Abstract
Complex recombinant antibody fragments for modulation of immune function such as tumor cell destruction have emerged at a rapid pace and diverse anticancer strategies are being developed to benefit patients. Despite improvements in molecule design and expression systems, the quantity and stability, e.g., of single-chain antibodies produced in cell culture, is often insufficient for treatment of human disease, and the costs of scale-up, labor, and fermentation facilities are prohibitive. The ability to yield mg/ml levels of recombinant antibodies and the scale-up flexibility make transgenic production in plants and livestock an attractive alternative to mammalian cell culture as a source of large quantities of biotherapeutics. Here, we report on the efficient production of a bispecific single-chain antibody in the serum of transgenic rabbits and a herd of nine cloned, transgenic cattle. The bispecific protein, designated r28M, is directed to a melanoma-associated proteoglycan and the human CD28 molecule on T cells. Purified from the serum of transgenic animals, the protein is stable and fully active in mediating target cell-restricted T cell stimulation and tumor cell killing.
Keywords: scFV, nuclear transfer, DNA microinjection, antimelanoma, anti-CD28
Since the pioneering work of Emil von Behring that led to the definition of “antitoxins,” serum and serum constituents have been used in the diagnosis and treatment of human disease. In recent years, transgenic animals expressing a protein of choice have been suggested as bioreactors for the production of defined, protein-based therapeutics. The basic concept for this “gene farming” approach was developed in the mid-1980s (1, 2) and gained new impetus with the successful generation of cloned farm animals by nuclear transfer (3, 4). The recent success achieved by the production of polyclonal human immunoglobulins from transchromosomic calves has revealed the potential of this technology; one of its particularly attractive prospects being the production of large amounts of specific human Ig after vaccination (5).
In addition to polyclonal or monoclonal antibodies in a physiological format, various artificial antibody derivatives generated by recombinant gene technology have been developed for therapeutic use (6, 7), e.g., bispecific single-chain antibodies redirecting immunologic effector cells toward tumor cells (8–10). However, such antibodies are often difficult and sometimes even impossible to produce in sufficient yield and quality using conventional expression systems, that is, cultured bacteria, insect cells, yeast, or mammalian cells carrying the gene of interest. This underlines the need for optimization of expression strategies involving transformed plants (11) or transgenic animals (12).
Here, we took advantage of the B cell compartment in animals as the native Ig factory. We expected the secretion of bispecific antibodies from circulating blood cells to yield a stable and continuous production system with the option of rapid upscaling. To establish such a system, rearranged VH and VL genes of a bispecific single-chain antibody, in a format that does not disrupt endogenous Ig gene rearrangements, were introduced into fertilized rabbit oocytes (13) and bovine fetal fibroblasts (14) to generate transgenic rabbits and cloned transgenic calves, respectively. The bispecific single-chain variable fragment (biscFV) molecule with anti-human CD28 × anti-human melanoma specificity (r28M) was previously shown to induce a TCR/CD3 independent, “supraagonistic” stimulation of the CD28 molecule on resting human T cells, which depends on tumor cell binding and results in tumor cell killing (15).
We explore here the production and purification of this recombinant protein from blood of transgenic animals with the aim to obtain sufficient amounts for clinical application.
Materials and Methods
All animal studies were carried out at Agrobiogen, in compliance with the approval of the registered projects involving experimental animals Ref. 211-2531-1/99 (rabbits) and Ref. 211-2531-92/2000 (cows) granted by the State Government of Oberbayern, Germany.
r28M Vector Transfer into Bovine Fetal Fibroblasts. Bovine fetal fibroblasts were obtained from a fetus of a pure-bred German Fleckvieh cow 207 (BFF207) slaughtered 42 days after artificial insemination. The cells were cultured in DMEM. Subconfluent cells were transfected with 1.7 μg of AhdI linearized 13.58-kb r28M expression cassette (construct III; including the vector-backbone), using lipofectamine (GIBCO) according to the manufacturer's guidelines. Forty-eight hours after transfection, the cells were split 1:10 (16, 17) and geneticin (G418, Sigma) was added to a final concentration of 1 mg/ml. At subconfluence, resistant colonies were split and used for DNA analysis or expansion in the presence of geneticin for additional 15 days. Positive integration of the bi-scFV r28M DNA was verified by PCR using a forward primer binding to the second scFV exon (5′-TGG GGG AAC TGA GGT TTC TG-3′) and a reverse primer binding to the poly(A)-region (5′-GTC CCA TTC GCC ATT CAG-3′).
Nuclear Transfer and Blastocyst Culture. The transfer of recombinant DNA containing fibroblast nuclei into enucleated oocytes was carried out as described (14). On day 6 or 7, in vitro cultured nuclear transfer blastocysts were then transferred nonsurgically to estrus synchronized recipients.
ELISA and Statistical Quantification of bi-scFV r28M. The concentration of bi-scFV r28M in animal sera was determined by ELISA [capture: goat-anti-mouse-IgG (Dianova, Hamburg, Germany); detection: horseradish peroxidase-conjugated Protein L (Pierce)]. Samples were titrated in duplicates of seven 3-fold serial dilutions and compared with the bi-scFV r28M purified from the cell culture (r28M standard). The OD450 values and log serum dilutions were plotted by using a nonlinear regression sigmoidal binding analysis according to the following equation: y = Vmax + (A1 - Vmax)/((1 + x/xo)p), where Vmax is the maximum binding, A1 is the initial binding, x is the log sera dilution or concentration of r28M standard, and p is the exponential binding order. The concentration of bi-scFV r28M in each serum sample was calculated from the titration curves at the point of 50% binding.
Fluorescence-Activated Cell Sorting Analysis. To analyze the binding capacity of the bispecific r28M, Jurkat cells expressing CD28 and Sk-Mel63 expressing the melanoma-associated proteoglycan were used in flow cytometry analysis. The cells were incubated with sera or purified material at various concentrations, washed, and stained with phycoerythrin-labeled F(ab′)2 fragments of a goat anti-mouse IgG antibody (Dianova) at dilutions recommended by the manufacturer. Cells were then analyzed in a FACSCalibur equipped with cellquest software (Becton Dickinson).
Functional Assays. Target cell-dependent T cell proliferation and tumor cell killing induced by the bispecific antibody fragment r28M were measured as described (15). Briefly, serum or purified bi-scFV r28M were incubated in triplicates in 96-well microtiter plates with x-irradiated (120 Gy) Sk-Mel63 tumor cells (5,000 cells per well) and peripheral blood mononuclear cells (PBMC) (50,000 cells per well) from healthy donors. During the last 16 h of a 3-day incubation period, [3H]thymidine was added (18,5 kBq per well). Cells were harvested on a filtermate and incorporated [3H]thymidine was measured.
Tumor cell killing was determined under identical experimental conditions except that in this case viable rather than irradiated tumor cells were used. The number of viable and adherent tumor cells was determined after washing the plates and adding the tetrazolium salt WST (Roche Diagnostics, Mannheim, Germany).
Photomicrographs of cocultures of PBMC and nonirradiated Sk-Mel63 incubated with sera from transgenic or control animals (rabbit and calf) were taken with an XR-X3000 camera (Ricoh, Eschborn, Germany) mounted on a Axiovert 25 microscope (Zeiss, Oberkochen, Germany).
Results
Construction and Optimization of Expression Cassettes. To decipher the relevant promoter sequences and intronic transcription elements that are essential for high-level expression of bi-scFV r28M, linearized constructs of three different expression cassettes were microinjected into the male pronucleus of fertilized rabbit oocytes (13).
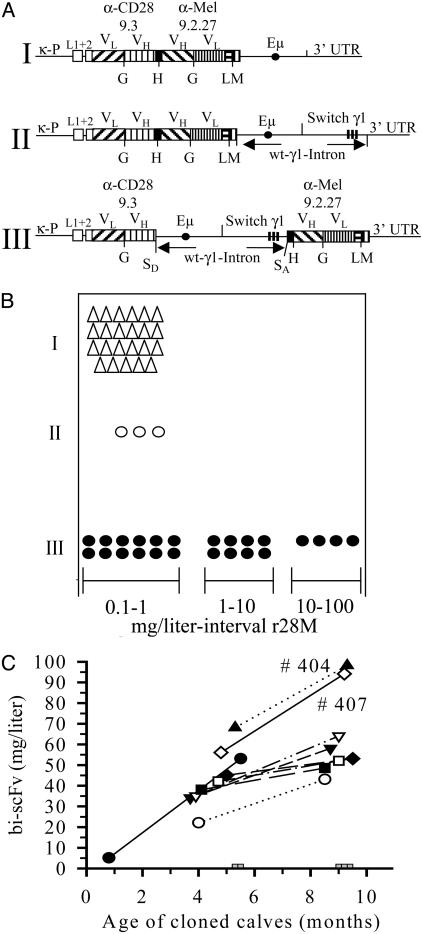
As shown in Fig. 1A, the constructs I and II share the same core μ-enhancer element but differ in the size of their 3′ sequence (γ1-switch). Previous investigations had revealed that the switch region located within the wt-γ1-intron of a human heavy-chain gene increases the level of Ig transgene expression in mice (18) but has only a marginal effect in stably transfected myeloma cell lines (19). Both gene constructs were used to generate transgenic rabbits. However, only a low level of bi-scFV r28M expression was achieved (Fig. 1B). We next proceeded to place the 5.5-kb wt-γ1-intron into a more “physiological” genomic environment: 3′ of the VH-region of scFV1 and 5′ of the CH1-linker to scFV2 (construct III, Fig. 1 A). This region does not contain any recombination elements and thus has no potential to affect the normal Ig gene repertoire. This is confirmed by the finding that endogenous IgG concentrations in transgenic animals, as monitored by ELISA, were similar to that of control rabbit sera (data not shown). For the confirmation of a correct assembly of 5′ and 3′ exon boundaries following the 1+2 splice rule for Ig genes, cytoplasmic mature mRNA was isolated. After cDNA synthesis with an oligo(dT) primer, a defined amplicon of 533 bp was generated by using specific primers for the 5′ and 3′ exons. Correct joining of splice donor and splice acceptor sites was verified after subcloning and sequencing (data not shown). This unique “spliced transcript” exerts a marked effect on the level of bi-scFV r28M expression (Fig. 1B).
Fig. 1.
Bi-scFV r28M constructs and expression in transgenic farm animals. (A) Structure of the microinjected recombinant DNA constructs (not to scale). Antibody domains and transcriptional control elements are denoted by boxed regions and lines, respectively. The 9.3 anti-CD28 scFV fragment (34) with 5′ sequences of signal peptide L1 and L2 interspersed by intron are joined by a linker derived from the elbow-region of the human Igγ CH1 domain to the 9.2.27 anti-melanoma scFV fragment (35) with 3′ N-terminal human IgγCL and a c-myc tag. Integrated copies of the bi-scFV r28M were detected in all three groups of transgenic founders without rearrangements or deletions as confirmed by PCR and DNA sequencing (results not shown). κ-P, murine kappa promoter 1.2 kb in length; L1 + 2, exon 1 and 2 coding for 19 aa leader-sequence; VL, variable light-chain domain; VH, variable heavy-chain domain; G, (G4S)3-linker; H, CH1-linker; L, CL-linker; M, c-myc-tag; Eμ, 1.85kb intronic element containing the μ-enhancer; Switch γ1, 3.65-kb intronic sequence containing the γ1 switch region; 3′ UTR, 1.8-kb 3′ untranslated region of murine γ1 locus; wt-γ1-Intron, 5.5-kb wild-type murine γ1 heavy-chain intronic sequence; SD, splice donor site; SA, splice acceptor site. (B) Expression of r28M protein in the serum of transgenic founder rabbits. The concentration (interval of mg/liter) of bi-scFV r28M in rabbits for the constructs shown in A was determined by flow cytometry analysis. Each symbol represents a founder animal. (C) Expression of recombinant bi-scFV r28M (construct III) in cloned calves. The concentration of bi-scFV r28M (mg/liter) was determined by ELISA; first (left symbol) and second (right symbol) time points for n = 9 animals, urine of cloned calves (gray squares) n = 5. Sera of control calves n = 5 gave zero values (data not shown).
Transgenic rabbits carrying construct I (n = 23) or construct II (n = 3) produce <1 mg/liter of bi-scFV r28M in the serum. Remarkably, several of the construct III transgenic founders (n = 24) display up to a 100-fold increase in the concentration of bi-scFV r28M: four animals in the range of 10–100 mg/liter and eight animals in the range of 1–10 mg/liter. In some F1 offspring of construct III transgenic founder rabbits with low expression levels, an increased expression comparable with that of high producing animals was found (data not shown). This phenomenon is most likely attributable to mosaicism of the founder animals. Bi-scFV r28M expressed in supernatants of construct III-transfected Sp2/0 myeloma cells was in the range of 1–3 mg/liter (15).
Expression in Cloned Calves. Having identified an optimized gene construct for expression of bi-scFV r28M in rabbit blood, we used the splice gene construct III to generate cloned transgenic calves (Fig. 4, which is published as supporting information on the PNAS web site). To this end, primary fetal fibroblasts derived at day 42 after implantation were transfected, selected in vitro, and used for nuclear transfer (14). Embryo transfer of the developed blastocysts resulted in a total of 13 pregnancies with 12 viable fetuses (Table 1). The protein was detected by flow cytometry in the blood of one fetus killed at day 46 (results not shown).
Table 1. Development of cloned embryos derived from transfected bovine fetal fibroblasts.
| Nuclear transfers | 309 |
| Blastocysts (%) | 96 (31) |
| No. of blastocyst transfers | 77 |
| No. of recipients | 31 |
| Pregnancy 28 days (%) | 13 (40) |
| No. of offspring | 11 |
| Percent of transgenic offspring | 100 |
Transfected primary fetal fibroblasts derived from a fetus at day 42 after implantation were used for cloning. The percentage of blastocysts was calculated from the number of blastocysts divided by the number of fused donor oocyte complexes or nuclear transfers. The average fusion rate in the laboratory was 95%. Embryo transfers resulted in 13 pregnant recipients. Two animals were killed on day 46 of pregnancy for collecting fetal cells and analyzing expression of the targeted gene; two pregnancies were lost on days 41 and 83. The remaining nine pregnant recipients delivered 11 calves, 9 of which were born alive and healthy; two calves died during parturition.
To quantify the amount of bi-scFV r28M and to monitor the expression levels in the blood at different time points, specific ELISA measurements were made of sera taken from cloned and control calves during the first 9 months postpartum (Fig. 1C). Each individual cloned calf showed a significant increase in bi-scFV r28M production with age, from 0.5–5 to 8–10 months postpartum. This is consistent with the maturation of cow splenic B cells and the increased Ig titer observed in mature calves compared with suckling newborns (20).
From the standpoint of pharmacokinetics of bispecific singlechain antibody fragments, it was of interest to know whether or not the cloned calves might excrete the 57-kDa bi-scFV r28M molecule. None of the five tested animals had detectable levels of recombinant protein in the urine (Fig. 1C, gray squares), whereas bi-scFV fragments were measurable in r28M spiked control urine (data not shown).
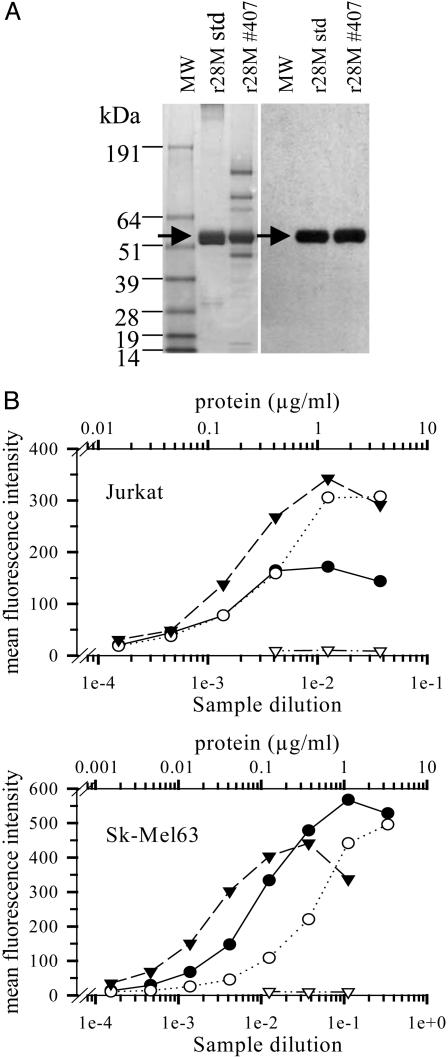
Purification and Characterization of the Transgenic Protein. To purify bi-scFV r28M from animal blood, 80 ml of serum from calf 407 was passed over a protein L agarose column, and bound material was eluted with 0.1 M glycine at pH 3. The advantage of protein L affinity chromatography for purification of r28M from calf serum is that the κ light chains of bovine immunoglobulins, unlike rabbit immunoglobulins, interact only weakly with protein L (21). The SDS/PAGE Coomassie staining (Fig. 2A) indicates that the 57-kDa bi-scFV r28M protein derived from cloned calves, after a single affinity chromatography step, is of 50–70% purity. The 57-kDa band is indistinguishable from material purified from supernatant of the transfected murine myeloma cell line Sp2/0. The identity and homogenicity of the protein was further confirmed by an immunoblot (Fig. 2 A) and mass spectrometric analysis.
Fig. 2.
Purification of bi-scFV r28M and determination of binding capacity. (A) (Left) SDS/PAGE Coomassie staining: bi-scFV r28M of calf 407 purified by pH3 elution from protein L agarose (Actigen, Halle-Zoersel, Belgium) and bi-scFV r28M standard. Each lane was loaded with 10 μg of protein. (Right) Immunoblot with a horseradish peroxidase-conjugated anti-c-myc monoclonal antibody 9E10 (dilution 1:5,000; Invitrogen). The arrow indicates the 57-kDa bi-scFV r28M molecule. (B) Binding of bi-scFV r28M in flow cytometry analysis stained with a polyclonal phycoerythrin-labeled goat anti-mouse antibody. Mean fluorescence intensity of cells incubated with defined concentrations (Upper, μg/ml) of (i) r28M standard (•) or purified material from calf 407 serum (○) and (ii) with dilutions of serum (Lower, sample dilution) from calf 407 (▾) or control calf serum ((▿)).
The binding capacities of serum from calf 407, bi-scFV r28M purified from calf 407, and the purified r28M standard were similar on Jurkat T cells and Sk-Mel63 melanoma cells (Fig. 2B). The activity of the diluted serum compared with the standard protein preparation is consistent with serum concentrations of 0.05–0.1 g/liter as determined by ELISA (Fig. 1C). No detectable binding was found if transgenic serum was incubated with antigen-negative cells (data not shown) or if antigen-positive cells were incubated with nontransgenic control calf serum (Fig. 2B, open triangles).
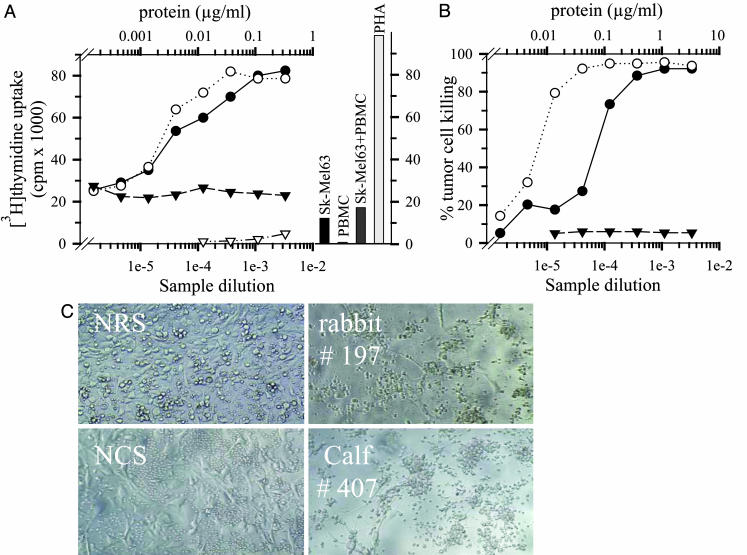
Induction of Target Cell-Dependent T Cell Activation and Tumor Cell Killing. To investigate whether the bi-scFV r28M obtained from calf serum retains its biological function after purification, we added samples of either the purified material or source serum to cocultures of human PBMC and Sk-Mel63 and measured the [3H]thymidine uptake after 3 days (Fig. 3A). As an example, unpurified serum derived from calf 407 as well as protein L purified material derived from it induced a strong dosedependent proliferation that was comparable to the pan clonal stimulator phytohemagglutinin (Fig. 3A). No stimulatory effect of bi-scFV r28M was observed if PBMC were cocultured with the mammary carcinoma cell line T47D lacking expression of the HMWG proteoglycan, which underlines the target cell dependence of r28M-induced T cell activation. No T cell proliferation was detectable with serum of a control animal. Cytotoxicity assays (Fig. 3B) demonstrated that the r28M-induced T cell activation resulted in an effective dose-dependent tumor cell killing. When either the serum of calf 407 or the purified material was used, melanoma cells were almost completely killed, whereas no cytotoxic effect was seen when a bovine control serum was used. This striking antitumor effect of bi-scFV r28M is also demonstrated by the photomicrographs in Fig. 3C. Tumor cell destruction was complete after 3 days of coculture with PBMC and bi-scFV r28M containing sera of either splice transgenic rabbit 197 or cloned transgenic calf 407. In contrast, tumor cells remained viable in the cocultures exposed to sera of control animals (Fig. 3C, NRS and NCS) or when antigen-negative tumor cells were used (data not shown).
Fig. 3.
Proliferation of human PBMC and killing of tumor target cells incubated with bi-scFV r28M. (A) Proliferation measured by [3H]thymidine incorporation of PBMC incubated for 3 days with x-irradiated Sk-Mel63 tumor cells and the following sources: (i) serum dilution of transgenic calf 407 (•) or control calf serum (▾) (lower scale; sample dilution) and (ii) bi-scFV r28M purified from sera of calf 407 (○) (upper scale; μg/ml). PBMC and serum of calf 407 (lower scale; sample dilution) were incubated with an antigen negative mammary carcinoma cell line T47D ((▿)). Additional controls shown as bars: [3H]thymidine incorporation of tumor cells, PBMC, PBMC and tumor cells, and phytohemagglutinin (5 μg/ml) activated PBMC as positive control. (B) Percentage of tumor cell killing of nonirradiated Sk-Mel63 cells incubated for 3 days with PBMC and the same samples as indicated in A. Survival of adherent tumor cells was determined by using the WST dye after removing the PBMC. (C) Killing of Sk-Mel63 tumor cells incubated with PBMC in the presence of serum from transgenic rabbit 197 and control rabbit serum (NRS, Upper) or cloned calf 407 and control calf serum (NCS, Lower).
The functional activity of the recombinant protein is maintained for at least several months upon storage in serum or PBS at 4°C. This holds true for both material isolated from serum and cell culture supernatant, respectively. Likewise, in both cases the presence of a dimeric protein version was indicated by gel filtration analysis (data not shown) (15).
To compare pharmacokinetic properties, we performed an experiment in which C57BL/6 mice were injected i.p. with 30 μg of bi-scFV purified from the cell culture or from transgenic animals, respectively. Serum concentration of the r28M protein was measured 1, 3, 6, 9, 24, and 30 h after injection by fluorescence-activated cell sorting analysis of CD28+ Jurkat cells. We found that the half-life of r28M either purified from serum or cell culture supernatant is ≈10–12 h (data not shown).
Discussion
Diverse expression systems are currently available for the experimental and industrial production of antibodies and their fragments. In applying the bacterial expression system, which is both simple and easy to scale up, complex proteins tend to fold incorrectly and aggregate in large, insoluble inclusion bodies. This requires refolding procedures that often lead to loss of function (22). A further disadvantage of prokaryotic expression is that posttranslational modifications such as glycosylation do not take place. Many of the problems associated with bacterial expression have been overcome through the use of insect cells, yeast, and plants, but because of the differences in glycosylation patterns (high-mannose, multiple branched oligosaccharides), the antibodies produced are often not suitable for therapeutic use in humans (6). In contrast, mammalian cells carry out authentic posttranslational modifications. Thus, mammalian cell culture is currently the prevailing method for the production of several antibodies in clinical use. Although efficient, this approach has intrinsic limitations. One disadvantage is that mammalian cell culture is costly and laborious. Consequently, the scale up and the capacity for scale up may well become limiting factors for clinical application in the near future (23, 24). Part of the problem is that, despite significant improvement, animal cells in culture still produce suboptimal amounts of recombinant protein because of the fact that a fermentor usually operates at relatively low cell densities and under nonoptimal metabolic conditions. This was perceived years ago and constituted the rationale for the generation of transgenic animals and transformed plants. Since then, the successful expression of antibodies in milk (25, 26) and serum (27, 28) of transgenic livestock as well as in transformed plants (29) has been described. More recently, cloned transgenic calves carrying a human artificial chromosome vector with the entire human IgH and Igλ loci have been generated (5). In all these cases, antibodies in a “physiological” multichain format were produced in sufficient yields of several grams per liter (26, 27, 30) or kg per acre (29). In case of antibody expression in serum, one has to keep in mind that a portion of the produced molecules may be hybrid antibodies if the animal IgG loci remain active.
Despite the significant progress that has been made with antibodies in a physiological format, there is a great demand for the production of more complex nonphysiological recombinant antibody derivatives such as bi-scFV. Such molecules hold the promise for increased therapeutic potential but also aggravate the problem of production. When cell culture systems are used, these molecules are generally more difficult to produce in sufficient amounts and good quality, even after time consuming and often unstable gene amplification steps (31).
Until now, it was unclear whether gene farming could improve this situation. The work presented here indicates that this does indeed seem to be the case. It demonstrates the production of a complex bispecific single-chain antibody in the serum of transgenic farm animals and, carrying the gene farming approach one step further, in the serum of cloned cattle. The transgenic protein could be isolated from the serum with good yield and retained biological activity. Concentration of the protein in the cow serum exceeds that obtained in tissue culture supernatants at least by a factor of fifty and the purified protein was active in driving tumor cell dependent T cell activation and tumor cell killing. Having established a successful “gene farming” procedure for the r28M protein, it should now be possible to evaluate its therapeutic potential in experimental clinical trials.
With respect to safety considerations applicable to the transmission of pathogenic substances and viruses, the U.S. Food and Drug Administration and the European Medicines Evaluation Agency have laid down well established regulations for therapeutic proteins derived from animal serum (www.emea.eu.int/pdfs/human/bwp/179302en.pdf and www.emea.eu.int/pdfs/human/bwp/335499en.pdf). Examples of such pharmaceutical products are anti-thymocyte globulin from rabbit serum or coagulation factors from bovine blood (refs. 32 and 33, www.pei.de/english/bse/fibrin_bse_engl.htm, and www.fda.gov/cber/efoi/approve.htm.). These latter proteins are components of fibrin sealants that are widely used in surgery. If concerns remain that the application of a particular purification procedure does not reliably remove prion proteins, animals could be bred under pathogen-free conditions and tested for prion disease with existing technology. Moreover, in the future, animals with an inactive prion protein gene may become available.
In conclusion, the gene farming approach described here underscores the fact that blood cells can be directed to produce high concentrations of fully active therapeutic molecules that are otherwise difficult to express. This can be achieved without compromising existing drug safety regulations or the animal's health and without input from fermentation facilities or the expensive assistance of technical staff. Future applications of transgene expression in blood may extend to the production of large quantities of additional Ig fusion proteins for diagnostic or therapeutic use.
Supplementary Material
Acknowledgments
We thank Wolfgang Marwan for initial assistance in cloning the melanoma-specific antibody, and we express our special thanks to Jörn Dengjel for carrying out the mass spectrometry. This work was partly supported by Paktis Antibody Services, Agrobiogen, Wilhelm Sander Foundation Grant 2001.114.1 (to G.J.), and the German Ministry for Education and Research Grant PTJ 0312625. L.G.-H. was funded in part by a joint project of Agrobiogen and Eberhard Karls University.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Bi-scFV, bispecific single-chain variable fragment; PBMC, peripheral blood mononuclear cells.
References
- 1.Hammer, R. E., Pursel, V. G., Rexroad, C. E., Jr., Wall, R. J., Bolt, D. J., Ebert, K. M., Palmiter, R. D. & Brinster, R. L. (1985) Nature 315, 680-683. [DOI] [PubMed] [Google Scholar]
- 2.Brem, G., Brenig, B., Goodman, H. M., Selden, R. C., Graf, F., Kruff, B., Springmann, K., Hondele, J., Meyer, J., Winnacker, E. L., et al. (1985) Zuchthyg 20, 251-252. [Google Scholar]
- 3.Campbell, K. H., McWhir, J., Ritchie, W. A. & Wilmut, I. (1996) Nature 380, 64-66. [DOI] [PubMed] [Google Scholar]
- 4.Wilmut, I., Schnieke, A. E., McWhir, J., Kind, A. J. & Campbell, K. H. (1997) Nature 385, 810-813. [DOI] [PubMed] [Google Scholar]
- 5.Kuroiwa, Y., Kasinathan, P., Choi, Y. J., Naeem, R., Tomizuka, K., Sullivan, E. J., Knott, J. G., Duteau, A., Goldsby, R. A., Osborne, B. A., et al. (2002) Nat. Biotechnol. 20, 889-894. [DOI] [PubMed] [Google Scholar]
- 6.Little, M., Kipriyanov, S. M., Le Gall, F. & Moldenhauer, G. (2000) Immunol. Today 21, 364-370. [DOI] [PubMed] [Google Scholar]
- 7.van Spriel, A. B., van Ojik, H. H. & van de Winkel, J. G. (2000) Immunol. Today 21, 391-397. [DOI] [PubMed] [Google Scholar]
- 8.Mallender, W. D. & Voss, E. W., Jr. (1994) J. Biol. Chem. 269, 199-206. [PubMed] [Google Scholar]
- 9.Gruber, M., Schodin, B. A., Wilson, E. R. & Kranz, D. M. (1994) J. Immunol. 152, 5368-5374. [PubMed] [Google Scholar]
- 10.Kurucz, I., Titus, J. A., Jost, C. R., Jacobus, C. M. & Segal, D. M. (1995) J. Immunol. 154, 4576-4582. [PubMed] [Google Scholar]
- 11.Stoger, E., Sack, M., Fischer, R. & Christou, P. (2002) Curr. Opin. Biotechnol. 13, 161-166. [DOI] [PubMed] [Google Scholar]
- 12.Houdebine, L. M. (2002) Curr. Opin. Biotechnol. 13, 625-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Besenfelder, U. & Brem, G. (1993) J. Reprod. Fertil. 99, 53-56. [DOI] [PubMed] [Google Scholar]
- 14.Zakhartchenko, V., Mueller, S., Alberio, R., Schernthaner, W., Stojkovic, M., Wenigerkind, H., Wanke, R., Lassnig, C., Mueller, M., Wolf, E., et al. (2001) Mol. Reprod. Dev. 60, 362-369. [DOI] [PubMed] [Google Scholar]
- 15.Grosse-Hovest, L., Hartlapp, I., Marwan, W., Brem, G., Rammensee, H. G. & Jung, G. (2003) Eur. J. Immunol. 33, 1334-1340. [DOI] [PubMed] [Google Scholar]
- 16.Schnieke, A. E., Kind, A. J., Ritchie, W. A., Mycock, K., Scott, A. R., Ritchie, M., Wilmut, I., Colman, A. & Campbell, K. H. (1997) Science 278, 2130-2133. [DOI] [PubMed] [Google Scholar]
- 17.McCreath, K. J., Howcroft, J., Campbell, K. H., Colman, A., Schnieke, A. E. & Kind, A. J. (2000) Nature 405, 1066-1069. [DOI] [PubMed] [Google Scholar]
- 18.Gram, H., Zenke, G., Geisse, S., Kleuser, B. & Burki, K. (1992) Eur. J. Immunol. 22, 1185-1191. [DOI] [PubMed] [Google Scholar]
- 19.Sigurdardottir, D., Sohn, J., Kass, J. & Selsing, E. (1995) J. Immunol. 154, 2217-2225. [PubMed] [Google Scholar]
- 20.Holloway, N. M., Tyler, J. W., Lakritz, J., Carlson, S. L., Tessman, R. K. & Holle, J. (2002) J. Vet. Intern. Med 16, 187-191. [DOI] [PubMed] [Google Scholar]
- 21.Akerstrom, B. & Bjorck, L. (1989) J. Biol. Chem. 264, 19740-19746. [PubMed] [Google Scholar]
- 22.Kipriyanov, S. M., Moldenhauer, G., Braunagel, M., Reusch, U., Cochlovius, B., Le Gall, F., Kouprianova, O. A., der Lieth, C. W. & Little, M. (2003) J. Mol. Biol. 330, 99-111. [DOI] [PubMed] [Google Scholar]
- 23.Dove, A. (2002) Nat. Biotechnol. 20, 777-779. [DOI] [PubMed] [Google Scholar]
- 24.Gura, T. (2002) Nature 417, 584-586. [DOI] [PubMed] [Google Scholar]
- 25.Castilla, J., Pintado, B., Sola, I., Sanchez-Morgado, J. M. & Enjuanes, L. (1998) Nat. Biotechnol. 16, 349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pollock, D. P., Kutzko, J. P., Birck-Wilson, E., Williams, J. L., Echelard, Y. & Meade, H. M. (1999) J. Immunol. Methods 231, 147-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidle, U. H., Lenz, H. & Brem, G. (1991) Gene 98, 185-191. [DOI] [PubMed] [Google Scholar]
- 28.Lo, D., Pursel, V., Linton, P. J., Sandgren, E., Behringer, R., Rexroad, C., Palmiter, R. D. & Brinster, R. L. (1991) Eur. J. Immunol. 21, 1001-1006. [DOI] [PubMed] [Google Scholar]
- 29.Ma, J. K., Hiatt, A., Hein, M., Vine, N. D., Wang, F., Stabila, P., van Dolleweerd, C., Mostov, K. & Lehner, T. (1995) Science 268, 716-719. [DOI] [PubMed] [Google Scholar]
- 30.Larrick, J. W., Yu, L., Naftzger, C., Jaiswal, S. & Wycoff, K. (2001) Biomol. Eng. 18, 87-94. [DOI] [PubMed] [Google Scholar]
- 31.Loffler, A., Kufer, P., Lutterbuse, R., Zettl, F., Daniel, P. T., Schwenkenbecher, J. M., Riethmuller, G., Dorken, B. & Bargou, R. C. (2000) Blood 95, 2098-2103. [PubMed] [Google Scholar]
- 32.Merion, R. M. (1999) Transplant. Proc. 31, 2208-2209. [DOI] [PubMed] [Google Scholar]
- 33.Clark, R. A. (2000) Expert Opin. Invest. Drugs 9, 2371-2392. [DOI] [PubMed] [Google Scholar]
- 34.Ledbetter, J. A., Martin, P. J., Spooner, C. E., Wofsy, D., Tsu, T. T., Beatty, P. G. & Gladstone, P. (1985) J. Immunol. 135, 2331-2336. [PubMed] [Google Scholar]
- 35.Schulz, G., Bumol, T. F. & Reisfeld, R. A. (1983) Proc. Natl. Acad. Sci. USA 80, 5407-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.