Abstract
We assessed the potential for vacant lots and other non-residential settings to serve as source environments for Aedes (Stegomyia) aegypti (L.) in Mérida City, México. Mosquito immatures were collected, during November 2011 – June 2013, from residential premises (n = 156 site visits) and non-residential settings represented by vacant lots (50), parking lots (18), and streets/sidewalks (28). Collections totaled 46,025 mosquito immatures of 13 species. Ae. aegypti was the most commonly encountered species accounting for 81.0% of total immatures, followed by Culex quinquefasciatus Say (12.1%). Site visits to vacant lots (74.0%) were more likely to result in collection of Ae. aegypti immatures that residential premises (35.9%). Tires accounted for 75.5% of Ae. aegypti immatures collected from vacant lots. Our data suggest that vacant lots should be considered for inclusion in mosquito surveillance and control efforts in Mérida City, as they often are located near homes, commonly have abundant vegetation, and frequently harbor accumulations of small and large discarded water-holding containers that we now have demonstrated to serve as development sites for immature mosquitoes. Additionally, we present data for associations of immature production with various container characteristics, such as storage capacity, water quality and physical location in the environment.
Keywords: Aedes aegypti, immatures, vacant lots, Mérida, Yucatán, México
Introduction
Dengue is the most important arboviral disease of humans in the subtropics and tropics. A recent study estimated that up to 390 million dengue virus (DENV) infections, including nearly 100 million cases with dengue disease manifestations, occur annually (Bhatt et al. 2013). The principal urban vector of DENV, Aedes (Stegomyia) aegypti (L.), is closely associated with human dwellings; eggs and immature stages can be found in a wide range of water-holding containers in the peridomestic environment while the females often feed and rest indoors (Scott et al. 2000, Barrera et al. 2006a, Focks and Alexander 2006, García-Rejón et al. 2008a, Tun-Lin et al. 2009, Weaver and Reisen 2010).
Previous studies from México, including some conducted in Mérida City, have examined which container types are most commonly infested with Ae. aegypti on residential premises – the primary urban environment targeted for mosquito surveillance and control (Lloyd et al. 1992, Winch et al. 1992, Arredondo-Jimenez and Valdez-Delgado 2006, Manrique-Saide et al. 2008, García-Rejón et al. 2011a, Villegas-Trejo et al. 2011). However, there is growing recognition in the Americas that non-residential urban environments (e.g., cemeteries, schools, commercial premises, vacant lots, and stormwater drains and catch basins) also can be important sources for production of Ae. aegypti (Lopes et al. 1993; Vezzani and Schweigmann 2002; Abe et al. 2005; da Silva et al. 2006; Morrison et al. 2006; Troyo et al. 2008; dos Reis et al. 2010; García-Rejón et al. 2011b, 2012; de Mendonca et al. 2011; Costa et al. 2012; Manrique-Saide et al. 2012, 2013). Moreover, water-holding containers may differ across urban environments with regards to such factors as container composition, water dynamics, water quality, storage capacity, temperature, and physical location. Previous workers have found that these variables can greatly influence Ae. aegypti productivity (Christophers 1960, Focks et al. 1993, Barrera et al. 2006b, Focks and Alexander 2006, García-Rejón et al. 2011a). Therefore it is important that mosquito surveillance and control programs include both residential premises and other urban environments for the production of Ae. aegypti, so that successful control is not compromised by an influx of mosquitoes from surrounding non-residential environments.
The primary aim of our research was to assess the potential for non-residential urban environments (such as, vacant lots, parking lots, and streets/sidewalk) to serve as source environments of water-holding containers for the production of Ae. aegypti in Mérida City. Vacant lots represent a non-residential urban environment of particular interest in this respect because they often are located near homes, commonly have abundant vegetation, and frequently harbor accumulations of small and large discarded items that hold water and may serve as development sites for Ae. aegypti immatures. Moreover, vacant lots also are easy to access, which facilitates their inclusion in mosquito surveillance and control efforts.
Materials and Methods
Study Area
Studies were conducted within Mérida City (population ~ 800,000) in the Yucatán Peninsula of southeastern México. The flat and low Yucatán Peninsula (elevation range, 0–250 m above sea level) has a bedrock dominated by limestone and is characterized by a subtropical climate. Mérida City’s climate and housing characteristics were further described in previous studies (García-Rejón et al. 2008a, b). Mean monthly maximum temperatures in Mérida range from 29°C in December to 34°C in July, and the majority of the rainfall occurs from May to October, with a peak from June to September.
Sampling of Mosquito Immatures
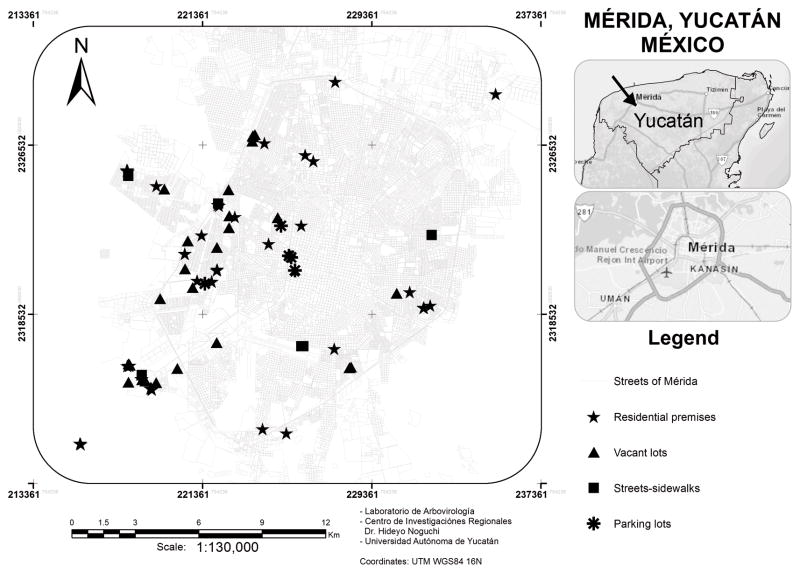
Sampling for mosquito immatures from different types of water-holding containers were undertaken from November 2011 to June 2013 in different parts of Mérida City (Figure 1). Urban environments examined included residential premises (n = 156 site visits) and non-residential settings represented by vacant lots (50), parking lots (18), and streets/sidewalks (28). As defined in this study, vacant (empty) lots did not have buildings but often had abundant vegetation and substantial accumulations of domestic trash items. Representative vacant lots are shown in Figure 2. In Mérida City, vacant lots occur sporadically and are much less common than residential premises. We therefore first located and sampled vacant lots, and thereafter randomly selected nearby residential premises (within ~100 m of the vacant lots) for sampling. Parking lots and streets/sidewalks included in the study were in the general areas where vacant lots were sampled. Most (>90%) of the sites were examined on a single occasion but some (6 vacant lots located near markets or schools and 8 residential premises and 2 parking lots adjacent to these vacant lots) were examined on two or more dates. Sampling site locations were recorded using a global positioning system receiver (Garmin, Olathe, KS).
Figure 1.
Figure 2.
Immature mosquito collections, including the classification of container types, were carried out as described previously by García-Rejón et al. (2011a). The surveys were carried out by trained entomologists from Universidad Autónoma de Yucatán. Briefly, mosquito immatures were collected –using nets, turkey basters, or pipettes – from various containers found in the urban environment, primarily disposable containers, tires, and buckets. We also collected immatures from stormwater drains and catch basins located on streets or sidewalks but those, more extensive collections will be presented in a separate publication.
Mosquito Rearing and Species Identification
Larvae and pupae were collected between 0900 and 1400 hours and transported to the Laboratorio de Arbovirología at Universidad Autónoma de Yucatán where early instar stages were reared (28 ± 1 °C water temperature and a photoperiod of 12:12, light:dark) to fourth instar for more accurate identification. Pupae were allowed to emerge as adults, and then identified to species. Species identification used the taxonomic keys of Carpenter and LaCasse (1955), Ibañez-Bernal and Martinez-Campos (1994), and Darsie and Ward (2005).
Container and Water Characteristics
Container types were classified, following the method of García-Rejón et al. (2011a) and included type of construction (Vezzani and Albicocco 2009) such as ceramic (made from mud, faience [glazed pottery], or cement), rubber, plastic, glass, or metal, also a two-way size of container classification – small disposable containers with a capacity <5 liters versus larger containers of ≥5 liters that used the national guidelines of México for surveillance of Ae. aegypti (http://www.pediatria.gob.mx/sgc/manussa_den.pdf). We also used an alternative three-way classification of water storage capacity, <1.5, 1.5–8.0, or >8.0 liters, to achieve three groupings with similar sample sizes as well as classifying the actual water volume in each container as <0.140, 0.140–0.499, 0.500–1.800, or >1.800 liters to achieve four groupings with similar sample sizes. Shading was classified as shade versus no shade, and we also noted presence or absence of organic matter (leaf litter and/or detritus) in the water as well as water color subjectively categorized as clear, lightly-colored, or dark-colored.
Data Presentation and Statistical Analyses
Summary values for collections of Ae. aegypti and selected entomological indices are shown by urban environment class in Table 1. The presented indices are: 1) the percentage of site visits resulting in collection of Ae. aegypti immatures; 2) the percentage of water-filled containers with Ae. aegypti immatures present (larvae and/or pupae); and 3) a pupal index representing the percentage of containers with Ae. aegypti pupae present out of all containers with Ae. aegypti immatures present.
Table 1.
Abundance of Ae. aegypti immatures in Mérida City by type of urban environment, from November 2011 to June 2013.
| Urban environment class | No. site visits | Containers | Total no. Ae. aegypti collected | Entomological indices | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total no. examined | No. (%)with water | Larvae | Pupae | % site visits with collection of Ae. aegypti immatures | % water-filled containers with Ae. aegypti immatures present | Pupal index (%)b | ||
| Residential premises | 156 | 1,920 | 383 (19.9) | 9,129 | 1,201 | 35.9 | 23.0 | 57.9 |
| Vacant lots | 50 | 1,060 | 337 (31.8) | 17,734 | 800 | 74.0 | 24.3 | 64.6 |
| Streets/sidewalksa | 28 | 84 | 24 (28.6) | 1,369 | 109 | 28.6 | 45.8 | 63.6 |
| Parking lots | 18 | 168 | 33 (19.6) | 6,799 | 159 | 61.1 | 33.3 | 63.6 |
| GRAND TOTAL | 252 | 3,232 | 777 (24.0) | 35,031 | 2,269 | |||
Including collections from various containers (primarily disposable containers, tires, and buckets) found on streets or sidewalks but not from stormwater drains and catch basins.
Percentage of containers with Ae. aegypti pupae present out of all containers with Ae. aegypti immatures present.
Statistical analyses were performed using the IBM SPSS Statistics version 19 software (IBM Corporation, Armonk, NY), and results were considered significant when P < 0.05. When necessary, data were log10-transformed to meet the assumptions of normality and homogeneity of variances. To compare the likelihood of residential premises versus vacant lots being infested with Ae. aegypti immatures, we used a 2 × 2 contingency table and the chi-square test statistic. A two-way analysis of variance (ANOVA) test was used to compare number of Ae. aegypti immatures by container type (restricted to disposable container, tire, and bucket) and urban environment class (restricted to residential premises and vacant lots). Significant ANOVA results were followed by a post-hoc Tukey test for multiple comparisons of means. Unpaired Student’s t-test or one-way ANOVA test were used to compare the numbers of Ae. aegypti immatures for infested containers of different water storage capacity (small disposable containers with a capacity <5 liters versus larger controllable containers with a capacity ≥5 liters), and for small disposable containers with a capacity <5 liters located in shade versus no shade, with water of different color, or with versus without organic matter in the water. These tests were conducted separately for residential premises and non-residential urban environments (vacant lots, parking lots, and streets/sidewalks combined).
Moreover, we used a principal component analysis (PCA), followed by a multiple linear regression model based on the factors emerging from the PCA, to determine associations between potentially explanatory independent variables (water volume, water storage capacity, size of container, shading, urban environment, organic matter, water color, container type, and container construction material) and, as the response variable, the number of Ae. aegypti pupae (log10-transformed) per pupal-infested container in urban environments (including residential premises, vacant lots, parking lots, and streets/sidewalks). We focused on pupae in this specific analysis because the pupal stage has lower mortality than the larval stage and pupal abundance therefore is a better proxy for the abundance of emerging adults compared with abundance of both immature stages combined (Tun-Lin et al. 1996, Focks and Chadee 1997, Focks and Alexander 2006, Knox et al. 2010).
Results
Summary of Mosquito Collections
We encountered a total of 3,232 containers during the study. Of these, 24.0% held water at the time they were examined and 5.9% yielded mosquito immatures. In total, we collected 46,025 immatures representing 13 species. The most abundant species was Ae. aegypti (37,300), followed by Culex quinquefasciatus Say (5,590), Culex interrogator Dyar and Knab (1,115), Culex thriambus Dyar (1,099), Culex lactator Dyar and Knab (363), Culex salinarius Coquillett (278), Culex nigripalpus Theobald (172), Limatus durhamii Theobald (35), Toxorhynchites rutilus (Coquillett) (30), Aedes (Ochlerotatus) trivittatus (Coquillett) (18), Culex coronator Dyar and Knab (14), Haemagogus equinus Theobald (7), and Aedes (Howardina) cozumelensis Díaz Nájera (4).
Collections of Immatures by Urban Environment Class
Containers from all urban environment classes yielded large numbers of Ae. aegypti immatures (Table 1). When collection was grouped by environment class, the percentage of site visits with collection of Ae. aegypti immatures differed significantly between classes (X2 = 22.19, d.f. = 1, P= 0.000). Notably, vacant lots (74.0%) had a greater percentage of sites infested with Ae. aegypti immatures that residential premises (35.9%). However, there was no significant difference between vacant lots and residential premises for the percentage of water-filled containers with Ae. aegypti immatures present (24.3 and 23.0%, respectively) (Table 1). Although the difference was not statistically significant, the average number of Ae. aegypti immatures collected per infested container tended to be greater for vacant lots versus residential premises (Table 2). There were no significant differences between urban environment class (F= 0.478, d.f. = 1, P = 0.49) or for the interaction between urban environment class and container type (F= 0.912, d.f.= 2, P = 0.91), but container type was found to be a significant source of variation (F= 5.86, d.f. = 2, P = 0.004).
Table 2.
Collections of Ae. aegypti and Cx. quinquefasciatus immatures in Mérida City by type of urban environment and container type, from November 2011 to June 2013.
| Urban environment class and Container type | Total no. containers/No. with water | No. water-filled containers with immatures (% by container type) | Total no. immatures collected | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Ae. aegypti | Cx. quinquefasciatus | Ae. aegypti | Cx. quinquefasciatus | ||||||
|
| |||||||||
| Larvaea | Pupaea | Larvaea | Pupaea | Larvae | Pupae | Larvae | Pupae | ||
| RESIDENTIAL PREMISES | |||||||||
| Disposable container | 832/161 | 44 (50.0) | 24 (47.1) | 5 (55.6) | 0 | 2,970 | 436 | 63 | 0 |
| Bucket | 298/73 | 10 (11.4) | 8 (15.7) | 0 (0) | 0 | 1,692 | 237 | 0 | 0 |
| Tire | 56/23 | 7 (8.0) | 4 (7.8) | 2 (22.2) | 0 | 1,244 | 272 | 8 | 0 |
| Flower pot | 558/25 | 6 (6.8) | 3 (5.9) | 1 (11.1) | 1 (100) | 680 | 96 | 210 | 2 |
| Vase | 41/38 | 13 (14.8) | 8 (15.7) | 1 (11.1) | 0 | 747 | 41 | 1,327 | 0 |
| Animal water dish | 28/16 | 6 (6.8) | 4 (7.8) | 0 (0) | 0 | 1,750 | 119 | 0 | 0 |
| Discarded toilet | 1/1 | 1 (1.1) | 0 (0) | 0 (0) | 0 | 43 | 0 | 0 | 0 |
| Other – Fruit shell | 106/46 | 1 (1.1) | 0 (0) | 0 (0) | 0 | 3 | 0 | 0 | 0 |
| TOTAL | 1,920/383 | 88 | 51 | 9 | 1 | 9,129 | 1,201 | 1,608 | 2 |
| VACANT LOTS | |||||||||
| Disposable container | 767/198 | 31 (37.8) | 20 (37.7) | 5 (25.0) | 0 (0) | 2,401 | 115 | 44 | 0 |
| Bucket | 48/12 | 3 (3.7) | 3 (5.7) | 1 (5.0) | 1 (20.0) | 1,159 | 11 | 131 | 130 |
| Tire | 168/94 | 40 (48.8) | 26 (49.1) | 13 (65.0) | 4 (80.0) | 13,344 | 640 | 2,394 | 244 |
| Discarded toilet | 9/7 | 6 (7.3) | 4 (7.5) | 0 (0) | 0 (0) | 629 | 34 | 0 | 0 |
| Other – Large diverse | 68/26 | 2 (2.4) | 0 (0) | 1 (5.0) | 0 (0) | 201 | 0 | 5 | 0 |
| TOTAL | 1,060/337 | 82 | 53 | 20 | 5 | 17,734 | 800 | 2,574 | 374 |
| STREETS/SIDEWALKS | |||||||||
| Disposable container | 39/6 | 1 (9.1) | 1 (14.3) | 0 (0) | 0 (0) | 27 | 41 | 0 | 0 |
| Bucket | 8/4 | 4 (36.4) | 2 (28.6) | 1 (33.3) | 1 (50.0) | 455 | 19 | 66 | 2 |
| Tire | 18/7 | 4 (36.4) | 3 (42.9) | 2 (66.7) | 1 (50.0) | 730 | 38 | 187 | 1 |
| Discarded toilet | 3/2 | 1 (9.1) | 1 (14.3) | 0 (0) | 0 (0) | 146 | 11 | 0 | 0 |
| Other – Hole in wall | 16/5 | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 11 | 0 | 0 | 0 |
| TOTAL | 84/24 | 11 | 7 | 3 | 2 | 1,369 | 109 | 253 | 3 |
| PARKING LOTS | |||||||||
| Disposable container | 28/5 | 3 (27.3) | 2 (28.6) | 2 (40.0) | 0 (0) | 808 | 15 | 303 | 0 |
| Bucket | 24/13 | 3 (27.3) | 2 (28.6) | 0 (0) | 0 (0) | 772 | 7 | 0 | 0 |
| Tire | 2/1 | 1 (9.1) | 0 (0) | 0 (0) | 0 (0) | 28 | 0 | 0 | 0 |
| Flower pot | 98/8 | 4 (36.4) | 3 (42.9) | 3 (60.0) | 1 (100) | 5,191 | 137 | 457 | 16 |
| Other – Large diverse | 16/6 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 0 | 0 | 0 |
| TOTAL | 168/33 | 11 | 7 | 5 | 1 | 6,799 | 159 | 760 | 16 |
| GRAND TOTAL | 3,232/777 | 192 | 118 | 37 | 9 | 35,031 | 2,269 | 5,195 | 395 |
Data for immatures are the same as for larvae (no containers had only pupae).
For other commonly encountered species, we note that vacant lots and parking lots together yielded substantial collections of immatures of Cx. quinquefasciatus (3,724), Cx. interrogator (1,115), Cx. thriambus (720), Cx. lactator (363), and Cx. salinarius (278).
Collections of Ae. aegypti and Cx. quinquefasciatus Immatures in Relation to Container Type
We did find a greater relative importance of tires to the overall production of Ae. aegypti immatures on vacant lots versus residential premises (accounting for 75.4 and 14.7%, respectively), and of the importance of flower pots for the overall production of Ae. aegypti immatures in parking lots (76.5%) (Table 2). In contrast to vacant lots and parking lots, none of the container types accounted for more than 33% of the overall production of Ae. aegypti on residential premises.
Abundance of Ae. aegypti Immatures in Relation to Selected Container Characteristics and Water Quality
Univariate Analyses of Immatures
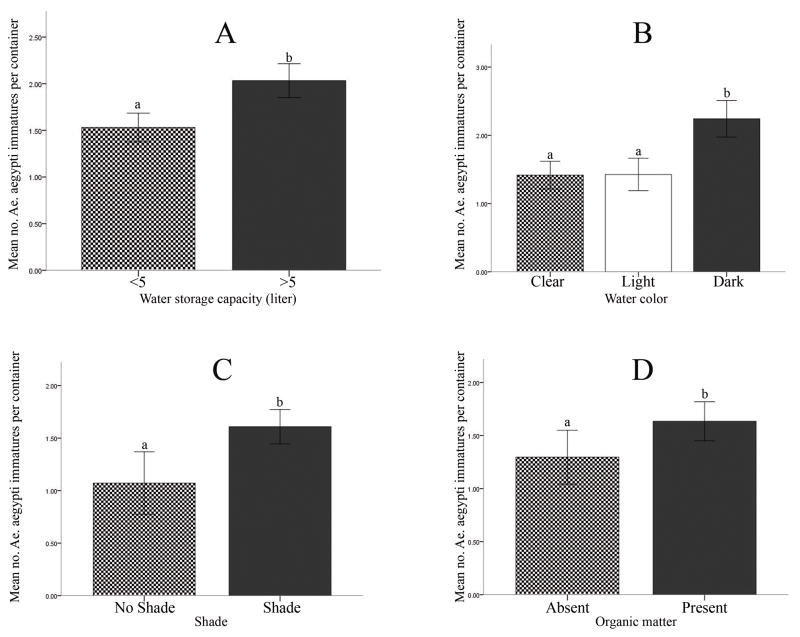
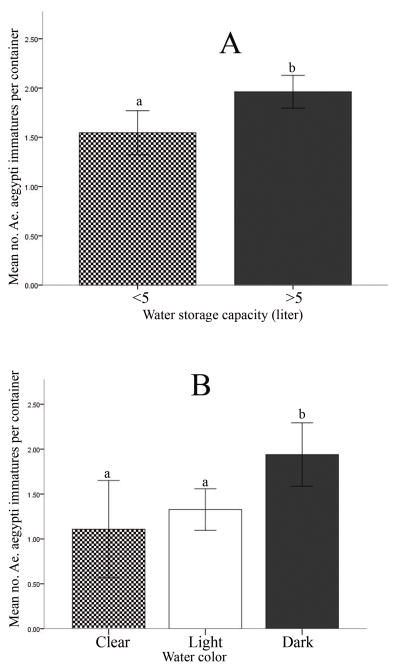
The mean number of Ae. aegypti immatures (log10-transformed) per infested container was greater for containers with larger water storage capacity (≥5 liters) compared with those of smaller capacity for residential premises (T = −4.13, d.f. = 86, P < 0.001) and non-residential environments (T = −2.97, d.f. = 102, P = 0.004) (Figures 3A and 4A). Abundance of immatures was greatest in dark colored water for residential premises (F = 9.54, d.f. = 55, P < 0.001) and non-residential environments (F = 5.97, d.f. = 35, P = 0.006) (Figures 3B and 4B). Mean number of Ae. aegypti immatures was significantly greater in containers located in shade (T = −2.59, d.f. = 53, P = 0.01) (Figure 3C), and for containers with organic matter present in the water (T = −2.09, d.f. = 53, P = 0.041) (Figure 3D).
Figure 3.
Comparison of the mean number of Ae. aegypti immatures (log10-transformed) per infested container on residential premises for: A) small disposable containers with a capacity <5 liters versus larger controllable containers with a capacity ≥5 liters, B) small disposable containers with a capacity <5 liters with water of different color, C) small disposable containers with a capacity <5 liters located in shade versus no shade, and D) small disposable containers with a capacity <5 liters with versus without organic matter in the water. Different letters above the error bars (standard error) indicate a significant difference (P<0.05).
Figure 4.
Comparison of the mean number of Ae. aegypti immatures (log10-transformed) per infested container in non-residential settings (vacant lots, parking lots, and streets/sidewalks) for: A) small disposable containers with a capacity <5 liters versus larger controllable containers with a capacity ≥5 liters, and B) small disposable containers with a capacity <5 liters with water of different color. Different letters above the error bars (standard error) indicate a significant difference (P<0.05).
Multivariate Analysis for Pupae
Principal component analysis resulted in four factors that explained >70% of the variation in the data (Table 3). These factors included container water storage capacity and water volume present in the containers (PC1), shading and urban environment class (PC2), presence/absence of organic matter in the containers and water color (PC3), and container type and material (PC4). The abundance of Ae. aegypti pupae was significantly associated with PC1 and PC3 (R2 = 0.17, F = 11.89, d.f. = 117, P < 0.001), indicating that container storage capacity, water volume present, water color, and presence/absence of organic matter in the water are important factors to determine pupal productivity in Mérida City.
Table 3.
Rotated factor pattern scores from nine principal components relating to container or water characteristics to explain the number of Ae. aegypti pupae encountered in pupal-infested containers.
| Variable | PC1a | PC2a | PC3a | PC4a |
|---|---|---|---|---|
| Three-way water storage capacity class (<1.5, 1.5–8.0, or >8.0 l) | 0.866b | 0.266 | 0.048 | 0.156 |
| Two-way size of container class (<5 or ≥5 l) | 0.857b | 0.310 | 0.096 | 0.067 |
| Classification for actual water volume in container | 0.831b | −0.157 | −0.058 | 0.007 |
| Shading present/absent | −0.079 | −0.796b | 0.207 | 0.057 |
| Urban environment class | 0.134 | 0.694b | 0.172 | 0.013 |
| Organic matter present/absent | −0.027 | −0.173 | 0.831b | −0.023 |
| Water color class | 0.076 | 0.179 | 0.767b | 0.199 |
| Container type | −0.042 | 0.079 | 0.028 | 0.837b |
| Container construction material | 0.220 | −0.135 | 0.132 | 0.742b |
The percentage variation for each component is: PC1 (29%), PC2 (17.5%), PC3 (12.6%), and PC4 (11.4%).
Scores >0.5.
Discussion
We demonstrate that water-filled containers in vacant lots and other non-residential urban environments (parking lots and sidewalks/streets) serve as sources for production of Ae. aegypti in Mérida City. We suggest that these non-residential urban environments, particularly vacant lots, should be considered for inclusion in mosquito surveillance and control efforts. Similar findings for vacant lots were reported previously from other Latin American countries, including Argentina (Costa et al. 2012), Brazil (Lopes et al. 1993, da Silva et al. 2006, dos Reis et al. 2010, de Mendonca et al. 2011), and Costa Rica (Troyo et al. 2008). Vacant lots have several charactersistics that make them potentially important sources for production of Ae. aegypti, including often being located near residential premises, commonly having abundant vegetation, and frequently harboring accumulations of small and large discarded items that may serve as adult harborage sites, oviposition sites for eggs, and development sites of immatures. The latter is related to dumping of household trash on vacant lots, which can be a substantial problem if these areas are not included in the municipal garbage collection program (Mazine et al. 1996).
In our study, the most important container types for production of Ae. aegypti differed between residential premises and non-residential settings. As in previous studies on residential premises in Mérida City (Winch et al. 1992, Manrique-Saide et al., 2008, García-Rejón et al. 2011a), we found that several different container types, including disposable containers, buckets and drinking troughs for animals, contributed substantially to production of Ae. aegypti immatures. In contrast, a single container type was found to produce >75% of immatures on vacant lots (tires) and in parking lots (flower pots). The importance of tires for production of mosquitoes in non-residential settings in Latin America was noted previously (Lopes et al. 1993, Morrison et al. 2006, Rubio et al. 2011). We also found greater abundance of Ae. aegypti immatures in larger containers that were shaded or had higher nutrient content (as indicated by dark color or presence of organic material); these results agree with previous findings from the Americas (Vezzani et al. 2005, Barrera et al. 2006b, Bisset Lazcano et al. 2006, Maciel-de-Freitas et al. 2007, Vezzani and Albicocco 2009, Beserra et al. 2010, Murrell et al. 2011, Wong et al. 2012).
We point out that in addition to Ae. aegypti, the non-residential settings produced large numbers of Culex immatures, primarily of the notorious nuisance biter and arbovirus vector Cx. quinquefasciatus. This underscores the importance of mosquito control in non-residential settings, as Cx. quinquefasciatus not only is a major pest of homes and other indoor environments in Mérida City (García-Rejón et al. 2008a, 2011b; Loroño-Pino et al. 2013) but also recently was found to carry viruses with unknown pathogenicity to humans, such as T’Ho virus, in the Yucatán Peninsula (Farfan-Ale et al. 2009, 2010).
Further studies are needed to quantify the relative production of Ae. aegypti on vacant lots versus residential premises in Mérida City, including data not only for average mosquito production in these respective environments (based on repeated sampling of individual sites across wet and dry seasons) but also for the size of the areas covered by residential premises versus vacant lots within the city. Nevertheless, it seems feasible that under a scenario of successful mosquito control on residential premises but exclusion of non-residential environments from the control effort, vacant lots and other non-residential settings may emerge as key sources for mosquito production. Because non-residential settings typically are easier to access compared with residential premises, they can readily be included in mosquito surveillance and control efforts.
Acknowledgments
We thank Jesus J. Baak-Baak, Mildred and Genny Lopez-Uribe, Carlos Cobá-Tún, Victor Rivero-Osorno, Damián Lavalle-Kantún, and María Puc-Tinal of Universidad Autónoma de Yucatán for assistance with fieldwork and mosquito identification, and the involved home owners for granting us permission to collect mosquitoes. The study was supported by the National Institutes of Health/National Institute of Allergy and Infectious Diseases (International Collaborations in Infectious Disease Research Program U01-AI-088647). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
References Cited
- Abe M, McCall PJ, Lenhart A, Villegas E, Kroeger A. The Buen Pastor cemetery in Trujillo, Venezuela: measuring dengue vector output from a public area. Trop Med Int Health. 2005;10:597–603. doi: 10.1111/j.1365-3156.2005.01428.x. [DOI] [PubMed] [Google Scholar]
- Arredondo-Jimenez JI, Valdez-Delgado KM. Aedes aegypti pupal/demographic surveys in southern Mexico: consistency and practicality. Ann Trop Med Parasitol. 2006;100(Suppl 1):S17–S32. doi: 10.1179/136485906X105480. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Clark GG. Use of the pupal survey technique for measuring Aedes aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg. 2006a;74:290–302. [PubMed] [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006b;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Beserra EB, Fernandes CRM, de Sousa JT, de Freitas EM, Santos KD. Efeito da qualidade da água no ciclo de vida e na atração para oviposição de Aedes aegypti (L.) (Diptera: Culicidae) Neotrop Entomol. 2010;39:1016–1023. doi: 10.1590/s1519-566x2010000600026. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset Lazcano JA, Marquetti MC, Portillo R, Rodriguez MM, Suarez S, Leyva M. Factores ecológicos asociados con la presencia de larvas de Aedes aegypti en zonas de alta infestación del municipio Playa, Ciudad de La Habana, Cuba. Rev Panam Salud Publica. 2006;19:379–384. doi: 10.1590/s1020-49892006000600003. [DOI] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico) University of California Press; Berkeley, CA: 1955. [Google Scholar]
- Christophers RS. Its Life History, Bionomics and Structure. Cambridge University Press; Cambridge, U.K: 1960. Aedes aegypti (L.), the Yellow Fever Mosquito. [Google Scholar]
- Costa F, Fattore G, Abril M. Diversity of containers and buildings infested with Aedes aegypti in Puerto Iguazú, Argentina. Cad Saúde Pública. 2012;28:1802–1806. doi: 10.1590/s0102-311x2012000900019. [DOI] [PubMed] [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. University Press of Florida; Gainesville, FL: 2005. [Google Scholar]
- da Silva VC, Scherer PO, Falcao SS, Alencar J, Cunha SP, Rodrigues IM, Pinheiro NL. Diversidade de criadouros e tipos de imóveis freqüentados por Aedes albopictus e Aedes aegypti. Rev Saúde Pública. 2006;40:1106–1111. doi: 10.1590/s0034-89102006000700021. [DOI] [PubMed] [Google Scholar]
- de Mendonca HFMS, Ferreira AL, dos Santos CB, Rezende HR, Ferreira GEM, Leite GR, Falqueto A. Breeding sites of Aedes aegypti in metropolitan vacant lots in Greater Vitoria, State of Espirito Santo, Brazil. Rev Soc Bras Med Trop. 2011;44:243–246. doi: 10.1590/s0037-86822011000200022. [DOI] [PubMed] [Google Scholar]
- dos Reis IC, Honorio NA, Codeco CT, Magalhaes MAFM, Lourenco-de-Oliveira R, Barcellos C. Relevance of differentiating between residential and non-residential premises for surveillance and control of Aedes aegypti in Rio de Janeiro, Brazil. Acta Trop. 2010;114:37–43. doi: 10.1016/j.actatropica.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Farfan-Ale JA, Lorono-Pino MA, García-Rejón JE, Hovav E, Powers AM, Lin M, Dorman KS, Platt KB, Bartholomay LC, Soto V, Beaty BJ, Lanciotti RS, Blitvich BJ. Detection of RNA from a novel West Nile-like virus and high prevalence of an insect-specific flavivirus in mosquitoes in the Yucatan Peninsula of Mexico. Am J Trop Med Hyg. 2009;80:85–95. [PMC free article] [PubMed] [Google Scholar]
- Farfan-Ale JA, Lorono-Pino MA, García-Rejón JE, Soto V, Lin M, Staley M, Dorman KS, Bartholomay LC, Hovav E, Blitvich BJ. Detection of flaviviruses and orthobunyaviruses in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector-Borne Zoonotic Dis. 2010;10:777–783. doi: 10.1089/vbz.2009.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focks DA, Alexander N. Findings and Recommendations. World Health Organization; Geneva, Switzerland: 2006. Multicountry Study of Aedes aegypti Pupal Productivity Survey Methodology. [Google Scholar]
- Focks DA, Chadee DD. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am J Trop Med Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- Focks DA, Haile DG, Daniels E, Mount GA. Dynamic life table model for Aedes aegypti (Diptera: Culicidae): analysis of the literature and model development. J Med Entomol. 1993;30:1003–1017. doi: 10.1093/jmedent/30.6.1003. [DOI] [PubMed] [Google Scholar]
- García-Rejón J, Loroño-Pino MA, Farfan-Ale JA, Flores-Flores L, Rosado-Paredes EP, Rivero-Cardenas N, Najera-Vazquez R, Gomez-Carro S, Lira-Zumbardo V, Gonzalez-Martinez P, Lozano-Fuentes S, Elizondo-Quiroga D, Beaty BJ, Eisen L. Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg. 2008a;79:940–950. [PubMed] [Google Scholar]
- García-Rejón JE, Farfan-Ale JA, Ulloa A, Flores-Flores LF, Rosado-Paredes E, Baak-Baak C, Lorono-Pino MA, Fernandez-Salas I, Beaty BJ. Gonotrophic cycle estimate for Culex quinquefasciatus in Merida, Yucatan, Mexico. J Am Mosq Contr Assoc. 2008b;24:344–348. doi: 10.2987/5667.1. [DOI] [PubMed] [Google Scholar]
- García-Rejón JE, Lopez-Uribe MP, Loroño-Pino MA, Farfan-Ale JA, Najera-Vazquez MR, Lozano-Fuentes S, Beaty BJ, Eisen L. Productive container types for Aedes aegypti immatures in Merida, Mexico. J Med Entomol. 2011a;48:644–650. doi: 10.1603/me10253. [DOI] [PubMed] [Google Scholar]
- García-Rejón JE, Loroño-Pino MA, Farfan-Ale JA, Flores-Flores LF, Lopez-Uribe MP, Najera-Vazquez MR, Nunez-Ayala G, Beaty BJ, Eisen L. Mosquito infestation and dengue virus infection in Aedes aegypti females in schools in Merida, Mexico. Am J Trop Med Hyg. 2011b;84:489–496. doi: 10.4269/ajtmh.2011.10-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Rejón JE, Lopez-Uribe MP, Loroño-Pino MA, Arana-Guardia R, Puc-Tinal M, Lopez-Uribe GM, Coba-Tun C, Baak-Baak CM, Machain-Williams C, Reyes-Solis GC, Lozano-Fuentes S, Saavedra-Rodriguez K, Black WC, IV, Beaty BJ, Eisen L. Aedes (Stegomyia) aegypti and Aedes (Howardina) cozumelensis in Yucatan State, Mexico, with a summary of published collection records for Ae. cozumelensis. J Vector Ecol. 2012;37:365–372. doi: 10.1111/j.1948-7134.2012.00240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez-Bernal S, Martinez-Campos C. Clave para la identificación de larvas de mosquitos comunes en las áreas urbanas y suburbanas de la República Mexicana (Díptera: Culicidae) Folia Entomol Mex. 1994;92(4):3–73. [Google Scholar]
- Knox TB, Nguyen YT, Vu NS, Kay BH, Ryan PA. Quantitative relationships between immature and emergent adult Aedes aegypti (Diptera: Culicidae) populations in water storage container habitats. J Med Entomol. 2010;47:748–758. doi: 10.1603/me09297. [DOI] [PubMed] [Google Scholar]
- Lloyd LS, Winch P, Ortega-Canto J, Kendall C. Results of a community-based Aedes aegypti control program in Merida, Yucatan, Mexico. Am J Trop Med Hyg. 1992;46:635–642. doi: 10.4269/ajtmh.1992.46.635. [DOI] [PubMed] [Google Scholar]
- Lopes J, da Silva MAN, Borsato AM, de Oliveira VDRB, de Oliveira FJA. Aedes (Stegomyia) aegypti L. e a culicideofauna associada em área urbana da região sul, Brasil. Rev Saúde Pública. 1993;27:326–333. doi: 10.1590/s0034-89101993000500002. [DOI] [PubMed] [Google Scholar]
- Loroño-Pino MA, García-Rejón JE, Machain-Williams C, Gomez-Carro S, Nuñez-Ayala G, Nájera-Vázquez MR, Losoya A, Aguilar L, Saavedra-Rodriguez K, Lozano-Fuentes S, Beaty MK, Black WC, IV, Keefe TJ, Eisen L, Beaty BJ. Towards a Casa Segura: a consumer product study of the effect of insecticide-treated curtains on Aedes aegypti and dengue virus infections in the home. Am J Trop Med Hyg. 2013;89:385–397. doi: 10.4269/ajtmh.12-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Marques WA, Peres RC, Cunha SP, Lourenco-de-Oliveira R. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz. 2007;102:489–496. doi: 10.1590/s0074-02762007005000056. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P, Davies CR, Coleman PG, Rebollar-Tellez E, Che-Medoza A, Dzul-Manzanilla F, Zapata-Peniche A. Pupal surveys for Aedes aegypti surveillance and potential targeted control in residential areas of Merida, Mexico. J Am Mosq Contr Assoc. 2008;24:289–298. doi: 10.2987/5578.1. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P, Uc V, Prado C, Carmona C, Vadillo J, Chan R, Dzib-Florez S, Che-Mendoza A, Barrera-Perez M, Sanchez EC, Arredondo-Jimenez JI. Storm sewers as larval habitats for Aedes aegypti and Culex spp. in a neighborhood of Merida, Mexico. J Am Mosq Contr Assoc. 2012;28:255–257. doi: 10.2987/12-6244R.1. [DOI] [PubMed] [Google Scholar]
- Manrique-Saide P, Arisqueta-Chable C, Geded-Moreno E, Herrera-Bojorquez J, Uc V, Chable-Santos J, Che-Mendoza A, Sanchez EC, Arredondo-Jimenez JI, Medina-Barreiro A. An assessment of the importance of subsurface catch basins for Aedes aegypti adult production during the dry season in a neighborhood of Merida, Mexico. J Am Mosq Contr Assoc. 2013;29:164–167. doi: 10.2987/12-6320R.1. [DOI] [PubMed] [Google Scholar]
- Mazine CAB, Macoris MLG, Andrighetti MTM, Yasumaro S, Silva ME, Nelson MJ, Winch PJ. Disposable containers as larval habitats for Aedes aegypti in a city with regular refuse collection: a study in Marília, São Paulo State, Brazil. Acta Trop. 1996;62:1–13. doi: 10.1016/s0001-706x(96)00013-7. [DOI] [PubMed] [Google Scholar]
- Morrison AC, Sihuincha M, Stancil JD, Zamora E, Astete H, Olson JG, Vidal-Ore C, Scott TW. Aedes aegypti (Diptera: Culicidae) production from non-residential sites in the Amazonian city of Iquitos, Peru. Ann Trop Med Parasitol. 2006;100(Suppl 1):S73–S86. doi: 10.1179/136485906X105534. [DOI] [PubMed] [Google Scholar]
- Murrell EG, Damal K, Lounibos LP, Juliano SA. Distributions of competing container mosquitoes depend on detritus types, nutrient ratios, and food availability. Ann Entomol Soc Am. 2011;104:688–698. doi: 10.1603/AN10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio A, Cardo MV, Vezzani D. Tire-breeding mosquitoes of public health importance along an urbanisation gradient in Buenos Aires, Argentina. Mem Inst Oswaldo Cruz. 2011;106:678–684. doi: 10.1590/s0074-02762011000600006. [DOI] [PubMed] [Google Scholar]
- Scott TW, Morrison AC, Lorenz LH, Clark GG, Strickman D, Kittayapong P, Zhou H, Edman JD. Longitudinal studies of Aedes aegypti (Diptera: Culicidae) in Thailand and Puerto Rico: population dynamics. J Med Entomol. 2000;37:77–88. doi: 10.1603/0022-2585-37.1.77. [DOI] [PubMed] [Google Scholar]
- Troyo A, Calderon-Arguedas O, Fuller DO, Solano ME, Avendano A, Arheart KL, Chadee DD, Beier JC. Seasonal profiles of Aedes aegypti (Diptera: Culicidae) larval habitats in an urban area of Costa Rica with a history of mosquito control. J Vector Ecol. 2008;33:76–88. doi: 10.3376/1081-1710(2008)33[76:spoaad]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun-Lin W, Kay BH, Barnes A, Forsyth S. Critical examination of Aedes aegypti indices: correlations with abundance. Am J Trop Med Hyg. 1996;54:543–547. doi: 10.4269/ajtmh.1996.54.543. [DOI] [PubMed] [Google Scholar]
- Tun-Lin W, Lenhart A, Nam VS, Rebollar-Tellez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14:1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Albicocco AP. The effect of shade on the container index and pupal productivity of the mosquitoes Aedes aegypti and Culex pipiens breeding in artificial containers. Med Vet Entomol. 2009;23:78–84. doi: 10.1111/j.1365-2915.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Schweigmann N. Suitability of containers from different sources as breeding sites of Aedes aegypti (L.) in a cemetery of Buenos Aires City, Argentina. Mem Inst Oswaldo Cruz. 2002;97:789–792. doi: 10.1590/s0074-02762002000600006. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Rubio A, Velazquez SM, Schweigmann N, Wiegand T. Detailed assessment of microhabitat suitability for Aedes aegypti (Diptera: Culicidae) in Buenos Aires, Argentina. Acta Trop. 2005;95:123–131. doi: 10.1016/j.actatropica.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Villegas-Trejo A, Che-Mendoza A, Gonzalez-Fernandez M, Guillermo-May G, Gonzalez-Bejarano H, Dzul-Manzanilla F, Ulloa-Garcia A, Danis-Lozano R, Manrique-Saide P. Control enfocado de Aedes aegypti en localidades de alto riesgo de transmisión de dengue en Morelos, México. Salud Publica Mex. 2011;53:141–151. doi: 10.1590/s0036-36342011000200007. [DOI] [PubMed] [Google Scholar]
- Weaver SC, Reisen WK. Present and future arboviral threats. Antiviral Res. 2010;85:328–345. doi: 10.1016/j.antiviral.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winch PJ, Barrientos-Sanchez G, Puigserver-Castro E, Manzano-Cabrera L, Lloyd LS, Mendez-Galvan JF. Variation in Aedes aegypti larval indices over a one year period in a neighborhood of Merida, Yucatan, Mexico. J Am Mosq Contr Assoc. 1992;8:193–195. [PubMed] [Google Scholar]
- Wong J, Morrison AC, Stoddard ST, Astete H, Chu YY, Baseer I, Scott TW. Linking oviposition site choice to offspring fitness in Aedes aegypti: consequences for targeted larval control of dengue vectors. PLoS Negl Trop Dis. 2012;6:e1632. doi: 10.1371/journal.pntd.0001632. [DOI] [PMC free article] [PubMed] [Google Scholar]