Abstract
Many systemic antimicrobials have been implicated to cause ocular adverse effects. This is especially relevant in multidrug therapy where more than one drug can cause a similar ocular adverse effect. We describe a case of progressive loss of vision associated with linezolid therapy. A 45-year-old male patient who was on treatment with multiple second-line anti-tuberculous drugs including linezolid and ethambutol for extensively drug-resistant tuberculosis (XDR-TB) presented to us with painless progressive loss of vision in both eyes. Color vision was defective and fundus examination revealed optic disc edema in both eyes. Ethambutol-induced toxic optic neuropathy was suspected and tablet ethambutol was withdrawn. Deterioration of vision occurred despite withdrawal of ethambutol. Discontinuation of linezolid resulted in marked improvement of vision. Our report emphasizes the need for monitoring of visual function in patients on long-term linezolid treatment.
Keywords: Ethambutol, linezolid, optic neuropathy
Linezolid is the first member of a new synthetic class of anti-microbials known as oxazolidinones with activity against many important pathogens including multidrug-resistant tubercle bacillus, methicillin-resistant staphylococcus and streptococcus. We report a case of toxic optic neuropathy due to linezolid occurring in a patient who was on concurrent linezolid and ethambutol therapy for extensively drug-resistant pulmonary tuberculosis (XDR-TB).
Case Report
A 45-year-old man presented to our outpatient department with painless progressive diminution of vision both eyes for the past 10 days. Medical history included XDR-TB on treatment with linezolid (600 mg/day), ethambutol (800 mg/day), moxifloxacin (400 mg/day), cycloserine (500 mg/day), ethionamide (500 mg/day), and kanamycin (750 mg/day) for the past 6 months. He was a non-smoker and non-alcoholic.
On examination, his visual acuity was 20/200 (OU), not improving with pin hole. Color vision was defective in both eyes. Anterior segment examination was unremarkable and pupils were 3 mm, round, regular, and reacting to light in both eyes (Direct and Indirect). There was no relative afferent pupillary defect (RAPD).
Fundus examination revealed hyperemic disc with blurred margins (OU) [Figs. 1 and 2]. Visual field evaluation by Humphrey field analyzer showed peripheral constriction and quadrantanopia in the right eye [Fig. 3] and low reliable fields in the left eye [Fig. 4]. Optical coherence tomography (OCT) revealed increased retinal nerve fiber layer (RNFL) thickness in both eyes [Fig. 5].
Figure 1.
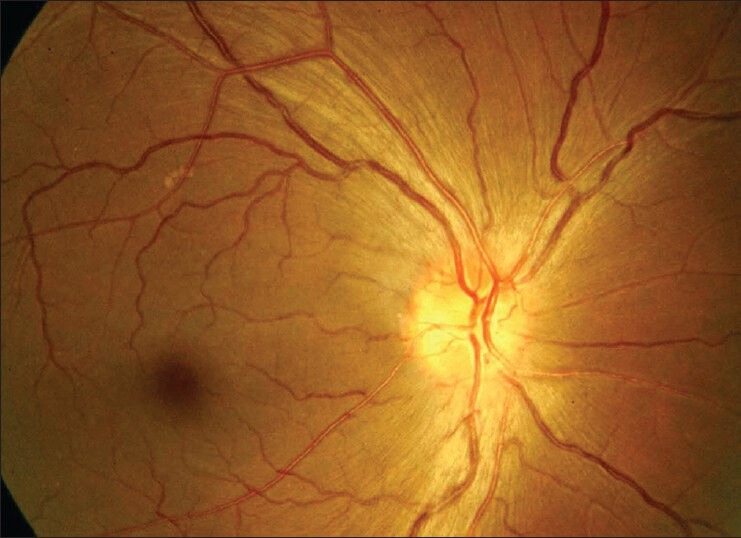
Fundus photograph showing disc edema in right eye
Figure 2.
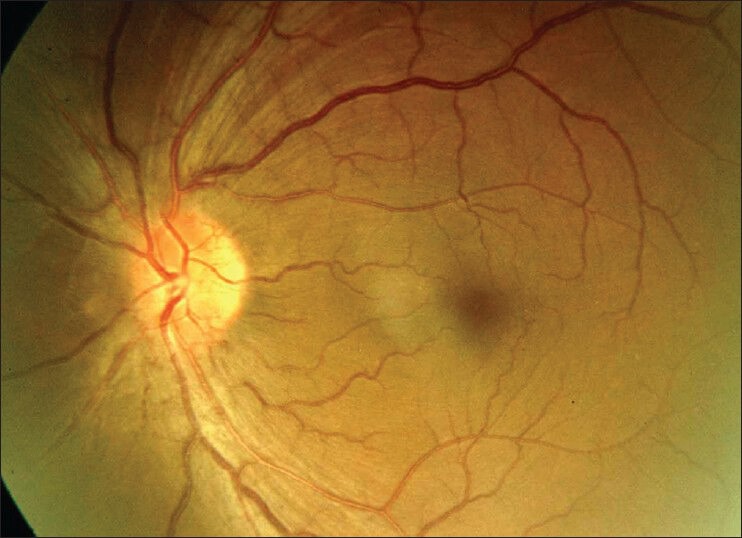
Fundus photograph showing disc edema in left eye
Figure 3.
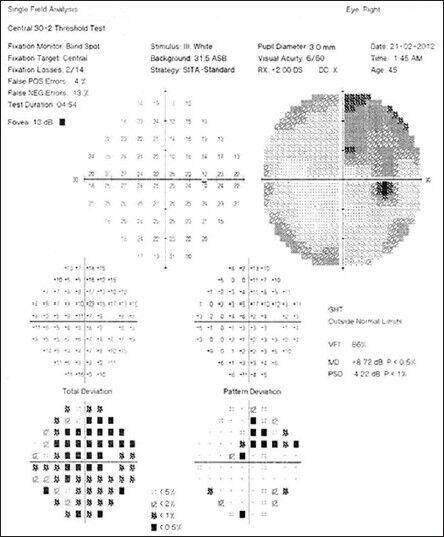
Visual field showing peripheral constriction and superotemporal quadrantanopia in right eye
Figure 4.
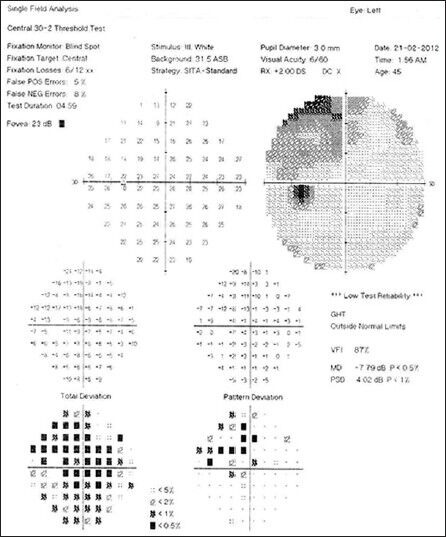
Visual field left eye showing quadrantanopia (low reliability)
Figure 5.
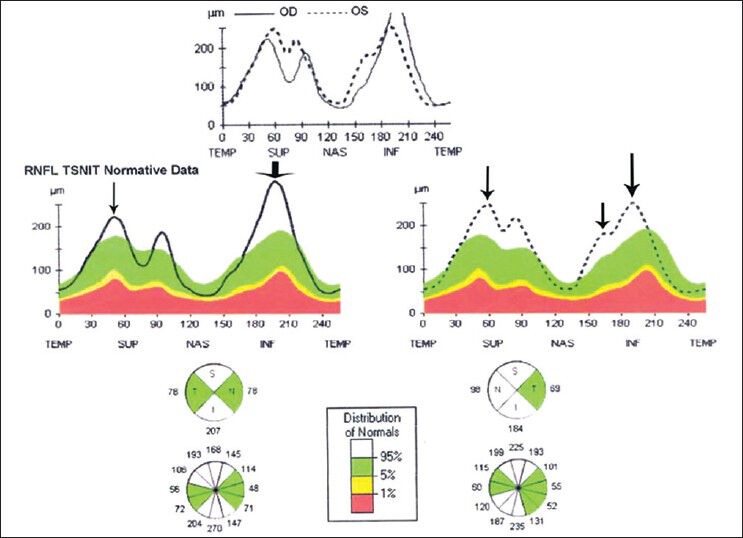
Stratus OCT showing increase in retinal nerve fiber layer thickness involving superior and inferior quadrants in right eye and superior, inferior, and nasal quadrants in left eye (arrows)
Ethambutol-induced toxic optic neuropathy was initially suspected and tablet ethambutol was discontinued after discussing with the treating physician. After two weeks, the patient's visual acuity had dropped to 20/400 in both eyes, and the fundus picture remained unchanged. Hence, the possibility of toxic optic neuropathy due to linezolid was considered as reported in the literature and linezolid was discontinued (A total cumulative dose of 126 g had been already consumed by the patient).
Rapid improvement in visual acuity was seen four days after discontinuation of linezolid. Color vision was restored to normal and patient's vision was restored to 20/20 after one month. Fundus examination revealed resolved optic disc edema with setting in of temporal pallor in both eyes [Figs. 6 and 7]. Follow-up of visual field showed partial improvement with residual quadrantanopia [Figs. 8a and b] and OCT demonstrated normalization of RNFL [Fig. 9]. Tablet ethambutol was restarted at the instance of the physician. The patient is under regular follow-up and no toxic effects have been noted at three months of follow-up.
Figure 6.
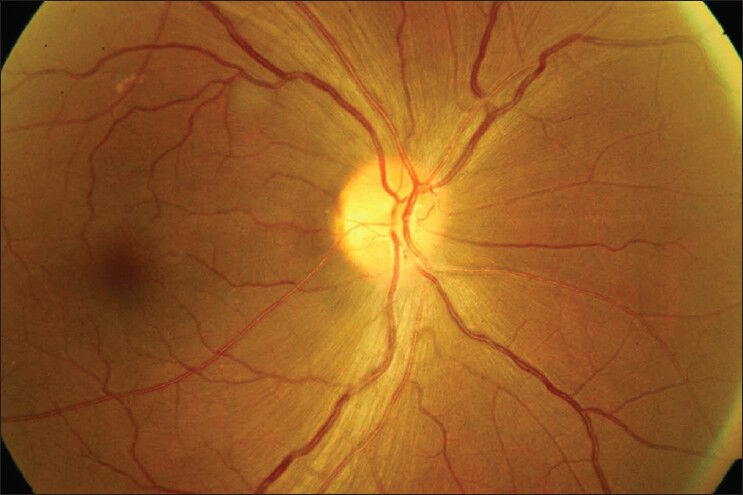
Fundus photograph showing resolved disc edema with temporal pallor of optic disc in right eye
Figure 7.
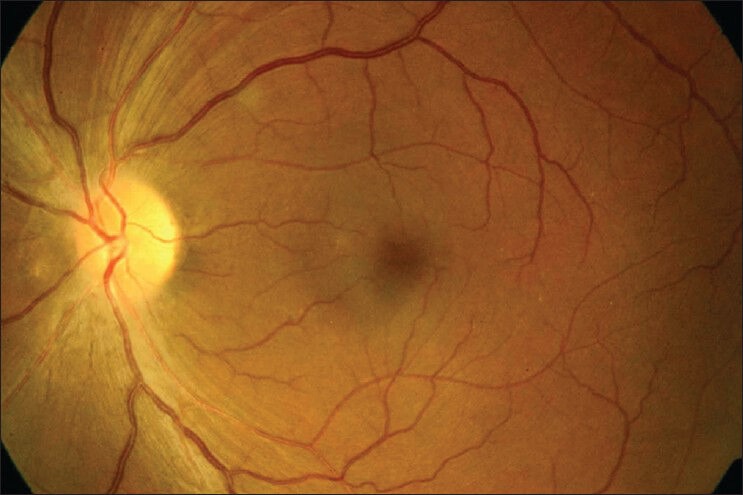
Fundus photograph left eye showing temporal pallor of optic disc
Figure 8a.
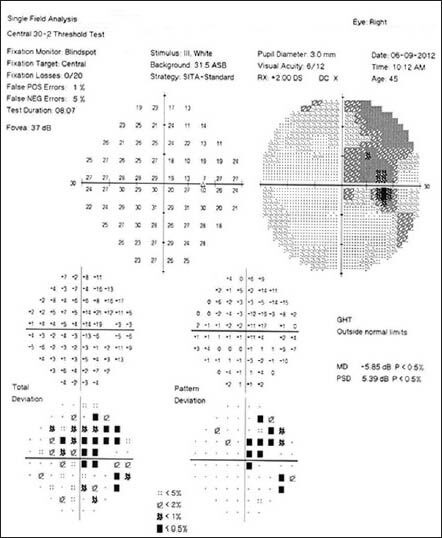
Follow-up visual field after discontinuation of linezolid showing partial recovery in right eye
Figure 8b.
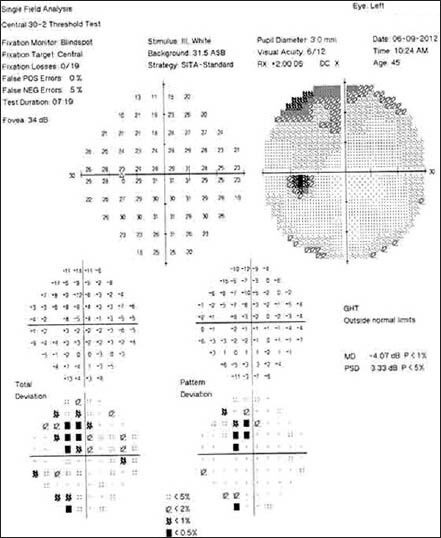
Follow-up visual field in left eye after discontinuation of linezolid
Figure 9.
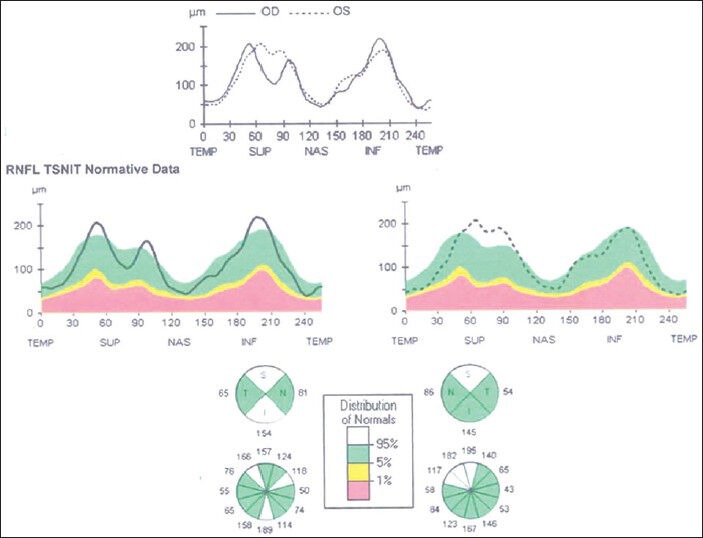
Stratus OCT showing reduction in retinal nerve fiber layer edema in both eyes
Discussion
Toxic optic neuropathies are characterized by gradual, progressive, painless, bilaterally symmetric visual loss affecting central vision, and causing central or centrocecal scotoma.
XDR-TB is defined as resistance to at least rifampicin and isoniazid among the first-line anti-TB drugs (which is the definition of multidrug-resistant TB) in addition to resistance to any fluoroquinolone and at least one of the three injectable second-line anti-TB drugs (capreomycin, amikacin, kanamycin).
Linezolid inhibits protein synthesis by preventing formation of the ribosome complex that initiates protein synthesis. Its unique binding site located on 23S ribosomal RNA of the 50s subunit results in no cross resistance with other drug classes. Hence, linezolid is being increasingly used for the treatment of infections caused by multidrug-resistant Gram-positive bacteria. Long-term linezolid interferes with bacterial ribosomes and also with mammalian ribosomes, thereby disrupting mitochondrial oxidative phosphorylation and protein synthesis.[1]
Linezolid is usually well tolerated with few described adverse effects. Serious adverse reactions demanding withdrawal of the drug include myelosuppression, peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome.[2] The safety of linezolid treatment has been established for use only up to 28 days.[3] There are several case reports of linezolid-induced optic or peripheral neuropathy in patients treated for a time period beyond 28 days.[4] Only two cases of toxic optic neuropathy have been reported following short-term linezolid treatment of 16 days.[5,6] Fundus picture can be varied, showing temporal pallor, disc edema, or essentially normal. Complete visual recovery has been reported in all cases except one.[5] Peripheral neuropathy has been reported to be irreversible.[7] The most common indication for long-term linezolid therapy in these patients has been infection with methicillin-resistant Staphylococcus aureus.
In our case, neuropathy occurred after linezolid had been used for six months at a dose of 600 mg per day for infection with Mycobacterium tuberculosis. The co-administration of ethambutol could have potentiated the toxicity. We attribute toxic optic neuropathy to linezolid in our patient because even two weeks after stopping ethambutol there was deterioration of vision, and it was only after withdrawal of linezolid that visual improvement started.
Linezolid is recommended by the World Health Organization (WHO) to treat drug-resistant tuberculosis as a medicine with “unclear efficacy”.[8] Linezolid, approved for Gram-positive infections in 2000 has been used off-label for drug-resistant tuberculosis. There is limited data available regarding the efficacy and safety of linezolid in multidrug-resistant TB since it is always administered as part of combination therapy.
Linezolid may improve the chance of bacteriological cure only in the most complicated XDR-TB cases. Its safety profile precludes its use in cases for which there are other alternatives.[9]
With increasing emergence of XDR-TB, for which treatment options are limited, physicians are compelled to resort to new drug therapies. Although ethambutol is the most common antitubercular drug implicated to cause toxic optic neuropathy, it is pertinent to be aware that if withdrawal of one drug does not show visual recovery or there is further deterioration of vision, the possibility of toxicity due to other drugs should be thought of. With our country bearing the brunt of tuberculosis, where multiple drug therapy is the rule, this is even more relevant. Ophthalmologists and physicians must be aware that monitoring of visual function is important in patients on long-term linezolid therapy and that early recognition of toxicity and discontinuation of drug results in complete visual recovery.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin Infect Dis. 2006;42:1111–7. doi: 10.1086/501356. [DOI] [PubMed] [Google Scholar]
- 2.Narita M, Tsuji BT, Yu VL. Linezolid- associated peripheral and optic neuropathy, lactic acidosis and serotonin syndrome. Pharmacotherapy. 2007;27:1189–97. doi: 10.1592/phco.27.8.1189. [DOI] [PubMed] [Google Scholar]
- 3.Rydalmere, NSW: Pharmacia Australia; 2002. Zyvox (linezolid) product information. [Google Scholar]
- 4.Javaheri M, Khurana RN, O’hearn TM, Lai MM, Sadun AA. Linezolid–induced optic neuropathy: A mitochondrial disorder? Br J Ophthalmol. 2007;91:111–5. doi: 10.1136/bjo.2006.102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azamfirei L, Copotoiu SM, Branzaniuc K, Szederjesi J, Copotoiu R, Berteanu C. Complete blindness after optic neuropathy induced by short-term linezolid treatment in a patient suffering from muscle dystrophy. Pharmacoepidemiol Drug Saf. 2007;16:402–4. doi: 10.1002/pds.1320. [DOI] [PubMed] [Google Scholar]
- 6.Joshi L, Taylor SR, Large O, Yacoub S, Lightman S. A case of optic neuropathy after short-term linezolid use in a patient with acute lymphatic leukemia. Clin Infect Dis. 2009;48:e73–4. doi: 10.1086/597298. [DOI] [PubMed] [Google Scholar]
- 7.Bressler AM, Zimmer SM, Gilmore JL, Somani J. Peripheral neuropathy associated with prolonged use of linezolid. Lancet Infect Dis. 2004;4:528–31. doi: 10.1016/S1473-3099(04)01109-0. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. [last accessed on June]. Available from: http://www.who.int/entity/tb/publications/2006/who_htm_tb_2008_402.pdf . [PubMed]
- 9.Migliori GB, Eker B, Richardson MD, Sotgiu G, Zellweger JP, Skrahina A, et al. A retrospective TBNET assessment of linezolid safety, tolerability and efficacy in multidrug- resistant tuberculosis. Eur Respir J. 2009;34:387–93. doi: 10.1183/09031936.00009509. [DOI] [PubMed] [Google Scholar]


