cells. All concentrations were performed in triplicates and the experiments repeated three times.
Caspase-3/7 is a key effector in the pathway of apoptosis, and its activation heralds the cell commitment to disassembly. Therefore, we analyzed the caspase-3/7 activity to study the molecular pathway of cell death. Sequential activation of caspase plays a central role in the execution phase of cell apoptosis.
The caspase-3/7 activity was detected using caspase detection kits (Carboxyfluorescein FLICA Apoptosis Detection kits; Immunochemistry Technologies, LLC, Bloomington, MN). The FLICA reagent has an optimal excitation range from 488 to 492 nm and an emission range from 515 to 535 nm. Apoptosis was quantified as the level of fluorescence emitted from FLICA probes bound to caspase. Nonapoptotic cells appeared unstained, whereas cells undergoing apoptosis fluoresced brightly.
At the designated time period, the wells were rinsed briefly with fresh culture media, replaced with 300 μL/well of 1 × FLICA solution in culture media, and incubated at 37°C for 1 h. Cells were washed with phosphate buffered saline. The following controls were included: Untreated HTM cells without FLICA to exclude auto fluorescence from cells; untreated HTM cells with FLICA for the comparison of the caspase activity of treated cells; tissue culture plate wells without cells with buffer alone to represent the background levels; tissue culture plate wells without cells with culture media with DMSO to exclude cross reaction of FLICA with DMSO with culture media; HTM with DMSO and FLICA to account for any cross fluorescence between untreated cells and DMSO.
Quantitative calculations of caspase activities were performed with a fluorescence image scanning unit instrument (FMBIO III; Hitachi, Yokohama, Japan). The caspase-3/7 activity was measured as average signal intensity of the fluorescence of the pixels in a designated spot-Mean Signal Intensity (msi). In addition, cultures were observed through an inverted fluorescent microscope (Leica, Solms, Germany) with a band pass filter (excitation 490 nm, emission 520 nm) to view the green fluorescence of caspase positive cells.
Apoptotic cell death is characterized by the cleavage of DNA into oligonucleosomal multimers of approximately 200 basepair fragments which can be visualized by an oligosomal ladder by 3% agarose gel electrophoresis. DNA fragmentation is an early sign of cellular apoptosis. Hence, we did DNA fragmentation assay, a sensitive assay to look for any apoptotic cell death, which could be noncaspase mediated.
HTM cells (1 × 106 cells/dish) were plated overnight in 60 mm dishes and then incubated for another 24 h with 125 and 250 μg/mL of TA-C and 250 and 500 μg/mL of TA-S and DMSO-equivalent cultures. These concentrations were chosen because the cell death at these concentrations was not complete. Still there were good numbers of viable cells or dying cells that expressed quantifiable caspase-3/7 activity. DNA was extracted (QIAamp DNA Micro Kit; Qiagen, Hilden, Germany) according to the manufacturer's instructions. Samples were separated by electrophoresis on 3% agarose gels and stained with 5% ethidium bromide. Images were captured with a fluorescence image scanning instrument (FMBIOIII; Hitachi, Yokohama, Japan).
LDH cytotoxicity assay is a common technique for measuring cell death. It quantifies the release of lactate dehydrogenase (LDH), a stable enzyme found in the cytoplasm, into the supernatant upon rupture of the plasma membrane. It is a colorimetric assay and measures the absorbance at a particular wavelength. The absorbance correlates positively with the number of compromised cell membranes as in necrosis.
LDH release rate was detected using LDH cytotoxicity assay kit-II (Biovision, Mountain View, CA). 2 × 104 of HTM cells were plated in 96-well plate. After 24 h of exposure to TA-C and TA-S, the plate was shaken well to ensure LDH was evenly distributed in the culture medium. The cells were centrifuged at 6000 rpm for 10 min to precipitate the cells. 50 μL/well of supernatant was transferred into an optically clear 96-well plate. The cells were then treated with 100 μL/well LDH reaction mix. LDH reaction mix was prepared by mixing the WST substrate mix with LDH assay buffer. After 30 min of incubation at room temperature, the LDH activity was quantified by multiwell spectrophotometer (Perkin Elmer, Wellesley, MA) at OD 450 nm filter. The plate was read at multiple time points until the consistent reading was observed.
Normative data were subjected to statistical analysis by ANOVA (Prism, ver. 3.0; GraphPad Software Inc., San Diego, CA). Multiple-comparison test was conducted with posthoc Tukey test. Error bars in the graphs represent SEM (Standard Error of Mean) with experiments performed in triplicate. P < 0.05 was considered statistically significant.
Results
HTM cells showed a progressive concentration dependent decrease in cell viability after exposure to both TA-C and TA-S [Figs. 1 and 2] [Table 1a].
Figure 1.
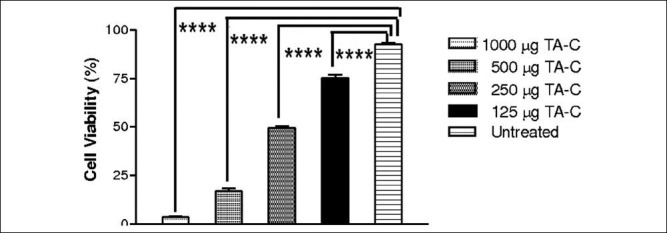
HTM cells showed a dose-dependent decrease in cell viability after treatment at all concentrations of TA-C for 24 h compared with untreated control cultures. Statistically significant (P < 0.0001)
Figure 2.
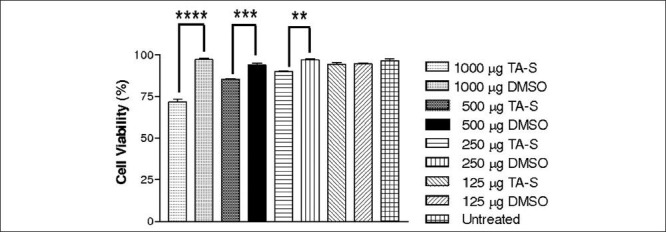
HTM cells showed a dose-dependent decrease in cell viability at all concentrations of TA-S except 125 μg/mL when treated for 24 h compared to respective DMSO-controls. **Statistically significant (P < 0.01) ***Statistically significant (P < 0.001) ****Statistically significant (P < 0.0001)
Table 1a.
The mean cell ciabilities of human trabecular meshwork cells after 24 hours exposure with triamcinolone acetonide treatment
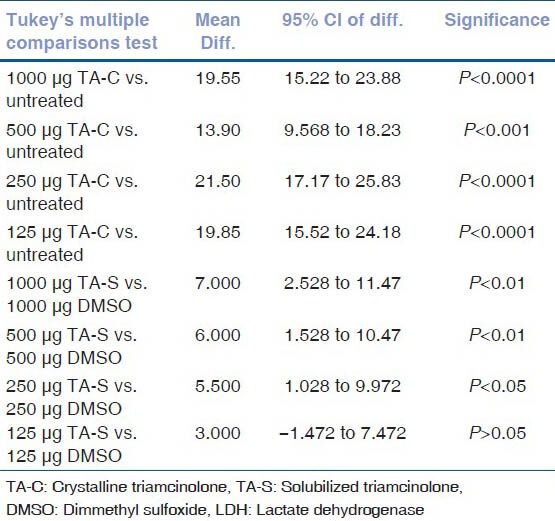
Cell viability of untreated HTM cell cultures was similar to DMSO equivalent cultures of TA-S at all the concentrations. There was significant loss of cell viability at all the concentration of TA-S concentrations except 125 μg/mL compared to untreated HTM cultures. TA-C showed higher loss of cell viability compared to TA-S at all the concentrations tested [Table 1b].
Table 1b.
Results of post hoc tukey test for cell viability
HTM cells showed no statistically significant increase in the caspase-3/7 activity at any concentration of TA-C treated cultures for 24 h [Fig. 3] [Tables 2a and b].
Figure 3.
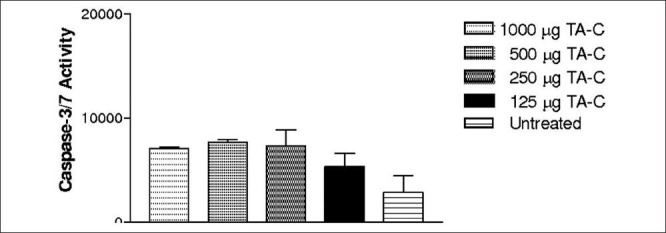
HTM cells did not show statistically significant caspase-3/7 activity at any concentration of TA-C
Table 2a.
The mean caspase-3/7 activity of human trabecular meshwork cells after 24 hours exposure with triamcinolone acetonide treatment
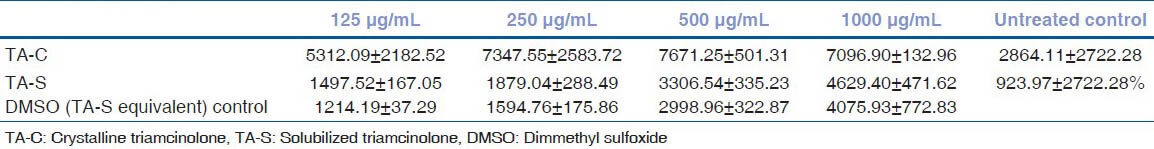
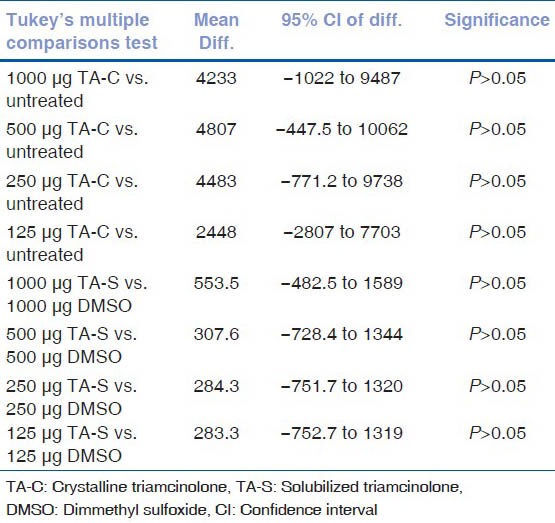
Table 2b.
Results of post hoc tukey test for caspase-3/7 activity
HTM cells showed no statistically significant increase in the caspase-3/7 activity at any concentration of TA-S compared to equivalent DMSO-treated cultures at 24 h [Fig. 4] [Tables 2a and b].
Figure 4.
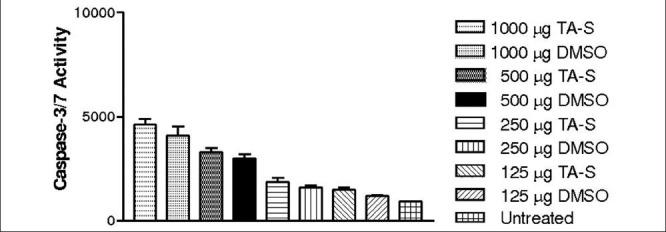
HTM cells did not show statistically significant caspase-3/7 activity at any concentration of TA-S and DMSO-equivalent cultures
Caspase-3/7 activity of untreated HTM cell cultures were similar to DMSO cultures at all the concentrations along with all the TAS concentrations tested [Table 2b].
To verify the findings of our caspase-3/7 assay, we performed DNA fragmentation assay with TA-C at concentration of 125 and 250 μg/mL. No 200 basepair (bp) banding pattern was noted. DNA fragmentation with TA-S at concentration of 250 and 500 μg/mL and DMSO-equivalent controls also did not show any 200 bp banding. This supports our caspase-3/7 findings of nonapoptotic cell death with TA-C and TA-S [Fig. 5a and b].
Figure 5.
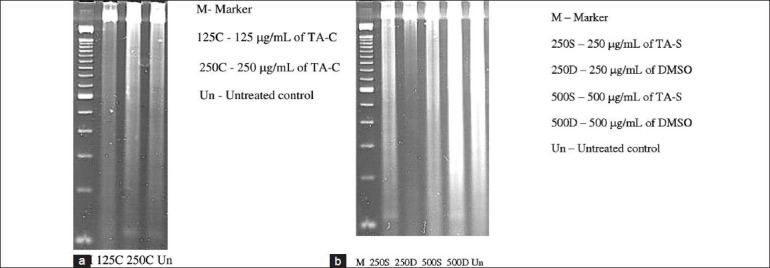
(a) DNA laddering showed no bands at 125 and 250 μg/mL concentration of TA-C cultures. (b) DNA laddering showed no bands at 500 and 250 μg/mL concentration of TA-S and DMSO-equivalents cultures
LDH release rates of untreated HTM cell cultures were similar to DMSO equivalent cultures of TA-S at all the concentrations except 1000 μg/mL (P < 0.05). There was significant release rate at 250, 500, and 1000 μg/mL of TA-S concentrations whereas 125 μg/mL release rate was not significant compared to untreated HTM cultures. TA-C showed higher LDH release compared to TA-S at all the concentrations tested [Figs. 6 and 7] [Tables 3a and b].
Figure 6.
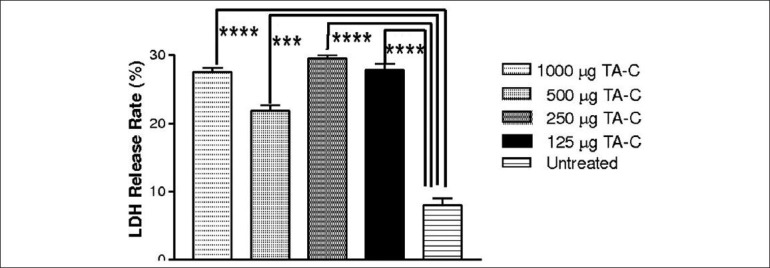
LDH release rate of HTM cells increased at all concentrations tested *** Statistically significant (P < 0.001), ****Statistically significant (P < 0.0001)
Figure 7.
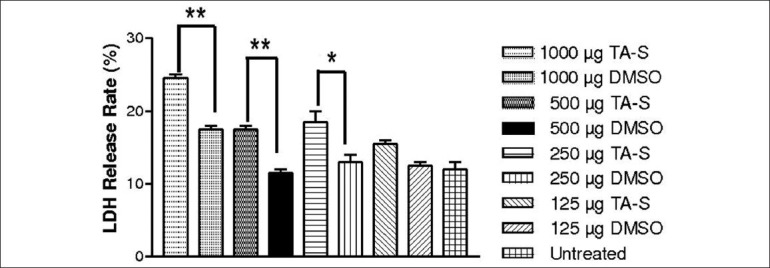
LDH release rate of HTM cells increased at concentration of all the concentrations except 125 μg/mL. *Statistically significant (P < 0.05) **Statistically significant (P < 0.01)
Table 3a.
Lactate dehydrogenase release rate of human trabecular meshwork cells after 24 hours exposure with triamcinolone acetonide treatment
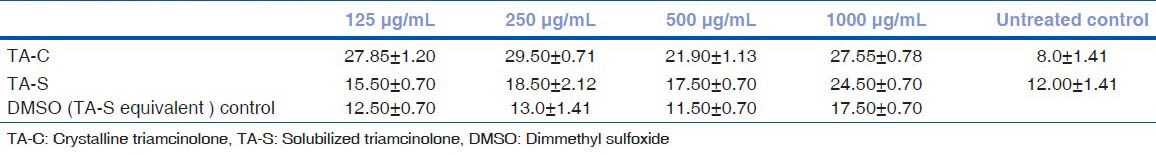
Table 3b.
Results of post hoc tukey test for LDH release rate
Discussion
In this study, we analyzed the effects of TA on HTM cells and noted loss of cell viability at all concentrations 125, 250, 500, and 1000 μg/mL of the commercially available TA solution, TA-C. However, when the TA crystals were solubilized in DMSO (TA-S), the loss of cell viability was less at the higher concentrations and absent at 125 μg/mL. On direct comparison of TA-S and TA-C, TA-S was found to be less toxic to HTM cells at all the concentrations compared to TA-C on cell viability and LDH release assay. This cytotoxicity of TA could be one of the contributing factors for ocular hypertension following IVTA.
Our cytotoxicity studies of TA with HTM cells corroborate the findings by Wang et al.[15] As in our case, Wang et al. found that after being treated with TA, the entire monolayer of cells were covered with TA-C crystals at 24 h. They also reported statistically significant loss in cell number upon treating with TA-C at concentration of 1 and 0.1 mg/mL. When the vehicle containing the preservatives was removed (similar to our TA-S solution), the cell-proliferation as measured by MTT assay was similar to control cultures thereby suggesting the drug without the preservatives to be much safer than the commercial TA. In our study, we demonstrated that although there was significant loss of cell viability with TA-S at higher concentrations (250, 500, and 1000 μg/mL), still it was safer at these concentrations than TA-C in terms of cell viability.
The relative safety of TA-S over TA-C could be due to the fact that TA-S is free from the vehicle containing the preservative, benzyl alcohol, which is found to be cytotoxic by itself. The persistence of loss of cell viability due to TA-S could be related to the direct chemical toxicity of the steroid.
We then studied the molecular pathways involved in the cell death. The activation of caspase-3/7 represents a cell commitment to disassemblage and is the hallmark of apoptosis. Caspase-3/7 assays showed no activity at any concentration of TA-C and TA-S, indicating noncaspase-mediated cell death. We then ran the DNA fragmentation assay with TA-C and TA-S, but did not find any banding pattern, which was consistent with lack of caspase-3/7 activity. This was in contrast to the apoptosis noted in a HTM cell line after TA treatment in a study by Wang et al., with TA.[15] This study examines the effect after 24 h and shows no significant caspase activity that is in agreement with the study by Wang et al.[15]; however, they have found significant apoptosis at day 3 and 5.
The contradictory mechanisms of cell death to the same cytotoxic stimuli are also observed in several other studies using bovine HTM cells and dexamethasone. Sibayan et al. did not notice any apoptosis with dexamethasone at concentration of 0.01-100 μM in bovine trabecular meshwork cells.[21] In contrast, Gu et al. noticed apoptosis with dexamethasone at concentration of 0.24-0.96 mmol·L in the same cell line.[22] Such a phenomenon is also seen with TA and ARPE-19 cells. Chang et al. has shown necrosis to be the main mode of cell death in ARPE-19 cells.[23] This is again in contrast to the studies by Yeung et al. who reported the increasing caspase-3/7 activity and hence apoptosis in ARPE-19 cells.[24] In disagreement with both these studies, Valamanesh et al. demonstrated that in rats the TA induced retinal cell death did not use a caspase-dependent or caspase-independent apoptotic pathways.[25]
Kong et al. suggested that chemical-induced oxidative stress can increase the expression of cytoprotective genes, which can safeguard the cells against apoptosis and enhance cell survival at some concentrations and not at others.[26]. Moreover, apoptotic caspase pathways can be cell-type specific and not stimuli specific.
Necrotic cell death is classically evaluated by the quantification of plasma membrane damage. Lactate dehydrogenase (LDH) is a stable enzyme, present in all cell types, and rapidly released into the cell culture medium upon damage of the plasma membrane. LDH cytotoxicity assay supported our earlier results with caspase-3/7 and DNA fragmentation assays. Statistically significant LDH release rate was observed at all concentrations with TA-C and at 250, 500, and 1000 μg/mL concentrations with TA-S. TA-C had significantly more LDH release at all the concentration compared to TA-S. This supports our hypothesis that the TA-induced HTM cell death was through the necrosis pathway.
The mechanism of raised IOP due to IVTA is not clear. The depletion of TM cells occurs with aging and also with continuous exposure to cytotoxic stimuli. The disruption of the trabecular meshwork beams affect the aqueous outflow and eventually could result in raised IOP.[27,28] Other theories as that the raise in IOP could be because of activation of steroid receptors.[29] or due to the deposition of glycosaminoglycans causing TM dysfunction.[30] Singh et al. showed the rise in IOP as a result of HTM obstruction by the particulate matter of crystalline TA.[31] We infer it could be due to the proposed cytotoxicity of TA on the HTM cells and subsequent TM dysfunction. There are some limitations to extrapolate our results directly to clinical practice.
Shorter incubation: We incubated the HTM cells for 24 h with TA, which would not explain the chronic exposure of TA crystals to the TM cells in vivo
Varying concentrations: In vivo, the aqueous levels of TA following IVTA vary with different time points postinjection and also on the amount injected. This could not be reproduced in our study design
HTM is a single cell type.
In spite of these variations, we still gained valuable information.
We could understand the molecular mechanism of necrotic cell death in TM cells on exposure to TA
HTM cells appeared to be very sensitive to TA compared to other cell types that did not decrease cell viability with the same levels of TA
The differential toxicity of the TA in its two different forms was studied. TA-C was found to have greater cytotoxic effects than TA-S. The relative safety of TA-S over TA-C supports the rationale behind the current use of preservative free TA for intravitreal injections
We could also get some clues to the question of raise in IOP following IVTA.
Footnotes
Source of Support: Supported by the Discovery Eye Foundation, Henry L. Guenther Foundation, The Iris and B. Gerald Cantor Foundation, The Skirball Molecular Ophthalmology Program, Poly and Michael Smith Foundation, Research to Prevent Blindness Foundation.
Conflict of Interest: None declared.
References
- 1.Jonas JB, Hayler JK, Söfker A, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative diabetic retinopathy. Am J Ophthalmol. 2001;131:468–71. doi: 10.1016/s0002-9394(00)00882-5. [DOI] [PubMed] [Google Scholar]
- 2.Lam DS, Chan CK, Tang EW, Li KK, Fan DS, Chan WM. Intravitreal triamcinolone for diabetic macular oedema in Chinese patients: Six-month prospective longitudinal pilot study. Clin Experiment Ophthalmol. 2004;32:569–72. doi: 10.1111/j.1442-9071.2004.00903.x. [DOI] [PubMed] [Google Scholar]
- 3.Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, ffytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular edema: An optical coherence tomography study. Ophthalmology. 2001;108:765–72. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 4.Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001;29:2–6. doi: 10.1046/j.1442-9071.2001.00360.x. [DOI] [PubMed] [Google Scholar]
- 5.Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000;20:244–50. [PubMed] [Google Scholar]
- 6.Baath J, Ells AL, Crichton A, Kherani A, Williams RG. Safety profile of intravitreal triamcinolone acetonide. J Ocul Pharmacol Ther. 2007;23:304–10. doi: 10.1089/jop.2006.125. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Kreissig I, Degenring R. Intraocular pressure after intravitreal injection of triamcinolone acetonide. Br J Ophthalmol. 2003;87:24–7. doi: 10.1136/bjo.87.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonas JB, Degenring RF, Kreissig I, Akkoyun I, Kamppeter BA. Intraocular pressure elevation after intravitreal triamcinolone acetonide injection. Ophthalmology. 2005;112:593–8. doi: 10.1016/j.ophtha.2004.10.042. [DOI] [PubMed] [Google Scholar]
- 9.Ip MS. Intravitreal injection of triamcinolone: An emerging treatment for diabetic macular edema. Diabetes Care. 2004;27:1794–7. doi: 10.2337/diacare.27.7.1794. [DOI] [PubMed] [Google Scholar]
- 10.Westfall AC, Osborn A, Kuhl D, Benz MS, Mieler WF, Holz ER. Acute endophthalmitis incidence: Intravitreal triamcinolone. Arch Ophthalmol. 2005;123:1075–7. doi: 10.1001/archopht.123.8.1075. [DOI] [PubMed] [Google Scholar]
- 11.Moshfeghi DM, Kaiser PK, Scott IU, Sears JE, Benz M, Sinesterra JP, et al. Acute endophthalmitis following intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2003;136:791–6. doi: 10.1016/s0002-9394(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 12.Chu KO, Ho TC, Chiang WY, Wang CC, Lam DS, Pang CP. Measuring triamcinolone acetonide in aqueous humor by gas chromatography-electron-capture negative-ion mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:199–204. doi: 10.1016/j.jchromb.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Dwinger MC, Pieper-Bodeewes I, Eter N, Holz FG. Variations in intraocular pressure (IOP) and necessity for paracentesis following intravitreal triamcinolone injection. Klin Monbl Augenheilkd. 2005;222:638–42. doi: 10.1055/s-2005-858459. [DOI] [PubMed] [Google Scholar]
- 14.Gillies MC, Islam FM, Zhu M, Larsson J, Wong TY. Efficacy and safety of multiple intravitreal triamcinolone injections for refractory diabetic macular oedema. Br J Ophthalmol. 2007;91:1323–6. doi: 10.1136/bjo.2006.113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang DY, Fan BJ, Yam GY, Lam DS, Pang CP. The cytotoxic and stress responses of human trabecular meshwork cells treated with triamcinolone acetonide. Mol Vis. 2008;14:105–13. [PMC free article] [PubMed] [Google Scholar]
- 16.Lee EW, Hariprasad SM, Mieler WF, Newman TL, Apte RS. Short-term intraocular pressure trends after intravitreal triamcinolone injection. Am J Ophthalmol. 2007;143:365–7. doi: 10.1016/j.ajo.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Jones R, 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: A brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163–7. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 18.Luthra S, Dong J, Gramajo AL, Chwa M, Kim DW, Neekhra A, et al. 7-Ketocholesterol activates caspases-3/7, -8, and -12 in human microvascular endothelial cells in vitro. Microvasc Res. 2008;75:343–50. doi: 10.1016/j.mvr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Narayanan R, Mungcal JK, Kenney MC, Seigel GM, Kuppermann BD. Toxicity of triamcinolone acetonide on retinal neurosensory and pigment epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:722–8. doi: 10.1167/iovs.05-0772. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Pirouzmanesh A, Patil J, Estrago-Franco MF, Zacharias LC, Pirouzmanesh A, et al. Evaluation of the toxicity of triamcinolone acetonide and dexamethasone sodium phosphate on human lens epithelial cells. J Ocul Pharmacol Ther. 2011;27:265–71. doi: 10.1089/jop.2010.0120. [DOI] [PubMed] [Google Scholar]
- 21.Sibayan SA, Latina MA, Sherwood ME, Flotte TJ, White K. Apoptosis and morphologic changes in drug-treated trabecular meshwork cells in vitro. Exp Eye Res. 1998;66:521–9. doi: 10.1006/exer.1997.0458. [DOI] [PubMed] [Google Scholar]
- 22.Gu Y, Zeng S, Qiu P, Peng D, Yan G. Apoptosis of bovine trabecular meshwork cells induced by dexamethasone. Zhonghua Yan Ke Za Zhi. 2002;38:302–4. [PubMed] [Google Scholar]
- 23.Chang YS, Wu CL, Tseng SH, Kuo PY, Tseng SY. Cytotoxicity of triamcinolone acetonide on human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2792–8. doi: 10.1167/iovs.06-1146. [DOI] [PubMed] [Google Scholar]
- 24.Yeung CK, Chan KP, Chan CK, Pang CP, Lam DS. Cytotoxicity of triamcinolone on cultured human retinal pigment epithelial cells: Comparison with dexamethasone and hydrocortisone. Jpn J Ophthalmol. 2004;48:236–42. doi: 10.1007/s10384-003-0053-8. [DOI] [PubMed] [Google Scholar]
- 25.Valamanesh F, Torriglia A, Savoldelli M, Gandolphe C, Jeanny JC, BenEzra D, et al. Glucocorticoids induce retinal toxicity through mechanisms mainly associated with paraptosis. Mol Vis. 2007;13:1746–57. [PubMed] [Google Scholar]
- 26.Kong AN, Yu R, Lei W, Mandlekar S, Tan TH, Ucker DS. Differential activation of MAPK and ICE/Ced-3 protease in chemical-induced apoptosis. The role of oxidative stress in the regulation of mitogen-activated protein kinases (MAPKs) leading to gene expression and survival or activation of caspases leading to apoptosis. Restor Neurol Neurosci. 1998;12:63–70. [PubMed] [Google Scholar]
- 27.Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond) 1987;1:204–10. doi: 10.1038/eye.1987.38. [DOI] [PubMed] [Google Scholar]
- 28.Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in primary open-angle glaucoma and nonglaucomatous normals. Ophthalmology. 1984;91:564–79. doi: 10.1016/s0161-6420(84)34248-8. [DOI] [PubMed] [Google Scholar]
- 29.Weinreb RN, Bloom E, Baxter JD, Alvarado J, Lan N, O’Donnell J, et al. Detection of glucocorticoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci. 1981;21:403–7. [PubMed] [Google Scholar]
- 30.Tane N, Dhar S, Roy S, Pinheiro A, Ohira A, Roy S. Effect of excess synthesis of extracellular matrix components by trabecular meshwork cells: Possible consequence on aqueous outflow. Exp Eye Res. 2007;84:832–42. doi: 10.1016/j.exer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Singh IP, Ahmad SI, Yeh D, Challa P, Herndon LW, Allingham RR, et al. Early rapid rise in intraocular pressure after intravitreal triamcinolone acetonide injection. Am J Ophthalmol. 2004;138:286–7. doi: 10.1016/j.ajo.2004.03.001. [DOI] [PubMed] [Google Scholar]


