Abstract
Deficits in odor identification have been the most frequently described in schizophrenia (SZ). A relationship between dysfunction in odor identification and negative symptoms of SZ has also been reported. Furthermore, deficit SZ (a subtype of the illness with primary, enduring negative symptoms) has been found to be associated with a particularly poor performance on odor identification tests indicating that deficits in smell identification could be differentially expressed in some subtypes of SZ. We describe correlations of performance on smell identification with positive and negative symptoms of SZ. Patients with SZ (n=15) and normal controls (n=19) were tested by the University of Pennsylvania Smell Identification Test (UPSIT). Psychopathology was assessed with the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS). SZ patients performed more poorly on the UPSIT test than did normal controls. Consistent with previous findings, we observed a correlation of SANS with UPSIT performance. In particular, specific subdomains of SANS, such as blunted affect, apathy and anhedonia, were associated with odor identification deficits. Furthermore, UPSIT score predict these subdomains of negative symptoms. No correlation was observed between positive symptom and odor identification deficits. Our study further reinforces a relation between olfactory identification deficit and negative symptoms in SZ and suggests that smell identification could be a candidate endophenotype relevant to negative symptoms of SZ.
Keywords: olfactory system, smell identification, UPSIT, schizophrenia, negative symptoms, endophenotype
1. Introduction
Schizophrenia (SZ) is a severe brain disorder characterized by positive symptoms (e.g., delusions and hallucinations), negative symptoms (e.g., affective flattening, apathy, and social withdrawal), and cognitive dysfunction. The disease typically affects adolescents and young adults, and the majority of the cases have a chronic course, resulting in abnormal mental functions and significant social disability (Sawa and Snyder, 2002; Thaker and Carpenter, 2001). Despite considerable research, neither the etiology nor pathogenesis that underlies such functional deficits is yet fully understood.
The relevance of the olfactory system in the study of the pathophysiology of SZ is underscored by several reports that patients with SZ exhibit significant deficits in olfactory function, including odor identification (Stedman and Clair, 1998), detection threshold sensitivity (Turetsky and Moberg, 2009), discrimination (Dunn and Weller, 1989), and memory (Wu et al., 1993). Among such olfactory dysfunctions, deficits in odor identification have been the most frequently described in SZ (Cascella et al., 2007). Studies with use of the University of Pennsylvania Smell Identification Test (UPSIT) (Doty et al., 1984), show that up to 80% of SZ patients have deficits in odor identification, whereas less than 15% of the general population show such deficits (Moberg et al., 1999). These deficits are seen early in the course of the illness and appear to be correlated with the duration of the disorder, although independent of normal aging effects (Kopala et al., 1995). The impact of possible confounding variables, such as gender, medication, or smoking history, has also not been significant (Brewer et al., 2001; Corcoran et al., 2005; Malaspina and Coleman, 2003; Moberg et al., 1999; Moberg et al., 2006; Roalf et al., 2006; Rupp et al., 2005a; Rupp et al., 2005b; Ugur et al., 2005). The relationship between smoking and odor identification tasks was even paradoxical in some studies indicating a “normalizing” effect of smoking (i.e. nicotine) on odor identification (Brewer et al., 1996; Malaspina and Coleman, 2003; McLean et al., 2004). A relationship between dysfunction in odor identification and negative symptoms of SZ has also been described (Brewer et al., 1996; Brewer et al., 2001; Coleman et al., 2002; Corcoran et al., 2005; Good et al., 2006; Malaspina and Coleman, 2003; Moberg et al., 2006; Stedman and Clair, 1998). Furthermore, deficit SZ (i.e., a subtype of the illness with primary, enduring negative symptoms) has been found to be associated with a particularly poor performance on the UPSIT (Malaspina and Coleman, 2003; Malaspina et al., 2002; Moberg et al., 2006; Strauss et al., 2009), indicating that deficits in smell identification could be differentially expressed in some subtypes of SZ.
Here we report results on the association of olfactory deficits with clinical variables in SZ. We found that specific subdomains of negative symptoms (apathy, anhedonia, and affective flattening) were significantly correlated with and predicted poor performance on the UPSIT in SZ patients.
2. Subjects and methods
2.1. Subjects and clinical measures
Nineteen subjects diagnosed with SZ according to criteria of the Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition (DSM-IV) (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV, 2000) and 20 age- and education-matched normal controls participated in the study. Patients were recruited from the outpatient psychiatric clinics of the Johns Hopkins Medical Institutions. Normal controls were recruited from the general population through flyers posted at the Hopkins Hospital and an ad-hoc ad placed in a local magazine. All subjects were administered the Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-IV) (First et al., 1997). All patients were assessed with the Scales for the Assessment of Positive and Negative Symptoms (SAPS and SANS) (Andreasen and Olsen, 1982) by a study psychiatrist that specializes in schizophrenia (NC). Subjects were excluded if they had a history of traumatic brain injury with loss of consciousness for > 1 h, a history of drug abuse within 6 months of the study or drug dependence within 12 months of the study, a history of untreated major medical illnesses. The study was approved by the Hopkins Institutional Review Board, and all subjects gave their written consent for their participation.
2.2. Olfactory identification test procedures
Olfactory identification assessments were conducted using the UPSIT (Doty et al., 1984), which is a scratch-and-sniff test consisting of four multiple-choice options for each of 40 items. In this study, the UPSIT was performed birhinally (both nostrils at a time) in a self-administered way. Participants were asked to scratch each microencapsulated patch containing the odor with a pencil, sniff it, and then select the name of the released odor from among four alternatives. The number of correct items is summed to determine an overall score (possible range, 0–40), with higher scores indicating better olfactory identification ability. Subjects were excluded if they had conditions like colds or allergies that would limit their ability to identify odors.
2.3. Data analysis
Univariate analysis of variance was used to compare SZ patients and controls on continuous variables. Chi-square was applied when necessary for categorical variables. Pearson’s r correlation was used to correlate demographic and each subdomain and total of SANS and SAPS with UPSIT scores. We exclude the subdomain of attention from SANS total, because this subdomain does not represent negative symptoms that reflect diminished sense of purpose, poverty of speech, diminished interest, and diminished social drive (Milev et al., 2005). To assess possible confounding effects of age, sex, smoking status, years of education, and duration of illness, each confounder was included individually in a multiple linear regression with UPSIT score. This modeling strategy was utilized because of the small sample size in the study. The estimated slopes from the multiple linear regression models were compared to those obtained from the simple linear regressions.
3. Results
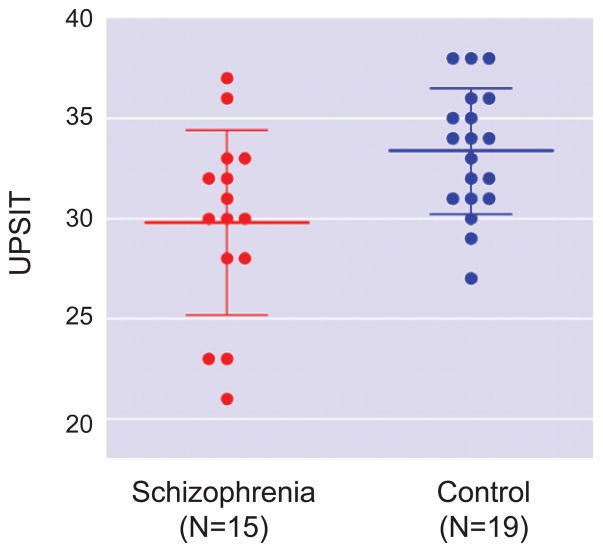
Table 1 shows the demographic characteristics of the study subjects. Of the 19 SZ patients recruited for the study, 15 had fully available data on the UPSIT test; while 19 of the 20 control subjects had full data sets. Univariate analysis of variance showed significant differences between patients and controls on the smell identification test (Fig. 1 and Table 1). SZ patients and controls did not differ significantly in age, sex, education, smoking status, and smoking history assessed by Brinkman Index (the number of cigarette packs smoked per day multiplied by the number of years of smoking) (Table 1). The differences on UPSIT score between smokers and nonsmokers were not statistically significant within each group. There was no correlation of the Brinkman index with UPSIT within each group as well as for the two groups combined together.
Table 1.
Characteristics of the patient and control groups.
| Characteristics1 | Schizophrenia (N=15) | Control (N=19) | p |
|---|---|---|---|
| Age (years) | 37.47 ± 10.67 | 36.00 ± 10.89 | 0.70 |
| Sex (male/female) | 9/6 | 14/5 | 0.402 |
| Education (years) | 11.93 ± 2.71 | 12.8 4 ± 1.86 | 0.26 |
| Current smoking status (smoker/nonsmoker) | 8/7 | 6/13 | 0.202 |
| Smoking history (Brinkman Index)3 | 8.18 ± 12.32 | 6.73 ± 8.69 | 0.72 |
| SANS (total) | 7.40 ± 3.44 | ||
| SAPS (total) | 4.40 ± 2.90 | ||
| Duration of illness (years) | 17.25 ± 9.69 | ||
| Neuroleptics | 14/15 | ||
| Antidepressants | 6/15 | ||
| Mood stabilizers | 1/15 | ||
| Anticholinergic agents | 3/15 | ||
| UPSIT score | 29.80 ± 4.62 | 33.37 ± 3.13 | 0.01 |
Values expressed as means ± standard deviations unless otherwise indicated.
χ2.
Brinkman Index = the number of cigarette packs smoked per day is multiplied by the number of years of smoking.
Fig. 1.
Differences between schizophrenia patients and controls on UPSIT. Univariate analysis of variance showed that UPSIT score of patients was significantly lower than that of controls. Cross bars indicate mean ± SD.
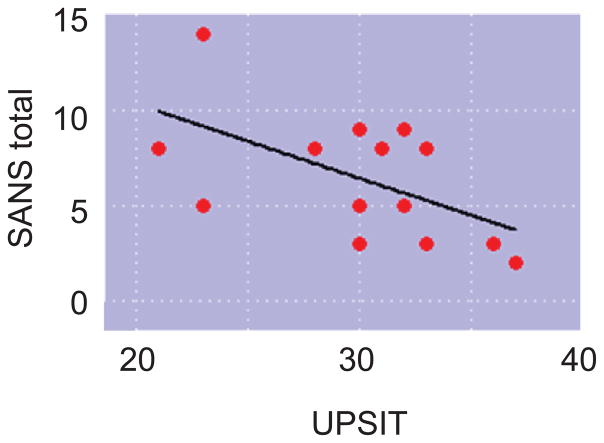
To test a possible relationship between UPSIT score and clinical traits, we used simple linear regression models to quantify the relationship of UPSIT score to SAPS or SANS measures. Individual SANS subdomains of apathy, anhedonia, and affective flattening as well as SANS total score were negatively associated with UPSIT score, even after controlling for age, sex, smoking status, years of education, and duration of illness (Fig. 2 and Table 2). No positive symptoms were significantly correlated with smell test scores.
Fig. 2.
Correlation between SANS total and UPSIT.
Table 2.
Regression analyses with use of UPSIT scores to predict negative symptoms.
| Predictors | Response | UPSIT β | SE | T | p | Mult. R2 |
|---|---|---|---|---|---|---|
| UPSIT | SANS Total score | −0.43 | 0.157 | −2.76 | 0.02 | 0.43 |
| UPSIT+Age | SANS Total score | −0.44 | 0.164 | −2.68 | 0.03 | 0.45 |
| UPSIT+Educ. | SANS Total score | −0.33 | 0.171 | −1.96 | 0.08 | 0.52 |
| UPSIT+Dur. | SANS Total score | −0.44 | 0.165 | −2.65 | 0.03 | 0.44 |
| UPSIT+Sex | SANS Total score | −0.44 | 0.173 | −2.55 | 0.03 | 0.43 |
| UPSIT+Smoke | SANS Total score | −0.47 | 0.170 | −2.80 | 0.02 | 0.47 |
| UPSIT | Apathy | −0.15 | 0.060 | −2.50 | 0.03 | 0.38 |
| UPSIT+Age | Apathy | −0.15 | 0.060 | −2.52 | 0.03 | 0.43 |
| UPSIT+Educ. | Apathy | −0.16 | 0.070 | −2.26 | 0.05 | 0.40 |
| UPSIT+Dur. | Apathy | −0.15 | 0.062 | −2.46 | 0.04 | 0.41 |
| UPSIT+Sex | Apathy | −0.15 | 0.066 | −2.24 | 0.05 | 0.39 |
| UPSIT+Smoke | Apathy | −0.15 | 0.067 | −2.23 | 0.05 | 0.38 |
| UPSIT | Anhedonia | −0.12 | 0.054 | −2.25 | 0.05 | 0.34 |
| UPSIT+Age | Anhedonia | −0.13 | 0.036 | −3.72 | 0.01 | 0.74 |
| UPSIT+Educ. | Anhedonia | −0.13 | 0.064 | −1.98 | 0.08 | 0.34 |
| UPSIT+Dur. | Anhedonia | −0.13 | 0.041 | −3.23 | 0.01 | 0.66 |
| UPSIT+Sex | Anhedonia | −0.13 | 0.060 | −2.13 | 0.06 | 0.34 |
| UPSIT+Smoke | Anhedonia | −0.12 | 0.061 | −1.95 | 0.08 | 0.34 |
| UPSIT | Affective flattening | −0.13 | 0.058 | −2.28 | 0.05 | 0.34 |
| UPSIT+Age | Affective flattening | −0.13 | 0.056 | −2.24 | 0.05 | 0.45 |
| UPSIT+Educ. | Affective flattening | −0.09 | 0.060 | −1.44 | 0.18 | 0.50 |
| UPSIT+Dur. | Affective flattening | −0.13 | 0.059 | −2.16 | 0.06 | 0.39 |
| UPSIT+Sex | Affective flattening | −0.13 | 0.064 | −1.99 | 0.08 | 0.35 |
| UPSIT+Smoke | Affective flattening | −0.17 | 0.054 | −3.11 | 0.01 | 0.54 |
Educ., Education (years); Dur., Duration of illness (years), Smoke, Current smoking status
4. Discussion
In this study, we found significantly lower UPSIT scores in SZ patients compared to healthy controls well-matched for age, education, and smoking status, which are consistent with the majority of published reports (see Table 3). Previous reports have also suggested that odor deficits are associated with negative symptoms. In the present study, we further characterize this correlation and report that subdomains of SANS, such as anhedonia, blunted affect and apathy, are specifically correlated with the odor deficit. Of importance, we found that those specific subdomains predict UPSIT score.
Table 3.
Association of olfactory identification deficit and negative symptoms in schizophrenia (2001-).
| References | Assessment of negative symptoms/deficit syndrome | Olfactory identification test | Sample size | Olfactory identification deficit in patients | Association | Notes |
|---|---|---|---|---|---|---|
| 1 | The Manchester Scale14 | UPSIT (baseline and 6 months later) | 74 patients with a first episode of psychosis, 38 controls | + | + | Olfactory identification deficit in patients remained stable at 6- month follow-up. |
| 2 | SDS | UPSIT | 67 patients with SZ (21 deficit syndrome, 46 non deficit syndrome) | + | + | Olfactory identification deficit in deficit group was significantly worse than in nondeficit patients. |
| 3 | SDS, PANSS | UPSIT | 70 patients with SZ or schizoaffective disorder, 68 controls | + | + | The association of olfactory identification deficit with diminished social drive explained its relationships with negative symptoms and deficit syndrome. |
| 4 | SANS, BPRS | UPSIT | 81 subjects at ultra-high risk for psychosis, 31 controls | + | − | A significantly lower UPSIT score was found in the ultra-high- risk subjects who later developed SZ spectrum disorder. |
| 5 | SADS-C+PD, SANS | UPSIT unirhinally | 15 antipsychotic drug- free patients with a first- episode of SZ spectrum disorder, 17 controls | + (both nostrils) | − | In patients, grooming and hygiene deficits were correlated with left nostril UPSIT score. |
| 6 | PANSS | UPSIT | 26 adolescents (age 11–17 years) with early onset psychosis | + | + | Olfactory identification deficit was more common in patients with schizophrenia and psychotic depression than in patients with psychosis NOS and bipolar disorder. |
| 7 | PANSS | Sniffin’ Sticks test15 unirhinally | 33 male patients with SZ, 40 controls | + (both nostrils) | − | |
| 8 | PANSS | Identify common odors from four descriptors unirhinally. | 30 male patients with SZ, 30 male controls | + (both nostrils) | − | |
| 9 | SANS, BPRS | Sniffin’ Sticks test15 unirhinally | 10 monozygotic twin pairs discordant for SZ or schizoaffective psychosis, 10 healthy monozygotic twin pairs | ± (trend) | − | Unaffected twins showed partial olfactory acuity and discrimination deficits. |
| 10 | SANS, BPRS | UPSIT unirhinally | 22 patients with SZ, 30 healthy fist-degree family members, 45 controls | + | − | There was no significant difference between patients and non affected family members in olfactory identification. |
| 11 | PANSS (baseline and 1 year later) | UPSIT (baseline) | 58 antipsychotic-drug- naïve patients | + | + | Better olfactory function predicted remission of negative symptoms. (one year after diagnosis, 44 SZ and schizoaffective disorder; 14 psychosis NOS) |
| 12 | SANS, BPRS | UPSIT | 21 patients with SZ (8 deficit syndrome, 13 non deficit syndrome), 20 controls | + | + | Olfactory identification deficit in deficit group was significantly worse than in nondeficit patients. |
| 13 | SDS, BPRS | B-SIT16 | 41 patients with SZ (15 deficit syndrome, 26 non deficit syndrome), 22 controls | + | + | Olfactory identification deficit in deficit group was significantly worse than in nondeficit patients and controls. There was no significant difference between non deficit and control groups in olfactory identification. |
| This study | SANS | UPSIT | 15 patients with SZ, 19 controls | + | + | Apathy, anhedonia, and affective flattening were associated with olfactory identification function. |
References:
5-point scale to assess symptoms of depression, anxiety, coherently expressed delusions, hallucinations, incoherence and irrelevance of speech, poverty of speech or mutism, flattened or incongruous affect, and psychomotor retardation.
16 common odor items, forced choice test.
12 items forced choice test.
SDS, the Schedule for the Deficit Syndrome; PANSS, the positive and negative syndrome scale; SANS, the Scales for the Assessment of Negative Symptoms; BPRS, the Brief Psychiatric Rating Scale; SADS-C+PD, the Schedule for Affective Disorders and Schizophrenia Change Version with Psychosis and Disorganization Items rating scale; UPSIT, the University of Pennsylvania Smell Identification Test; B-SIT, the University of Pennsylvania Brief Smell Identification Test; SZ, schizophrenia; NOS, Not Otherwised Specified.
Olfactory identification deficits might reflect abnormalities of both peripheral and central olfactory circuitry. Several studies have suggested that the processing of olfactory information is involved in several brain regions, including right orbitofrontal cortex and amygdala, which also play a role in mediating emotional experience and expression (Davidson and Slagter, 2000; Francis et al., 1999; Gur et al., 1994; Moberg et al., 2003; Rolls, 1999; Zald and Pardo, 1997; Zatorre et al., 1992). In addition, recent evidence indicates that there are abnormalities in olfactory epithelium and olfactory receptor neurons in patients with SZ (Arnold et al., 2001; Arnold et al., 1998; Borgmann-Winter et al., 2009; Feron et al., 1999; McCurdy et al., 2006).
The sample of this study is relatively small in size. Nonetheless, we could replicate a basic tenet of an association between UPSIT score and negative symptoms of SZ. There have been few exceptions to this association (Brewer et al., 2003; Roalf et al., 2006; Rupp et al., 2005a; Rupp et al., 2005b; Szeszko et al., 2004; Ugur et al., 2005). However, these negative studies have used different methods from the UPSIT to assess odor identification (Rupp et al., 2005a; Rupp et al., 2005b; Ugur et al., 2005), UPSIT was administered unirhinally (Roalf et al., 2006; Szeszko et al., 2004), or the study’s population consisted of subjects at “ultra high-risk” for psychosis (Brewer et al., 2003). Successful replication of this association with a small sample size may have particular significance in relation to some biological studies of SZ. Molecular profiling with patient-derived neurons and induced pluripotent stem cells is very important for exploring biomarkers and disease mechanisms for SZ (Kano et al., 2009; Sawa and Cascella, 2009; Tajinda et al., 2009). However, a drawback of studies with these cells/tissues may be the difficulty of achieving a large cohort. Studying UPSIT score in association with molecular profiles of these patient materials may shed important biological insight in negative symptoms of SZ.
Acknowledgments
We thank Ms. Yukiko Lema and Dr. Pamela Talalay for preparing the figures/manuscript and critical reading of the manuscript, respectively. This work was supported by NIH grants (MH-084018, MH-069853) (A.S.), grants from Stanley, CHDI (A.S), HighQ (A.S), S-R (A.S), and NARSAD (A.S.), as well as a support from Astellas (A.S.)
Footnotes
The paper has not been published elsewhere and is not under review with another journal. All co-authors have agreed to the submission of the final manuscript.
References
- American Psychiatric Association., and American Psychiatric Association. Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Andreasen NC, Olsen S. Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry. 1982;39:789–794. doi: 10.1001/archpsyc.1982.04290070025006. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Han LY, Moberg PJ, et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58:829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Smutzer GS, Trojanowski JQ, et al. Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer’s disease and schizophrenia. Ann N Y Acad Sci. 1998;855:762–775. doi: 10.1111/j.1749-6632.1998.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Borgmann-Winter KE, Rawson NE, Wang HY, et al. Human olfactory epithelial cells generated in vitro express diverse neuronal characteristics. Neuroscience. 2009;158:642–653. doi: 10.1016/j.neuroscience.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WJ, Edwards J, Anderson V, et al. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021–1031. doi: 10.1016/0006-3223(95)00594-3. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Pantelis C, Anderson V, et al. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Wood SJ, McGorry PD, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- Cascella NG, Takaki M, Lin S, et al. Neurodevelopmental involvement in schizophrenia: the olfactory epithelium as an alternative model for research. J Neurochem. 2007;102:587–594. doi: 10.1111/j.1471-4159.2007.04628.x. [DOI] [PubMed] [Google Scholar]
- Coleman E, Goetz RR, Leitman D, et al. Odor identification impairments in schizophrenia: relationship with demographic measures, clinical variables, and diagnostic subtypes. CNS Spectr. 2002;7:43–48. doi: 10.1017/s1092852900022252. [DOI] [PubMed] [Google Scholar]
- Corcoran C, Whitaker A, Coleman E, et al. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283–293. doi: 10.1016/j.schres.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Slagter HA. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment Retard Dev Disabil Res Rev. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, et al. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Dunn TP, Weller MP. Olfaction in schizophrenia. Percept Mot Skills. 1989;69:833–834. doi: 10.1177/00315125890693-121. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Hirning MH, et al. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40:211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders - Clinical Version (SCID-CV) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Francis S, Rolls ET, Bowtell R, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10:453–459. doi: 10.1097/00001756-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Good KP, Whitehorn D, Rui Q, et al. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am J Psychiatry. 2006;163:932–933. doi: 10.1176/ajp.2006.163.5.932. [DOI] [PubMed] [Google Scholar]
- Gur RC, Skolnick BE, Gur RE. Effects of emotional discrimination tasks on cerebral blood flow: regional activation and its relation to performance. Brain Cogn. 1994;25:271–286. doi: 10.1006/brcg.1994.1036. [DOI] [PubMed] [Google Scholar]
- Kano S, Ishii S, Cascella NG, et al. Society of Biological Psychiatry. Vancouver, Canada: 2009. Molecular Profiling of Schizophrenia by use of Multiple Tissue/Cell Samples: Olfactory Neurons and Induced Pluripotent Stem (iPS) Cells. [Google Scholar]
- Kopala LC, Good K, Honer WG. Olfactory identification ability in pre- and postmenopausal women with schizophrenia. Biol Psychiatry. 1995;38:57–63. doi: 10.1016/0006-3223(94)00224-Q. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E. Olfaction and social drive in schizophrenia. Arch Gen Psychiatry. 2003;60:578–584. doi: 10.1001/archpsyc.60.6.578. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Coleman E, Goetz RR, et al. Odor identification, eye tracking and deficit syndrome schizophrenia. Biol Psychiatry. 2002;51:809–815. doi: 10.1016/s0006-3223(01)01319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy RD, Feron F, Perry C, et al. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82:163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- McLean D, Feron F, Mackay-Sim A, et al. Paradoxical association between smoking and olfactory identification in psychosis versus controls. Aust N Z J Psychiatry. 2004;38:81–83. doi: 10.1111/j.1440-1614.2004.01301.x. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, et al. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. doi: 10.1176/appi.ajp.162.3.495. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, et al. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, et al. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444–1461. doi: 10.1080/13803390500434409. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Arnold SE, Doty RL, et al. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160:1784–1789. doi: 10.1176/appi.ajp.160.10.1784. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Turetsky BI, Owzar K, et al. Unirhinal olfactory function in schizophrenia patients and first-degree relatives. J Neuropsychiatry Clin Neurosci. 2006;18:389–396. doi: 10.1176/jnp.2006.18.3.389. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The brain and emotion. Oxford University Press; New York: 1999. [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, et al. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005a;74:149–161. doi: 10.1016/j.schres.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Kemmler G, et al. Various bilateral olfactory deficits in male patients with schizophrenia. Schizophr Bull. 2005b;31:155–165. doi: 10.1093/schbul/sbi018. [DOI] [PubMed] [Google Scholar]
- Sawa A, Cascella NG. Peripheral olfactory system for clinical and basic psychiatry: a promising entry point to the mystery of brain mechanism and biomarker identification in schizophrenia. Am J Psychiatry. 2009;166:137–139. doi: 10.1176/appi.ajp.2008.08111702. [DOI] [PubMed] [Google Scholar]
- Sawa A, Snyder SH. Schizophrenia: diverse approaches to a complex disease. Science. 2002;296:692–695. doi: 10.1126/science.1070532. [DOI] [PubMed] [Google Scholar]
- Stedman TJ, Clair AL. Neuropsychological, neurological and symptom correlates of impaired olfactory identification in schizophrenia. Schizophr Res. 1998;32:23–30. doi: 10.1016/s0920-9964(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Ross SA, et al. Olfactory Hedonic Judgment in Patients With Deficit Syndrome Schizophrenia. Schizophr Bull. 2009 doi: 10.1093/schbul/sbn178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeszko PR, Bates J, Robinson D, et al. Investigation of unirhinal olfactory identification in antipsychotic-free patients experiencing a first-episode schizophrenia. Schizophr Res. 2004;67:219–225. doi: 10.1016/S0920-9964(03)00218-4. [DOI] [PubMed] [Google Scholar]
- Tajinda K, Ishizuka K, Colantuoni C, et al. Neuronal biomarkers from patients with mental illnesses: a novel method through nasal biopsy combined with laser-captured microdissection. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.73. in press. [DOI] [PubMed] [Google Scholar]
- Thaker GK, Carpenter WT., Jr Advances in schizophrenia. Nat Med. 2001;7:667–671. doi: 10.1038/89040. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ. An Odor-Specific Threshold Deficit Implicates Abnormal Intracellular Cyclic AMP Signaling in Schizophrenia. Am J Psychiatry. 2009;166:226–233. doi: 10.1176/appi.ajp.2008.07071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugur T, Weisbrod M, Franzek E, et al. Olfactory impairment in monozygotic twins discordant for schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255:94–98. doi: 10.1007/s00406-004-0536-8. [DOI] [PubMed] [Google Scholar]
- Wu J, Buchsbaum MS, Moy K, et al. Olfactory memory in unmedicated schizophrenics. Schizophr Res. 1993;9:41–47. doi: 10.1016/0920-9964(93)90008-7. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94:4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatorre RJ, Jones-Gotman M, Evans AC, et al. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]