Abstract
Background
Randomized controlled trials comparing different vasectomy occlusion techniques are lacking. Thus, this multicenter randomized trial was conducted to compare the probability of the success of ligation and excision vasectomy with, versus without, fascial interposition (i.e. placing a layer of the vas sheath between two cut ends of the vas).
Methods
The trial was conducted between December 1999 and June 2002 with a single planned interim analysis. Men requesting vasectomies at eight outpatient clinics in seven countries in North America, Latin America, and Asia were included in the study. The men were randomized to receive vasectomy with versus without fascial interposition. All surgeons performed the vasectomies using the no-scalpel approach to the vas. Participants had a semen analysis two weeks after vasectomy and then every four weeks up to 34 weeks. The primary outcome measure was time to azoospermia. Additional outcome measures were time to severe oligozoospermia (<100 000 sperm/mL) and vasectomy failure based on semen analyses.
Results
We halted recruitment after the planned interim analysis, when 841 men had been enrolled. Fascial interposition decreased time to azoospermia (hazard ratio [HR], 1.35; P < 0.0001) and time to severe oligozoospermia (HR, 1.32; P < 0.0001) and reduced failures based on semen analysis by about half, from 12.7% (95% confidence interval [CI], 9.7 to 16.3) to 5.9% (95% CI, 3.8 to 8.6) (P < 0.0001). Older men benefited less from fascial interposition than younger men in terms of the speed of achieving azoospermia. However, the number of vasectomy failures was reduced to a similar degree in all age groups. Slightly more adverse events occurred in the fascial interposition group, but the difference was not significant. These failure rates may appear high to practitioners in countries such as the USA, but they are similar to results from other careful studies of ligation and excision techniques.
Conclusion
Fascial interposition significantly improves vasectomy success when ligation and excision is the method of vas occlusion. A limitation of this study is that the correlation between postvasectomy sperm concentrations and risk of pregnancy is not well quantified.
Background
Vasectomy failures are considered rare, but the specific failure rates associated with different vasectomy techniques have not been well documented [1]. Reviews have cited pregnancy rates after vasectomy of 0–2.2% [2,3]. However, recently reported data based on observational studies and individual surgeons' reports suggest that failures are more common than expected following vasectomies performed using ligation and excision [4-7].
The use of fascial interposition (i.e. placing a layer of the vas sheath between the two cut ends of the vas) may improve vasectomy success rates. However, no randomized controlled trials have evaluated fascial interposition, and reports in the literature are conflicting [8,9]. Furthermore, a vasectomy trainer has observed significant individual variations in how this technique is performed (personal communication, Ronald Reynolds, Ohio, March 4, 2003). The Royal College of Obstetricians and Gynecologists' evidence-based recommendations on vasectomy note that the evidence in favor of fascial interposition is at the lowest level (i.e. expert opinion) [10]. Also, the use of fascial interposition might lead to more adverse events, since it involves additional tissue manipulation and thus prolongs operating time [11].
Vasectomy success is commonly defined as two consecutive azoospermic specimens, but many laboratories do not centrifuge specimens for routine clinical testing. Centrifugation of semen samples can detect small numbers of nonmotile sperm that persist for long periods of time, and these sperm may have little clinical significance [12,13]. Though not explicitly stated, a World Health Organization study of hormonal contraception classified counts of <100,000 sperm/mL as azoospermic [14], but the generally accepted definition of azoospermia is the complete absence of sperm.
Surgeons' experience [15] and guidelines recently published by the British Andrology Society [16] suggest that low concentrations of nonmotile sperm (e.g. <100,000 sperm/mL) are of less concern than higher concentrations. Thus, it may be important to define vasectomy success not only by complete absence of sperm but also by very low sperm concentrations.
Thus we conducted this randomized controlled trial to compare the probability of success of ligation and excision of a vas segment with, versus without, fascial interposition. We considered three outcomes in our analysis: (a) time to azoospermia, the complete absence of sperm; (b) time to severe oligospermia, <100,000 sperm/mL; and (c) vasectomy failure based on the results of semen analyses. We followed the CONSORT guidelines for reporting clinical trials [17].
Interim results from this study were presented at a plenary session of the annual meeting of the Association of Reproductive Health Professionals, Washington, DC, USA, December 14, 2001. A description of the interim analysis decision process that led to the halting of the study has been published [18]. A revision of the Royal College of Obstetricians and Gynaecologists' recommendations [10] was issued in January, 2004. The new version cites the published interim results of this study [18] and has changed the evaluation of the evidence against ligation and excision alone from the lowest level, "C" based on expert opinion, to the highest level, "A" based on at least one randomized controlled trial.
Methods
Study sites and participants
The study was conducted at eight outpatient clinics in urban locations in Brazil, El Salvador, Mexico (two clinics), Nepal, Panama, Sri Lanka, and the USA, and was conducted in compliance with the Helsinki Declaration. Family Health International's (FHI's) institutional review board (IRB) approved the study on August 28, 1998, and it was also approved by individual IRBs at five of the eight sites. Because the remaining three sites did not have their own IRBs, FHI's IRB acted as their primary IRB. All participants gave written informed consent.
Men who requested a vasectomy at the study clinics were screened for eligibility and invited to join the study if they met all requirements. To be eligible for the study, men had to (a) satisfy the local clinic's criteria for vasectomy and (b) be willing to provide a prevasectomy semen sample and additional semen samples per the planned follow-up schedule. Men were compensated for the time and inconvenience of returning for frequent semen analyses. The compensation amounts were determined separately for each site. Exclusion criteria included (a) a history of previous vasectomy or other genital surgery; (b) clinical evidence of an acute illness including sexually transmitted infections; (c) history of a bleeding disorder; and (d) a large varicocele or other scrotal mass.
Participants were randomized to the fascial interposition or non-fascial interposition group. We used a block randomization method with randomly permuted block sizes of four, eight, and 12 participants. For allocation concealment, FHI provided the randomization assignments to the investigators in sequentially numbered, opaque sealed envelopes that were opened just prior to the vasectomy procedure. The surgeons knew the technique being used, but the laboratory staff who were conducting the semen analyses were not aware of the group assignments.
Vasectomy techniques
Prior to the study, the researchers – all experienced vasectomy surgeons – attended an investigators meeting where the vasectomy techniques were standardized with the help of two experienced vasectomy trainers. All surgeons used the no-scalpel approach to the vas [19]. The vas was then occluded using two silk sutures, and an approximately 1-cm segment of vas between the ligatures was excised. For the fascial interposition technique, a suture was used to contain the testicular end of the vas inside the fascial sheath; the prostatic end remained outside (Figure 1).
Figure 1.
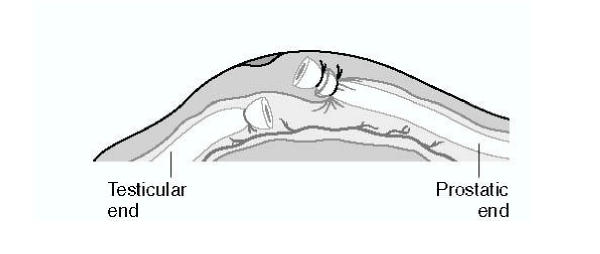
Standardized fascial interposition technique A suture is used to position the stump of the prostatic end outside of the fascial sheath and the stump of the testicular end inside the fascial sheath. Source: EngenderHealth: No-Scalpel Vasectomy: An Illustrated Guide for Surgeons. 3rd edition. New York: EngenderHealth; 2003. Reprinted with permission.
The investigators completed questionnaires immediately following each procedure, noting any difficulty experienced with parts of the vasectomy procedure (e.g. isolating the vas, using the fascial interposition technique). At two-week follow-up visits and at any unscheduled visits during the first six weeks, the investigators examined each patient and completed a questionnaire noting findings such as sperm granulomas, hematomas, epididymitis, or wound infection. The diagnosis of sperm granuloma was based on the clinical finding of swelling at the vasectomy site. It was not based on histology. We defined wound infection as a clinical sign of infection (i.e. local inflammation) for which a clinician had prescribed antibiotics.
Semen analysis
We performed standardized semen analyses two weeks postvasectomy and then every four weeks through 34 weeks, or until success or failure. For men without earlier vasectomy failure (i.e. those classified as vasectomy success or indeterminate), we performed semen analysis again at 52 weeks.
Laboratory methods were based on procedures recommended by the World Health Organization [20]. Briefly, participants were asked to produce a semen sample in a private room at the clinic. Semen was examined within one hour of collection. An aliquot was examined by phase-contrast microscopy at high power magnification (400×) to estimate sperm concentration. Based on the estimated concentration, dilutions were prepared to assess exact sperm concentration and motility. The concentration and motility of each sample were determined using a Neubauer hemocytometer. Samples with very low sperm numbers (<5 sperm per high power field) on the initial exam were centrifuged for 15 minutes at 600 g and then assessed for sperm concentration and motility as described above. Each laboratory conducted periodic quality-control tests.
Study outcomes
The primary study outcome for vasectomy success was time to azoospermia, defined as two consecutive azoospermic specimens taken at least two weeks apart. Two other study outcomes were (1) time to azoospermia or to severe oligozoospermia in two consecutive specimens taken at least two weeks apart, and (2) vasectomy failure based on the results of semen analysis. We defined severe oligozoospermia as 1 to <100,000 sperm/mL.
Throughout the text and tables, the second outcome (time to azoospermia or to severe oligozoospermia) is referred to as severe oligozoospermia.
We defined the date of vasectomy success as the date of the first of two azoospermic or severely oligospermic semen samples. Because older men take longer to reach azoospermia, we analyzed the data by age group in addition to examining overall effects. In the analyses of time to success, once a man met the definition of a vasectomy failure, he was classified as such and was considered as remaining a failure through 34 weeks, whether or not he continued clinical follow-up visits.
To define vasectomy failure, we used a criterion for early failure based on a report by Alderman [21]: the presence of more than 5 million motile sperm/mL at week 14 or later. We initially defined late failure as any motile sperm at 26 weeks or later. After the study began, we amended this because of the concern that motility could be difficult to evaluate in semen samples with very low sperm concentrations. The amended criterion specified the presence of more than 100,000 sperm/mL with any sperm motility. If a participant's semen analyses did not meet the primary criterion for success (azoospermia) or the criterion for failure, his outcome was indeterminate.
Partway through the study, the protocol was amended to gather extended follow-up data (up to 52 weeks) on men who were classified as vasectomy failures. This amendment facilitated clinicians' follow-up of men classified as early failures, to determine whether they would become azoospermic with longer follow-up. For men who became clinical successes with longer follow-up, their statuses were not changed for the life-table analyses of time to success.
Sample size estimation
We chose a study size of 1200 men, with 150 to be enrolled at each of the eight sites. We based this sample size on data showing that the cumulative chance of success through 22 weeks is about 82% for vas occlusion without fascial interposition, when success is defined as two consecutive azoospermic samples at least two weeks apart [7]. Assuming that the cumulative chance of success through 34 weeks of follow-up is 85% when fascial interposition is not used, and 90% when fascial interposition is used, enrolling at least 1156 participants would assure 90% power to detect a difference between the groups, with a one-sided test and an alpha of 0.05.
Statistical analysis methods
Cox proportional hazards regression was used to estimate anticipated improvement in the hazard ratio [HR] for successful vasectomy for the fascial interposition group versus the non-fascial interposition group, controlling for age and for surgeon experience (three surgeon groups based on prior experience with fascial interposition). A one-sided test with an alpha of 0.05 was used. We chose to use a one-sided test because of expert opinion that fascial interposition might improve vasectomy effectiveness and was very unlikely to have a negative effect. An age-by-treatment interaction was found in this primary comparison, so both overall and age-specific HRs were determined to more adequately explain the impact of fascial interposition on time to vasectomy success.
Kaplan-Meier product limit estimates, with 95% confidence intervals [CIs], of the probabilities of success at each scheduled week of follow-up through week 34 were determined overall, by treatment group and, because of the age-by-treatment interaction, by treatment group and age group. Peto's standard error [22] was used to compute the 95% CIs.
The proportions of vasectomy failures in the treatment groups were estimated and compared using logistic regression, controlling for age and surgeon experience.
A two-way analysis of variance that controlled for surgeon experience and participant age was used to compare duration of surgical procedure. Incidence of any surgical difficulty and of adverse events related to vasectomy were compared using a Mantel-Haenszel test, stratified by surgeon experience. The treatment-by-surgeon experience interactions for these comparisons were tested using the Breslow-Day test.
Interim analysis
We conducted a single planned interim analysis after the first 400 participants had completed 10 weeks of follow up. The results were reviewed by a data safety monitoring board (DSMB) composed of three independent and experienced researchers: a urologist, a statistician, and a clinical epidemiologist. The DSMB reviewed only the primary study outcome (i.e. time to azoospermia). Though a difference by age group complicated the DSMB's review of the results [18], the interim analysis showed a significant overall effect in favor of fascial interposition at the predefined significance level (P < 0.01). As a result, we halted recruitment in May 2001, but follow up continued for all enrolled participants.
Results
Baseline population data and participant disposition
Recruitment began in December 1999. 841 men (419 allocated to the fascial interposition group and 422 to the non-fascial interposition group) were enrolled and had vasectomies before recruitment was halted in May 2001; they constituted the treated population. 826 men returned for at least one semen analysis; they constituted the primary analysis population (Figure 2). The baseline data (Table 1) included all participants who returned for at least one semen analysis. The final follow-up visits were completed in June 2002. Unless specified, the efficacy results presented below are from the primary analysis population, and the safety results are from the treated population. 15 men did not return for any semen analyses and were excluded from the primary analysis population. 24 men were lost to follow up before reaching success, being declared a failure, or completing 34 weeks of follow-up (Figure 2).
Figure 2.
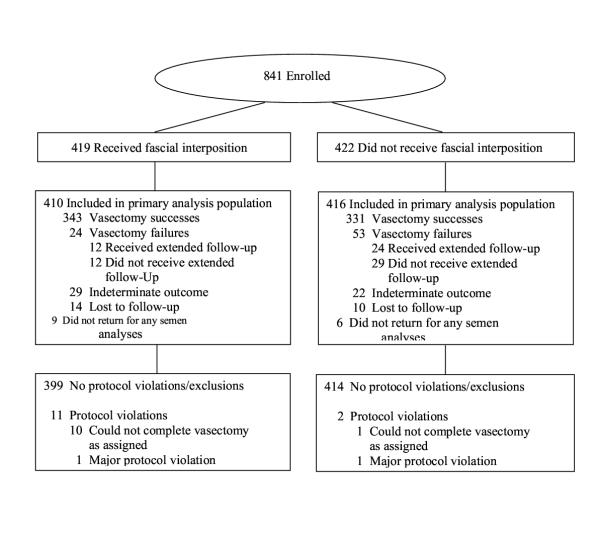
Participant disposition flow chart
Table 1.
Baseline characteristics
| Number (%) | ||
| Characteristic | With fascial interposition (n = 410) | Without fascial interposition (n = 416) |
| Age group, years | ||
| <30 | 109 (26.6) | 85 (20.4) |
| 30 to 34 | 125 (30.5) | 126 (30.3) |
| 35 to 39 | 95 (23.2) | 104 (25.0) |
| 40+ | 81(19.8) | 101 (24.3) |
| Marital status | ||
| Married | 344 (83.9) | 363 (87.2) |
| Partnered | 52 (12.7) | 49 (11.8) |
| Not partnered | 14 (3.4) | 4 (.96) |
| Number of children | ||
| 0 | 20 (4.9) | 11 (2.6) |
| 1 | 22 (5.4) | 20 (4.8) |
| 2 | 210 (51.2) | 206 (49.5) |
| 3+ | 158 (38.5) | 179 (43.0) |
| BMI > 30 | ||
| No | 369 (90.0) | 364 (87.5) |
| Yes | 41 (10.0) | 52 (12.5) |
| Prior condom use | ||
| No | 213 (52.0) | 239 (57.4) |
| Yes | 197 (48.0) | 177 (42.5) |
BMI: Body mass index
Time to success based on semen analyses
For the primary analysis population, the survival curves for time to azoospermia and time to severe oligozoospermia showed highly significant differences between the two groups, in favor of fascial interposition (Figure 3). The HRs were 1.35 for azoospermia and 1.32 for severe oligozoospermia (P < 0.0001 for both outcomes).
Figure 3.
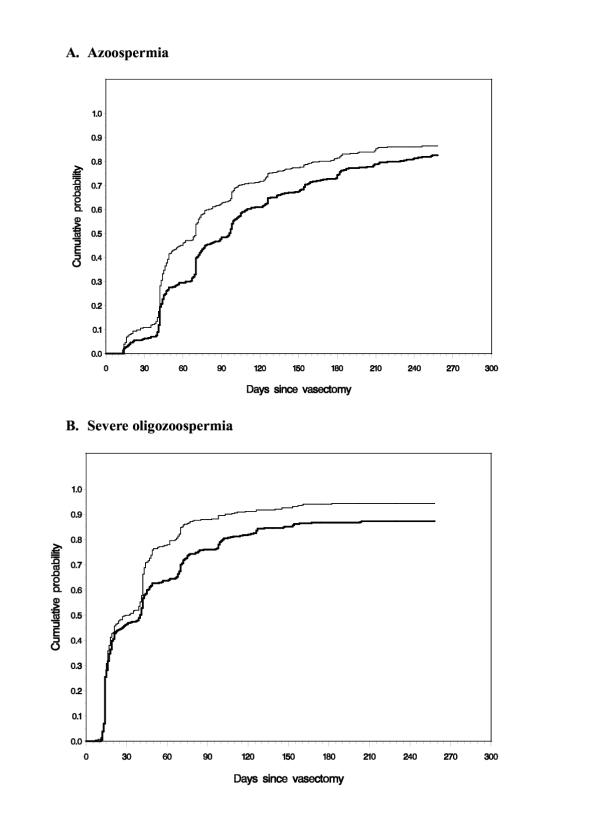
Cumulative event probabilities by treatment group Thin line, no-scalpel vasectomy with fascial interposition. Thick line, no-scalpel vasectomy without fascial interposition. 3.A: Time to success as defined by azoospermia. 3.B: Time to success as defined by severe oligozoospermia, <100,000 sperm/mL.
When time to azoospermia was examined by age group, younger men benefited the most from fascial interposition, especially when evaluated 14 weeks postvasectomy (Table 2). For men under age 30 years, those in the fascial interposition group were more than twice as likely to reach azoospermia than were those in the non-fascial interposition group (HR, 2.3; P < 0.0001). The difference between groups gradually disappeared with age. By age 40 years and older, men reached azoospermia at about the same rate whether they were in the fascial interposition group or the non-fascial interposition group (HR, .98; P = 0.54).
Table 2.
Kaplan-Meier product limit estimates of probabilities of success
| Age group, years | Probability of success (95% CI) | |
| With fascial interposition | Without fascial interposition | |
| Azoospermia, 14 weeks | ||
| Overall | 71% (67, 76) | 61% (56, 66) |
| <30 | 91% (85, 97) | 62% (51, 72) |
| 30 to 34 | 71% (63, 80) | 64% (56, 73) |
| 35 to 39 | 69% (59, 79) | 64% (54, 73) |
| 40+ | 48% (37, 59) | 53% (44, 63) |
| Azoospermia, 34 weeks | ||
| Overall | 86% (82, 91) | 83% (78, 87) |
| <30 | 95% (90, 99) | 80% (71, 90) |
| 30 to 34 | 85% (77, 93) | 81% (74, 86) |
| 35 to 39 | 89% (81, 97) | 88% (81, 95) |
| 40+ | 75% (59, 91) | 81% (72, 90) |
| Severe Oligozoospermia, 14 weeks | ||
| Overall | 91% (88, 94) | 82% (78, 86) |
| <30 | 94% (89, 99) | 77% (68, 87) |
| 30 to 34 | 89% (83, 95) | 82% (75, 89) |
| 35 to 39 | 91% (85, 97) | 84% (76, 91) |
| 40+ | 91% (85, 97) | 83% (76, 90) |
| Severe Oligozoospermia, 34 weeks | ||
| Overall | 94% (92, 97) | 87% (84, 91) |
| <30 | 95% (90, 99) | 86% (80, 96) |
| 30 to 34 | 92% (87, 97) | 87% (81, 93) |
| 35 to 39 | 96% (91, 100) | 89% (82, 96) |
| 40+ | 95% (89, 100) | 86% (79, 93) |
CI: Confidence interval
When time to severe oligozoospermia was examined by age group, men younger than 35 years were significantly more likely to reach oligozoospermia than men of the same age in the non-fascial interposition group (Table 2). HRs were 1.7 (P = 0.0003) for men younger than 30 years and 1.3 (P = 0.03) for men 30 to 34 years old. Men 35 years and older still benefited from fascial interposition, but differences in time to oligozoospermia between the fascial interposition and non-fascial interposition groups were not statistically significant (HR, 1.17 and P = 0.15 for men 35 to 39 years old; HR, 1.21 and P = 0.11 for men at least 40 years old).
In summary, the advantage of fascial interposition was more consistent across age groups when based on time to oligozoospermia than when based on time to azoospermia.
With respect to median time to success, men reached severe oligozoospermia six to eight weeks before they reached azoospermia. Among men in the fascial interposition group, the median times to success were four weeks using the oligozoospermia endpoint and 10 weeks using the azoospermia endpoint. Among men in the non-fascial interposition group, the median times to success were six and 14 weeks, respectively.
Failures based on semen analyses
By the 34-week visit, 77 vasectomy failures occurred: 24 (5.9%) of 410 vasectomies in the fascial interposition group (confidence interval [CI], 3.8 to 8.6) and 53 (12.7%) of 416 vasectomies in the non-fascial interposition group (CI, 9.7 to 16.3) (P = 0.0008). While the time-to-success results showed differences in the benefit of fascial interposition by age group, this was not the case with failures. All age groups had fewer failures with fascial interposition than without (Table 3).
Table 3.
Vasectomy failures based on semen analysis, by treatment and age group
| Age group, years | Number and percentage of failures (95% CI) | |||
| With fascial interposition | Without fascial interposition | |||
| Overall | 24 | 5.9% (3.8, 8.6) | 53 | 12.7% (9.7, 16.3) |
| <30 | 5 | 4.6% (1.5, 10.4) | 12 | 14.1% (7.5, 23.4) |
| 30 to 34 | 10 | 7.9% (3.8, 14.0) | 19 | 15.1% (9.3, 22.5) |
| 35 to 39 | 6 | 6.3% (2.3, 13.1) | 10 | 9.6% (4.7, 17.0) |
| 40+ | 3 | 3.6% (0.7, 10.1) | 12 | 11.9% (6.3, 19.8) |
CI: Confidence interval
Pregnancies and later follow up
Four pregnancies were reported during the study period (two in each group). Semen analyses performed close to the dates of conception showed that the semen of all four men contained motile sperm. The estimated time between vasectomy and the date of conception ranged from 41 to 91 days. Based on subsequent semen analyses, one case in each group was a success and one a failure. Three of the four pregnancies occurred at one center.
Among the 77 men with vasectomy failures, 36 (46.8%) accepted an offer of extended follow up (12 from the fascial interposition group and 24 from the non-fascial interposition group). 10 (27.8%) of these 36 men eventually became azoospermic or severely oligospermic (three of 12 in the fascial interposition group and seven of 24 in the non-fascial interposition group), generally between 22 and 42 weeks postvasectomy. At the 52-week visit, persistent sperm were found in 24 men who had been classified as vasectomy successes or indeterminate. 17 of the 24 had sperm concentrations of less than 100,000 sperm/mL. Of the 7 who had higher concentrations, two had been classified as successes (likely with subsequent late recanalization): one had concentrations of 100,000 sperm/mL and the other 400,000 sperm/mL. The remaining five had been classified as indeterminate and had concentrations ranging from 125,000 to 27 million sperm/mL. Six of the seven were in the non-fascial interposition group.
We collected supplementary follow-up data from 46 (59.8%) of the 77 men who had vasectomy failures. 29 of these men had undergone repeat vasectomies; 15 men had not had a repeat vasectomy at last contact; and the wives of two men had undergone postpartum tubal ligations.
Surgical procedures
Surgery took approximately two to three minutes longer when it included fascial interposition than when it did not. Mean surgical times were 14.3 minutes for the fascial interposition group versus 11.7 minutes for the non-fascial interposition group (P < 0.0001). The median surgical times were 12 minutes for the fascial interposition group versus 10 minutes for the non-fascial interposition group. The range of operating times was 5–60 minutes versus 4–75 minutes, respectively. Only three sites had cases with operating times longer than 30 minutes. At one of those sites, teaching activities contributed to the longer operating times.
The fascial interposition technique posed some difficulty for surgeons in 58 (13.8%) of 419 cases. However, surgeons in only nine (2.1%) of the 419 cases were unable to perform the fascial interposition procedure on one or both vas. Surgical difficulties with other aspects of the vasectomy procedure were not affected by the use of fascial interposition (data not shown).
Safety results
As indicated by reported analgesic use, postsurgical pain was mild, brief, and virtually identical in both groups. Among 820 men with data on postsurgical pain, 528 (64.4%) reported taking no analgesics. 231 (28.2%) reported analgesic use for one to three days, 47 (5.7%) for up to one week, and 14 (1.7%) for more than a week.
In the early follow-up period, within six weeks of surgery, 74 (17.7%) of the 419 men in the fascial interposition group versus 62 (14.7%) of the 422 men in the non-fascial interposition group reported one or more related adverse events. Although slightly more adverse events occurred in the fascial interposition group, the difference was not significant (P = 0.23).
The two diagnoses responsible for the slight difference in adverse events between the fascial interposition and the non-fascial interposition groups were sperm granuloma in 41 (9.8%) versus 29 (6.9%) of the cases and epididymitis or orchitis in 12 (2.9%) versus six (1.4%) of the cases, respectively. Three of the eight centers accounted for virtually all of the reports of sperm granuloma. Common events that occurred in equal numbers between groups were scrotal pain or swelling in 22 cases (2.6%) and hematomas in 12 (1.4%). The hematomas were small (2 cm or less in diameter), and none required drainage. Infections occurred in four (0.48%) of the cases (two in each group).
675 (89%) of 757 men classified as vasectomy successes or indeterminate attended the 12-month follow-up visit. At that visit, we asked the men if they had had any scrotal pain within the past three months. 652 (97%) reported no pain. In the fascial interposition group, nine (2.6%) of 346 reported pain (seven mild and two moderate); in the non-fascial interposition group, 14 (4.3%) of 326 (11 mild and three moderate). No men reported severe pain.
Three serious adverse events were considered related to the vasectomy. One man in each treatment group was hospitalized for epididymo-orchitis (one with onset one day and one with onset two months postvasectomy); and a man in the fascial interposition group was hospitalized with a painful granuloma of the spermatic cord eight months postvasectomy. Two of the three men were hospitalized for only one night. Two other men were hospitalized for events involving the genitourinary system: elective hernia surgeries one month and one year postvasectomy. These events were considered unrelated to vasectomy.
Discussion
To our knowledge, this is the first large randomized controlled trial of a vas occlusion technique. We found that the use of fascial interposition leads to more rapid achievement of both azoospermia and oligozoospermia and reduces vasectomy failures by about half when ligation and excision is used as the method of vas occlusion. All vasectomies in this study were done using the no-scalpel vasectomy approach to the vas, but our findings should be generalizable to procedures done with the standard incision approach, as it is unlikely that the approach to the vas would affect failure rates.
Five participants who had significant sperm concentrations at the 52-week visit had their vasectomies classified as indeterminant. If these five had been classified as failures, the benefit shown by fascial interposition would have been even higher. Thus, the advantage of fascial interposition clearly outweighs the additional two to three minutes needed to perform the procedure. Our results confirm some expert opinion on the value of fascial interposition and that ligation and excision should not be used alone given the high failure rate [10].
Slightly more adverse events did occur in the fascial interposition group than in the non-fascial interposition group during the six weeks after surgery, but the difference was not clinically or statistically significant. One could speculate that the slightly longer duration of the procedure and the presence of an additional piece of suture when fascial interposition is used might have contributed to this nonsignificant difference. Serious adverse events were rare in both groups.
We also found that time to severe oligozoospermia was a more useful outcome measure than was time to azoospermia. The analyses based on severe oligozoospermia provided results that were more consistent with the ultimate outcome of vasectomy success or failure. This was apparently because of the very slow and inconsistent achievement of azoospermia among older men who eventually achieved success. The slower achievement of azoospermia in older men was probably related to their having less frequent ejaculations than younger men (data not shown).
The percentages of men with vasectomy failures based on semen analyses (5.9% with and 12.7% without fascial interposition) appear inconsistent with the commonly quoted pregnancy rates of 1% or less after vasectomy. However, despite the large amount of published data on the failure rates of various vasectomy techniques, there are relatively few detailed semen analysis data from well-designed studies. Interpreting the published data is difficult because most data are from retrospective reviews of individual physicians' experiences; follow-up details and semen analysis methods vary widely and are often not carefully described; and various outcome definitions are used. Prospectively gathered data with frequent semen analyses are unusual. In fact, a broad range of failure rates based on semen analysis and pregnancy have been reported for vasectomy by ligation and excision; including rates as high as 3–10% [4-7].
Data from Nepal showed a cumulative pregnancy rate of 4.2% at 36 months postvasectomy in a program where ligation and excision was the most common method of vas occlusion and where postvasectomy semen analyses were not available [4]. Data from China have shown higher pregnancy rates than in Nepal, 9.5% at five years, but these data are difficult to interpret [5]. In a Canadian study of clip ligation and excision, using two clips per vas, careful follow up showed a failure rate of 8.7% based on semen analyses [6]. Data from a study of ligation and excision without fascial interposition in Mexico found a failure rate of 11.5% based on semen analysis through 24 weeks [7], similar to the 12.7% found in this study.
Since extended follow up in our study led to azoospermia in some men classified as vasectomy failures, and since women's age and fertility status are independent determinants of pregnancy, pregnancy rates would be lower than failure rates based on semen analyses. None-the-less, vasectomy failure and pregnancy rates in high-resource settings such as the USA [3] are estimated to be much lower than those cited above. Possible reasons for this difference include: (a) the availability of routine semen analysis provides rapid feedback to surgeons so that they can improve their occlusion techniques – or stop doing vasectomies – if they see too many vasectomy failures among their clients; (b) semen analysis identifies many vasectomy failures; (c) most surgeons in the USA use cautery (71%), usually in conjunction with clips or ligation [23]; and (d) men from the USA are generally older – and have older, less fertile wives – than men from Nepal and China at the time of vasectomy. Nazerali et al [4] found that pregnancies decreased with increasing spousal age.
In light of our results, and given the feedback that clinicians routinely receive in the USA from laboratory results and from the medicolegal system, it seems unlikely that the estimated 18% of surgeons who report using ligation and excision in the USA [23] currently use the standardized techniques that we studied.
Our results underscore the importance of the details of vas occlusion techniques for vasectomy success. While surgical experience is frequently cited as a key determinant of vasectomy success, our study suggests that the details of the vas occlusion technique are at least as important. Goldstein [11] suggests that intraluminal cautery is the most effective method of vas occlusion, based on the report by Schmidt [24]. However, data from randomized controlled trials are lacking.
Our study outcomes were based on semen analysis data rather than on pregnancy data so that we could minimize sample size, study duration, and pregnancy risk. Despite the 77 vasectomy failures identified by semen analyses, only four pregnancies were reported (two in each group). The number of pregnancies was probably small because of the frequent reinforcement of the counselling messages at the time of the semen analyses: continue using a back-up contraceptive method until azoospermia is achieved and – in cases of failure – do not rely on the vasectomy. We are not currently planning to gather longer-term follow-up data on the study participants for the possible later occurrence of additional pregnancies.
Counselling should always include information on the possibility of vasectomy failure. However, as shown by this study, vasectomy failure rates may differ substantially for different vas occlusion techniques. Where semen analyses are not available, clinicians have often recommended a waiting period of 20 ejaculations or three months before a man should rely on his vasectomy for contraception. Barone et al [7] have shown that three months is a better criterion than 20 ejaculations when ligation and excision is used. The results of this study are consistent with that recommendation (data not shown).
Conclusions
We have shown that the use of fascial interposition in conjunction with ligation and excision decreases time to azoospermia, decreases time to severe oligozoospermia, and reduces vasectomy failure rates by about half when failures are defined by semen analyses. Vasectomy success depends on the technique of vas occlusion that is used at least as much as on the surgical experience of the operator. Limitations of this study are that: (a) these findings are not necessarily applicable to vas occlusion by other methods; and (b) the correlation between postvasectomy sperm concentrations and risk of pregnancy is not well quantified.
Competing interests
None declared.
Authors' contributions
DS participated in the conception, design and analysis of the study, and drafted the manuscript. BI participated in the conception, design, and analysis of the study, and was primarily responsible for managing the study implementation. MH participated in the design of the study and was primarily responsible for the final statistical analysis. MC participated in the design of the study and was primarily responsible for the interim statistical analysis. MB participated in the conception, design, management and analysis of the study. All authors reviewed and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors would like to thank the lead investigators at each site – the Investigator Study Group – without whom this study could not have been conducted: Priya da Silva (Sri Lanka), Tika Man Vaidya (Nepal), José Braulio E. Otero (Mexico), Elsimar M. Coutinho (Brazil), Samuel Castro (El Salvador), Alfonso A. Lavergne F. (Panama) and Richard Berger (USA).
Partial support for this study was provided by FHI with funds from the US Agency for International Development (USAID) Cooperative Agreement # CCP-A-00-95-00022-02 and by EngenderHealth with funds from USAID Cooperative Agreement # HRN-A-00-98-00042-00. However, the views expressed in this article do not necessarily reflect those of FHI, EngenderHealth, or USAID.
We would like to thank Apichart Nirapathpongporn of Thailand and Magdalena Lozano Balderas of Mexico for helping standardize the vasectomy procedure at the investigators meeting, as well as Charles Muller and Kim Pomeroy for helping train the laboratory technicians. We would also like to thank the following clinical investigators and study coordinators whose work was essential to the study's success: Soma Kulatunge, Kusum Gunaratne (Sri Lanka); Jeewan Sharma, Mahendra Shrestha, Deepak Limbu (Nepal); Horacio Herrera, Miguel Angel Alvar, Magdalena Torres, Servando Arellano, Arturo Gonzales, Pilar Saldaña (Mexico); Mauricio Sánchez, Gabriel Atta, Celia Athayde (Brazil); Carlos Pérez, Melvin Sonia de Morales (El Salvador); Carlos Duque, Mirna Villarreal (Panama); and Bill Neighbor and Ivan Rothman (USA).
Contributor Information
David Sokal, Email: dsokal@fhi.org.
Belinda Irsula, Email: birsula@fhi.org.
Melissa Hays, Email: mhays@fhi.org.
Mario Chen-Mok, Email: mchen@fhi.org.
Mark A Barone, Email: mbarone@engenderhealth.org.
References
- Schwingl PJ, Guess HA. Safety and effectiveness of vasectomy. Fertil Steril. 2000;73:923–936. doi: 10.1016/S0015-0282(00)00482-9. [DOI] [PubMed] [Google Scholar]
- Liskin L, Pile JM, Quillin WF. Population Reports: Male Sterilization Series D. Vol. 4. Baltimore: John Hopkins University, Population Information Program; 1983. [Google Scholar]
- Trussell J, Kowal D. The essentials of contraception. In: Hatcher RA, Trussel J, Stewart F, Cates W, Stewart GK, Guest F, Kowal D, editor. In Contraceptive Technology. 17. New York: Ardent Media; 1998. pp. 211–247. [Google Scholar]
- Nazerali H, Thapa S, Hays M, Pathak LR, Pandey KR, Sokal DC. Vasectomy effectiveness in Nepal: a retrospective study. Contraception. 2003;67:397–401. doi: 10.1016/S0010-7824(03)00028-3. [DOI] [PubMed] [Google Scholar]
- Wang D. Contraceptive failure in China. Contraception. 2002;66:173–178. doi: 10.1016/S0010-7824(02)00334-7. [DOI] [PubMed] [Google Scholar]
- Labrecque M, Nazerali H, Mondor M, Fortin V, Nasution M. Effectiveness and complications associated with 2 vasectomy occlusion techniques. J Urol. 2002;168:2495–2498. doi: 10.1016/S0022-5347(05)64176-6. [DOI] [PubMed] [Google Scholar]
- Barone MA, Nazerali H, Cortes M, Chen-Mok M, Pollack AE, Sokal D. A prospective study of time and number of ejaculations to azoospermia after vasectomy by ligation and excision. J Urol. 2003;170:892–896. doi: 10.1097/01.ju.0000075505.08215.28. [DOI] [PubMed] [Google Scholar]
- Esho JO, Cass AS. Recanalization rate following methods of vasectomy using interposition of fascial sheath of vas deferens. J Urol. 1978;120:178–179. doi: 10.1016/s0022-5347(17)57094-9. [DOI] [PubMed] [Google Scholar]
- Rhodes DB, Mumford SD, Free MJ. Vasectomy: efficacy of placing the cut vas in different fascial planes. Fertil Steril. 1980;33:433–438. [PubMed] [Google Scholar]
- Royal College of Obstetricians and Gynaecologists (RCOG) Male and Female Sterilization Evidence-Based Clinical Guidelines. Vol. 4. London: RCOG; 1999. [Google Scholar]
- Goldstein M. Surgical management of male infertility and other scrotal disorders. In: Walsh PC, Retik AB, Vaughan ED, Wein AJ, editor. In Campbell's Urology. 8. Philadelphia: W.B. Saunders Company; 2002. pp. 1532–1587. [Google Scholar]
- Davies AH, Sharp RJ, Cranston D, Mitchell RG. The long-term outcome following "special clearance" after vasectomy. Br J Urol. 1990;66:211–212. doi: 10.1111/j.1464-410x.1990.tb14907.x. [DOI] [PubMed] [Google Scholar]
- Lemack GE, Goldstein M. Presence of sperm in the pre-vasectomy reversal semen analysis: incidence and implications. J Urol. 1996;155:167–169. doi: 10.1097/00005392-199601000-00063. [DOI] [PubMed] [Google Scholar]
- World Health Organization Task Force on Methods for the Regulation of Male Fertility Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril. 1996;65:821–829. [PubMed] [Google Scholar]
- Benger JR, Swami SK, Gingell JC. Persistent spermatozoa after vasectomy: a survey of British urologists. Br J Urol. 1995;76:376–379. [PubMed] [Google Scholar]
- Hancock P, McLaughlin E. British Andrology Society guidelines for the assessment of post vasectomy semen samples (2002) J Clin Pathol. 2002;55:812–816. doi: 10.1136/jcp.55.11.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomized trials. JAMA. 2001;285:1987–1991. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Chen-Mok M, Bangdiwala SI, Dominik R, Hays M, Irsula B, Sokal DC. Termination of a randomized controlled trial of two vasectomy techniques. Control Clin Trials. 2003;24:78–84. doi: 10.1016/S0197-2456(02)00267-2. [DOI] [PubMed] [Google Scholar]
- Li SQ, Goldstein M, Zhu J, Huber D. The no-scalpel vasectomy. J Urol. 1991;145:341–344. doi: 10.1016/s0022-5347(17)38334-9. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) WHO Laboratory Manual for the Examination of Human Semen and Sperm–Cervical Mucus Interaction. 3. New York: Cambridge University Press; 1992. [Google Scholar]
- Alderman PM. The lurking sperm. A review of failures in 8879 vasectomies performed by one physician. JAMA. 1988;259:3142–3144. doi: 10.1001/jama.259.21.3142. [DOI] [PubMed] [Google Scholar]
- Harris EK, Albert A. Survivorship Analysis for Clinical Studies. New York: M. Dekker; 1991. [Google Scholar]
- Haws JM, Morgan GT, Pollack AE, Koonin LM, Magnani RJ, Gargiullo PM. Clinical aspects of vasectomies performed in the United States in 1995. Urology. 1998;52:685–691. doi: 10.1016/S0090-4295(98)00274-X. [DOI] [PubMed] [Google Scholar]
- Schmidt SS. Vasectomy. Urol Clin North Am. 1987;14:149–154. [PubMed] [Google Scholar]


