Abstract
Aim:
To evaluate the prevalence and causes of low vision and blindness in an urban south Indian population.
Settings and Design:
Population-based cross-sectional study. Exactly 3850 subjects aged 40 years and above from Chennai city were examined at a dedicated facility in the base hospital.
Materials and Methods:
All subjects had a complete ophthalmic examination that included best-corrected visual acuity. Low vision and blindness were defined using World Health Organization (WHO) criteria. The influence of age, gender, literacy, and occupation was assessed using multiple logistic regression.
Statistical Analysis:
Chi-square test, t-test, and multivariate analysis were used.
Results:
Of the 4800 enumerated subjects, 3850 subjects (1710 males, 2140 females) were examined (response rate, 80.2%). The prevalence of blindness was 0.85% (95% CI 0.6–1.1%) and was positively associated with age and illiteracy. Cataract was the leading cause (57.6%) and glaucoma was the second cause (16.7%) for blindness. The prevalence of low vision was 2.9% (95% CI 2.4–3.4%) and visual impairment (blindness + low vision) was 3.8% (95% CI 3.2–4.4%). The primary causes for low vision were refractive errors (68%) and cataract (22%).
Conclusions:
In this urban population based study, cataract was the leading cause for blindness and refractive error was the main reason for low vision.
Keywords: Blindness, cataract, India, low vision, population
Blindness is an important public health problem in India.[1,2,3] A nation-wide survey was conducted recently to evaluate the impact of the World Bank supported Cataract Blindness Control Project in the country. According to this report, the blindness rates in the country have decreased mainly due to the National Blindness Control Program.[3] Blindness remains a major health and social issue in a vast country like India which has a population of over 1 billion and where providing access to health care and education remains a challenge. Continued collection of information on low vision and blindness will help improve understanding of the problem and assist in developing newer strategies. The purpose of the present study is to report the prevalence and causes of low vision and blindness in an urban population in Chennai, India.
Materials and Methods
The details of the study design and sampling plan are published elsewhere.[4] In brief, the Chennai Glaucoma Study (CGS) was designed to estimate the prevalence of glaucoma in a rural and an urban population in South India. This study was approved by the Institutional Ethics Review Board and was performed in accordance with the tenets of the Helsinki Declaration for research involving human subjects. The present study includes only urban subjects and was conducted between May 2002 and May 2004.
The sample, for the urban component of the study, was selected using a multistage random cluster sampling procedure. According to the 1991 census (the most current information available at the time), the total population of Chennai was 3.8 million. Considering that 22% of the population was over 40 years, the approximate number of persons in Chennai aged over 40 was 0.85 million. The city is divided into 10 administration zones, comprising 155 divisions. One division was randomly selected from each of the 10 zones and five divisions were randomly picked from these 10 divisions. A simple random sample consisting of 960 individuals aged 40 years or older from each of the five randomly selected divisions was enumerated. A total of 4800 subjects were enumerated. Trained social workers performed the enumeration by a door-to-door survey. During enumeration, the demographic information was collected by a household questionnaire. All the eligible subjects were allotted a unique nine-digit identification number and were invited to come to the base hospital for a detailed ophthalmic examination.
After obtaining the written informed consent, all subjects were made to undergo a comprehensive ophthalmic examination. The ophthalmic examination consisted of recording the best-corrected visual acuity using the modified ETDRS chart, applanation tonometry, gonioscopy, grading of lens opacities using LOCS II[5] for those with a minimum pupillary dilation of 6 mm, stereoscopic evaluation of the optic nerve head and macula using + 78 diopter lens at the slit lamp, a detailed retinal examination with a binocular indirect ophthalmoscope using a +20 diopter lens optic disc fundus photography.
The presenting and best-corrected visual acuity was measured using the logarithm of minimum angle of resolution (logMAR) 4-m charts. Landolt's C chart was used for those who could not read English. Monocular visual acuity was determined with current spectacle prescription, if any, and pinhole acuity was assessed in eyes with visual acuity less than 20/20 (logMAR 0.0). Streak retinoscopy and subjective refraction were performed on all subjects. The best-corrected visual acuity was ascertained and the value recorded. If the visual acuity could not be measured, we used the following tests sequentially: counting fingers, hand movements, and light perception. Automated threshold visual field test using SITA standard 30-2 program (Model 750, Humphrey Instruments, San Leandro, CA, USA) was performed for subjects with glaucoma, optic atrophy, retinitis pigmentosa, and glaucoma suspects. After the completion of examination, the diagnosis was recorded using International Classification of Diseases-9.[6] If more than one disease was present, the disease that was most likely to have a significant effect on vision was considered as the cause for blindness. The definitions for low vision and blindness that we used were similar to the World Health Organization (WHO) definitions. Blindness was defined as best-corrected distance visual acuity of less than 3/60 and/or less than 10° visual field in the better eye. Low vision was defined as a best-corrected distance visual acuity of less than 6/18 but equal to or better than 3/60 in the better eye. We did not include visual fields in this group. We classified people with at least primary education as literates and people with no formal education as illiterates.[7]
Statistical analysis was carried out using SPSS for Windows (SPSS Inc., Chicago, IL, USA). Significance was assessed at the p < 0.05 level for all parameters. Univariate analysis for gender, literacy, and occupation was carried out using the Chi-square test; age between the two groups was compared using the independent t-test. Multivariate logistic regression analysis was done after adjusting for age (age group of 40–49 years was used as the reference age group) and gender. Blindness was the dependent variable.
Results
A total of 3850 subjects, of the enumerated 4800, participated in the study (response rate, 80.2%). Of these, 1710 (44.4%) subjects were males and 2140 (55.6%) were females. Out of 950 non-participants (19.8%), 577 were males (60.7%) and 373 (39.3%) were females. The mean age of the study population was 54.8 ± 10.6 years, which was slightly higher than that of the non-participants (53.8 ± 10.9 years). Exactly 3018 (78.4%) subjects were literates and 832 (21.6%) were illiterates.
Blindness and visual impairment rates
Thirty-three subjects [male: female = 14 (42.4%): 19 (57.6%)] were found to be blind as per our definitions and their mean age was 68.6 ± 13.1 years. In 31 subjects (94%), blindness was diagnosed based on visual acuity measurements, and it was based on visual field changes in two subjects (6%). Thirty (91%) out of the 33 blind subjects were non-manual workers, 16 (48.5%) were literates, and 17 (51.5%) were illiterates. The blindness rate was 0.5% (16 out of 3018) among the literates and 2% (17 out 832) in the illiterates. The prevalence of blindness in this study population was 0.85% (95% CI 0.6–1.1%). The prevalence of low vision in this population was 2.9% (95% CI 2.4–3.4%) and visual impairment (blindness + low vision) was 3.8% (95% CI 3.2–4.4%).
The visual acuity data of the study population is given in Table 1. Eighty-six (2.2%) of the 3850 subjects had visual acuity of less than 3/60 on presentation. With refraction, the visual acuity of 55 subjects improved, resulting in 31 subjects (0.8%) with less than 3/60 vision. Low vision was seen in 353 (9.2%) subjects on presentation and in 112 (2.9%) subjects after refractive error correction. The association of age, gender, occupation, and literacy with blindness is shown in Table 2. After adjusting for gender, blindness was found to be significantly associated with increasing age. Using the 40-49 year age group as a reference population, the Odds Ratio (OR) for the 50–59 age group increased from 1.35 (95% CI 0.3–6.8%) to 67.3 (95% CI 15.5–291.8%) for subjects above 80 years. Illiteracy was positively associated with blindness (OR 2.4, 95% CI 1.1–5.2%). There was no association with gender or occupation. The causes of blindness are enumerated in Table 3; cataract was leading cause for blindness [38 (57.6%) eyes], followed by glaucoma [11 (16.7%) eyes]. In 29 subjects, the cause for blindness was the same in both eyes. Cataract was responsible for blindness in 18 subjects (62.1%) and glaucoma in 5 subjects (17.2%). Fig. 1 gives the causes for low vision; the primary cause was refractive error (68%), followed by cataract (22%).
Table 1.
Visual acuity data of subjects with low vision and blindness
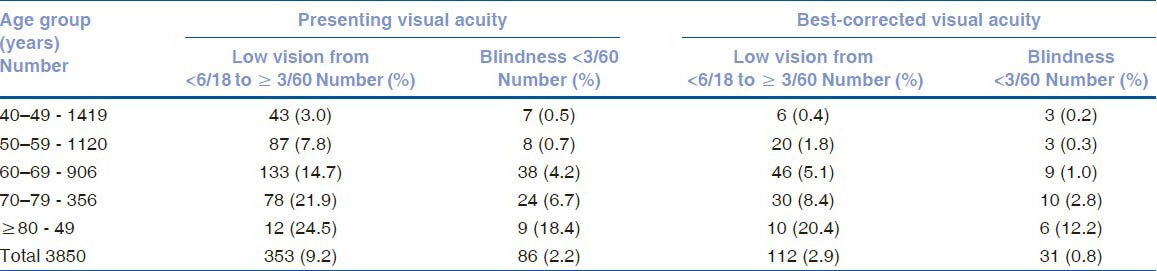
Table 2.
Effect of age, gender, occupation, and literacy on blindness
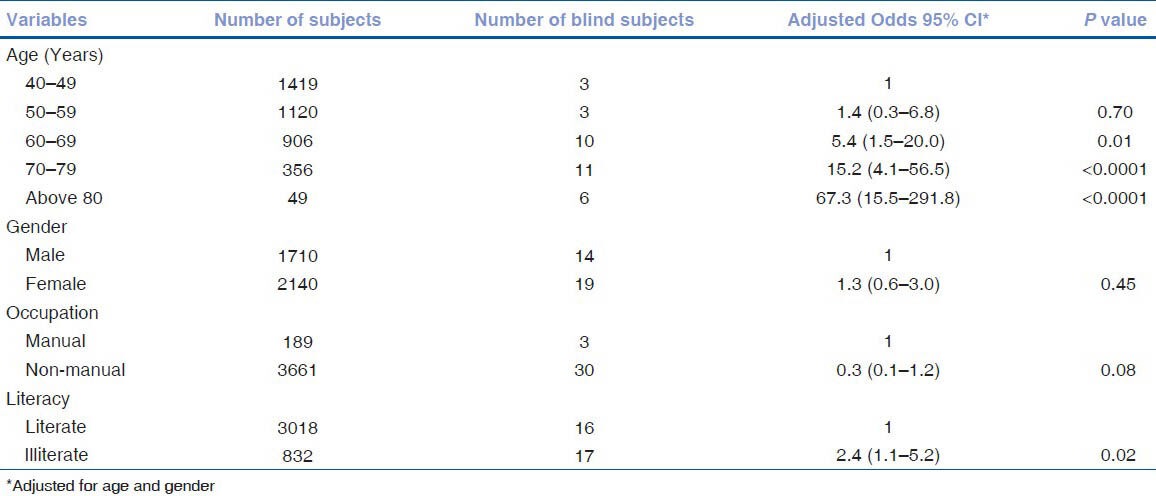
Table 3.
Causes for blindness
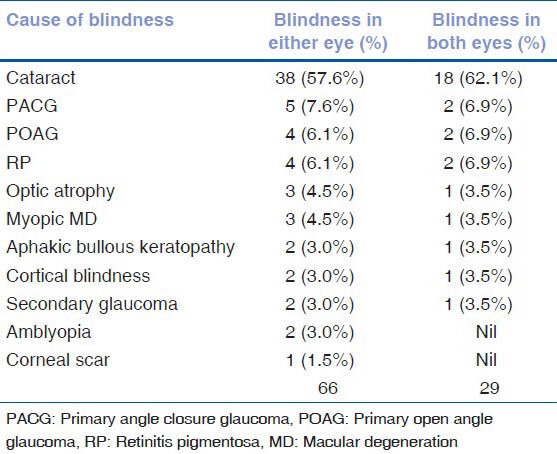
Figure 1.
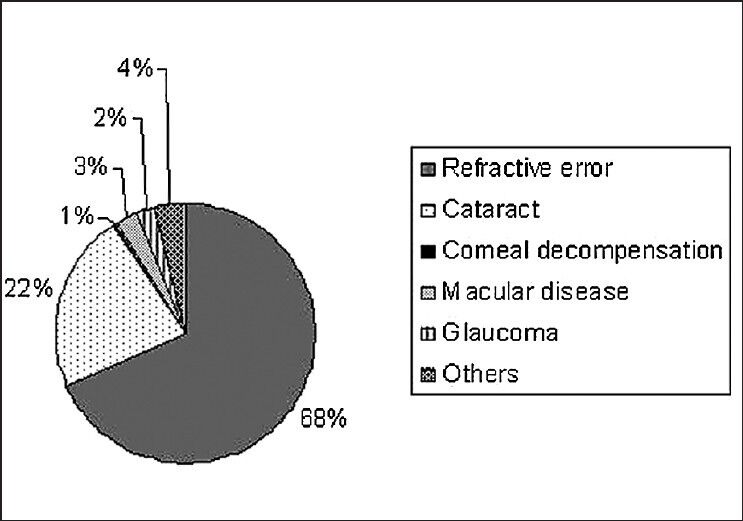
Causes for low vision
The age- and gender-adjusted (based on provisional population totals, census of India 2001)[8] prevalence of blindness among subjects ≥ 40 years in the urban Tamil Nadu population was 1.07% (95% CI 0.7–1.4%). It was 3.02% (95% CI 2.5–3.6%) for low vision and 4.09% (95% CI 3.5–4.7%) for visual impairment.
Discussion
Chennai is one of the four metropolitan cities in India and is popularly known as the “health city” of India. In such an urban population, we found the prevalence of blindness to be 0.85% (95% CI 0.6–1.1%), low vision 2.9% (95% CI 2.4–3.4%), and visual impairment (blindness + low vision) 3.8% (95% CI 3.2–4.4%).
We reported the prevalence of blindness in the rural population to be 3.36% (95% CI 2.8–3.9%) using the same methodology.[1] This fourfold rural and urban difference in blindness rates clearly suggests that probably the urban population has better access to ophthalmic care. The reported prevalence of blindness using the WHO definition in an urban population aged 40 and above was 0.2% (95% CI 0.1–1.0%) in Beijing[9] and 0.14% (95% CI 0.06–0.32%) in Tajami.[10] It appears from these reports that our urban population blindness rate is higher than the urban blindness rates in other Asian countries. This could probably be due to the differences in socioeconomic conditions, health care practices, and access to vision care.
Previous studies have shown that age is a risk factor for blindness.[1,2,3,11,12,13,14] In this study, the OR for blindness increased from 1.4 for 50–60 years age group to 67.3 for subjects aged 80 and above. If appropriate measures are not taken to control the reversible causes for blindness, blindness rates will continue to increase. Illiteracy was positively associated with blindness in our population; those in the low socioeconomic stratum are less likely to have access to education and health care, resulting in higher blindness rates. Gender differences in the prevalence of blindness in the Indian population were reported earlier.[3,13] We did not see any such difference in gender either in our rural population[1] or in the current urban population. Unlike the nation-wide survey which suggested that unemployed people were likely to have higher rates of blindness, we did not notice any difference in the blindness rates among different occupational groups.[2]
The causes of blindness vary across the world. There are a number of studies suggesting that the leading cause of blindness in the White population is age-related macular degeneration.[15,16,17] Cataract seems to be the leading cause of blindness in Africa and in developing countries.[1,2,3,11,12,13,14,18] In India, cataract has been documented to be the cause of bilateral blindness in 50–80% of blind people.[1,2,3] Our study population was similar with cataract being the main cause for blindness in 57.6%. This is lower than the reported cataract blindness rates in our rural population (78.6%). There are many reasons for the high rate of cataract blindness in the country, such as increasing life expectancy, lack of awareness and access to vision care, among others. We do not have the details of the study population cataract surgery data. However, 4.5% of the causes of blindness(in 3 out of 66 eyes - corneal scar in one eye and bullous keratopathy in 2 eyes) were possibly related to cataract surgery. This again is lower than the reported causes for blindness following cataract surgery in our rural population (7.2%). One of the reasons for this rural–urban difference could be the fact that our urban population has better access to vision care.[19] The second leading cause for blindness in this present study was glaucoma. This finding is similar to other population studies in India that have shown glaucoma as the second leading cause of blindness in the adults.[20] Unlike cataract, glaucoma results in irreversible blindness which can potentially be prevented if diagnosed early. The high rates of blindness due to glaucoma in India can be explained partially by the large proportion of undiagnosed disease in the population. In population-based studies across the country, more than 90% of glaucoma patients were diagnosed during the study examination.[20] The causes for poor detection rates were overdependence on intraocular pressure measurements to diagnose glaucoma and the lack of a comprehensive eye examination by eye care professionals. Unless we improve our ability to diagnose glaucoma in the country, glaucoma detection rates cannot be improved. The low detection rates will continue to increase the blindness rates due to glaucoma. With an increase in life expectancy, in India, the number of people at risk of developing glaucoma will increase, thereby resulting in more blindness due to glaucoma. There is a need to sensitize eye care professionals to adopt a comprehensive eye examination that includes slit-lamp biomicroscopy, applanation tonometry, gonioscopy, indirect ophthalmoscopy, and stereoscopic evaluation of the optic disc. This definitely will improve detection rates of any eye disease, which in turn will minimize visual impairment, resulting in blindness. The public too should be educated about the importance of undergoing a comprehensive eye examination.
The primary cause for low vision in our population was refractive errors. Low vision on presentation was seen in 353 (9.2%) subjects, and with appropriate refraction in two-thirds of this group (241 subjects, 6.3%), vision improved. This high proportion of low vision due to uncorrected refractive errors is similar to other reports from different parts of the world including India.[21,22] The second cause for low vision was cataract.
In conclusion, we report the prevalence and causes of low vision and blindness in an urban South Indian population in subjects aged 40 and above. Our results suggest that cataract is the main cause of blindness and the prevalence is lower than the prevalence reported for the rural population. Glaucoma is the leading cause of irreversible blindness. An increase in ophthalmic care and public education on the need for comprehensive examination is needed to minimize the irreversible blindness rates in this part of the world. Uncorrected refractive errors and cataract were the main causes for low vision.
Acknowledgments
Authors thank the Chennai Willingdon Corporate Foundation, Chennai for their financial support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vijaya L, George R, Arvind H, Baskaran M, Raju P, Ramesh SV, et al. Prevalence and causes of blindness in a rural south Indian population. Br J Ophthalmol. 2006;90:407–10. doi: 10.1136/bjo.2005.081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy GV, Vashist P, John N, Pokharel G, Ellwein LB. Prevelence and causes of visual impairment and blindness in older adults in an area of India with a high cataract surgical rate. Ophthalmic Epidemiol. 2010;17:185–95. doi: 10.3109/09286586.2010.483751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy GV, Gupta SK, Bachani D, Jose R, John N. Current estimates of blindness in India. Br J Ophthalmol. 2005;89:257–60. doi: 10.1136/bjo.2004.056937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvind H, Paul PG, Raju P, Baskaran M, George R, Balu S, et al. Methods and design of the Chennai glaucoma study. Ophthalmic Epidemiol. 2003;10:337–48. doi: 10.1076/opep.10.5.337.17320. [DOI] [PubMed] [Google Scholar]
- 5.Chylack LT, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107:991–7. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 6.9th ed. Geneva, Switzerland: World Health Organization; 1977. Manual of the international classification of diseases, injuries, and causes of death. [Google Scholar]
- 7.Paul PG, George R, Baskaran M, Arvind H, Raj M, Augustian , et al. A comparison of participants and non-participants in the Chennai glaucoma study—rural population. Ophthalmic Epidemiol. 2005;2:1–8. doi: 10.1080/09286580590932798. [DOI] [PubMed] [Google Scholar]
- 8.Census of India. [Accessed May 7,2007]. Available from: http://www.censusindia.net/agedata .
- 9.Xu L, Wang Y, Li Y, Wang Y, Cui T, Li J, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: The Beijing eye study. Ophthalmology. 2006;113:1134–41. doi: 10.1016/j.ophtha.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Iwase A, Araie M, Tomidokoro A, Yamamoto T, Shimizu H, Kitazawa Y. Prevalence and causes of low vision and blindness in a Japanese adult population: The Tajami study. Ophthalmology. 2006;113:1354–62. doi: 10.1016/j.ophtha.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Dandona L, Dandona R, Naduvilath TJ, McCarty CA, Srinivas M, Mandal P, et al. Burden of moderate visual impairment in an urban population in southern India. Ophthalmology. 1999;106:497–504. doi: 10.1016/S0161-6420(99)90107-0. [DOI] [PubMed] [Google Scholar]
- 12.Dandona L, Dandona R, Srinivas M, Giridhar P, Prasad MN, Vilas K, et al. Moderate visual impairment in India: The Andhra Pradesh eye disease study. Br J Ophthalmol. 2002;86:373–7. doi: 10.1136/bjo.86.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dandona L, Dandona R, Srinivas M, Giridhar P, Vilas K, Prasad MN, et al. Blindness in the Indian state of Andhra Pradesh. Invest Ophthalmol Vis Sci. 2001;42:908–16. [PubMed] [Google Scholar]
- 14.Thulisiraj RD, Nirmalan PK, Ramakrishnan R, Krishnadas R, Manimekalai TK, Bapurajan NP. Blindness and vision impairement in a rural south Indian population: The Aravind comprehensive eye survey. Ophthalmology. 2003;110:1491–8. doi: 10.1016/S0161-6420(03)00565-7. [DOI] [PubMed] [Google Scholar]
- 15.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia: The Blue Mountains eye study. Ophthalmology. 1996;103:357–64. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 16.Klaver CC, Wolfs RC, Vingerling JR, Hofman A, de Jong PT. Age - specific prevalence and causes of blindness and visual impairment in an older population: The Rotterdam Study. Arch Ophthalmol. 1998;116:653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 17.VanNewkirk M, Weih L, McCarty CA, Taylor HR. Causes-specific prevalence of bilateral visual impairment in Victoria, Australia: The Visual Impairment Project. Ophthalmology. 2001;108:960–7. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 18.Abdull MM, Sivasubramaniam S, Murthy GV, Gilbert C, Abubakar T, Ezelum C, et al. Nigeria National Blindness and Visual Impairement Study Group. Causes of blindness and visual impairement in Nigeria: National blindness and visual impairement survey. Invest Ophthalmol Vis Sci. 2009;50:4114–20. doi: 10.1167/iovs.09-3507. [DOI] [PubMed] [Google Scholar]
- 19.Rao GN. Ophthalmology in India. Arch Ophthalmol. 2000;118:1431–2. doi: 10.1001/archopht.118.10.1431. [DOI] [PubMed] [Google Scholar]
- 20.George R, Ramesh SV, Vijaya L. Glaucoma in India: Estimated burden of disease. J Glaucoma. 2010;19:391–7. doi: 10.1097/IJG.0b013e3181c4ac5b. [DOI] [PubMed] [Google Scholar]
- 21.Raju P, Ramesh SV, Arvind H, George R, Baskaran M, Paul PG, et al. Prevalence of refractive errors in a rural South Indian population. Invest Ophthalmol Vis Sci. 2004;45:4268–72. doi: 10.1167/iovs.04-0221. [DOI] [PubMed] [Google Scholar]
- 22.Raju P, George R, Ramesh SV, Arvind H, Baskaran M, Govindaswamy K, et al. Comparison of refractive errors and factors associated with spectacle use in a rural and urban South Indian population. Indian J Ophthalmol. 2008;56:139–44. doi: 10.4103/0301-4738.39119. [DOI] [PMC free article] [PubMed] [Google Scholar]


