Abstract
Low levels of the vitamin D-regulated antimicrobial peptide cathelicidin (LL-37) may negatively impact the immune status of human immunodeficiency virus-1 (HIV-1) infected individuals (HIV+). We compared plasma LL-37 levels in healthy controls (HIV−) and HIV+ individuals on or off antiretroviral therapies (ARTs) (ART+ and ART−, respectively), and evaluated the relationship between vitamin D and LL-37 levels. In this cross-sectional study, levels of LL-37, 25-hydroxycholecalciferol [25(OH)D3] and 1,25-dihydroxycholecalciferol [1,25(OH)2D3] were measured from an initial cohort of 18 healthy controls and 10 HIV+/ART− individuals. Because this cohort lacked HIV+/ART+ subjects, LL-37 was also quantified from a second cohort of 10 HIV+/ART− and 13 HIV+/ART+ individuals. LL-37 levels were significantly lower in the HIV+/ART− group compared to the healthy controls (P = 0.01). A direct relationship was observed between LL-37 and both 25(OH)D3 and 1,25(OH)2D3. The level of 25(OH)D3 was predictive of higher LL-37 (P = 0.04) and for any given level of 25(OH)D3, HIV+/ART− subjects averaged 20 % lower LL-37 compared to the healthy controls (P = 0.045). For any given level of 1,25(OH)2D3, HIV+/ART− subjects averaged 25 % lower LL-37 compared to the healthy controls (P = 0.018), although 1,25(OH)2D3 was not predictive of higher LL-37 (P = 0.28). Finally, LL-37 levels were significantly lower in the HIV+/ART− group compared to the HIV+/ART+ group from the second cohort (P = 0.045). Untreated HIV infection may contribute to lower LL-37 levels, independent of vitamin D levels. ART treatment may potentially mitigate this decrease in LL-37 levels.
Introduction
Human cathelicidin (LL-37) is an antimicrobial peptide that is transcriptionally induced by active 1,25-dihydroxycholecalciferol [1,25(OH)2D3] vitamin D complexed to the vitamin D receptor (VDR) (Wang et al., 2004). 1,25(OH)2D3 is produced when the CYP27B1 enzyme hydroxylates 25-hydroxycholecalciferol [25(OH)D3], the major circulating form of vitamin D. In healthy individuals, LL-37 levels correlate with 25(OH)D3 levels (Bhan et al., 2011; Dixon et al., 2012). LL-37 demonstrates potent antimicrobial activity against a wide assortment of bacterial, mycobacterial, protozoal, fungal and viral pathogens (Durr et al., 2006). Gombart et al. (2009) were the first to report a link between low cathelicidin levels and increased risk of death due to infectious agents. Jeng et al. (2009) subsequently showed a positive association between plasma LL-37 levels and vitamin D status in critically ill patients with sepsis. Meanwhile, serum LL-37 concentrations did not correlate with vitamin D status in individuals with tuberculosis (Yamshchikov et al., 2010). In ex vivo macrophage studies, cathelicidin and vitamin D induction of the autophagic pathway was found to be a key mechanism by which Mycobacterium tuberculosis and human immunodeficiency virus (HIV) are eliminated (Campbell & Spector, 2012a, b). Yet, there are no data concerning the relationship between plasma cathelicidin and vitamin D levels in HIV-infected individuals in the absence of tuberculosis.
Vitamin D deficiency has been frequently observed in HIV-infected individuals (Bang et al., 2010; Vescini et al., 2011; Viard et al., 2011; Crutchley et al., 2012). Antiretroviral therapy (ART) may further diminish vitamin D levels (Van Den Bout-Van Den Beukel et al., 2008). In HIV-infected individuals, low levels of vitamin D are linked to increased incidence of opportunistic infections and worse clinical outcomes (Allavena et al., 2012). It is reasonable to speculate that low vitamin D levels, and subsequently low cathelicidin levels, may contribute to the increased susceptibility of HIV-infected individuals to bacterial infections even before the progression to AIDS.
Currently, there are no data regarding plasma LL-37 levels or the association between circulating vitamin D and LL-37 levels in HIV-infected individuals. Thus, we measured LL-37, 25(OH)D3 and 1,25(OH)2D3 levels from a cohort of untreated HIV-infected (HIV+/ART−) individuals and healthy controls to elucidate the relationship between circulating LL-37 levels, vitamin D and the presence or absence of HIV infection. Since this initial cohort did not contain HIV+ individuals being treated with ART (HIV+/ART+), LL-37 levels were also measured in a second cohort, which included both HIV+/ART− and HIV+/ART+ subjects to determine the impact of ART on LL-37 levels.
Methods
Study population.
Blood samples were analysed from two separate cohorts enrolled at the same geographical location. Cohort 1 consisted of 18 healthy HIV-negative controls and 10 HIV+/ART− individuals. Peripheral blood was collected between September 2009 and January 2010 into BD Vacutainer sodium citrate cell preparation tubes from which plasma and peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved at −80 °C. Because this cohort did not include HIV+/ART+ subjects, LL-37 concentrations were measured in a second cohort of 10 HIV+/ART− and 13 HIV+/ART+ individuals using banked cryopreserved plasma from blood collected in BD Vacutainer sodium heparin tubes. Participants of cohort 2 were enrolled between February and June 2010.
All subjects were adult residents of Colorado not receiving vitamin D supplementation. Inclusion criteria for healthy controls included self-reported HIV-negative status and no high-risk behaviours for HIV infection. All HIV+ subjects were under the care of the University of Colorado Hospital Infectious Disease Group Practice. Inclusion criteria for HIV+ subjects were: (i) documented HIV-1 antibody, (ii) not receiving ART (or off-treatment for at least 3 months) or receiving ART with good virological suppression (<100 HIV-1 RNA copies ml−1) for a minimum of 6 months or longer, and (iii) CD4+ T cell counts >250 cells mm−3. The exclusion criteria for all subjects were: (i) acute illness, (ii) currently pregnant or lactating, and (iii) known blood or bleeding disorders. Studies were conducted in accordance with the Declaration of Helsinki and the United States Department of Health and Human Services, and approved by the Colorado Multiple Institutional Review Board with the informed consent of each participant.
Measurement of plasma vitamin D and LL-37 levels.
Plasma 25(OH) and 1,25(OH)2D3 were measured by the Colorado Clinical and Translational Sciences Institute Core (Colorado, USA) via direct competitive chemiluminescence immunoassay (DiaSorin Liaison automated chemiluminescence instrument) and competitive radio-immunoassay (DiaSorin RIA method), respectively. This laboratory is certified for proficiency by the Vitamin D External Quality Assessment Scheme (DEQAS). The 25(OH) assay measures both D2 and D3 forms. 25(OH) levels were tested in single determinations using the Liaison method, which has passed all four DEQAS proficiency challenges each year for the past 4 years when run in singlet. The 1,25(OH)2D3 levels were measured in duplicate and the intra-assay coefficient of variation was acceptable per DEQAS proficiency evaluations.
A human LL-37 ELISA kit (Hycult Biotech) was used to quantify LL-37 from plasma according to the manufacturer’s instructions using thawed specimens. The lower limit of assay detection was 1 ng ml−1. LL-37 levels of subjects in cohort 1 and cohort 2 were not quantified on the same day and separate ELISA kits were used. LL-37 measurements were done in duplicate according to the information from the manufacturer and the intra-assay coefficient of variation is ≤10 % (Goleva et al., 2012; Levinson et al., 2012).
Immunoblotting.
Immunoblotting was used to detect VDR and CYP27B1 in PBMCs from healthy controls and HIV+/ART− subjects. Cell lysates (15 µg) were separated by 4–12 % Tris-MES, SDS, EDTA (MES)-PAGE and transferred to PVDF using the iBLOT transfer system (Life Technologies). Proteins were immunostained with antibodies to VDR (Santa Cruz Biotechnology) or CYP27B1 (Santa Cruz Biotechnology). Detection was performed using horseradish peroxidase-conjugated secondary antibodies followed by Supersignal West Femto detection (Thermo). Electrophoretically resolved protein bands were quantified by scanning digital densitometry using a Kodak 1D Imager and software analysis. VDR or CYP27B1 signals were normalized to that of β-actin.
Statistical analyses.
Statistical analyses were performed using R version 2.13.2 and GraphPad Prism software 6.0 for MAC. Circulating LL-37, 25(OH)D3 and 1,25(OH)2D3 levels were natural log (loge) transformed for normality. Two-sided tests with a significance level of 0.05 were used to determine statistical significance. Continuous outcomes are reported as means or geometric means with corresponding 95 % confidence intervals (95 % CIs). Pearson’s correlations were utilized. Given the small group sizes in each cohort, Fisher’s exact test was used to evaluate categorical outcomes between groups. Ordinary least-squares regression was used to predict LL-37 values from vitamin D and HIV status.
Results
Cohort 1: HIV+/ART− individuals have significantly lower plasma LL-37 levels than healthy controls
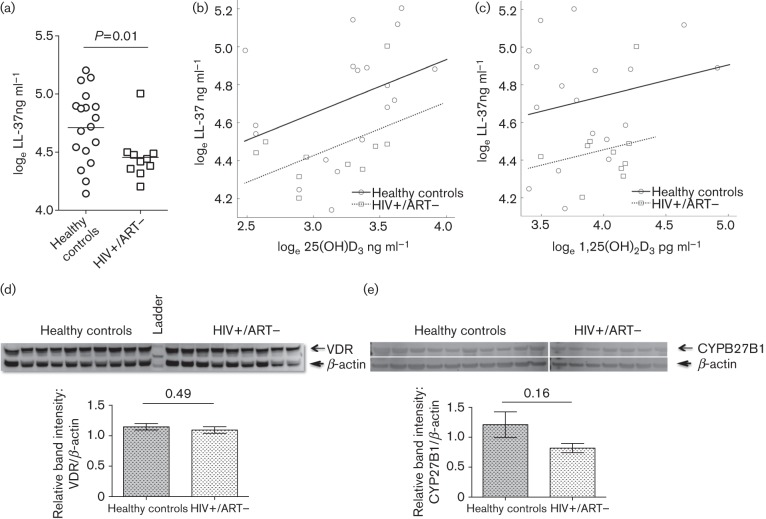
The HIV+/ART− group (none with an AIDS diagnosis) was composed of significantly more males (70 %) compared to the healthy controls (22 %) (P = 0.02). Age (P = 0.30) and race (P = 0.40) were not significantly different between the two groups (Table 1). For the HIV+/ART− subjects, geometric mean CD4+ T cell count was 620 cells µl−1 and the mean log10 viral load was 3.96 HIV-1 RNA copies ml−1. Plasma loge LL-37 ng ml−1 levels were 23 % lower (95 % CI 5.5 %, 37.2 %) in the HIV+/ART− subjects (mean 4.46) compared to the healthy controls (mean 4.72) (P = 0.01) (Fig. 1a). There was no significant correlation between log10 viral load and log10 LL-37 levels in HIV+/ART− subjects (r = 0.38; 95 % CI −0.32, 0.82; P = 0.27). Circulating vitamin D levels were not significantly different between the two groups. Mean plasma loge 25(OH)D3 levels for the healthy controls were 3.25 ng ml−1 (95 % CI 3.05, 3.46) and 3.11 ng ml−1 (95 % CI 2.85, 3.38) for the HIV+/ART− participants (P = 0.37). The mean plasma loge 1,25(OH)2D3 level was 3.87 pg ml−1 (95 % CI 3.67, 4.08) in the healthy controls and 4.01 pg ml−1 (95 % CI 3.84, 4.18) in the HIV+/ART− subjects (P = 0.26).
Table 1. Characteristics of cohort 1 participants.
| Characteristic | Statistic | Healthy controls (n = 18) | HIV+/ART− (n = 10) | P value† |
| Sex (male) | n (%) | 4 (22 %) | 7 (70 %) | 0.02* (FET) |
| Age (year) | Mean (95 % CI) | 36.7 (30.5, 42.8) | 41.3 (34.5, 48.1) | 0.30 (t-mean) |
| Race (white) | n (%) | 14 (78 %) | 6 (60 %) | 0.40 (FET) |
| CD4+ T cell count (cells µl−1) | Geometric mean (lower CI, upper CI) | nd | 620 (494, 779) | na |
| Viral load (copies ml−1) | log10 (lower CI, upper CI) | nd | 3.96 (3.56, 4.36) | na |
| % with AIDS diagnosis | n (%) | nd | 0 | na |
| loge LL-37 ng ml−1 | Mean (lower CI, upper CI) | 4.72 (4.57, 4.88) | 4.46 (4.31, 4.61) | 0.01* (t-mean) |
na, Not applicable; nd, not determined.
Statistical analysis used: FET, Fisher’s exact test for categorical data; t-mean, t-test for comparing mean values (assuming equal distribution). *, P<0.05
Fig. 1.
Relationship between plasma LL-37 and vitamin D levels in healthy and HIV+/ART− subjects of cohort 1. (a) loge LL-37 ng ml−1 levels of the healthy-control and HIV+/ART− subjects were compared. (b, c) The loge LL-37 ng ml−1 level from each healthy-control (circles) and HIV+/ART− (squares) subject was plotted versus loge 25(OH)D3 ng ml−1 (b) or loge 1,25(OH)2D3 pg ml−1 (c). Linear regression lines are denoted by the solid and dotted lines for the healthy-control and HIV+/ART− groups, respectively. (d, e) Detection of VDR and CYP27B1 by immunoblot. Total PBMC lysate from healthy controls and HIV+/ART− subjects were probed for VDR (d) and CYP27B1 (e). Representative blots are shown. Absolute band intensities were measured and relative band intensities were calculated from the ratio of VDR or CYP27B1 band densities to that of β-actin. VDR or CYP27B1 levels (P = 0.49 and P = 0.16, respectively) were similar between the two groups.
To explore the relationship between circulating LL-37 and vitamin D levels for the HIV+/ART− subjects and healthy controls, each LL-37 value was modelled as a function of its corresponding 25(OH)D3, and 1,25(OH)2D3 levels. As shown in Fig. 1(b, c), there was a direct relationship between circulating 25(OH)D3 or 1,25(OH)2D3 levels and LL-37 in both the HIV+/ART− (dotted lines) and healthy-control (solid lines) groups. For a one loge increase in 25(OH)D3, LL-37 increased by 36.7 % (95 % CI 2.1 %, 71.9 %; P = 0.040) and for any given level of 25(OH)D3, HIV+/ART− participants averaged 20 % lower LL-37 than the healthy controls (95 % CI 1.2 %, 35.1 %; P = 0.045) as shown in Fig. 1(b). For a one loge increase in 1,25(OH)2D3, LL-37 increased by 17.4 % (95 % CI −12.5 %, 48.9 %; P = 0.28) and for any given level of 1,25(OH)2D3, HIV+/ART− participants averaged 25 % lower LL-37 than healthy controls (95 % CI 5.8 %, 39.9 %; P = 0.018) as shown in Fig. 1(c). The effect of loge 25(OH)D3 or loge 1,25(OH)2D3 on loge LL-37 did not vary by HIV status (P = 0.86 and P = 0.70, respectively). Race, sex and age were considered as potential confounders. After adjusting for race, the magnitude of the effect of 25(OH)D3 increased with non-whites estimated to have 11 % higher loge LL-37 (95 % CI −13.5 %, 42.1 %; P = 0.41). Similarly, the magnitude of the effect of 1,25(OH)2D3 increased with non-whites estimated to have 9 % higher loge LL-37 (95 % CI −16.9, 44.1; P = 0.52). Separately adjusting for sex and age did not have a clinically relevant impact on the estimates of loge 25(OH)D3) or loge 1,25(OH)2D3 or HIV status, and they did not significantly add to the model (P>0.53 and P>0.14, respectively).
To determine if the lower LL-37 levels in the HIV+/ART− subjects could be due to lower expression of VDR and CYP27B1, these two proteins were detected by immunoblotting whole-cell lysates prepared from PBMCs of HIV+/ART− and healthy subjects of cohort 1. After densitometry and normalization for β-actin expression density, the semi-quantification of VDR and CYP27B1 was not significantly different between the groups (Fig. 1d, e).
Cohort 2: HIV+/ART− individuals have significantly lower plasma LL-37 levels than HIV+/ART+ individuals
The data from cohort 1 indicated that HIV+/ART− individuals have lower LL-37 levels than healthy-control participants. To assess whether ART affects LL-37, plasma LL-37 levels were also measured from a second cohort composed of HIV+/ART− and HIV+/ART+ subjects. Sex and age were not significantly different between the groups (P = 0.65 and P = 0.98, respectively) (Table 2).
Table 2. Characteristics of cohort 2 participants.
| Characteristic | Statistic | HIV+/ART− (n = 10) | HIV+/ART+ (n = 13) | P value† |
| Sex (male) | n (%) | 6 (60 %) | 10 (77 %) | 0.65 (FET) |
| Age (year) | Mean (95 % CI) | 45.1 (36.4, 53.8) | 45.0 (41.0, 49.0) | 0.98 (t-mean) |
| Race (white) | n (%) | 5 (50 %) | 9 (69 %) | 0.42 (FET) |
| CD4+ T cell count (cells µl−1) | Geometric mean (lower CI, upper CI) | 594 (441, 800) | 484 (384, 611) | 0.23 (t-Welch) |
| Viral load (copies ml−1) | log10 (lower CI, upper CI) | 3.41 (2.90, 3.93) | 1.70‡ (1.65, 1.75) | <0.0001 (t-Welch) |
| % with AIDS diagnosis | n (%) | 0 | 8 (62 %) | 0.003** (FET) |
| loge LL-37 ng ml−1 | Mean (lower CI, upper CI) | 2.87 (2.42, 3.32) | 3.40 (3.08, 3.72) | 0.045* (t-Welch) |
Statistical analysis used: FET, Fisher’s exact test for categorical data; t-mean, t-test for comparing mean values (assuming equal distribution); t-Welch, t-tests with Welch’s correction for comparing geometric means. *, P<0.05; 0.003.
Censored observations set to the lower limit of detection for assay.
Whites composed 50 and 69 % of these groups, respectively (P = 0.42). CD4+ T cell counts did not differ significantly between the HIV+ subjects on or off ART (P = 0.23). The mean log10 viral load of the HIV+/ART− group of cohort 2 was 3.41 HIV-1 RNA copies ml−1 (95 % CI 2.90, 3.93). This viral load was not significantly different from cohort 1 (P = 0.08). There was no significant correlation between log10 viral load and log10 LL-37 levels in HIV+/ART− subjects (r = −0.19; 95 % CI −0.73, 0.50; P = 0.59). As expected, HIV+/ART+ individuals showed significantly lower viral loads compared to the HIV+/ART− group (P<0.0001). In the HIV+/ART+ group, 62 % were previously diagnosed with AIDS, although all had CD4+ T cells above 250 cells mm−3 at the time of the study.
The same brand of ELISA kit was used to quantify LL-37 from both cohorts. In a regression model that utilized data from both cohorts, loge LL-37 ng ml−1 was estimated to be 1.42 (95 % CI 1.19, 1.65) higher in cohort 1 compared to cohort 2 (P<0.001). This assay effect did not differ by HIV-status group (P = 0.91). We attribute this difference to variation in the methods used to collect and process the plasma. Using all available data, race, age and sex were considered as potential confounders; although they did not have a clinically relevant impact on group estimates and failed to achieve statistical significance (P>0.52). Using only cohort 2 data, plasma loge LL-37 levels were 41.5 % lower (95 % CI 1.4 %, 65.2 %) in the HIV+/ART− subjects (mean 2.87) compared to HIV+/ART+ subjects (mean 3.40) (P = 0.045) (Fig. 2).
Fig. 2.
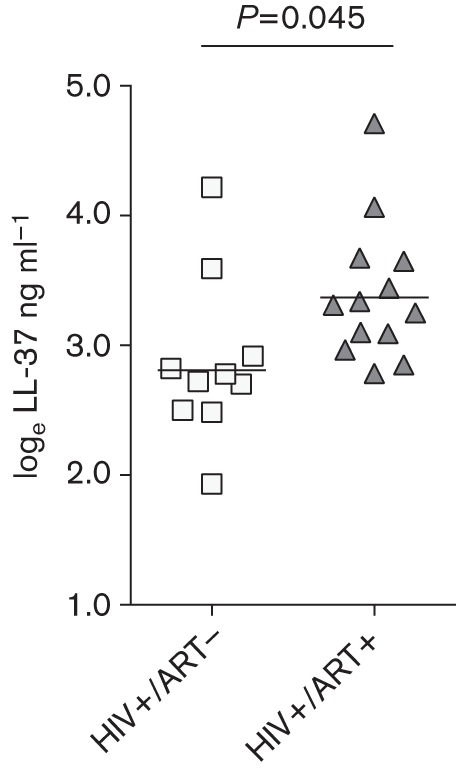
LL-37 levels among cohort 2 participants. loge LL-37 ng ml−1 levels of the HIV+/ART− and HIV+/ART+ subjects were compared.
Discussion
To our knowledge, this is the first study to evaluate plasma LL-37 levels in HIV+ individuals. Cohort 1 results demonstrated three novel findings: (i) LL-37 levels were significantly lower in HIV+/ART− subjects compared to the healthy controls, (ii) there was a direct relationship between circulating LL-37 levels and both 25(OH)D3 and 1,25(OH)2D3 for both the HIV+/ART− subjects and healthy controls, and (iii) LL-37 levels were consistently lower for any given vitamin D level in individuals with untreated HIV infection compared to healthy controls. Finally, cohort 2 results indicated that LL-37 levels were significantly lower in HIV+/ART− individuals compared to HIV+/ART+ individuals.
HIV+ individuals are more commonly affected by microbial infections compared to healthy individuals. For example, tuberculosis and invasive Streptococcus pneumoniae infections are 50 and 100 times more likely to develop in HIV+ individuals, respectively, compared to HIV− individuals (Redd et al., 1990; Mahmood, 2010). Multiple mechanisms have been proposed for the increased susceptibility to infections in HIV+ individuals, including impaired cell-mediated responses and antibody production (Belec et al., 1995; Twigg et al., 1996), deficiencies in neutrophil and macrophage function (Howell et al., 1997) and unsafe sexual practices (Fowler et al., 1997). Our findings suggest that diminished LL-37 levels may also be a new potential mechanism to explain the heightened susceptibility of untreated HIV+ individuals to microbial infections. Furthermore, infection rates in HIV+ individuals have been shown to decrease following ART treatment (Velasco et al., 2009; Segal et al., 2011). Our study showing that the HIV+/ART+ subjects had significantly higher levels of LL-37 than HIV+/ART− subjects suggests ART may mitigate the HIV-induced decrease in LL-37. A prospective study of LL-37 levels before and after initiation of ART would be important to confirm this.
This study has several limitations. First, the sample sizes of the cohorts were small and not fully matched; yet, we found significant differences in LL-37 levels between the HIV+/ART− subjects and healthy controls and between the HIV+/ART+ and HIV+/ART− subjects. It would be important to validate these findings in a larger number of individuals. Second, we cannot exclude the possibility of HIV infection in the healthy-control participants since they were not tested for HIV infection, although the relatively low prevalence of undiagnosed HIV infection in Colorado (0.04 % of the population) renders it unlikely that healthy controls had undiagnosed HIV infection. Third, we did not monitor parameters such as body mass index, duration of HIV infection, kidney, liver and parathyroid diseases, or calcium disorders; it is possible that one or more of these factors can influence LL-37 production.
In cohort 1, non-whites showed a trend toward greater effect of vitamin D on LL-37 levels than whites. Our inability to further separate non-whites from whites down to ethnicity and our small cohort size precludes definitive conclusions about the impact of race on the relationship between LL-37 and vitamin D. It would be important to validate these findings in a larger group of healthy controls and HIV+ subjects including both ART− and ART+ individuals. Cohort 1 also showed differences in gender composition. To date, we are unaware of any reports that suggest an association between gender and LL-37 levels, but it would be prudent to recruit an equal number of males and females in future studies to determine whether LL-37 levels differ by sex.
In cohort 2, an important caveat is that we did not have vitamin D levels for these participants and therefore could not examine the relationship between vitamin D and LL-37 levels in HIV+/ART+ individuals. Additionally, the cross-sectional design of this study did not permit us to definitively conclude that ART leads to normalization of LL-37 levels; a longitudinal study would be required to confirm or refute these findings. We were also unable to examine the mechanisms for lower LL-37 in HIV+/ART− individuals compared to HIV+/ART+ subjects. Cellular studies can help to elucidate whether synthesis, turnover and/or secretion of LL-37 are affected with in vitro HIV infection, with or without addition of ART. Additionally, cohort 2 showed an inverse relationship between viral load and circulating LL-37 levels, yet the opposite was observed for cohort 1. Further research needs to be conducted to investigate this discrepancy.
In conclusion, this study indicates LL-37 levels were directly associated with vitamin D levels in both the healthy controls and HIV+/ART− subjects, although LL-37 levels were consistently lower in the HIV+/ART− group. Our findings also suggest, but do not prove, that ART may reverse the depressed circulating levels of LL-37 due to untreated HIV infection. A case-control study from a large cohort could help demonstrate that reduced LL-37 level in HIV+ individuals is truly related to increased risk for bacterial infections. A better understanding of the role of LL-37 in predisposing HIV-1 infected individuals to microbial infections could lead to novel therapies to augment LL-37 levels and improve clinical outcomes in HIV-1 infected individuals.
Acknowledgements
We extend our appreciation to all study participants for their enthusiasm and support. This study was supported, in part, by the National Center for Advancing Translational Sciences at the National Institutes of Health Colorado (CTSI grant number UL1 TR000154), and through the National Heart, Lung, and Blood Institute at the National Institutes of Health and institutional funds (Division of Pulmonary Sciences and Critical Care Medicine, University of Colorado) for Dr Sonia Flores. Jennifer R. Honda PhD is supported by the Colorado HIV-1 Research Training Program through the NRSA NIH Infectious Disease (T32-AI007447-19) Training Award, the Tim Gill Endowment for AIDS Research, and the Pulmonary and Critical Care Medicine (T32 HL 7085-83) Training Award.
Abbreviations:
- 1,25(OH)2D3
1,25-dihydroxycholecalciferol
- 25(OH)D3
25-hydroxycholecalciferol
- ART
antiretroviral therapy
- CI
confidence interval
- DEQAS
Vitamin D External Quality Assessment Scheme
- HIV
human immunodeficiency virus
- PBMC
peripheral blood mononuclear cell
References
- Allavena C., Delpierre C., Cuzin L., Rey D., Viget N., Bernard J., Guillot P., Duvivier C., Billaud E., Raffi F. (2012). High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J Antimicrob Chemother 67, 2222–2230 10.1093/jac/dks176 [DOI] [PubMed] [Google Scholar]
- Bang U. C., Shakar S. A., Hitz M. F., Jespersen M. S., Andersen O., Nielsen S. D., Jensen J. E. (2010). Deficiency of 25-hydroxyvitamin D in male HIV-positive patients: a descriptive cross-sectional study. Scand J Infect Dis 42, 306–310 10.3109/00365540903463981 [DOI] [PubMed] [Google Scholar]
- Belec L., Meillet D., Gaillard O., Prazuck T., Michel E., Ngondi Ekome J., Pillot J. (1995). Decreased cervicovaginal production of both IgA1 and IgA2 subclasses in women with AIDS. Clin Exp Immunol 101, 100–106 10.1111/j.1365-2249.1995.tb02284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhan I., Camargo C. A., Jr, Wenger J., Ricciardi C., Ye J., Borregaard N., Thadhani R. (2011). Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol 127, 1302–1304 10.1016/j.jaci.2010.12.1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. R., Spector S. A. (2012a). Autophagy induction by vitamin D inhibits both Mycobacterium tuberculosis and human immunodeficiency virus type 1. Autophagy 8, 1523–1525 10.4161/auto.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. R., Spector S. A. (2012b). Vitamin D inhibits human immunodeficiency virus type 1 and Mycobacterium tuberculosis infection in macrophages through the induction of autophagy. PLoS Pathog 8, e1002689. 10.1371/journal.ppat.1002689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crutchley R. D., Gathe J., Jr, Mayberry C., Trieu A., Abughosh S., Garey K. W. (2012). Risk factors for vitamin D deficiency in HIV-infected patients in the south central United States. AIDS Res Hum Retroviruses 28, 454–459 [DOI] [PubMed] [Google Scholar]
- Dixon B. M., Barker T., McKinnon T., Cuomo J., Frei B., Borregaard N., Gombart A. F. (2012). Positive correlation between circulating cathelicidin antimicrobial peptide (hCAP18/LL-37) and 25-hydroxyvitamin D levels in healthy adults. BMC Res Notes 5, 575. 10.1186/1756-0500-5-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürr U. H., Sudheendra U. S., Ramamoorthy A. (2006). LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim Biophys Acta 1758, 1408–1425 10.1016/j.bbamem.2006.03.030 [DOI] [PubMed] [Google Scholar]
- Fowler M. G., Melnick S. L., Mathieson B. J. (1997). Women and HIV. Epidemiology and global overview. Obstet Gynecol Clin North Am 24, 705–729 10.1016/S0889-8545(05)70340-5 [DOI] [PubMed] [Google Scholar]
- Goleva E., Searing D. A., Jackson L. P., Richers B. N., Leung D. Y. (2012). Steroid requirements and immune associations with vitamin D are stronger in children than adults with asthma. J Allergy Clin Immunol 129, 1243–1251 10.1016/j.jaci.2012.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A. F., Bhan I., Borregaard N., Tamez H., Camargo C. A., Jr, Koeffler H. P., Thadhani R. (2009). Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis 48, 418–424 10.1086/596314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell A. L., Groveman D. S., Wallace P. K., Fanger M. W. (1997). HIV-1-infected monocytes and monocyte-derived macrophages are impaired in their ability to produce superoxide radicals. Int J Clin Lab Res 27, 111–117 10.1007/BF02912444 [DOI] [PubMed] [Google Scholar]
- Jeng L., Yamshchikov A. V., Judd S. E., Blumberg H. M., Martin G. S., Ziegler T. R., Tangpricha V. (2009). Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med 7, 28. 10.1186/1479-5876-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson P., Choi R. Y., Cole A. L., Hirbod T., Rhedin S., Payne B., Guthrie B. L., Bosire R., Cole A. M. & other authors (2012). HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS ONE 7, e31996. 10.1371/journal.pone.0031996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood S. A. I. (2010). TB and HIV/AIDS in Bangladesh. J AIDS HIV Res 2, 66–78 [Google Scholar]
- Redd S. C., Rutherford G. W., III, Sande M. A., Lifson A. R., Hadley W. K., Facklam R. R., Spika J. S. (1990). The role of human immunodeficiency virus infection in pneumococcal bacteremia in San Francisco residents. J Infect Dis 162, 1012–1017 10.1093/infdis/162.5.1012 [DOI] [PubMed] [Google Scholar]
- Segal L. N., Methé B. A., Nolan A., Hoshino Y., Rom W. N., Dawson R., Bateman E., Weiden M. D. (2011). HIV-1 and bacterial pneumonia in the era of antiretroviral therapy. Proc Am Thorac Soc 8, 282–287 10.1513/pats.201006-044WR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg H. L., III, Spain B. A., Soliman D. M., Bowen L. K., Heidler K. M., Wilkes D. S. (1996). Impaired IgG production in the lungs of HIV-infected individuals. Cell Immunol 170, 127–133 10.1006/cimm.1996.0142 [DOI] [PubMed] [Google Scholar]
- Van Den Bout-Van Den Beukel C. J., Fievez L., Michels M., Sweep F. C., Hermus A. R., Bosch M. E., Burger D. M., Bravenboer B., Koopmans P. P., Van Der Ven A. J. (2008). Vitamin D deficiency among HIV type 1-infected individuals in The Netherlands: effects of antiretroviral therapy. AIDS Res Hum Retroviruses 24, 1375–1382 10.1089/aid.2008.0058 [DOI] [PubMed] [Google Scholar]
- Velasco M., Castilla V., Sanz J., Gaspar G., Condes E., Barros C., Cervero M., Torres R., Guijarro C., COMESEM Cohort (2009). Effect of simultaneous use of highly active antiretroviral therapy on survival of HIV patients with tuberculosis. J Acquir Immune Defic Syndr 50, 148–152 10.1097/QAI.0b013e31819367e7 [DOI] [PubMed] [Google Scholar]
- Vescini F., Cozzi-Lepri A., Borderi M., Re M. C., Maggiolo F., De Luca A., Cassola G., Vullo V., Carosi G. & other authors (2011). Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J Acquir Immune Defic Syndr 58, 163–172 10.1097/QAI.0b013e31822e57e9 [DOI] [PubMed] [Google Scholar]
- Viard J. P., Souberbielle J. C., Kirk O., Reekie J., Knysz B., Losso M., Gatell J., Pedersen C., Bogner J. R. & other authors (2011). Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS 25, 1305–1315 10.1097/QAD.0b013e328347f6f7 [DOI] [PubMed] [Google Scholar]
- Wang T. T., Nestel F. P., Bourdeau V., Nagai Y., Wang Q., Liao J., Tavera-Mendoza L., Lin R., Hanrahan J. W. & other authors (2004). Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 173, 2909–2912 10.4049/jimmunol.173.5.2909 [DOI] [PubMed] [Google Scholar]
- Yamshchikov A. V., Kurbatova E. V., Kumari M., Blumberg H. M., Ziegler T. R., Ray S. M., Tangpricha V. (2010). Vitamin D status and antimicrobial peptide cathelicidin (LL-37) concentrations in patients with active pulmonary tuberculosis. Am J Clin Nutr 92, 603–611 10.3945/ajcn.2010.29411 [DOI] [PMC free article] [PubMed] [Google Scholar]