Abstract
The growing evidence linking social connectedness and chronic diseases such as cancer calls for a better understanding of the underlying biophysiological mechanisms. This study assessed the associations between social network ties and multiple measures of inflammation in a nationally representative sample of adults with a history of cancer (N = 1,075) from the National Health and Nutrition Examination Survey III (1988–94). Individuals with lower social network index (SNI) scores showed significantly greater inflammation marked by C-reactive protein and fibrinogen, adjusting for age and sex. Compared to fully socially integrated individuals (SNI 4), those who were more socially isolated or had a SNI score of 3 or less exhibited = increasingly elevated inflammation burdens. Specifically, the age- and sex-adjusted odds ratios (95%CI) for SNIs of 3, 2, and 0–1 were 1.49 (1.08, 2.06), 1.69 (1.21, 2.36), and 2.35 (1.62, 3.40), respectively (p < .001). Adjusting for other covariates attenuated these associations. The SNI gradients in the risks of inflammation were particularly salient for the lower socioeconomic status groups and remained significant after adjusting for other social, health behavioral, and illness factors. This study provided initial insights into the immunological pathways by which social connections are related to morbidity and mortality outcomes of cancer in particular and aging-related diseases in general.
Introduction
It is well recognized that social, environmental, and biological factors can jointly contribute to health risk and survival. There is rigorous evidence that social stressors such as a lack of social network connections not only increase general susceptibility to developing disease (Berkman and Syme 1979; House, Landis, and Umberson 1988) but are also tied to poorer prognoses for existing conditions such as cardiovascular disease and cancer (Ertel, Glymour, and Berkman 2008; Everson-Rose and Lewis 2005). A growing body of research has begun to reveal the biophysiological mechanisms underlying the links between social disconnectedness and disease. Innate immunity as indicated by chronic, low-grade, systemic inflammation has been shown to be a major physiological determinant of health and longevity and plays an important role in aging-related diseases (Finch 2007). In addition, positive links between social isolation and impaired immunity have been found in both animal and human studies. For instance, social isolation promotes immune dysfunction by slowing wound healing in rats (Hermes et al. 2006); enhancing the peripheral production of pro-inflammatory cytokines (Dowd et al. 2007); and increasing levels of several inflammatory markers, including C-reactive protein (CRP), fibrinogen, and serum albumin (Yang et al. 2013).
Although a wide variety of illnesses and conditions have been linked to social network ties (see, e.g., the review by Yang et al. [2013]), cancer has been less studied at the population level. However, this area of study deserves more attention, because cancer is the second-leading cause of death in the United States, and there is increasing evidence that social disconnectedness is consequential for cancer-related outcomes (see, e.g., the review by Penwell and Larkin [2010]). Animal studies have showed robust and consistent relationships between social isolation and tumor growth and metastases (McClintock et al. 2005; Reiche, Nunes, and Morimoto 2004). In humans, higher levels of social embeddedness and support have been found to be associated with better survival from breast (Kroenke et al.2013), colorectal (Ikeda et al. 2013), and lung cancer (Kemeny and Schedlowski 2007). Social isolation, on the other hand, appears to increase the risks of mortality from breast (Kroenke et al. 2006), lung, and other cancers (Yang et al. 2013). Although social disconnectedness has been related to poor immunocompetence in general, how it is related to inflammation for individuals afflicted with cancer is not clear. Thus it is particularly important to investigate this association in the context of cancer.
Chronic systemic inflammatory response is a key contributor to all stages of carcinogenesis, including initiation, promotion, and progression (Grivennikov, Greten, and Karin 2010; Karin and Greten 2005), and cancer prognosis (Caruso et al. 2004; Marx 2004). It has been estimated that chronic inflammation accounts for up to 15 percent of all cancer cases (Marx 2004). Higher CRP concentrations have been found among individuals living with colorectal (Erlinger et al. 2004), kidney, and all-cause cancers (Johnson and Master 2010). In patients diagnosed with cancer, increasing levels of inflammation marked by CRP concentration and low serum albumin have predicted poor cancer prognosis across multiple sites, independent of stage (McMillan 2009; Hickman et al. 1980); increased metastasis; and decreased cancer-specific survival (Canna et al. 2004, Jabs et al. 2005; Pierce et al. 2009; Yang et al. 2013). An elevated plasma fibrinogen level has also been shown to increase cancer mortality in men (Yano et al. 2001; Yang et al. 2013).
To the extent that cancer is characterized as a pro-inflammatory condition and various inflammatory markers carry prognostic potential, clinicians have focused on the investigation of these markers in relation to other malignant factors in order to improve patient outcomes. It is largely unknown how nonmalignant factors such as social stressors could also contribute to cancer etiology and prognosis through their relation to inflammation. A leading hypothesis is that the deleterious physiological consequences of chronic stress exposure could strongly influence cancer risk and progression (Reiche, Nunes, and Morimoto 2004). Recent findings from lab animals and humans suggest that social behavioral stress processes can influence cancer biology via the effects of immune responses to stress that are regulated by the neuroendocrine system and alter the functional activity of tumor cells (Antoni et al. 2006; Lutgendorf, Sood, and Antoni 2010). It is believed that increased autonomic nervous system, hypothalamic-pituitary-adrenal (HPA) axis, and sympathetic nervous system (SNS) activities in response to stress can alter inflammatory gene expression and promote inflammation in the tumor microenvironment (de Visser, Eichten, and Coussens 2006). Antoni and colleagues (2006) have suggested that this response constitutes the molecular mechanism that underlies the epidemiological finding that social behavioral stressors such as low social support exert their most pronounced effects on cancer by facilitating the growth of established tumors. It is thus possible that adverse changes in immune responses and impairment of the immune functions induced by social isolation stress, while harmful to all, are particularly detrimental to cancer patients because they weaken the immune surveillance crucial for preventing tumor growth and survival and create a permissive environment for cancer progression (Reiche, Nunes, and Morimoto 2004; Kemeny and Schedlowski 2007). On the other hand, social relationships may also impact disease risk through behavioral mechanisms, whereby individuals conform to other network members’ social norms and control; receive health-enhancing knowledge; adopt preventive lifestyles through smoking cessation, better diet, and exercise; and utilize health care (Cacioppo and Hawkley 2003; Smith and Christakis 2008; Umberson, Crosnoe, and Reczek 2010).
Furthermore, social relationship deficits and a lack of ties may influence inflammatory status following a diagnosis of cancer. An aging-related condition largely attributable to genetic dysregulation, cancer remains one of the most malicious diseases known to mankind. The onset of cancer initiates a traumatic medical and psychosocial event that can radically change an individual’s stress response process, making him or her more vulnerable to the impact of his or her social context. Any absence of connections with family, friends, or confidants, or isolation from social organizations and activities can magnify the stressful impact of cancer through a heightened inflammatory response (Johnson and Master 2010). This suggests that the association between social disconnectedness and inflammation may be stronger in people diagnosed with cancer than it is in the general population. In fact, a recent study shows that unmarried older adults who reported having a history of cancer were significantly more likely to have elevated CRP as compared to their married counterparts, while there was no difference between unmarried and married adults without a history of cancer (Johnson and Master 2010). In addition, because inflammation marked by CRP generally increases during aging in humans and is associated with cardiovascular disease (Danesh et al. 2004; Ross 1999), an increasing number of cancer survivors will also be subject to the risk of inflammation-related comorbidity and mortality. Understanding how social stress relates to inflammatory status in individuals with cancer can inform researchers and clinicians on the importance of providing psychosocial interventions, such as increasing social ties and engagement during the prognostic stage, that can lead to improved cancer prevention and treatment and better long-term survival.
The association between social network ties and inflammation among populations with malignant disease is not well documented or understood and merits further investigation. In addition, several other gaps exist in our knowledge about this relationship. First, the measurements of both social network ties and inflammation used in prior studies have been limited. Examining one specific sphere of an individual’s social network, such as marital status, cannot fully capture his or her degree of social disconnection. Prior research has also found that the number of network ties that an individual has across multiple social life domains has a more significant health impact than ties in any specific domain (Berkman and Syme 1979). Also, rather than examining one inflammatory marker such as CRP, a more comprehensive assessment of multiple markers is needed to characterize the status of systemic inflammation. In addition, the possibility that the presence of multiple risk factors may contribute to morbidity beyond the impact of individual markers (Yang and Kozloski 2011; Zajacova, Dowd, and Aiello 2009) should also be investigated.
Second, previous studies of cancer cases have largely been restricted to animal or small clinical samples that are homogeneous in terms of social demographic characteristics or cancer sites (e.g., Caucasian ovarian cancer patients; see Penwell and Larkin [2010]). It is thus difficult to generalize the findings of such studies to the general or a disadvantaged population in which a deficit in social connectedness may have more pronounced effects. For example, African Americans have higher prevalence and mortality rates for many cancers, as well as more social isolation and loneliness associated with perceived discrimination and neighborhood stress (McClintock et al. 2005). Also, the availability of social connections may also depend on socioeconomic status (SES), because people of lower SES have fewer social network ties and fare worse in terms of health and survival (Stringhini et al. 2012). In sum, previous studies have shown race and SES to be associated with measures of both social network ties and cancer outcomes. However, no research has evaluated whether the nature or strength of the relationship between social connections and inflammation in populations with cancer may be more pronounced for racial minorities and socioeconomically disadvantaged individuals.
This study filled in gaps in the extant research by examining the association between social network ties and inflammation using a nationally representative sample of the U.S. adult population with a history of cancer. It utilized the Berkman Social Network Index (SNI) to measure individuals’ quantity of social network ties across four domains (marital status, frequency of social contacts, religious attendance, and organizational membership). It included multiple markers of inflammation (CRP, fibrinogen, and serum albumin), as well as an overall inflammation burden index to more comprehensively evaluate the specific and cumulative inflammatory correlates of social relationship ties. Finally, it further explored the group-specific association by race and SES.
Data and Methods
Study Population
Data for the study were drawn from the National Health and Nutrition Examination Survey (NHANES) III conducted by the National Center for Health Statistics between 1988 and 1994 (National Center for Health Statistics [NCHS] 1996a; NCHS 1996b). The ongoing NHANES uses a multistage stratified sampling design and includes a representative sample of the noninstitutionalized U.S. population aged 20 years and older who participated in household interviews and clinical examinations, with an oversample of older persons and minorities (Centers for Disease Control and Prevention [CDC] 1996a). The ascertainment of cancer status is based on the survey question: “Has a doctor ever told you that you had cancer?” Respondents who replied yes to this question and also had nonmissing biomarker data were included in this study. In our initial analyses, we excluded skin cancer from the cancer sample. That led to a substantial reduction in sample size and less statistical power but not substantively different findings. Therefore, we retained these individuals in the final sample. We excluded those who had missing data on any variable included in the analyses. The final study sample consisted of 1,075 individuals.
Measures
Markers of Inflammation
We examined three biomarkers of inflammation: CRP, plasma fibrinogen, and serum albumin (see Table 1). The laboratory measurements and assay procedures for all these markers are described elsewhere (CDC 1996b). Data on fibrinogen were not available for respondents under the age of 40, so the final analysis was restricted to those aged 40 years or older. We used continuous scales of these markers, with log transformation of CRP because of its skewness, in linear regression analysis. We then constructed an inflammation burden index, which is a commonly used count index of individual markers validated by principal component analyses in previous studies (Yang and Kozloski 2011; Yang et al. 2013). As suggested by studies of allostatic load (Seeman et al. 2001) and infection burden index (Zajacova, Dowd, and Aiello 2009), a composite measure of inflammation reflects the presence of multiple risk factors and hence an overall burden of inflammation that has considerable predictive power in accounting for the health effects of complex factors like social relationships (Yang et al. 2013). We used the top quartiles for each continuous variable as cut points for dysregulation and then added in the positive indicators (CDC 1996a). Alternatively, we used a clinical cut point for CRP greater than 3 mg/dL, which may have been overly stringent and may have led to a low prevalence rate for community-dwelling respondents in this study. Thus we reported results based on the former measure. The inflammation burden index ranges from 0 to 3.
Table 1.
Sample characteristics (weighted) by social network index, NHANES III 1988–1994
| Social network index (SNI), mean (SD) or % |
||||||
|---|---|---|---|---|---|---|
| All (N = 1,075) | 4 (High) (N = 207) |
3 (N = 374) | 2 (N = 304) | 0–1(Low) (N = 190) |
p valuec | |
| Markers of inflammation | ||||||
| C-reactive protein (mg/dL) | 0.51 (0.81) | 0.42 (0.46) | 0.59 (1.07) | 0.46 (0.61) | 0.56 (0.78) | .049 |
| Plasma fibrinogen (mg/dL) | 315.87 (87.37) | 307.80 (62.00) | 317.93 (91.95) | 314.94 (94.81) | 325.10 (94.41) | .007 |
| Serum albumin (ug/mL) | 4.08 (0.37) | 4.08 (0.30) | 4.04 (0.41) | 4.10 (0.34) | 4.13 (0.39) | .144 |
| Inflammation burden Index | ||||||
| 0 | 47.21 | 51.98 | 45.90 | 48.35 | 41.14 | |
| 1 | 32.34 | 34.38 | 32.38 | 29.23 | 34.65 | .001 |
| 2 | 13.96 | 11.54 | 14.61 | 14.56 | 15.00 | |
| 3 | 6.49 | 2.11 | 7.10 | 7.86 | 9.21 | |
| Demographic and social factors | ||||||
| Age | 64.69 (12.64) | 64.58 (11.76) | 63.84 (12.71) | 64.49 (12.92) | 67.35 (12.90) | <.001 |
| Sex (1 = male) | 42.3 | 45.46 | 38.27 | 44.53 | 47.29 | .186 |
| Race (1 = nonwhite) | 5.24 | 3.05 | 5.81 | 7.12 | 6.6 | .278 |
| Education | 12.27 (3.25) | 13.55 (2.77) | 12.47 (2.95) | 11.77 (3.43) | 10.67 (3.41) | <.001 |
| Family income (in thousands) | 26.18 (13.59) | 28.75 (11.48) | 27.77 (13.75) | 24.65 (13.92) | 22.61 (13.68) | <.001 |
| Health behaviors | ||||||
| Cigarette smoking (never) | 40.76 | 36.58 | 48.12 | 36.92 | 32.97 | |
| Former | 43.32 | 53.47 | 39.07 | 40.1 | 41.98 | <.001 |
| Current | 15.92 | 9.94 | 12.82 | 22.98 | 25.06 | |
| Alcohol drinking (1 = none) | 56.47 | 43.26 | 63.76 | 54.61 | 57.85 | <.001 |
| Physical activitya (1 = none) | 27.1 | 19.00 | 21.88 | 32.98 | 42.64 | <.001 |
| Body mass index (BMI) | 26.61 (5.14) | 26.69 (4.37) | 26.32 (4.75) | 26.67 (5.74) | 27.13 (5.94) | .823 |
| Obesity (≥30 kg/m2) | 22.32 | 19.52 | 20.73 | 24.19 | 29.69 | .109 |
| Health status and medication | ||||||
| Number of chronic illnessesb | 1.60 (1.45) | 1.33 (1.19) | 1.67 (1.49) | 1.61 (1.55) | 1.83 (1.51) | .075 |
| Self-rated health (1 = poor; 5 = excellent) |
2.22 (1.11) | 2.52 (1.07) | 2.22 (1.04) | 2.21 (1.12) | 1.76 (1.17) | <.001 |
| Cholesterol medication (1 = yes) | 6.92 | 10.17 | 6.33 | 7.07 | 3.94 | .146 |
Notes:
Physical activities include jogging, biking, swimming, doing aerobics, dancing, doing exercises, doing garden work, lifting weights, or other exercises.
Includes 13 self-reported chronic illnesses: angina, arthritis, asthma, bronchitis, diabetes, emphysema, heart attack, heart failure, stroke, hip fracture, osteoporosis, spine fracture, and wrist fracture.
from χ2 test for difference in means between individuals with high to low levels of SNI.
Social Network Index (SNI)
We used the Berkman SNI, which has been widely adopted as a valid and reliable quantitative measure of a key aspect of social relationship or the degree of social integration and isolation. This index summarizes the quantity of social ties across four domains: marital status, frequency of interactions with friends and family, religious attendance, and membership in social organizations (Berkman and Syme 1979). We generated a dichotomous variable for each of these domains that signified either the presence or absence of a tie based on prior studies of the NHANES III data (Ford, Loucks, and Berkman 2006; Yang et al. 2013). Marital status was coded 1 if the respondent was currently married or cohabiting with someone. Frequency of contact was assessed using two questions: “In a typical week, how many times do you talk on the phone with family, friends, or neighbors?” and “How often do you get together with friends or relatives per year?” Frequent contact was coded as 1 if the respondent reported more than 156 contacts per year. Frequent religious attendance was coded as 1 if the answer to the question “How often do you attend church or religious services per year?” was four or more times. Social organization membership was coded as 1 if the answer to the question “Do you belong to any clubs or organizations such as church groups, unions, fraternal or athletic groups, or school groups?” was positive. These variables were then summed to determine an individual’s SNI, which could range from 0 to 4, with 0–1 indicating social isolation and 4 indicating the highest level of social integration.
Control Variables
We adjusted for an array of other factors that have been shown to correlate with inflammation in prior research (Yang et al. 2013). Demographic and socioeconomic factors included age (in years), sex (0 = female; 1 = male), race/ethnicity (0 = white; 1 = black or other), education (years of school completed), and family income (in thousands of dollars). Health behaviors included cigarette smoking status (0 = never; 1 = former or current smokers), the frequency of alcohol (beer, wine, or hard liquor) consumption per month (0 = any drink; 1 = none), physical activity based on the frequency of participation in nine or more leisure activities during the past month (0 = any; 1 = none), and body mass index (BMI) based on measured weight and height (0 = not obese or BMI < 30; 1 = obese or BMI ≥ 30). Health status included number of chronic illnesses as reported by a doctor and as listed in Table 1, self-rated health status (1 = poor, 2 = fair, 3 = good, 4 = very good, 5 = excellent), and utilization of cholesterol medication (0 = no; 1 = yes).
Statistical Analyses
Multivariate regression models were used to examine the associations between SNI and various measures of inflammation. The SNI was operationalized as a categorical variable in all models to account for nonlinearity in the relationship between social network ties and inflammation. We tested the relationship between SNI and continuous scales of the three inflammatory markers using the linear regression models and between SNI and dichotomous variables of these markers using the logistic models. We here present results from the former specification because it yielded more statistically significant coefficients and better model fit based on the Bayes information criterion (BIC). We then examined the correlation of SNI and inflammation burden index using the ordinal logit models. For both sets of analyses, we adjusted for age and sex and all other covariates in a stepwise fashion. The control variables were retained in the final models if their coefficients were statistically significant or their inclusion affected the regression coefficients of other variables. We also conducted these analyses by race and SES across samples of whites and nonwhites, individuals with less than and above a high school level of education, and individuals from the lower three and top quartiles of family income. All statistical analyses were performed using Stata 12.1 and adjusted for the complex survey designs using sampling weights.
Results
Descriptive statistics (weighted) for all covariates by level of SNI are shown in Table 1, with the p values reported from a chi-squared test of the differences between higher and lower SNI scores. Individuals with lower social integration and SNI scores tended to have significantly higher levels of inflammation as marked by CRP, fibrinogen, and the overall inflammation burden than those with high social integration and SNI scores. In addition, they tended to be older, have less education and family income, be smokers, be physically inactive, and report worse general health.
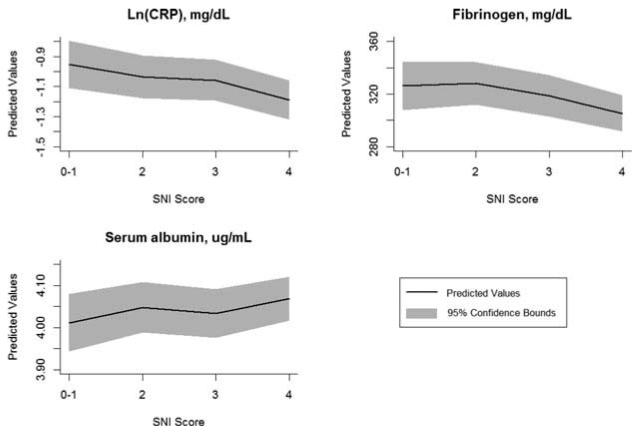
Table 2 shows the regression coefficient estimates for the SNI variables and the corresponding p values from chi-squared tests of the group difference in the models for the three individual markers of inflammation. Consistent with the bivariate associations shown in Table 1, a higher level of social integration was related to lower levels of inflammation, adjusting for age and sex. Figure 1 shows the predicted values of CRP, fibrinogen, and albumin by the SNI score based on Model 1. Increasing SNI scores were accompanied by significantly lower values of logged CRP (p = .028) and fibrinogen (p = .038) and higher values of serum albumin, indicating less inflammation (although this = finding was not statistically significant). Such associations were attenuated by other social, behavioral, and illness-related factors but were no longer statistically significant when adjusted for all covariates in Model 2. Results for race and SES subgroups were similar to those for the total sample qualitatively but showed significant associations only among whites and lower-income quartiles in the models for fibrinogen.
Table 2.
The associations between social network index and inflammatory markers
| ln(CRP) |
Fibrinogen |
Albumin |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| b | (s.e.) | P value | b | (s.e.) | P value | b | (s.e.) | P value | |
| Model 1: adjusted for age and sex | |||||||||
| SNI = 4 (High) | ref | ref | ref | ||||||
| 3 | 0.13 | 0.07 | 13.29 | 7.94 | −0.04 | 0.03 | |||
| 2 | 0.16 | 0.07 | 0.028 | 22.64 | 8.26 | 0.038 | −0.02 | 0.03 | 0.380 |
| 0-1 (Low) | 0.24 | 0.08 | 20.82 | 9.26 | −0.06 | 0.04 | |||
| Model 2: adjusted for all covariates | |||||||||
| SNI = 4 (High) | ref | ref | ref | ||||||
| 3 | 0.09 | 0.07 | 10.69 | 7.97 | −0.02 | 0.03 | |||
| 2 | 0.09 | 0.07 | 0.413 | 18.31 | 8.44 | 0.190 | −0.01 | 0.03 | 0.751 |
| 0-1 (Low) | 0.13 | 0.08 | 13.53 | 9.59 | −0.03 | 0.04 | |||
Notes: p values are from the χ2 test for the group difference across SNI scores of 0–4; covariates in Model 2 include age, sex, race, education, income, smoking, drinking, physical activity, BMI, chronic illnesses, self-rated health, and cholesterol medication.
Figure 1.
Predicted values of inflammatory markers by social network index score. Higher albumin values indicate less inflammation.
Table 3 presents results for the ordinal logit models of the inflammation burden index. In the overall sample, individuals with lower SNI scores were more likely to have significantly higher inflammation burdens compared to those with higher SNI scores. Model 1 shows that, adjusting for age and sex, compared to fully socially integrated individuals with a SNI score of 4, those who were more socially isolated or had a SNI score of 3 or less exhibited increasingly elevated inflammation burdens. Specifically, the odds ratios (ORs) (with 95% CI in parentheses) for SNIs of 3, 2, and 0–1 were 1.49 (1.08, 2.06), 1.69 (1.21, 2.36), and 2.35 (1.62, 3.40), respectively (p < .001). In Model 2, the ORs of each SNI category were slightly reduced, but the SNI gradient in the inflammation burden remained statistically significant after adjusting for all other covariates (p = .03).
Table 3.
Ordinal logit models of the associations between social network index and the inflammation burden index
| Model 1 |
Model 1 |
||||||
|---|---|---|---|---|---|---|---|
| SNI | OR | (95% CI) | p value | OR | (95% CI) | p value | |
| All | 4 | 1.00 | 1.00 | ||||
| (N = 1,075) | 3 | 1.49 | (1.08, 2.06) | 1.32 | (0.95, 1.83) | ||
| 2 | 1.69 | (1.21, 2.36) | <.001 | 1.43 | (1.01, 2.03) | .030 | |
| 0-1 | 2.35 | (1.62, 3.40) | 1.81 | (1.22, 2.68) | |||
| Race | |||||||
| White | 1.00 | <.001 | 1.00 | .013 | |||
| Black or Other | 1.83 | (1.32, 2.54) | 1.54 | (1.10, 2.17) | |||
| Education | |||||||
| <12 years | 1.00 | .872 | 1.00 | .085 | |||
| 12+ years | 1.02 | (0.80, 1.30) | 1.25 | (0.97, 1.61) | |||
| Income | |||||||
| Lower quartiles | 1.00 | .076 | 1.00 | .482 | |||
| Top quartile | 0.79 | (0.61, 1.02) | 0.91 | (0.70, 1.18) | |||
| Race | SNI | ||||||
| White | 4 | 1.00 | 1.00 | ||||
| (N = 918) | 3 | 1.41 | (1.00, 1.99) | 1.27 | (0.89, 1.80) | ||
| 2 | 1.63 | (1.14, 2.34) | .001 | 1.43 | (0.98, 2.07) | .091 | |
| 0-1 | 2.21 | (1.49, 3.28) | 1.69 | (1.11, 2.58) | |||
| Black or Other | 4 | 1.00 | 1.00 | ||||
| (N = 157) | 3 | 1.71 | (0.65, 4.47) | 2.08 | (0.73, 5.88) | ||
| 2 | 1.64 | (0.62, 4.32) | .248 | 1.91 | (0.65, 5.60) | .390 | |
| 0-1 | 3.02 | (1.01, 9.03) | 2.75 | (0.86, 8.84) | |||
| Education | SNI | ||||||
| <12 years | 4 | 1.00 | 1.00 | ||||
| (N = 442) | 3 | 2.25 | (1.15, 4.41) | 2.22 | (1.12, 4.40) | ||
| 2 | 2.47 | (1.27, 4.78) | .017 | 2.25 | (1.14, 4.42) | .049 | |
| 0-1 | 3.01 | (1.52, 5.96) | 2.61 | (1.30, 5.28) | |||
| 12+ years | 4 | 1.00 | 1.00 | ||||
| (N = 633) | 3 | 1.30 | (0.89, 1.89) | 1.13 | (0.77, 1.66) | ||
| 2 | 1.45 | (0.96, 2.20) | .007 | 1.25 | (0.81, 1.92) | .216 | |
| 0-1 | 2.41 | (1.46, 3.98) | 1.73 | (1.02, 2.93) | |||
| Income | |||||||
| Lower quartiles | 4 | 1.00 | 1.00 | ||||
| (N = 842) | 3 | 1.49 | (1.04, 2.15) | 1.28 | (0.88, 1.87) | ||
| 2 | 1.79 | (1.23, 2.60) | <.001 | 1.46 | (0.98, 2.18) | .041 | |
| 0-1 | 2.49 | (1.65, 3.76) | 1.87 | (1.20, 2.89) | |||
| Top quartile | 4 | 1.00 | 1.00 | ||||
| (N = 233) | 3 | 1.49 | (0.76, 2.93) | 1.33 | (0.66, 2.68) | ||
| 2 | 1.37 | (0.670, 2.80) | .580 | 1.20 | (0.57, 2.52) | .835 | |
| 0-1 | 1.74 | (0.75, 4.03) | 1.45 | (0.59, 3.60) | |||
Notes: Model 1 adjusted for age and sex; Model 2 adjusted for all covariates; p values from χ2 test for the group difference across SNI scores of 0–4; inflammation burden index is the sum of the positive indicators of three inflammation markers and ranges from 0 to 3, as shown in Table 1.
The main effect coefficients for race in models including the=SNI showed significantly higher inflammation burdens for nonwhites, a category including individuals classified as black or other. The main effects for education and income were less significant, adjusting for the SNI. Patterns of the SNI associations with the inflammation burden for the overall sample held for race- and SES-stratified samples but also showed some variation in significance and magnitude across subgroups. The ORs of elevated inflammation burdens for each SNI level were larger for nonwhites than whites, suggesting a possible stronger association between social isolation and inflammation. However, the ORs for the nonwhite sample based on only 157 individuals were not statistically significant.
The results by SES suggest that the relationship between social isolation and inflammation was specific to each education and income stratum. The SNI gradient in the inflammation burden was large and highly significant for people across all levels of education but more salient for respondents with less than a high school education. For instance, the odds of an elevated inflammation burden were estimated to be three times higher (OR [95% CI] = 3.01 [1.52, 5.96]) for socially isolated individuals with a SNI score of 0–1 and more than twice as large for individuals with SNI scores of 2 or 3 than for those with a SNI score of 4 in the low-education group in Model 1 (p = .017). Similarly, the increase in the inflammation risk with fewer social connections was particularly pronounced in the lower three income quartiles but was not significant for the top income quartile. Adjusting for all other covariates, the SNI and inflammation links remained significant for the lower SES groups in Model 2. For the higher SES groups, such associations were largely mediated by behavioral and health-related factors, including cigarette smoking, drinking, physical activity, number of chronic illnesses, and self-rated health. We tested the interaction effects between the SNI and race, education, and income but did not find significant results. This may have been due to the lack of statistical power for some subsamples such as black/other and top income quartile. The differences reported here are thus suggestive rather than definitive.
Discussion
The emerging understanding of the role of immune disorder in the pathogenesis of malignancies highlights the importance of identification of not only malignant but also nonmalignant factors and processes that contribute to immune regulations and cancer outcomes. This is the first study to correlate one important but relatively understudied social factor—social network ties—with multiple measures of inflammation in a nationally representative sample of adults with a history of malignant disease. It provides some initial insights into the immunological pathways by which social connections are related to morbidity and mortality outcomes of cancer in particular and chronic diseases of aging in general.
We extended previous research on the links between specific social relationship ties such as marital status and single biomarkers such as CRP in demographically homogeneous samples of adults with a history of cancer. We found that greater social isolation defined by lower numbers of social network ties was associated with multiple markers of innate immunity such as an elevated CRP concentration, higher plasma fibrinogen, and a greater overall burden of inflammation in a diverse sample of Americans living with cancer. In the case of the inflammation burden index, the magnitude of the association increased as the degree of social isolation increased, resembling a dose-response relationship. The SNI gradient remained significant for the risk of an elevated inflammation burden after adjusting for confounding factors, suggesting that the observed associations were independent of social, health behavioral, and other illness-related factors included in the analyses. These cross-sectional correlations seem to be consistent with the expectation that social disconnectedness may constitute chronic stress that can promote systemic inflammation and cancer progression, whereas more social connections may convey physiological benefits such as the downregulation of inflammatory response crucial for improved prognosis and survival.
We found that the SNI associations with the cumulative burden of inflammation were stronger and more significant than those with individual markers such as albumin. This suggests the advantage of an analysis including multiple biomarkers. Each marker reflects different stages of the inflammatory process and hence may bear a different relationship to social isolation. Each marker is also affected by other physiological processes distinct from inflammation. For example, low serum albumin levels indicate not only catabolism associated with inflammation, but also liver disease impairing its synthesis, nephrotic syndrome increasing its excretion, and malnutrition impairing protein intake (Doweiko and Nompleggi 1991). Therefore, a composite measure of inflammatory markers is more indicative of the existence of systemic inflammation than is any one marker alone (Yang et al. 2013). The finding that the summary index of inflammation had considerable power in accounting for the immunological effects of complex factors like social relationships is highly consistent with previous research on the conceptualization and methodology of allostatic load (Crimmins and Seeman 2004). This finding may also have clinical implications, because it suggests the necessity for physicians to track overall burden of inflammation above and beyond single risk factors in assessment of patient immune function in response to daily challenges in social life.
Using a heterogeneous population-based sample, we also discovered certain segments of the population whose immune functions may be more vulnerable to deficits in social network ties. Although the increment in the risks of elevated inflammation burden with decreasing numbers of social network ties was observed for all race, education, and income subgroups, the risks for each SNI category were larger among nonwhites, less educated individuals, and individuals in the lower income quartiles compared to their white, better-educated, and top–income quartile counterparts. These risks could not be completely attributed to differences in the other social, health behavioral, and illness-related conditions included in the study because the SNI gradients in the inflammation burden remained statistically significant for the lower SES groups after adjusting for these factors. The more pronounced social isolation gradients in inflammation risks for the lower SES groups could reflect a cumulative stress process whereby individuals disadvantaged in terms of socioeconomic resources and with cancer may be more susceptible to adverse physiological stress response to the absence of other forms of social capital, such as social network ties, that may have otherwise buffered such a response. This process could potentially operate in other disadvantaged subpopulations such as blacks, who generally have a lower SES than whites. The estimated SNI association with inflammation in this group, however, did not reach statistical significance. This may have been a function of the small size of the nonwhite sample in the current dataset reducing statistical power. In the absence of statistically significant interaction effects between the SNI and stratifying variables, the group differences are only suggestive and need corroboration by future research. What the study does present are the potentially large benefits of social integration for some major markers of aging-related disease that transcend race and socioeconomic strata. It suggests that the efforts to reduce inflammation burdens should not be isolated to particular subgroups.
This study has limitations that should be addressed in future investigations. First, although we assessed the extent of social engagement through a more comprehensive measure than those used in previous studies, the SNI represents quantitative and structural aspects of social relations and may not completely capture the quality of such relations or actual support received from these relations. The latter aspects of social relations were not available in the NHANES data. Recent studies that have assessed both quantitative and qualitative measures of social relations, however, show that an individual’s number of social network ties is more significantly related to disease and mortality than are appraisals of relationship quality such as perceived social support (Stringhini et al. 2012) and loneliness (Steptoe et al. 2013). It is unknown whether the relative importance of these two kinds of measures holds in determining immune dysregulation in individuals with a history of cancer. Additional data are needed to test the possibility that qualitative and functional dimensions of social affiliations may also be influential in immune functioning or may modify the immune impact of quantity of social relations.
Second, the biomarkers of immune function in general and inflammation in particular in the NHANES data are also limited. It is possible that other markers more directly related to tumor progression, such as inflammatory markers involved in angiogenesis that feed tumor growth, are also correlated with social stressors. Preliminary evidence has been found on the associations between social ties and support and some of these markers, including vascular endothelial growth factor (VEGF) as a cytokine fueling angiogenesis (Lutgendorf et al. 2002), tumor necrosis factor (TNF-α) as a reactive oxygen cytokine (Marucha et al.2005), and natural killer cell cytotoxicity (NKCC) (Lutgendorf et al. 2005). Because these studies were clinically based and restricted to small samples of Caucasian female ovarian and breast cancer patients, the challenge remains to test these relations in a broader population with a history of cancer.
Third, the number of cancer cases included in this study, although larger than that used in any previous studies of psychosocial stressors and immune functions, was relatively low compared to the number of individuals typically included in survey studies because the data were drawn from a community-dwelling sample. The analysis thus lacked sufficient power for the estimation of associations across different cancer sites. In addition, the data on cancer status were self-reported and lacked information on the time since a cancer diagnosis. The analyses thus pertained to those respondents who survived to be included in the NHANES III. Previous studies using similar survey data suggest that such limitations did not alter study findings that the inflammatory response to stressors are elevated following a prior diagnosis of cancer but only called into question the duration of this response (Johnson and Master 2010). The potential heterogeneity in those with a history of cancer regarding the degree to which inflammatory response grows or diminishes with time is a topic for future research. More detailed information on histology, stage of the malignancy, and treatments would further enhance the understanding of additional mechanisms by which social isolation engenders immune functions.
Last but not least, like previous research, this study was based on cross-sectional data and does not lend itself to causal inference. This constrained the interpretation of the relationship between social network ties and inflammation as correlational. That is, we cannot rule out the possibility of a reciprocal relationship. For instance, inflammation can increase such illness behaviors as withdrawal from social interactions and depression (Maier and Watkins 1998). Living with cancer and increased inflammation burdens can intensify the tendency toward greater social isolation. Interestingly, however, we noted in additional analysis that the cancer sample reported greater numbers of social ties and hence was more socially integrated than the noncancer sample. It is likely that having cancer made individuals more in need of social connections, such as those maintained through religious activities or interactions with and support from family and friends. Although it is not possible to test the reverse causation in this study, the associations found here are largely consistent with those from previous studies on different samples (both animals and humans). Further studies using longitudinal data are nonetheless needed to corroborate this finding.
In conclusion, the knowledge of biophysiological mechanisms relating specific social behavioral factors to disease progression and survival, when enriched by longitudinal studies, will provide the basis for new behavioral therapeutic approaches to the treatment of immune-related disorders including cancer. Intervention strategies for protecting patients, particularly those who are socially disadvantaged, from isolation stress may be particularly effective in sustaining or restoring immune function and may become an adjunct to standard clinical care and medical management of malignant and other aging-related diseases.
Acknowledgments
Funding
This study was supported by grant #K01AG036745 from the National Institute of Aging (awarded to the first author) and University Cancer Research Funds at the Lineberger Comprehensive Cancer Center (awarded to all authors). We are also grateful to the Carolina Population Center (R24 HD050924) for general support.
References
- Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, Stefanek M, Sood AK. The influence of bio-behavioral factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6(3):240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. Am J Epidemiol. 1979;109(2):186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. Social isolation and health, with an emphasis on underlying mechanisms. Perspect Biol Med. 2003;46(3 suppl):S39–S52. [PubMed] [Google Scholar]
- Canna K, McMillan DC, McKee RF, McNicol A-M, Horan PG, McArdle CS. Evaluation of a cumulative prognostic score based on the systemic inflammatory response in patients undergoing potentially curative surgery for colorectal cancer. Brit J Cancer. 2004;90(9):1707–1709. doi: 10.1038/sj.bjc.6601757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann NY Acad Sci. 2004;1028:1–13. doi: 10.1196/annals.1322.001. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . The Third National Health and Nutrition Examination Survey (NHANES III, 1988–94) reference manuals and reports. Centers for Disease Control and Prevention National Center for Health Statistics; Atlanta, GA: [Accessed July 1, 2009]. 1996a. at http://www.cdc.gov/nchs/nhanes/nh3data.htm. [Google Scholar]
- Centers for Disease Control and Prevention [Accessed July 1, 2009];Laboratory procedures used for NHANES III, 1988–1994. 1996b at http://www/cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/labman.pdf.
- Crimmins EM, Seeman TE. Integrating biology into the study of health disparities. Pop Develop Rev. 2004;30:89–107. [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, et al. C-reactive Protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350(14):1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Haan MN, Blythe L, Moore K, Aiello AE. Socioeconomic gradients in immune response to latent infection. Am J Epidemiol. 2007;167(1):112–120. doi: 10.1093/aje/kwm247. [DOI] [PubMed] [Google Scholar]
- Doweiko JP, Nompleggi DJ. Reviews: role of albumin in human physiology and pathophysiology, part III: albumin and disease states. J Parenter Enteral Nutr. 1991;15(4):476–483. doi: 10.1177/0148607191015004476. [DOI] [PubMed] [Google Scholar]
- Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ. C-reactive protein and the risk of incident colorectal cancer. JAMA. 2004;291(5):585–590. doi: 10.1001/jama.291.5.585. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Glymour MM, Berkman LF. Effects of social integration on preserving memory function in a nationally representative US elderly population. Am J Public Health. 2008;98(7):1215–1220. doi: 10.2105/AJPH.2007.113654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular disease. Ann Rev Public Health. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Finch CE. The biology of human longevity: inflammation, nutrition, and aging in the evolution of lifespans. Academic Press; Burlington, MA: 2007. [Google Scholar]
- Ford ES, Loucks EB, Berkman LF. Social intergration and concentrations of C-reactive protein among U.S. adults. Ann Epidemiol. 2006;16(2):78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes GL, Rosenthal L, Montag A, McClintock MK. Social isolation and the inflammatory response: sex differences in the enduring effects of a prior stressor. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R273–R282. doi: 10.1152/ajpregu.00368.2005. [DOI] [PubMed] [Google Scholar]
- Hickman DM, Miller RA, Rombeau JL, Twomey PL, Frey CF. Serum albumin and body weight as predictors of postoperative course in colorectal cancer. J Parenter Enteral Nutr. 1980;4(3):314–316. doi: 10.1177/014860718000400315. [DOI] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241(4865):540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Ikeda A, Kawachi I, Iso H, Iwasaki M, Inoue M, Tsugane S. Social support and cancer incidence and mortality: the JPHC study cohort II. Cancer Cause Control. 2013;24(5):847–860. doi: 10.1007/s10552-013-0147-7. [DOI] [PubMed] [Google Scholar]
- Jabs WJ, Busse M, Krüger S, Jocham D, Steinhoff J, Doehn C. Expression of C-reactive protein by renal cell carcinomas and unaffected surrounding renal tissue. Kidney Int. 2005;68(5):2103–2110. doi: 10.1111/j.1523-1755.2005.00666.x. [DOI] [PubMed] [Google Scholar]
- Johnson TV, Master VA. Non-malignant drivers of elevated C-reactive protein levels differ in patients with and without a history of cancer. Mol Diagn Ther. 2010;14(5):295–303. doi: 10.1007/BF03256385. [DOI] [PubMed] [Google Scholar]
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Schedlowski M. Understanding the interaction between psychosocial stress and immune-related diseases: a stepwise progression. Brain Behav Immun. 2007;21(8):1009–1018. doi: 10.1016/j.bbi.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24(7):1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- Kroenke CH, Quesenberry C, Kwan ML, Sweeney C, Castillo A, Caan BJ. Social networks, social support, and burden in relationships, and mortality after breast cancer diagnosis in the Life After Breast Cancer Epidemiology (LACE) study. Breast Cancer Res Treat. 2013;137(1):261–271. doi: 10.1007/s10549-012-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Johnsen EL, Cooper B, Anderson B, Sorosky JI, Buller RE, Sood AK. Vascular endothelial growth factor and social support in patients with ovarian carcinoma. Cancer. 2002;95(4):808–815. doi: 10.1002/cncr.10739. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Anderson B, McGinn S, Maiseri H, Dao M, Sorosky JI, De Geest K, Ritchie J, Lubaroff DM. Social support, psychological distress, and natural killer cell activity in ovarian cancer. J Clin Oncol. 2005;23(28):7105–7113. doi: 10.1200/JCO.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood AK, Antoni MH. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105(1):83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Crespin TR, Shelby RA, Andersen BL. TNF-α levels in cancer patients relate to social variables. Brain Behav Immun. 2005;19(6):521–525. doi: 10.1016/j.bbi.2005.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx J. Inflammation and cancer: the link grows stronger. Science. 2004;306(5698):966–968. doi: 10.1126/science.306.5698.966. [DOI] [PubMed] [Google Scholar]
- McClintock MK, Conzen SD, Gehlert S, Masi C, Olopade F. Mammary cancer and social interactions: identifying multiple environments that regulate gene expression through the life span. J Gerontol B Psychol Sci Soc Sci. 2005;60(special issue 1):32–41. doi: 10.1093/geronb/60.special_issue_1.32. [DOI] [PubMed] [Google Scholar]
- McMillan DC. Systemic inflammation, nutritional status and survival in patients with cancer. Curr Opin Clin Nutr Metab Care. 2009;12(3):223–226. doi: 10.1097/MCO.0b013e32832a7902. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics . Third National Health and Nutrition Examination Survey, 1988–1994: NHANES III household adult data file. Centers for Disease Control and Prevention; Hyattsville, MD: 1996a. CD-ROM, series 11, no. 1A. Public Use Data File Documentation no. 77560. [Google Scholar]
- National Center for Health Statistics . Third National Health and Nutrition Examination Survey, 1988-1994: NHANES III examination data file. Centers for Disease Control and Prevention; Hyattsville, MD: 1996b. CD-ROM, series 11, no. 1A. Public Use Data File Documentation no. 76200. [Google Scholar]
- Penwell LM, Larkin KT. Social support and risk for cardiovascular disease and cancer: a qualitative review examining the role of inflammatory processes. Health Psychol Rev. 2010;4(1):42–55. [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, Baumgartner KB, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiche EMV, Nunes SOV, Morimoto HK. Stress, depression, the immune system, and cancer. Lancet Oncol. 2004;5(10):617–625. doi: 10.1016/S1470-2045(04)01597-9. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340(2):115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci USA. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KP, Christakis NA. Social networks and health. Annu Rev Sociol. 2008;34:405–429. [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci U S A. 2013;110(15):5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S, Berkman L, Dugravot A, Ferrie JE, Marmot M, Kivimaki M, Singh-Manoux A. Socioeconomic status, structural and functional measures of social support, and mortality: the British Whitehall II Cohort Study, 1985-2009. Am J Epidemiol. 2012;175(12):1275–1283. doi: 10.1093/aje/kwr461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D, Crosnoe R, Reczek C. Social relationships and health behavior across the life course. Annu Rev Sociol. 2010;36:139–157. doi: 10.1146/annurev-soc-070308-120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kozloski M. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci. 2011;66A(5):493–500. doi: 10.1093/gerona/glr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, McClintock MK, Kozloski M, Li T. Social isolation and mortality: the role of chronic inflammation and sex differences. J Health Soc Behav. 2013;54(2):183–203. doi: 10.1177/0022146513485244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano K, Grove JS, Chen R, Rodriguez BL, Curb JD, Tracy RP. Plasma fibrinogen as a predictor of total and cause-specific mortality in elderly Japanese-American men. Arterioscler Thromb Vasc Biol. 2001;21(6):1065–1070. doi: 10.1161/01.atv.21.6.1065. [DOI] [PubMed] [Google Scholar]
- Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. 2009;64A(2):272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]