Abstract
Considerable progress has been made in understanding the roles of Helicobacter pylori in inflammation and gastric cancer; however, far less is known about the roles of enterohepatic Helicobacter species (EHS) in carcinogenesis and their zoonotic or pathogenic potential. We determined the prevalence of EHS infection in a cohort of geriatric rhesus monkeys in which intestinal adenocarcinoma (IAC) is common and investigated the association between EHS infection and IAC. The cohort consisted of 36 animals, 14 of which (age 26–35 years) had IAC. Of the 36 rhesus, 35 (97 %) were positive for EHS using PCR or bacterial isolation from faeces, colonic or tumour tissues. Only a single rhesus, which had IAC, was negative for EHS by all detection methods. The EHS identified by 16S rRNA sequencing in this study were from three Helicobacter taxa: Helicobacter macacae (previously rhesus monkey taxon 1), Helicobacter sp. rhesus monkey taxon 2, previously described from strain MIT 99-5507, and Helicobacter sp. rhesus monkey taxon 4, related to Helicobacter fennelliae. Thirteen of 14 monkeys with IAC were positive for either H. macacae (7/13, 54 %), EHS rhesus monkey taxon 4 (4/13, 31 %) or a mixture of the two EHS (2/13, 15 %). These results indicate that EHS are prevalent among aged rhesus macaques with IAC. Using Helicobacter genus-specific florescent in situ hybridization, EHS were detected on the surface of colonic epithelia of infected monkeys. All Helicobacter isolates, including H. macacae, effectively adhered to, invaded, and significantly induced proinflammatory genes, including IL-8, IL-6, TNF-α and iNOS, while downregulating genes involved in the function of inflammasomes, particularly IL-1β, CASPASE-1, NRLP3, NLRP6 and NLRC4 in the human colonic T84 cell line (P<0.0001). These results suggest that EHS may represent an aetiological agent mediating diarrhoea, chronic inflammation, and possibly intestinal cancer in non-human primates, and may play a role in similar disease syndromes in humans. Downregulation of inflammasome function may represent an EHS strategy for long-term persistence in the host and play a role in inducing pathological changes in the host’s lower bowel.
Introduction
Enterohepatic Helicobacter species (EHS) are Gram-negative, helical microaerophilic proteobacteria that have been implicated in the development of gastrointestinal diseases in humans and animals (Fox, 2002; Fox et al., 2002; Ceelen et al., 2005; Goldman & Mitchell, 2010). Unlike the stomach-colonizer Helicobacter pylori, which is a known aetiological agent for gastritis and gastric adenocarcinoma in humans, EHS reside in the host’s intestinal and hepatobiliary tracts, with the potential of eliciting pathological changes in infected tissues (Goldman & Mitchell, 2010; Fox et al., 2011).
In experimentally and naturally infected cases, several EHS can cause inflammatory conditions and neoplasia in various species of animals (Cimolai et al., 1987; Burnens et al., 1994; Burman et al., 1995; Cahill et al., 1998; Foley et al., 1998, 1999; Avenaud et al., 2000; Ceelen et al., 2005; Thomson et al., 2011). In susceptible mouse strains, chronic infection with Helicobacter hepaticus induces hepatitis, hepatic adenoma and hepatocellular carcinoma (Fox et al., 1996b; García et al., 2011). In immunocompromised mice, EHS infection causes typhlocolitis, enterocolitis and neoplasia of the lower bowel (Foltz et al., 1998; Chin et al., 2000; Erdman et al., 2009; Mangerich et al., 2012).
Several EHS, including Helicobacter pullorum, H. cinaedi, H. fennelliae, H. canadensis, H. bilis, H. winghamensis and H. canis, have been isolated from the gastrointestinal tract of human patients with ulcerative colitis, proctitis and diarrhoea (Hung et al., 1997; Foley et al., 1999; Hsueh et al., 1999; Fox et al., 2000; Melito et al., 2001; Hansen et al., 2011). One of the EHS, H. cinaedi, has been shown to cause bacteraemia, gastroenteritis, osteomyelitis and cellulitis in immunocompetent and immunocompromised humans and has recently been shown to persistently colonize the intestinal tract of humans (Kitamura et al., 2007; Hansen et al., 2011; Oyama et al., 2012). Importantly, experimental inoculation of infant pigtail macaques with the human isolates H. cinaedi or H. fennelliae resulted in colitis and bacteraemia (Flores et al., 1990). These findings raise the possibility that chronic EHS infection may lead to development of inflammatory bowel disease (IBD) and neoplasia in humans (Haggerty et al., 2005; Thomson et al., 2011). Furthermore, it has been previously observed that there is zoonotic transmission infection with EHS, particularly between humans and pets (Fox, 2002).
A novel EHS has been isolated from colitis-affected cotton-top tamarins, which are predisposed to developing IBD and colon cancer (Saunders et al., 1999). We previously surveyed a cohort of rhesus and cynomolgus monkeys in which idiopathic colitis was endemic, and found that many of them were infected with multiple EHS, including Helicobacter macacae (Fox et al., 2001b, 2007). Another colony of rhesus, where colitis was not endemic, also had Helicobacter spp. isolated from their faeces. Ten years after this initial survey, one of the monkeys in the non-colitis cohort, but persistently infected with H. macacae, developed intestinal adenocarcinoma (IAC) (Marini et al., 2010), the most common neoplasia in geriatric (>20 years of age) rhesus macaques (Uno et al., 1998; Valverde et al., 2000; Rodriguez et al., 2002). This observation led us to hypothesize that chronic EHS infection produces colitis, but also persistent EHS infection, similar to H. hepaticus-infected Rag−/− mice, which develop colon carcinoma, could play a role in the genesis of intestinal neoplasia (Erdman et al., 2009; Mangerich et al., 2012). In this study, we surveyed a cohort of ageing rhesus monkeys, in which IAC is common, to determine whether infection with EHS is prevalent among these animals. Recognizing that rhesus EHS were detected in a diarrhoeic child (Haggerty et al., 2005) and that EHS isolated from humans can experimentally cause disease in non-human primates, we also explored the pathogenic potential of the rhesus EHS using in vitro assays.
Methods
Rhesus monkey population.
The study population consisted of 36 geriatric rhesus macaques (7 males and 29 females) with ages ranging from 26 to 35 years, 14 of which were diagnosed with IAC. Thirty animals were positive for simian T-cell leukaemia virus (STLV) by serology and one monkey was positive for both STLV and simian retrovirus (SRV). All animals were negative for simian immunodeficiency virus and B virus, as well as for enteric pathogenic bacteria, Shigella spp. and Salmonella spp.
Bacteria isolation and culture.
EHS isolation from faecal, colonic and tumour samples was performed as previously described (Fox et al., 2001b). Briefly, the faecal samples were suspended in Brucella broth and the suspension was filtered through a 0.65 µm Acrodisc syringe filter (M.D. Laboratory Supplies) to enrich for EHS. The filtrate was spotted on the surface of the blood agar plates (Remel). Colon and tumour samples were homogenized in Brucella broth and the mixtures were filtered as mentioned above and spotted on blood agar media. After 7–10 days of incubation under microaerobic conditions (10 % H2, 10 % CO2, 80 % N2) in a vented jar at 37 °C, the plates were inspected for evidence of EHS growth. The bacteria were collected with a sterilized cotton swab and resuspended in PBS. Their genomic DNA was isolated using the High Pure PCR Template Preparation kit (Roche Molecular Biochemicals) (Fox et al., 2007). All Helicobacter isolates were preserved at −80 °C in Brucella broth containing 20 % glycerol (v/v) for subsequent analyses.
Molecular techniques.
Faecal samples were collected, and stored at −80 °C until they were used for analysis. Faecal DNA was obtained using the Qiagen DNA stool mini extraction kit according to the manufacturer’s recommendations. Faecal PCR was performed using Helicobacter genus 16S rRNA gene-specific primers (C05 and C97), which amplify an ~1200 bp conserved region of the 16S rRNA, as previously described (Fox et al., 2007). The identity of the 16S rRNA PCR products was determined by a combination of RFLP analysis, DNA sequencing and blast analysis of the DNA sequences against the NCBI DNA sequence database. RFLP analyses were performed using AluI and HhaI restriction endonucleases (New England Biolabs) as previously described (Fox et al., 2007). DNA sequencing was accomplished using the ABI prism Genetic Analyzer 3500 (Life Technologies). Full 16S rRNA sequences were obtained, sequences were aligned in our RNA database, and neighbour-joining trees were created as previously described (Dewhirst et al., 2005). To determine the presence of the cytolethal distending toxin (CDT) gene in the genome of rhesus monkey EHS, a set of degenerate primers VAT2, WMI1 and DHF1, previously used to identify CDT genes in other Helicobacter species, were used (Chien et al., 2000).
In vitro cell culture, cell adhesion, gentamicin protection assays.
T84 human colonic cells were obtained from the American Type Culture Collection (ATCC). A stock culture of T84 (a human colonic epithelial cell line; ATCC, CCL 6) was maintained in a 1 : 1 mixture of Han’s F12 and Dulbecco’s modified Eagle’s medium (DMEM : F12; ATCC) supplemented with 5 % (v/v) fetal bovine serum, penicillin (100 U ml−1) and streptomycin (100 µg ml−1) according to ATCC’s recommendation. T84 cells (106 per well) were seeded into 12-well cell culture plates and incubated at 37 °C in a humidified incubator aerated with 5 % CO2 for 48 h. Adhesion and gentamicin protection assays were performed according to a previously published procedure (Lertpiriyapong et al., 2012) with minor modifications. Briefly T84 cells were inoculated with EHS at 50 m.o.i. in 1 ml fresh culture medium. EHS were propagated on blood agar under microaerobic conditions and collected at 48 h post-incubation, which corresponds to the mid-exponential phase of growth. Viable organisms were predominantly spiral in shape, and contamination by other types of bacteria was not noted as visualized by Gram staining. The plates were centrifuged at 200 g to facilitate bacterial cell adhesion and incubated under microaerobic conditions. EHS was subjected to heat treatment by boiling in water at 100 °C for 20 min. After 2 h of incubation for cell adhesion assays and 4 h of incubation for the gentamicin protection assays, T84 cells were lysed with 0.1 % saponin and the bacteria were enumerated by 10-fold serial dilution and plating onto blood agar. The gentamicin concentration used in the gentamicin protection assay was 250 µg ml−1. An insignificant number of EHS (4 to 5 c.f.u. in 1 ml of media) were recovered from the supernatant with this concentration of gentamicin and 4 h duration of treatment.
CDT assay.
In vitro assays for CDT activity and preparation of cell sonicates of EHS were performed according to our previously described method using HeLa cells (CCL-2, ATCC) (Young et al., 2000b). Briefly, HeLa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with glutamine and 10 % fetal calf serum. HeLa cells (104) were seeded into each well of 24-well cell culture plates. The cells were allowed to acclimate for 2 h. Ten microlitres of filtered sterilized supernatant fractions of cell sonicate of rhesus EHS containing approximately 5 µg ml−1 of total protein, as measured using a BCA protein assay kit (Thermo Scientific), suspended in 1× PBS was then added to each well. As negative and positive controls, HeLa cells were also treated with 1× PBS and filtered, sterilized supernatant fractions of cell sonicate of H. hepaticus, previously shown to produce CDT using this in vitro assay, at the same concentration of total protein, respectively. The cells were inspected for CDT activity, indicated by evidence of cell rounding and enlargement as well as the presence of multinucleated giant cells at 48 h of incubation.
Histology.
Tumour samples and colonic tissues were collected during surgical resection, preserved in 10 % neutral formalin buffer, and sectioned as previously described (Marini et al., 2010). Tissue sections were then evaluated by a board-certified veterinary pathologists (plural) (T. W. M., S. M.).
Florescent in situ hybridization (FISH) for Helicobacter spp.
Fluorescent in situ hybridization assays were performed as previously described, with modifications (Bridgeford et al., 2008). For whole cell in situ hybridization, cells were fixed with 4 % paraformaldehyde, washed with PBS and air-dried. For paraffin-embedded tissue, the sections were deparaffinized by passage through xylene (three passages for 10 min each), 100 % ethanol (three passages for 2 min each) and 95 % ethanol (three passages for 2 min each) and air-dried. Two probes specific for the entire genus Helicobacter, HEL 274/HEL 717 (Chan et al., 2005) were used for the FISH assay. The probes were labelled with cy3, reconstituted with sterile water, and diluted to a working concentration of 10 ng µl−1 with hybridization buffer (20 mM Tris/HCl, 0.9 M NaCl, 0.1 % SDS, 30 % formamide, pH 7.2). Fifty microlitres of hybridization buffer with probe was applied to the slides. Sections were covered in Parafilm to minimize evaporation and placed in a hybridization chamber at 48 °C overnight. Washing buffers I (20 mM Tris/HCl, 0.9 M NaCl, 0.01 % SDS) and II (20 mM Tris/HCl, 0.9 M NaCl) were applied and incubated at 48 °C for 15 min each. Slides were rinsed in double-distilled water, air-dried and mounted in Vectashield Hardmount with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories). Slides were examined using a Zeiss Axioskop 2 fluorescence microscope. A slide was designated positive if the distinctive spiral organisms fluoresced under the Rhod filter.
Gene expression analysis in T84 cells.
To study inflammatory gene expression, T84 cells were seeded into a 12-well cell culture plate at 106 cells per well and incubated for 48 h. T84 cells were washed three times with 1× PBS and then inoculated with EHS suspended in DMEM : F12 medium free of antibiotics and fetal bovine serum at an m.o.i. of 50. After 2 h of microaerobic culture, the cells were suspended in 1 ml Trizol reagent (Invitrogen). RNA extraction with Trizol was performed according to the manufacturer’s recommendations. cDNA was synthesized from 3 µg total RNA using the High Capacity cDNA Archive kit (Applied Biosystems). mRNA levels were quantified with TaqMan gene expression assays (Applied Biosystems) in an ABI Prism Sequence Detection System 7700. Expression levels of inflammatory genes, interleukin-6 (IL-6; HS00985639_M1), IL-8 (HS00174103_M1), IL-18, tumour necrosis factor-α (TNF-α; HS99999043_M1), CASPASE-1 (CASP1: HS00354836-M1), inducible nitric oxide synthase (iNOS; HS01075529_M1), IL-1β (HS01555410_M1), nucleotide-binding oligomerization-domain protein-like receptor proteins 3 (NLRP3; HS00918082_M1), NLRP6 (HS00373246_M1) and NLR family, CARD domain-containing 4 (NLRC4; HS00892666_M1), in T84 cells were evaluated using quantitative PCR. mRNA levels of inflammatory mediators were normalized to the mRNA level of endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH; HS99999905_M1) using the standard ΔCT method (User Bulletin no.2, Applied Biosystems). Samples were run in duplicate or triplicate in two independent experiments.
Statistical analysis.
A non-parametric two-tailed Mann U Whitney was used for statistical analyses of mRNA expression levels using GraphPad Prism 5.0 (GraphPad Software).
Results
EHS are highly prevalent among a population of geriatric rhesus monkeys with a high IAC incidence
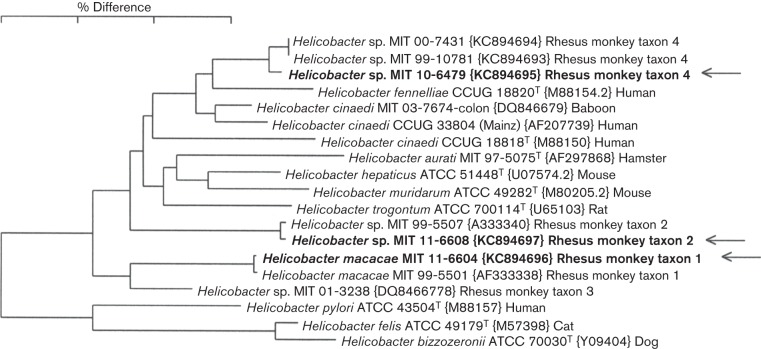
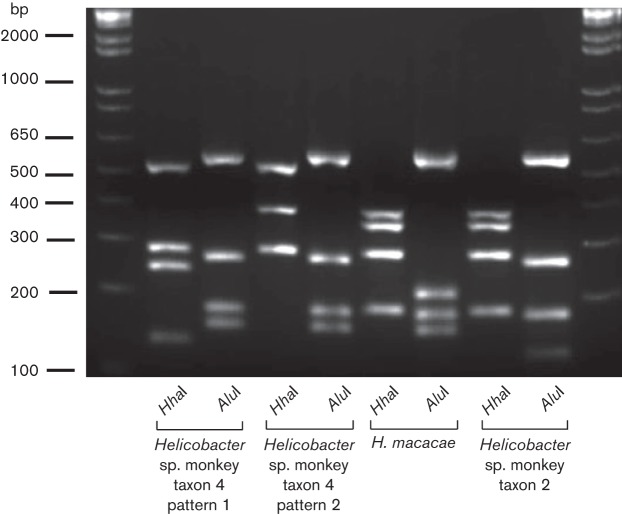
To investigate the association between EHS infection and IAC, we surveyed a population of geriatric rhesus macaques in which IAC is common, and determined EHS prevalence. Initially, the infection status of a cohort of 36 rhesus monkeys was evaluated by faecal PCR and bacterial isolation. From 36 animals, 34 faecal samples were available for the analysis. Thirty-two of 34 (94 %) faecal samples were positive for Helicobacter spp. by faecal PCR (Table 1), whereas only 27/34 (79 %) faecal samples were positive by EHS culture. Two animals that were negative by faecal PCR were also negative by faecal EHS isolation. Analyses using a combination of RFLP, 16S rRNA gene sequencing, and sequence analyses revealed that these isolates fall into four taxa. Specific isolates were >99 % similar to H. macacae, previously isolated from rhesus with idiopathic colitis, normal rhesus and a rhesus with colonic adenocarcinoma (Fox et al., 2001b, 2007; Marini et al., 2010) (previously designated rhesus monkey taxon 1). Helicobacter sp. MIT 99-5507 rhesus monkey taxon 2 from a rhesus macaque with idiopathic colitis (Fox et al., 2001b), and Helicobacter sp. rhesus monkey taxon 4, which is most closely related to H. fennelliae (98 % similarity), were also identified. The relationship between these three Helicobacter spp. isolates based on their 16S rRNA sequences is depicted in Fig. 1. Interestingly, one animal was confirmed positive for two EHS, H. macacae and the H. fennelliae-like Helicobacter sp. rhesus taxon 4, by faecal PCR, faecal bacterial isolation and DNA sequencing, whereas the remainder of the monkeys were positive for a single Helicobacter species. The RFLPs generated by using AluI and HhaI restriction endonucleases corresponding to each EHS are shown in Fig. 2 and were consistent with our previous report (Marini et al., 2010). Two RFLP patterns represented the H. fennelliae-like EHS. This was due to a single base pair substitution (T to C) which created an additional HhaI site (Fig. 2).
Table 1. Helicobacter spp. isolation from rhesus monkeys.
| Sample no. | Animal ID | Bacterial isolate ID | Faecal PCR | Bacterial isolation from faeces | ID of EHS |
| 1 | 04-R118* | MIT11-6586 | + | − | na* |
| 2 | 04-R122 | MIT11-6587 | + | − | na* |
| 3 | 05-R538 | MIT10-6478 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 4 | 07-R110 | MIT11-6588 | + | + | H. macacae, taxon 1 |
| 5 | 07-R111 | MIT11-6589 | + | − | na* |
| 6 | 07-R722* | MIT11-6590 | − | − | na* |
| 7 | 07-R924 | MIT10-6480 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 8 | 07-R928 | MIT10-6479 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 9 | 09-R908 | MIT11-6591 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 10 | 07-R740 | MIT11-6592 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 11 | 07-R736 | MIT11-6593 | + | + | H. macacae, taxon 1 |
| 12 | 07-R732 | MIT11-6594 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 13 | 07-R730* | MIT11-6595 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 14 | 09-R904 | MIT11-6596 | + | − | na* |
| 15 | 09-R905 | MIT11-6597 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 16 | 09-R903 | MIT11-6598 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 17 | 09-R902 | MIT11-6599 | + | − | na* |
| 18 | 09-R901 | MIT11-6600 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 19 | 04-R123 | MIT11-6601 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 20 | 07-R728* | MIT11-6602 | + | + | H. macacae, taxon 1 |
| 21 | 04-R128 | MIT11-6603 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 22 | 04-R126 | MIT11-6604 | + | + | H. macacae, taxon 1 |
| 23 | 04-R432* | MIT11-6605 | + | + | H. macacae, taxon 1 |
| 24 | 09-R906 | MIT11-6606 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 25 | 09-R907 | MIT11-6607 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 26 | 09-R927 | MIT11-6608 | + | + | Helicobacter spp. MIT 99-5507 monkey taxon 2 |
| 27 | 09-R929 | MIT11-6609 | + | + | H. macacae, taxon 1 |
| 28 | 04-R430 | MIT11-6610 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 29 | 07-R729* | MIT11-6611 | − | − | na* |
| 30 | 07-R727* | MIT11-6612 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 31 | 07-R108 | MIT11-6613 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 32 | 07-R129 | MIT11-6614 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 |
| 33 | 09-R910 | MIT11-6615 | + | + | Helicobacter spp. MIT 99-10781 monkey taxon 4 and H. macacae, taxon 1 |
| 34 | 09-R923 | MIT11-6616 | + | + | H. macacae, taxon 1 |
| % Positive | 94 % | 79 % |
These animals had IAC.
Fig. 1.
Dendrogram depicting the relationship, based on the full-length sequences of 16S rRNA gene, between the three rhesus Helicobacter isolates identified in this study and the most closely related named Helicobacter species. The scale bar shows a 3% sequence difference.
Fig. 2.
RFLP patterns of Helicobacter sp. monkey taxon 4, H. macacae and Helicobacter sp. monkey taxon 2 generated by AluI and HhaI.
EHS were detected in tumours of rhesus macaques with IAC
Of the 36 animals evaluated, 14 (40 %) were diagnosed with IAC by exploratory and histological evaluation. These tumours were detected primarily in the ileo-caeco-colic junction (Fig. 3). The age of the affected monkeys ranged from 26 to 35 years, although 9/14 (64 %) animals were 30 years or older (Table 2). Twelve of the affected monkeys were females and two were males; all of which were positive for STLV, and one animal was positive for both STLV and SRV (Table 2). Surgical removal of the tumour was the treatment of choice in all of the IAC-affected monkeys. Resection of the colonic tumours extended life as long as 6 years (Table 2). To definitively determine whether EHS were intimately associated with the tumour tissue, bacterial isolation was performed from tumour tissue in 10 of 14 animals available for analysis. EHS were recovered from the IAC of 9/10 of the affected monkeys. EHS were isolated from the tumour tissue of an animal, 07-R722, which was initially tested negative by both faecal PCR and faecal bacterial isolation. However, EHS were not recovered from one animal, 07-R729, the only other animal that initially tested negative by both faecal PCR and faecal bacterial isolation. Interestingly, by bacterial culture from tumour tissue, two animals with IAC were found to be co-infected with both H. macacae and H. fennelliae-like EHS (Table 1).
Fig. 3.

(a) Intact resected section of intestine, including distal ileum, ileo-caeco-colic (ICC) junction (caecum and proximal portion of ascending colon). Beneath the anterior serosal adipose pad noted over the ICC, there are multifocal areas that are expanded by an internal mass that includes the ICC, caecum, ileum and colon. The tumour does not expand grossly past this anatomical area. (b) Anterior midline dissection of intestine, including distal ileum, ICC junction, caecum and proximal portion of ascending colon. Intestinal wall of the ileum, ICC, caecum and ileo-caecal and caeco-colic valve (sphincters) are thickened by as much as 6–8 mm. Mucosa is eroded with focal ulcers, with blood noted within the lumen and lining the intestinal mucosa. Tumour is circumferential and causes stenosis.
Table 2. Prevalence of EHS in rhesus monkeys with IAC.
| Sample | Animal ID | Age at time IAC diagnosed/treated (years) | Survival after surgical resection IAC | Status of EHS isolated from colonic/tumour/faecal samples | Gender | Helicobacter species |
| 1 | 02-R028 | 26 | 5 years | + | M | H. macacae |
| 2 | 04-R118 | 32 | 2 years | + | F | H. macacae |
| 3 | 04-R432 | 32 | 2 years | + | F | H. macacae |
| 4 | 04-R127 | 25 | 6 years | + | F | Helicobacter sp. MIT 99-10781 monkey taxon 4 |
| 5 | 07-R722 | 31 | 4 months | + | F | H. macacae |
| 6 | 07-R727 | 30 | 4 months | + | F | H. macacae |
| 7 | 07-R730 | 27 | 5 months | + | F | H. macacae and Helicobacter sp. MIT 99-10781 monkey taxon 4 |
| 8 | 07-R728 | 30 | 1 year | + | F | H. macacae |
| 9 | 07-R729 | 28 | 3 years | − | F | na |
| 10 | 04-R128 | 31 | 4 years | + | F | H. macacae and Helicobacter sp. MIT 99-10781 monkey taxon 4 |
| 11 | 09-R903 | 31 | na | + | F | Helicobacter sp. MIT 99-10781 monkey taxon 4 |
| 12 | 09-R923 | 31 | na | + | M | H. macacae |
| 13 | 07-R108 | 29 | 3 years | + | F | Helicobacter sp. MIT 99-10781 monkey taxon 4 |
| 14 | 04-R430 | 35 | 6 years | + | F | Helicobacter sp. MIT 99-10781 monkey taxon 4 |
Tumour characteristics
From the 14 monkeys previously diagnosed by biopsy as having intestinal IACs, post-mortem specimens from 5 animals (04-R432, 07-R728, 07-R727, 09-R923 and 07-R108) were available for examination as part of this study. Of these, one animal had undergone prior resection of the ileal-caeco-colic tumour and hence histological assessment in this instance only included non-neoplastic colon. In cases with obvious tumour, the masses were often localized to the ileo-caecal junction or involved the caecum and colon to varying degrees. Grossly, these were characterized either by discrete nodular thickening at the ileo-caecal origin or by variable degrees of thickening of the caeco-colonic mucosa with associated mucosal ulceration, haemorrhage and/or mucus-filled cysts in the mucosa extending to the serosa and mesentery with variable serosal thickening and mesenteric adhesions.
Histologically, the four grossly observed tumours had a varying appearance and all were classified as intestinal carcinomas. The tumour of 09-R923 was localized to the caeco-colic junction with involvement of both the adjacent caecum and ascending colon. This tumour was histologically classified as a well-differentiated mucinous cystic adenocarcinoma. There were numerous variably distended cystic mucus-filled neoplastic glands with partial cell loss extending into the muscularis, serosa and adjacent mesentery in association with a background of prominent mucosal ulceration/necrosis/haemorrhage and a mixed population of inflammatory cells as well as oedema and fibrosis (Fig. 4a, b). The tumour of 07-R728 was classified as tubular, moderately differentiated adenocarcinoma with invasive spread into the muscularis, serosa and adjacent mesentery (Fig. 4c, d). There was also mild to moderate lympho-plasmacytic and histiocytic inflammation in the non-neoplastic segments of the colon and caecum.
Fig. 4.
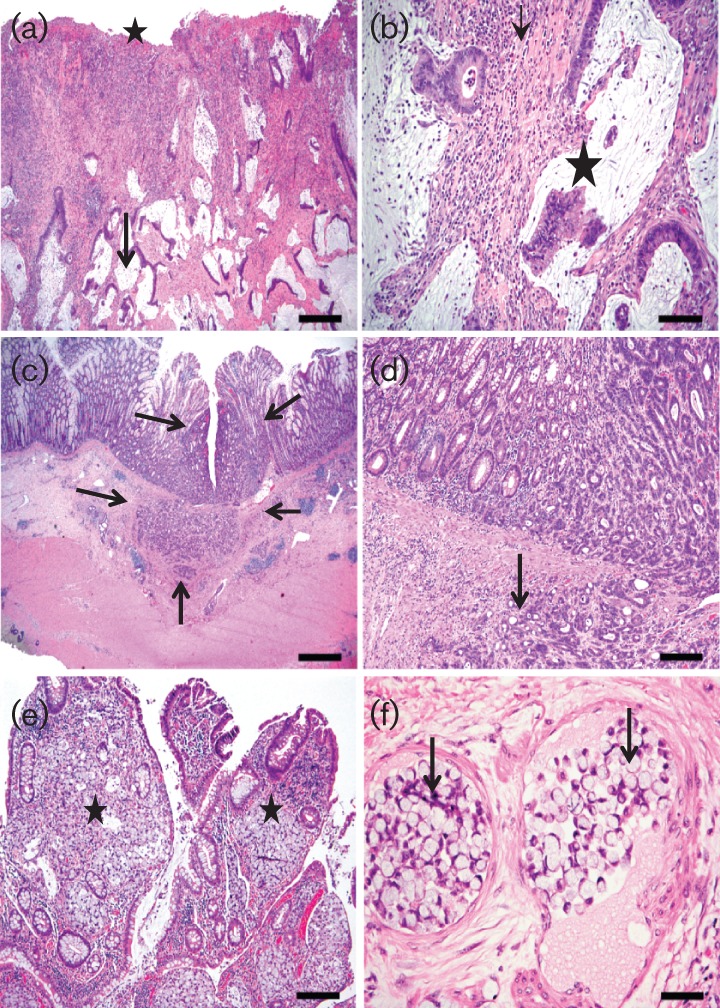
Representative haematoxylin–eosin images of the various types of caeco-colonic carcinomas observed in different macaques. (a, b) Low- (a) and high- (b) magnification images of the neoplastic caeco-colic junction (09-R923) diagnosed as mucinous cystic invasive adenocarcinoma. At low magnification (a) there are numerous cystic mucus-filled invasive glands deep in the submucosa (arrow) with associated mucosal necrosis/ulceration (star), inflammation and fibrosis. The higher-magnification image (b) shows invasive mucus-filled glands with partial cell loss (star), rafts of neoplastic cells in lumen and encircling fibrous connective-tissue and inflammatory aggregates. (c, d) Low- and high-magnification haematoxylin–eosin images of the colonic tumour from a macaque (07-R726), showing the origin and borders (arrows) of a moderately differentiated tubular adenocarcinoma with submucosal invasion (arrows) and associated lymphoid aggregates. (e, f) Low- and high-magnification haematoxylin–eosin images of the neoplastic ileo-caecal junction of a macaque (04-R432) classified as signet ring cell adenocarcinoma. The low-magnification image (e) shows expansion of lamina propria of the villi by clusters of neoplastic pale mucus-filled signet ring cells (stars) whereas the high-magnification image (f) shows submucosal intravascular plugs (arrows) of neoplastic signet ring cells. Bars: (a) 400 µm; (b) 80 µm; (c) 800 µm; (d) 80 µm; (e) 160 µm; (f) 40 µm.
The tumour from 04-R432 involved the ileal-caeco-colic junction and was classified histologically as signet ring cell adenocarcinoma characterized by multifocal effacement of the lamina propria by mucus-filled cells with marginated nucleus thus imparting a signet ring cell appearance. The neoplastic cells in this mass showed multifocal invasion into the submucosa, muscularis and mesentery as well as prominent intravascular neoplastic plugs in the affected regions (Fig. 4e, f). Inflammatory aggregates were also noted within the neoplastic foci. In addition, there was also multifocal to coalescing moderate lympho-plasmacytic ileitis and typhlocolitis admixed with variable numbers of macrophages, neutrophils and eosinophils as well as oedema and ulceration.
The tumour of 07-R727 was classified as a poorly differentiated invasive carcinoma arising from the base of the colonic glandular crypts with formation of neoplastic cords and nests of cells extending into submucosa (not shown) with associated mild diffuse lympho-histiocytic colitis. The intestine of one animal, 07-R108, with a history of prior surgical tumour resection did not have any discernible mass at necropsy and on histological examination there was moderate diffuse plasmacytic mucosal inflammation involving the caecum and colon, with mild associated epithelial hyperplasia (image not shown).
EHS are capable of adhering to and invading a T84 colonic cell line
Previously, EHS from rhesus monkeys were identified in the stools of diarrhoeic children (Haggerty et al., 2005), suggesting that EHS could be of zoonotic importance. To succeed as an enteric pathogen, EHS must be able to establish colonization within its host, which may require the ability to adhere to and/or invade host mucosal epithelia. We investigated whether the three rhesus EHS were capable of adhering to and invading human colonic epithelia. As illustrated in Fig. 5, spiral organisms representing the three EHS were detected by FISH on the surface of T84 cells after 2 h post-inoculation (Fig. 5a–c). FISH-positive bacteria could not be detected on the T84 cell surface with heat-treated EHS (Fig. 5d), indicating that cell adhesion requires live bacteria. The highest efficacy of cell adhesion, assessed by FISH, was observed for Helicobacter sp. monkey taxon 2. To confirm the FISH results, cell adhesion assays were conducted. Consistent with the FISH results, all EHS isolates adhered to T84 cells. Helicobacter sp. monkey taxon 2 adhered most effectively to T84 cells, followed by Helicofobacter sp. monkey taxon 4 and H. macacae (Helicobacter sp. monkey taxon 2, 2.3 % of the inoculum±0.17 se versus Helicobacter sp. monkey taxon 4, 0.3 %±0.02 %, and H. macacae, 0.02 %±0.0003). Additionally, all of the rhesus EHS invaded T84 cells, although at varying degrees of effectiveness (Helicobacter sp. monkey taxon 2, 0.001 %±0.0002; Helicobacter sp. monkey taxon 4, 0.04 %±0.03; H. macacae, 0.03 %±0.0007).
Fig. 5.
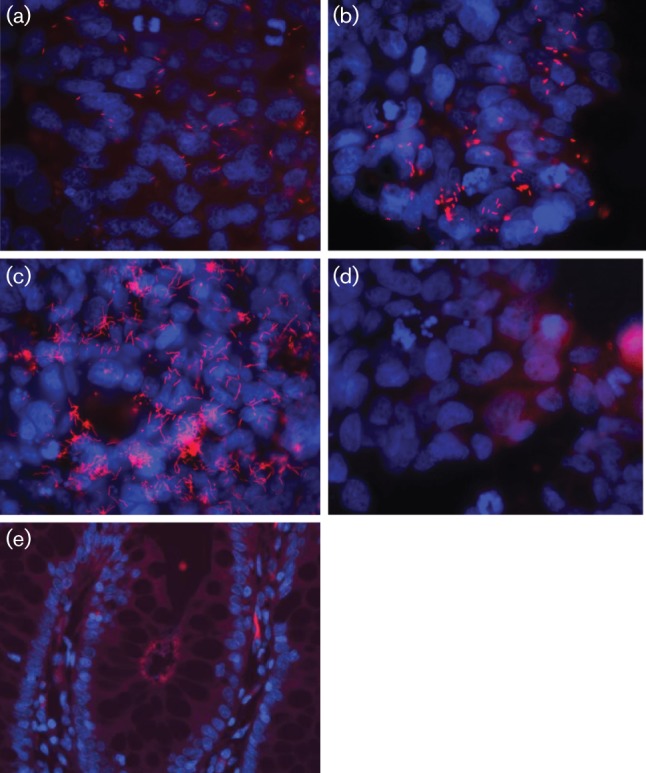
FISH staining showing the presence of rhesus Helicobacter (pink fluorescent spots) adhering to the surface of T84 cells. (a) H. macacae, (b) Helicobacter sp. MIT 10-6479 rhesus monkey taxon 4, (c) Helicobacter sp. MIT 10-6608 rhesus monkey taxon 2, (d) heat-treated Helicobacter sp. MIT 10-6608 rhesus monkey taxon 2 with in vitro T84 cells, and in (e) intimate association with colonic epithelium in the rhesus intestine.
Spiral Helicobacter could also be observed adhering to the surface of the colonic epithelia at high density in the region of the tumour tissue (Fig. 5e), confirming the intimate association of these EHS with the neoplastic tissues.
EHS induce a strong pro-inflammatory gene expression in T84 cells
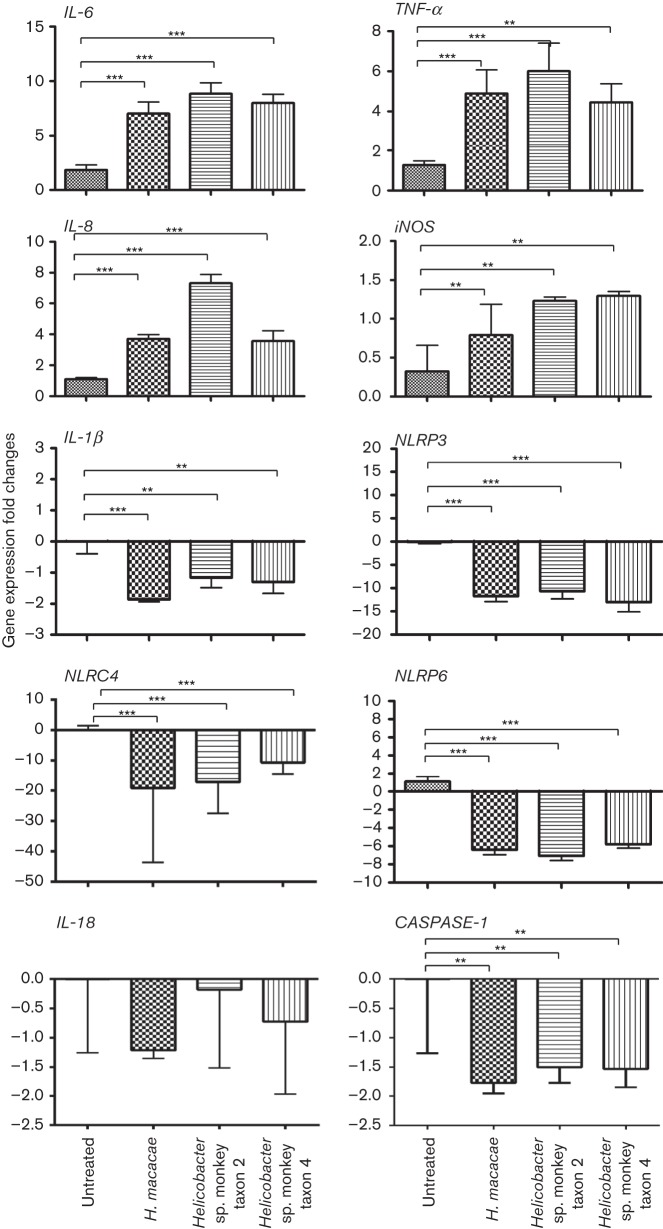
Chronic inflammation is a widely accepted mechanism mediating many cancer types (Grivennikov et al., 2010; Grivennikov & Karin, 2010; Eiró & Vizoso, 2012; Candido & Hagemann, 2013). Importantly, there is strong evidence that chronic H. pylori infection promotes gastric cancer in humans by inducing host pro-inflammatory responses (Bornschein et al., 2010). To determine whether the three rhesus EHS were capable of inducing inflammatory responses after establishing colonization, T84 cells were inoculated with the rhesus EHS, and the mRNA levels of pro-inflammatory genes, particularly IL-8, IL-6 and TNF-α, were measured 2 h post-inoculation. Significant inductions of IL-8, IL-6 and TNF-α were detected in EHS-treated cells (P<0.0001) compared with untreated cells (Fig. 6). Similarly, iNOS expression levels were significantly elevated in the T84 treated cells compared with the untreated control. These results suggest that EHS colonization is associated with transcriptional upregulation of pro-inflammatory genes as well as the nitric oxide synthase gene.
Fig. 6.
Expression levels of pro-inflammatory and inflammasome genes in T84 colonic cell lines not incubated and co-incubated with H. macacae, Helicobacter sp. monkey taxon 2, and Helicobacter sp. monkey taxon 4. Results are based on two independent experiments. Two independent assays were performed in triplicate. P value: **≤0.05, ***≤0.001.
EHS downregulate genes involved in inflammasome signalling in T84 cells
It has been shown that H. macacae can persistently infect rhesus macaques for a decade after initial isolation of the organism (Marini et al., 2010). Similar persistence of EHS has also been reported for other EHS in other hosts (Fox et al., 1996a; García et al., 2011). Certain bacterial pathogens have the capability to interfere with inflammasome function, thereby preventing the host from recognizing and eliminating the bacterial pathogens (Rathinam et al., 2012). Inflammasomes, particularly NLRP3 and NLRP6, expressed abundantly in intestinal epithelial cells, are targeted by multiple pathogenic bacteria, and are crucial for maintaining intestinal homeostasis and preventing the development of colitis and tumorigenesis in mouse models (Kempster et al., 2011; Huber et al., 2012). Recognizing the importance of inflammasomes in pathogen elimination, intestinal inflammation and tumorigenesis, we investigated the impact of all three rhesus monkey EHS, H. macacae, Helicobacter sp. monkey taxon 2 and Helicobacter sp. monkey taxon 4, on known genes involved in inflammasome function, particularly NLRP3, NLRC4 and NLRP6. We also analysed genes downstream of NLRP3, NLRC4 and NLRP6, specifically CASPASE-1, IL-1β and IL-18. At 2 h post-inoculation, CASPASE-1 and IL-1β were moderately downregulated, while NLRP3, NLRP6 and NLRC4 (P<0.0001) were strongly downregulated in EHS-treated T84 cells compared with untreated controls. IL-18 expression was unchanged in the treated compared with untreated T84 cells (Fig. 6). These results indicate that EHS are capable of modulating inflammasome signalling in the intestinal epithelial cells at the transcriptional level.
Rhesus EHS lack CDT activity in vitro
EHS species, such as H. hepaticus, H. cineadi, H. pullorum and H. bilis, contain CDT, which has been demonstrated to induce DNA damage and cell death (Sugai et al., 1998; Cortes-Bratti et al., 1999; Young et al., 2000a; Taylor et al., 2003; Shen et al., 2009). To determine whether CDT was present in the three EHS isolated from rhesus, PCR amplifications of the cdt gene and in vitro assay for CDT activity were performed. PCR failed to amplify cdt genes from any of the EHS species investigated. Moreover, in two independent assays performed, none of rhesus EHS was capable of inducing cell rounding and enlargement, indicating that the three rhesus EHS are unlikely to contain CDT.
Discussion
In this study, we demonstrated that EHS are prevalent among geriatric rhesus monkeys with IAC. These animals were infected with at least three distinct EHS. Moreover, in some animals, two distinct EHS co-colonized individual monkeys, consistent with our previous reports (Fox et al., 2001a, b). The significance of EHS co-infection requires further investigation. However, it is worth noting that in mouse models of EHS-induced colon cancer, co-infection with two or more species of EHS increased the prevalence of colon cancer in experimentally infected IL-10-deficient mice (Chichlowski et al., 2008).
We found that tumour or colonic tissue bacterial isolation was sensitive in detecting EHS in rhesus monkeys. Using this method, we were able to confirm an animal positive for EHS when faecal PCR and faecal bacterial isolation were both negative. However, faecal PCR appears to be sensitive as an initial screen to identify Helicobacter-positive animals. Additionally, we observed that surgical resection of IAC at the early stage is associated with a favourable prognosis and extended the life span of an affected monkey for as long as 6 years, which is consistent with a previous report (Valverde et al., 2000).
Interestingly, all animals with IAC were positive for STLV. Although STLV has been associated with lymphoma, it has not been associated with intestinal neoplasia in rhesus monkeys. The human counterpart of STLV, HTLV, has only been shown to be the cause of adult T-cell leukaemia/lymphoma and of tropical spastic paraparesis/HTLV-1-associated myelopathy, as well as dermatitis, uveitis, arthropathy and polymyositis (Proietti et al., 2005). No evidence to date suggests that STLV is involved in the pathogenesis of intestinal neoplasms; however, considering that STLV has been associated with lymphoma in non-human primates and humans, and their presence can modulate lymphocytic function and cytokine profiles (Lapin & Yakovleva, 2014), it is possible that STLV could synergize with Helicobacter spp. to promote colonic tumorigenesis. Further investigation is needed to delineate this relationship.
The zoonotic potential of EHS has been reported (Fox, 2002). Thus far, several species of EHS isolated from animals have the ability to infect humans (Fox, 2002). Intriguingly, the rhesus EHS previously isolated by our laboratory, Helicobacter sp. MIT 99-5507 rhesus monkey taxon 2, which was also identified among rhesus monkeys in this study, was detected in the stool of a child with diarrhoea (Haggerty et al., 2005). Moreover, H. cinaedi and H. fennelliae, which were shown to cause diarrhoea and bacteraemia when inoculated into infant pig-tailed macaques, have been isolated from humans with proctitis, proctocolitis and enteritis (Russell et al., 1990). H. cinaedi is also isolated from rhesus monkeys with and without clinical signs (Fox et al., 2001a; Fernandez et al., 2002). However, rhesus without diarrhoea did not have colonic biopsies to ascertain if histological colitis was present, a common feature in rhesus monkeys (Fernandez et al., 2002). H. cinaedi has also been identified in the stools of symptomatic and asymptomic patients (Oyama et al., 2012).
Little is known about the pathogenic mechanism of EHS in non-human primates and humans; however, considerable knowledge has been gained in the past decades from studies involving mouse models of IBD and colon cancer (Erdman et al., 2009; Fox et al., 2011). Consistent with previous reports, IAC is detected primarily in the ileo-caeco-colic junction in the affected monkeys (Uno et al., 1998; Valverde et al., 2000; Rodriguez et al., 2002; Erdman et al., 2003, 2009). This anatomical location of IAC is consistent with EHS colonizing this site. In fact, EHS-associated colon cancer in mice has a high propensity to localize in the ileo-caeco-colic junction (Fox et al., 2011). However, EHS also colonize other regions of the lower bowel, as does H. macacae.
EHS have also been shown to have abilities to modulate host adaptive and innate immune responses as well as nitric oxide synthesis pathways, leading to inflammation and the subsequent genetic mutations necessary for carcinogenesis (Erdman et al., 2009). Furthermore, production of cytotoxin, particularly CDT, exerts its effect by arresting the target cell in the G1 and G2 phase of the cell cycle, resulting in cell death. CDT has been demonstrated in H. hepaticus, H. cinaedi, H. pullorum and other EHS in vitro (Chien et al., 2000; Young et al., 2000b).� However, unlike these EHS, all three rhesus EHS identified in this study do not appear to produce secreted protein with CDT activity in vitro. Nevertheless, our in vitro studies demonstrated that the rhesus EHS have the ability to adhere to and invade human T84 colonic epithelia, supporting the view that these EHS are able to establish colonization within the gastrointestinal tract. Aside from adherence and invasion capability, we also found that upon adhesion to the intestinal epithelia these EHS induce a significant upregulation of pro-inflammatory genes including IL-6, IL-8, TNF-α and iNOS in T84 cells. IL-6, IL-8, TNF-α and NO production have been shown to play pivotal roles in inflammation-mediated carcinogenesis (Erdman et al., 2009; Grivennikov & Karin, 2010). Importantly, IL-6 and TNF-α, by stimulating downstream ROS pathways and pathways involved in recruiting immune cells and induction of nitric oxide production, are known to be potent enhancers of cancer progression and cancer cell growth and survival (Grivennikov & Karin, 2010). The upregulation of iNOS expression detected in this study is also consistent with the view that IL-6 and TNF-α induction promotes nitric oxide production (Erdman et al., 2009; Grivennikov et al., 2010; Mangerich et al., 2012). As many neoplastic conditions in humans and animals have been linked to bacterial infection and chronic inflammation, decades of infection with EHS, which continuously stimulates mucosal inflammatory responses via upregulation of IL-6, IL-8, TNF-α and iNOS, may contribute to colitis-associated carcinogenesis in rhesus monkeys.
Remarkably, while pro-inflammatory genes were stimulated by EHS in the colonic epithelial cells during the 2 h post-infection, genes involved in inflammasome function, including CASPASE-1, IL-1β, NLRP3, NLRP6 and NLRC4, were significantly downregulated during in vitro EHS infection. Multiple EHS have the capability to persistently colonize the host intestinal tract (Fox et al., 1996b; Shen et al., 2009; Ge et al., 2011). H. macacae can also persistently infect rhesus macaques (Marini et al., 2010). However, the mechanism of chronic persistence of EHS is unclear. Several bacterial pathogens, such as Legionella pneumophila and Mycobacterium tuberculosis, develop mechanisms to modulate inflammasome functions, thereby preventing the host from effectively eliminating the organism (Master et al., 2008; Lamkanfi & Dixit, 2011). Inflammasomes are multiprotein oligomers known to be crucial in the recognition and the destruction of pathogenic bacteria by triggering an inflammation-induced cell death called pyroptosis. Downregulation of key genes involved in inflammasome function, including NLRP3, NLRCP4 and NLRP6, and their downstream targets, CASPASE-1 and IL-1β, in EHS-treated T84 cell lines may represent a mechanism mediating EHS persistence and sustained inflammation in the host. Moreover, recent studies suggest that NLRP3 is crucial for establishment of intestinal homeostasis and protection against colitis. NLRP3 deficiency leads to loss of epithelial integrity, resulting in systemic dispersion of commensal bacteria, massive leukocyte infiltration and increased chemokine production in the colon (Zaki et al., 2010). An association of EHS infection and NLRP3 downregulation may in part explain why EHS infection can lead to inflammatory bowel conditions in humans and animals. Similar to NLRP3, NLRP6 also has recently been shown to be a suppressor of colitis. In addition, NLRP6 appears to function as a crucial regulator of colonic microbial ecology. The lower bowel of mice deficient in NLRP6 showed an increased representation of members of colitogenic bacteria including Prevotellaceae as well as the uncultured candidate division TM7 (TM for Torf, mittlere Schicht = peat, middle layer) and a reduction in members of the genus Lactobacillus (Elinav et al., 2011). Also of interest was the observation that the intestinal microbiome contained Helicobacter sp. (Elinav et al., 2011). It is appreciated that several EHS can be responsible for chronic inflammation in the colon of immunodysregulated mice (Fox et al., 1999; Maggio-Price et al., 2006; Whary et al., 2006; Shen et al., 2009; Ranatunga et al., 2012). Both Prevotellaceae and TM7 have been implicated in periodontal disease and the latter in inflammatory bowel diseases in humans. Further investigations using animal models should reveal whether EHS-mediated transcriptional downregulation of NLRP3 and NLRP6 can alter intestinal microbiota composition, modulate the intestinal microbiome and promote intestinal inflammation and carcinogenesis. Similarly, NLRC4 has been shown to play a crucial role in protecting the gut as mice deficient in NLRC4 displayed more severe disease upon treatment with dextran sulphate, sodium and when challenged with Salmonella infection (Carvalho et al., 2012). Moreover, NLRC4-deficient mice had an increased bacterial burden after pulmonary infection with L. pneumophila, suggesting the importance of NLRC4 in limiting bacterial colonization (Pereira et al., 2011). Thus, in addition to the negative effect of NLRP3 and NLRP6 downregulation, suppressive effects of EHS on NLRC4 could further exacerbate inflammation and promote tumorigenesis. Considering the emerging evidence that EHS play a role in inflammatory conditions and cancer, our findings support the hypothesis that chronic EHS infections may participate in the development of gastrointestinal cancer in animals and humans (Thomson et al., 2011).
Acknowledgements
This study was supported by NIH grants T32 RR007036 (J. G. F.), P30-ES002109 (MIT Center for Environmental Health Sciences) and P01-CA26731. We thank Alyssa Terestre for assisting with manuscript preparation.
Abbreviations:
- CDT
cytolethal distending toxin
- EHS
enterohepatic Helicobacter species
- FISH
florescent in situ hybridization
- IAC
intestinal adenocarcinoma
- IBD
inflammatory bowel disease
- STLV
simian T-cell leukaemia virus
- SRV
simian retrovirus
Footnotes
Present address: East Carolina University, Brody School of Medicine, Department of Comparative Medicine 600 Moye Boulevard, LSB 217, Greenville, NC 27834, USA.
References
- Avenaud P., Marais A., Monteiro L., Le Bail B., Bioulac Sage P., Balabaud C., Mégraud F. (2000). Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer 89, 1431–1439 [DOI] [PubMed] [Google Scholar]
- Bornschein J., Kandulski A., Selgrad M., Malfertheiner P. (2010). From gastric inflammation to gastric cancer. Dig Dis 28, 609–614 10.1159/000320061 [DOI] [PubMed] [Google Scholar]
- Bridgeford E. C., Marini R. P., Feng Y., Parry N. M., Rickman B., Fox J. G. (2008). Gastric Helicobacter species as a cause of feline gastric lymphoma: a viable hypothesis. Vet Immunol Immunopathol 123, 106–113 10.1016/j.vetimm.2008.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman W. J., Cohn D. L., Reves R. R., Wilson M. L. (1995). Multifocal cellulitis and monoarticular arthritis as manifestations of Helicobacter cinaedi bacteremia. Clin Infect Dis 20, 564–570 10.1093/clinids/20.3.564 [DOI] [PubMed] [Google Scholar]
- Burnens A. P., Stanley J., Morgenstern R., Nicolet J. (1994). Gastroenteritis associated with Helicobacter pullorum. Lancet 344, 1569–1570 10.1016/S0140-6736(94)90376-X [DOI] [PubMed] [Google Scholar]
- Cahill R. J., Foltz C. J., Fox J. G., Dangler C. A., Powrie F., Schauer D. B. (1997). Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun 65, 3126–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candido J., Hagemann T. (2013). Cancer-related inflammation. J Clin Immunol 33 (Suppl 1), 79–84 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- Carvalho F. A., Nalbantoglu I., Aitken J. D., Uchiyama R., Su Y., Doho G. H., Vijay-Kumar M., Gewirtz A. T. (2012). Cytosolic flagellin receptor NLRC4 protects mice against mucosal and systemic challenges. Mucosal Immunol 5, 288–298 10.1038/mi.2012.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceelen L., Decostere A., Verschraegen G., Ducatelle R., Haesebrouck F. (2005). Prevalence of Helicobacter pullorum among patients with gastrointestinal disease and clinically healthy persons. J Clin Microbiol 43, 2984–2986 10.1128/JCM.43.6.2984-2986.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan V., Crocetti G., Grehan M., Zhang L., Danon S., Lee A., Mitchell H. (2005). Visualization of Helicobacter species within the murine cecal mucosa using specific fluorescence in situ hybridization. Helicobacter 10, 114–124 10.1111/j.1523-5378.2005.00298.x [DOI] [PubMed] [Google Scholar]
- Chichlowski M., Sharp J. M., Vanderford D. A., Myles M. H., Hale L. P. (2008). Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med 58, 534–541 [PMC free article] [PubMed] [Google Scholar]
- Chien C. C., Taylor N. S., Ge Z., Schauer D. B., Young V. B., Fox J. G. (2000). Identification of cdtB homologues and cytolethal distending toxin activity in enterohepatic Helicobacter spp. J Med Microbiol 49, 525–534 [DOI] [PubMed] [Google Scholar]
- Chin E. Y., Dangler C. A., Fox J. G., Schauer D. B. (2000). Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor αβ mutant mice. Comp Med 50, 586–594 [PubMed] [Google Scholar]
- Cimolai N., Gill M. J., Jones A., Flores B., Stamm W. E., Laurie W., Madden B., Shahrabadi M. S. (1987). “Campylobacter cinaedi” bacteremia: case report and laboratory findings. J Clin Microbiol 25, 942–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Bratti X., Chaves-Olarte E., Lagergård T., Thelestam M. (1999). The cytolethal distending toxin from the chancroid bacterium Haemophilus ducreyi induces cell-cycle arrest in the G2 phase. J Clin Invest 103, 107–115 10.1172/JCI3831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewhirst F. E., Shen Z., Scimeca M. S., Stokes L. N., Boumenna T., Chen T., Paster B. J., Fox J. G. (2005). Discordant 16S and 23S rRNA gene phylogenies for the genus Helicobacter: implications for phylogenetic inference and systematics. J Bacteriol 187, 6106–6118 10.1128/JB.187.17.6106-6118.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiró N., Vizoso F. J. (2012). Inflammation and cancer. World J Gastrointest Surg 4, 62–72 10.4240/wjgs.v4.i3.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E., Strowig T., Kau A. L., Henao-Mejia J., Thaiss C. A., Booth C. J., Peaper D. R., Bertin J., Eisenbarth S. C. & other authors (2011). NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman S. E., Poutahidis T., Tomczak M., Rogers A. B., Cormier K., Plank B., Horwitz B. H., Fox J. G. (2003). CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol 162, 691–702 10.1016/S0002-9440(10)63863-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman S. E., Rao V. P., Poutahidis T., Rogers A. B., Taylor C. L., Jackson E. A., Ge Z., Lee C. W., Schauer D. B. & other authors (2009). Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A 106, 1027–1032 10.1073/pnas.0812347106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez K. R., Hansen L. M., Vandamme P., Beaman B. L., Solnick J. V. (2002). Captive rhesus monkeys (Macaca mulatta) are commonly infected with Helicobacter cinaedi. J Clin Microbiol 40, 1908–1912 10.1128/JCM.40.6.1908-1912.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores B. M., Fennell C. L., Kuller L., Bronsdon M. A., Morton W. R., Stamm W. E. (1990). Experimental infection of pig-tailed macaques (Macaca nemestrina) with Campylobacter cinaedi and Campylobacter fennelliae. Infect Immun 58, 3947–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Solnick J. V., Lapointe J. M., Jang S., Pedersen N. C. (1998). Identification of a novel enteric Helicobacter species in a kitten with severe diarrhea. J Clin Microbiol 36, 908–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley J. E., Marks S. L., Munson L., Melli A., Dewhirst F. E., Yu S., Shen Z., Fox J. G. (1999). Isolation of Helicobacter canis from a colony of Bengal cats with endemic diarrhea. J Clin Microbiol 37, 3271–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz C. J., Fox J. G., Cahill R., Murphy J. C., Yan L., Shames B., Schauer D. B. (1998). Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter 3, 69–78 10.1046/j.1523-5378.1998.08006.x [DOI] [PubMed] [Google Scholar]
- Fox J. G. (2002). The non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseases. Gut 50, 273–283 10.1136/gut.50.2.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Li X., Yan L., Cahill R. J., Hurley R., Lewis R., Murphy J. C. (1996a). Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of Helicobacter-induced carcinogenesis. Infect Immun 64, 1548–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Yan L., Shames B., Campbell J., Murphy J. C., Li X. (1996b). Persistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticus. Infect Immun 64, 3673–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Gorelick P. L., Kullberg M. C., Ge Z., Dewhirst F. E., Ward J. M. (1999). A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL-10-deficient mice. Infect Immun 67, 1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Chien C. C., Dewhirst F. E., Paster B. J., Shen Z., Melito P. L., Woodward D. L., Rodgers F. G. (2000). Helicobacter canadensis sp. nov. isolated from humans with diarrhea as an example of an emerging pathogen. J Clin Microbiol 38, 2546–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Handt L., Sheppard B. J., Xu S., Dewhirst F. E., Motzel S., Klein H. (2001a). Isolation of Helicobacter cinaedi from the colon, liver, and mesenteric lymph node of a rhesus monkey with chronic colitis and hepatitis. J Clin Microbiol 39, 1580–1585 10.1128/JCM.39.4.1580-1585.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Handt L., Xu S., Shen Z., Dewhirst F. E., Paster B. J., Dangler C. A., Lodge K., Motzel S., Klein H. (2001b). Novel Helicobacter species isolated from rhesus monkeys with chronic idiopathic colitis. J Med Microbiol 50, 421–429 [DOI] [PubMed] [Google Scholar]
- Fox J. G., Shen Z., Xu S., Feng Y., Dangler C. A., Dewhirst F. E., Paster B. J., Cullen J. M. (2002). Helicobacter marmotae sp. nov. isolated from livers of woodchucks and intestines of cats. J Clin Microbiol 40, 2513–2519 10.1128/JCM.40.7.2513-2519.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Boutin S. R., Handt L. K., Taylor N. S., Xu S., Rickman B., Marini R. P., Dewhirst F. E., Paster B. J. & other authors (2007). Isolation and characterization of a novel helicobacter species, “Helicobacter macacae,” from rhesus monkeys with and without chronic idiopathic colitis. J Clin Microbiol 45, 4061–4063 10.1128/JCM.01100-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. G., Ge Z., Whary M. T., Erdman S. E., Horwitz B. H. (2011). Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol 4, 22–30 10.1038/mi.2010.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García A., Zeng Y., Muthupalani S., Ge Z., Potter A., Mobley M. W., Boussahmain C., Feng Y., Wishnok J. S., Fox J. G. (2011). Helicobacter hepaticus-induced liver tumor promotion is associated with increased serum bile acid and a persistent microbial-induced immune response. Cancer Res 71, 2529–2540 10.1158/0008-5472.CAN-10-1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Z., Feng Y., Muthupalani S., Eurell L. L., Taylor N. S., Whary M. T., Fox J. G. (2011). Coinfection with enterohepatic Helicobacter species can ameliorate or promote Helicobacter pylori-induced gastric pathology in C57BL/6 mice. Infect Immun 79, 3861–3871 10.1128/IAI.05357-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman C. G., Mitchell H. M. (2010). Helicobacter spp. other than Helicobacter pylori. Helicobacter 15 (Suppl 1), 69–75 10.1111/j.1523-5378.2010.00780.x [DOI] [PubMed] [Google Scholar]
- Grivennikov S. I., Karin M. (2010). Inflammation and oncogenesis: a vicious connection. Curr Opin Genet Dev 20, 65–71 10.1016/j.gde.2009.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S. I., Greten F. R., Karin M. (2010). Immunity, inflammation, and cancer. Cell 140, 883–899 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggerty T. D., Perry S., Sanchez L., Perez-Perez G., Parsonnet J. (2005). Significance of transiently positive enzyme-linked immunosorbent assay results in detection of Helicobacter pylori in stool samples from children. J Clin Microbiol 43, 2220–2223 10.1128/JCM.43.5.2220-2223.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen R., Thomson J. M., Fox J. G., El-Omar E. M., Hold G. L. (2011). Could Helicobacter organisms cause inflammatory bowel disease? FEMS Immunol Med Microbiol 61, 1–14 10.1111/j.1574-695X.2010.00744.x [DOI] [PubMed] [Google Scholar]
- Hsueh P. R., Teng L. J., Hung C. C., Chen Y. C., Yang P. C., Ho S. W., Luh K. T. (1999). Septic shock due to Helicobacter fennelliae in a non-human immunodeficiency virus-infected heterosexual patient. J Clin Microbiol 37, 2084–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S., Gagliani N., Zenewicz L. A., Huber F. J., Bosurgi L., Hu B., Hedl M., Zhang W., O’Connor W., Jr & other authors (2012). IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 10.1038/nature11535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung C. C., Hsueh P. R., Chen M. Y., Teng L. J., Chen Y. C., Luh K. T., Chuang C. Y. (1997). Bacteremia caused by Helicobacter cinaedi in an AIDS patients. J Formos Med Assoc 96, 558–560 [PubMed] [Google Scholar]
- Kempster S. L., Belteki G., Forhead A. J., Fowden A. L., Catalano R. D., Lam B. Y., McFarlane I., Charnock-Jones D. S., Smith G. C. (2011). Developmental control of the Nlrp6 inflammasome and a substrate, IL-18, in mammalian intestine. Am J Physiol Gastrointest Liver Physiol 300, G253–G263 10.1152/ajpgi.00397.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Kawamura Y., Ohkusu K., Masaki T., Iwashita H., Sawa T., Fujii S., Okamoto T., Akaike T. (2007). Helicobacter cinaedi cellulitis and bacteremia in immunocompetent hosts after orthopedic surgery. J Clin Microbiol 45, 31–38 10.1128/JCM.01507-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M., Dixit V. M. (2011). Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol 187, 597–602 10.4049/jimmunol.1100229 [DOI] [PubMed] [Google Scholar]
- Lapin B. A., Yakovleva L. A. (2014). Spontaneous and experimental malignancies in non-human primates. J Med Primatol 43, 100–110 10.1111/jmp.12098 [DOI] [PubMed] [Google Scholar]
- Lertpiriyapong K., Gamazon E. R., Feng Y., Park D. S., Pang J., Botka G., Graffam M. E., Ge Z., Fox J. G. (2012). Campylobacter jejuni type VI secretion system: roles in adaptation to deoxycholic acid, host cell adherence, invasion, and in vivo colonization. PLoS ONE 7, e42842 10.1371/journal.pone.0042842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggio-Price L., Treuting P., Zeng W., Tsang M., Bielefeldt-Ohmann H., Iritani B. M. (2006). Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res 66, 828–838 10.1158/0008-5472.CAN-05-2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangerich A., Knutson C. G., Parry N. M., Muthupalani S., Ye W., Prestwich E., Cui L., McFaline J. L., Mobley M. & other authors (2012). Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc Natl Acad Sci U S A 109, E1820–E1829 10.1073/pnas.1207829109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini R. P., Muthupalani S., Shen Z., Buckley E. M., Alvarado C., Taylor N. S., Dewhirst F. E., Whary M. T., Patterson M. M., Fox J. G. (2010). Persistent infection of rhesus monkeys with ‘Helicobacter macacae’ and its isolation from an animal with intestinal adenocarcinoma. J Med Microbiol 59, 961–969 10.1099/jmm.0.019117-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Master S. S., Rampini S. K., Davis A. S., Keller C., Ehlers S., Springer B., Timmins G. S., Sander P., Deretic V. (2008). Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe 3, 224–232 10.1016/j.chom.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melito P. L., Munro C., Chipman P. R., Woodward D. L., Booth T. F., Rodgers F. G. (2001). Helicobacter winghamensis sp. nov., a novel Helicobacter sp. isolated from patients with gastroenteritis. J Clin Microbiol 39, 2412–2417 10.1128/JCM.39.7.2412-2417.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama K., Khan S., Okamoto T., Fujii S., Ono K., Matsunaga T., Yoshitake J., Sawa T., Tomida J. & other authors (2012). Identification of and screening for human Helicobacter cinaedi infections and carriers via nested PCR. J Clin Microbiol 50, 3893–3900 10.1128/JCM.01622-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M. S., Morgantetti G. F., Massis L. M., Horta C. V., Hori J. I., Zamboni D. S. (2011). Activation of NLRC4 by flagellated bacteria triggers caspase-1-dependent and -independent responses to restrict Legionella pneumophila replication in macrophages and in vivo. J Immunol 187, 6447–6455 10.4049/jimmunol.1003784 [DOI] [PubMed] [Google Scholar]
- Proietti F. A., Carneiro-Proietti A. B., Catalan-Soares B. C., Murphy E. L. (2005). Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24, 6058–6068 10.1038/sj.onc.1208968 [DOI] [PubMed] [Google Scholar]
- Ranatunga D. C., Ramakrishnan A., Uprety P., Wang F., Zhang H., Margolick J. B., Brayton C., Bream J. H. (2012). A protective role for human IL-10-expressing CD4+ T cells in colitis. J Immunol 189, 1243–1252 10.4049/jimmunol.1103421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam V. A., Vanaja S. K., Fitzgerald K. A. (2012). Regulation of inflammasome signaling. Nat Immunol 13, 333–342 10.1038/ni.2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez N. A., Garcia K. D., Fortman J. D., Hewett T. A., Bunte R. M., Bennett B. T. (2002). Clinical and histopathological evaluation of 13 cases of adenocarcinoma in aged rhesus macaques (Macaca mulatta). J Med Primatol 31, 74–83 10.1034/j.1600-0684.2002.01001.x [DOI] [PubMed] [Google Scholar]
- Russell R. G., Sarmiento J. I., Fox J., Panigrahi P. (1990). Evidence of reinfection with multiple strains of Campylobacter jejuni and Campylobacter coli in Macaca nemestrina housed under hyperendemic conditions. Infect Immun 58, 2149–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders K. E., Shen Z., Dewhirst F. E., Paster B. J., Dangler C. A., Fox J. G. (1999). Novel intestinal Helicobacter species isolated from cotton-top tamarins (Saguinus oedipus) with chronic colitis. J Clin Microbiol 37, 146–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Z., Feng Y., Rogers A. B., Rickman B., Whary M. T., Xu S., Clapp K. M., Boutin S. R., Fox J. G. (2009). Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infect Immun 77, 2508–2516 10.1128/IAI.00166-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai M., Kawamoto T., Pérès S. Y., Ueno Y., Komatsuzawa H., Fujiwara T., Kurihara H., Suginaka H., Oswald E. (1998). The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun 66, 5008–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Ge Z., Shen Z., Dewhirst F. E., Fox J. G. (2003). Cytolethal distending toxin: a potential virulence factor for Helicobacter cinaedi. J Infect Dis 188, 1892–1897 10.1086/379837 [DOI] [PubMed] [Google Scholar]
- Thomson J. M., Hansen R., Berry S. H., Hope M. E., Murray G. I., Mukhopadhya I., McLean M. H., Shen Z., Fox J. G. & other authors (2011). Enterohepatic Helicobacter in ulcerative colitis: potential pathogenic entities? PLoS ONE 6, e17184 10.1371/journal.pone.0017184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H., Alsum P., Zimbric M. L., Houser W. D., Thomson J. A., Kemnitz J. W. (1998). Colon cancer in aged captive rhesus monkeys (Macaca mulatta). Am J Primatol 44, 19–27 [DOI] [PubMed] [Google Scholar]
- Valverde C. R., Tarara R. P., Griffey S. M., Roberts J. A. (2000). Spontaneous intestinal adenocarcinoma in geriatric macaques (Macaca sp.). Comp Med 50, 540–544 [PubMed] [Google Scholar]
- Whary M. T., Danon S. J., Feng Y., Ge Z., Sundina N., Ng V., Taylor N. S., Rogers A. B., Fox J. G. (2006). Rapid onset of ulcerative typhlocolitis in B6.129P2-IL10tm1Cgn (IL-10−/−) mice infected with Helicobacter trogontum is associated with decreased colonization by altered Schaedler’s flora. Infect Immun 74, 6615–6623 10.1128/IAI.01091-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. B., Chien C. C., Knox K. A., Taylor N. S., Schauer D. B., Fox J. G. (2000a). Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J Infect Dis 182, 620–623 10.1086/315705 [DOI] [PubMed] [Google Scholar]
- Young V. B., Knox K. A., Schauer D. B. (2000b). Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect Immun 68, 184–191 10.1128/IAI.68.1.184-191.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki M. H., Boyd K. L., Vogel P., Kastan M. B., Lamkanfi M., Kanneganti T. D. (2010). The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 32, 379–391 10.1016/j.immuni.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]