Abstract
Phasic increases in brain dopamine are required for cue-directed reward seeking. While compelling within the framework of appetitive behavior, the view that illicit drugs hijack reward circuits by hyper-activating these dopamine transients is inconsistent with established psychostimulant pharmacology. However, recent work reclassifying amphetamine (AMPH), cocaine, and other addictive dopamine-transporter inhibitors (DAT-Is) supports transient hyper-activation as a unifying hypothesis of abused drugs. We argue here that reclassification also identifies generating burst firing by dopamine neurons as a keystone action. Unlike natural rewards, which are processed by sensory systems, drugs act directly on the brain. Consequently, to mimic natural reward and exploit reward circuits, dopamine transients must be elicited de novo. Of available drug targets, only burst firing achieves this essential outcome.
Dopamine, psychostimulants and reinforcement
A long-held tenet in the pharmacology of abused drugs is that, despite marked differences in cellular targets, all classes of these substances increase brain levels of extracellular dopamine [1]. Drug-induced dopamine elevations occur to the greatest extent in the nucleus accumbens (NAc), a brain region that is critical for translating motivational input into behavioral output [2,3]. This shared outcome of a hyper-dopamine state is thought to mediate the initial reinforcing properties of abused drugs (Box 1), the general focus of this Opinion piece. Not unexpectedly, extensive work has been directed at refining this general scheme, and abused drugs have been classified on the basis of specific mechanisms for targeting dopamine neurons [4,5]. Moreover, there is an emergent hypothesis that abused drugs hijack reward circuits by hyper-activating extracellular phasic (~1-2 s) signals called dopamine transients [6,7]. While attractive with regard to the processing of natural rewards by phasic dopamine signaling in appetitive behavior, this hypothesis is inconsistent with currently accepted mechanisms for how addictive DAT-Is, including AMPH, methamphetamine, cocaine, and methylphenidate (Ritalin®), act on dopamine neurons.
Text Box 1. Drug addiction.
Drug addiction is ultimately characterized by compulsive drug seeking and taking despite negative consequences and relapse following periods of abstinence [6,93,94]. The transition to addiction begins with goal-directed drug use that is reinforced by rewarding, often hedonic, drug effects. Later stages in the transition to addiction are characterized by an escalation in drug use and difficulty limiting drug intake (i.e., drug abuse). Such behaviors progress to compulsive drug seeking and taking in a subset of susceptible individuals following extended drug abuse [93]. Because relapse is prone to occur even following extended periods of drug abstinence and long after withdrawal symptoms have subsided, addiction is hypothesized to represent a disorder of learning and memory [6] arising from drug-induced neuroadaptations in brain circuits controlling motivated behavior [95]. Drug-induced alterations in the dopamine reward circuit are critical for the transition through each stage of the addiction process. The initial reinforcing effects of abused drugs are dependent on these substances targeting midbrain dopamine neurons originating in the ventral tegmental area (VTA) and projecting to the nucleus accumbens (NAc) [95,96]. Acute drug exposure acts preferentially on dopamine neurons innervating the medial shell of the NAc [28], which increases extracellular dopamine here to a greater extent than in the NAc core and dorsal striatum [1,28,97]. Drug-induced dopamine elevations in the NAc shell support the behavioral-invigorating or motivational effects of abused drugs, particularly the psychostimulants [98,99]. Acute drug exposure also elicits long-term potentiation at glutamatergic synapses onto dopamine neurons [79,80,100]. This drug-induced strengthening of excitatory input may increase the incidence of burst firing [101] and support the progressive manifestation of synaptic plasticity in striatal regions that occurs following repeated drug exposure and acts to strengthen drug-seeking behaviors [102,103]. Reciprocal feedback between the striatum and midbrain dopamine neurons [104] also results in a ventromedial to dorsolateral-directed progression in the primary striatal region controlling behavior following chronic drug exposure [98,105,106]. This process begins with the initial activation of dopamine neurons projecting to the NAc shell, which projects back to and recruits dopamine neurons innervating the NAc core. Dopamine input to the NAc core is particularly important for associating drug rewards with discrete cues and for these cues to motivate drug-seeking [94,107]. These cue-drug associations are also critical for the maintenance and escalation of drug intake, and driving relapse [94,108]. This “spiraling” feedback loop continues following prolonged drug intake so that dopamine neurons projecting to the dorsolateral striatum gain greater control, which supports drug seeking and taking transitioning from a behavior that is goal-directed to one that is ultimately habitual and compulsive [94,98]. It should be noted that while dopamine input to striatal regions is critical to the addiction process, numerous other brain regions and neurotransmitter systems are clearly necessary for addiction to manifest [93,109,110].
In this Opinion piece, we highlight recent work calling for reclassifying these psychostimulants. We argue that this reclassification reconciles dopamine theories of appetitive behavior and the hijacking of reward circuits by abused drugs with a mechanistic understanding of psychostimulant action on dopamine neurons. We begin by summarizing the role of phasic dopamine signaling in appetitive behavior, the emergent hypothesis that abused drugs usurp this process, and the traditional view of drug action on dopamine neurons. On the basis of reclassifying DAT-Is and by virtue of eliciting dopamine transients de novo, we then argue that generating burst firing by dopamine neurons is the keystone action by which abused drugs hijack reward circuits.
Phasic dopamine signaling plays a critical role in appetitive behavior
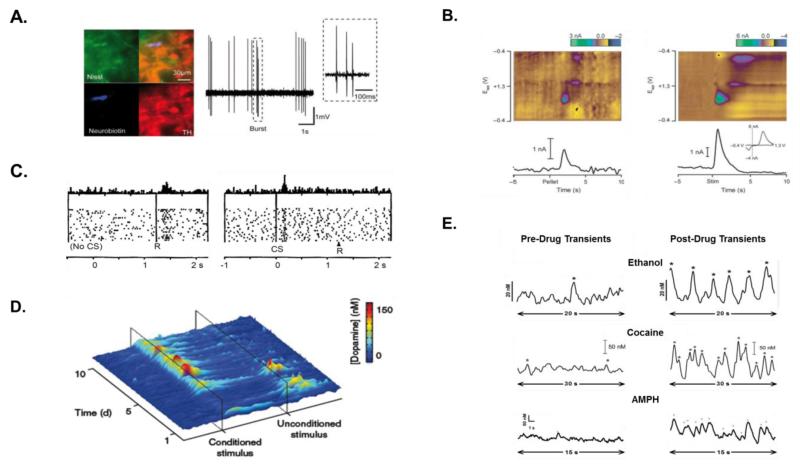
Intrinsic properties coupled with converging input from numerous excitatory and inhibitory afferents enable dopamine neurons to signal in two general modes: tonic and phasic [8-11]. During tonic dopamine signaling, slow and irregular firing contributes to a low ambient level of extracellular dopamine that binds high-affinity D2 dopamine receptors and supports movement, cognition, and motivation. In contrast, during phasic dopamine signaling, rapid and synchronous burst firing elicits dopamine concentration spikes called transients (Figures 1A and B) that activate low-affinity D1 dopamine receptors. These transients are monitored with fast-scan cyclic voltammetry (FSCV) and are faithfully reproduced by electrical stimulation, which fosters analysis of underlying mechanisms for dopamine release and uptake (Box 2). Similar to burst firing by dopamine neurons, natural rewards evoke dopamine transients that are transferred to predictive cues following associative learning (Figure 1C). The conditioned transfer highlights the functional link between these two components of phasic dopamine signaling, somatodendritic burst firing eliciting dopamine transients in terminal fields. Dopamine transients also occur “spontaneously”, i.e., in the absence of overt environmental stimuli (Figure 1D, left) and are pharmacologically evoked by abused drugs (Figure 1D, right), the specific focus of this Opinion piece.
Figure 1. Phasic dopamine signaling.
A. Electrophysiological recoding of an identified dopamine neuron in vivo. (left) The recorded neuron was labeled with a neurobiotin tracer (blue) and identified with a green fluorescent Nissel stain (green). The neurochemical phenotype was confirmed by labeling with an antibody against tyrosine hydroxylase (TH, red). (right) The dopamine neuron fired in a bursting pattern (outlined box). B. Extracellular phasic dopamine signals recorded with FSCV at a CFM. (Left) Dopamine transients evoked by an unpredicted food reward (“Pellet”) at time 0 s. (Right) Transient-like signals evoked by brief (0.4 s) electrical stimulation (“Stim”) applied to dopamine axons at time 0 s. (Top) Color plots display sequential voltammograms indicating that dopamine is evoked by the stimulation and food reward (measured current in color, z-axis; applied voltage, y-axis; time, x-axis). (Bottom) Current measured by the CFM at the peak oxidation potential for dopamine (i.e., dopamine current) versus time. (Inset) Individual voltammograms also identify the signal evoked by stimulation and food reward as dopamine. C. (Left) Burst firing by dopamine neurons in response to an unpredicted juice reward. (Right) Burst firing by dopamine neurons transfers to the reward-predicting conditioned stimulus once the cue-reward contingency is learned. Each panel shows the peri-event time histogram (top) and raster plot (bottom) of neuronal activity from the same neuron. CS, conditioned stimulus; R, reward. D. Dopamine transients measured by FSCV in response to food reward (Unconditioned stimulus) and a predictive cue (Conditioned stimulus) during Pavlovian conditioning. Heat map shows the transfer of dopamine transients elicited by the food reward to the conditioned stimulus. E. Drug-induced activation of dopamine transients measured by FSCV in awake animals recorded by FSCV at a CFM. Recordings reflect fluctuations in dopamine concentration versus time. Dopamine transients (identified by asterisks) recorded before (left) and after (right) administration of ethanol (2 g/kg, i.v.), cocaine (0.33 mg, i.v.), or AMPH (1 mg/kg, i.p.). Reproduced with permission from [76] (A.), [86] (B.), [87] (C.), [36] (D.), and [27,31,42] (E.).
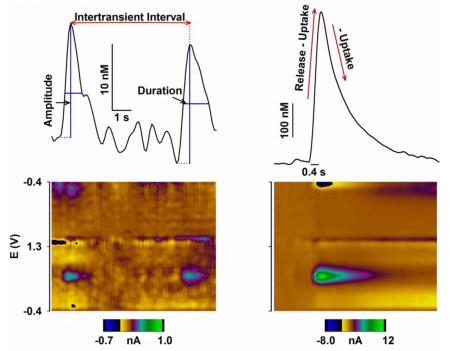
Text Box 2. Analysis of dopamine transients.
Dopamine transients are characterized by amplitude, duration (i.e., width at half amplitude), and frequency (i.e., inverse of inter-transient interval or ITI) (Box 2, left) [10]. These descriptive measures are not fundamental, but rather reflect burst firing of dopamine neurons, vesicular dopamine release, and dopamine uptake. Unfortunately, interactions between these three neural mechanisms preclude definitive assignment to changes in dopamine transients. However, insight into mechanism is provided by independent assessment of burst firing monitored by electrophysiology (Figure 1A) and dopamine release and uptake from electrically evoked phasic dopamine signals monitored by FSCV (Box 2, right). This latter analysis resolves the respective contributions of dopamine release and uptake by fitting evoked phasic signals to equations that describe the rising phase as a balance between release and uptake and falling phase to uptake [10]. As described in the text, considerable evidence suggests that the drug-induced increases in burst firing by dopamine neurons and frequency of dopamine transients are tightly associated. Moreover, direct comparisons of electrically evoked phasic signals and dopamine transients suggest that transient amplitude is relatively insensitive to dopamine uptake but highly dependent on dopamine release, while transient duration is more sensitive to dopamine uptake than release [111]. In excellent agreement, cocaine- and AMPH-induced increases in the amplitude of electrically evoked phasic signals better correlate with up-regulated dopamine release than inhibited dopamine uptake [42,56,57,60].
Compelling evidence obtained from monitoring burst firing by dopamine neurons [12,13] and dopamine transients [14,15] supports a critical role for phasic dopamine signaling in appetitive behavior by encoding key attributes of natural rewards, such as timing, cost, magnitude, probability, and uncertainty. Dopamine transients also exhibit the requisite temporal precision and amplitude to promote plasticity of corticostriatal synapses that is associated with reward learning [16,17]. At least two general, not necessarily mutually exclusive, conceptual models have emerged to integrate these phenomena. First, phasic dopamine signaling serves a teaching function in reinforcement learning by providing a “reward prediction error” describing the difference between expected and received reward [11]. In this manner, unexpected or greater than expected rewards phasically increase dopamine and reinforce behavior, expected rewards cause no change in dopamine and behavior, and absent or worse than expected rewards phasically decrease dopamine and suppress behavior (but see [18]). Second, phasic dopamine signaling attributes “incentive salience” or “wanting” to reward predicting cues, which underlies their ability to motivate behaviors directed toward obtaining rewards and to act as conditioned reinforcers [19,20]. Consistent with both theories, recent work using transgenic and optogenetic approaches for selectively manipulating neuronal activity indicate that phasic dopamine signaling is necessary and sufficient for forming cue-reward associations and for cue-directed reward seeking [21-24]
Abused drugs hijack reward circuits by hyper-activating dopamine transients
An emergent hypothesis is that abused drugs activate dopamine transients to a greater degree than natural rewards, leading to overvaluation of cues predicting drug availability [6,7]. Indeed, abused drugs from broad classes, including ethanol, cocaine, nicotine, and cannabinoids, have now been demonstrated to augment dopamine transients (Figure 1D, right) [25-28]. While drug-evoked dopamine transients resemble those occurring naturally [29], abused drugs evoke a quantitatively greater response. The robust nature of this activation is strikingly demonstrated during drug self-administration, which emulates voluntary drug taking by humans. Indeed, transient frequency is increased ~10-fold for the duration of repeated cocaine injections [30,31]. These effects are considered pharmacological in nature and mediated by central drug actions [32] (but see [33]). Thus, unlike natural rewards, which are processed by sensory systems and afferent input to dopamine neurons and whose neuronal responses are subject to modification during associative learning, abused drugs act directly on the brain [7,11]. However, cues predicting cocaine delivery also elicit dopamine transients [30,34,35] in a similar manner to cues predicting food reward [19,36], which reflects learned associations and non-pharmacological effects. Thus, although natural rewards and abused drugs both activate phasic dopamine signaling, qualitative and quantitative aspects of this activation differ.
Distinct actions of abused drugs on phasic dopamine signaling are thought to drive aberrant learning of cue-drug associations, leading to the hijacking of reward circuits. For example, the sheer number of pharmacologically evoked dopamine transients should increase the probability of learned associations between drug taking and environmental stimuli [7]. The robust drug-induced increase in phasic dopamine signaling should also confer to abused drugs a higher reward magnitude compared to natural rewards, resulting in cue-evoked dopamine transients with correspondingly greater amplitude [7,13,14]. Additionally, persistent positive prediction errors should be produced by abused drugs directly targeting the brain and reliably and robustly eliciting dopamine transients even if drug delivery is expected [37,38]. Consistent with aberrant reward learning, drug-paired cues maintain cocaine seeking in the absence of cocaine delivery for up to a year after only a single session of cocaine self-administration, which is sharply contrasted with responding to cues previously paired with a highly palatable food reward that extinguishes within 3 months [39]. While hyper-activation of dopamine transients usurping reward circuits thus fits well with dopamine theory of appetitive behavior, this hypothesis is not supported by established psychostimulant pharmacology. In the next section, we summarize the traditional view of drug action on dopamine neurons and identify key discrepancies for addictive DAT-Is.
Actions of abused drugs on dopamine neurons: traditional view
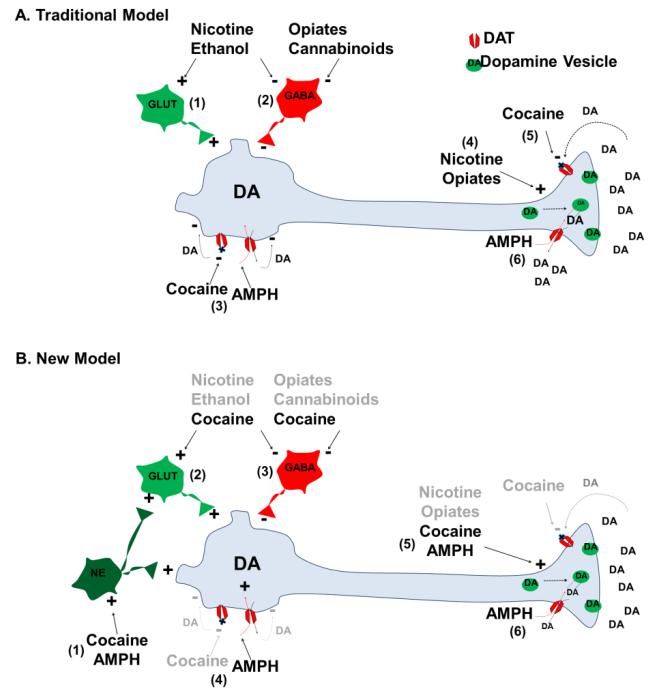
Abused drugs have traditionally been classified on the basis of three functional targets on dopamine neurons: firing of action potentials, vesicular dopamine release, and dopamine uptake [4,5]. Activation of each target is thought to increase brain levels of extracellular dopamine [1]. In general, (1) ethanol, nicotine, cannabinoids, and opiates increase burst firing by dopamine neurons; (2) nicotine and opiates up-regulate vesicular dopamine release; (3) cocaine- and AMPH-like psychostimulants inhibit dopamine uptake (Figure 2A - for details of these mechanisms see figure legend and Box 3). However, important mechanistic differences exist for these two subclasses of addictive DAT-Is. For example, cocaine-like blockers bind and allosterically inhibit DAT. In contrast, AMPH-like releasers are substrates of DAT and reverse its function, releasing intracellular dopamine into the extracellular space independently of action potentials. This reverse dopamine transport or efflux is driven by AMPH redistributing dopamine from vesicular to cytosolic compartments, which also disrupts exocytotic dopamine release. Both cocaine- and AMPH-like DAT-Is additionally suppress dopamine-cell firing by elevating extracellular dopamine that activates somatodendritic D2 dopamine autoreceptors.
Figure 2. Actions of abused drugs on dopamine neurons.
This figure summarizes the actions of abused drugs. It is important to note that these actions may differ across heterogenous subsets of midbrain dopamine neurons (see Box 3 and Outstanding Questions). Facilitation and inhibition are indicated by “+” and “-”, respectively. Abbreviations: GLUT, glutamate; NE, norepinephrine; DA, dopamine. A. Traditional Model. (1) Nicotine and ethanol enhance burst firing by dopamine neurons via enhancing excitatory glutamatergic drive [75,78]. (2) Nicotine [75] and ethanol [77] share with opiates and cannabinoids [88] the ability to disinhibit firing by reducing GABAergic input. Nicotine also activates firing directly via nicotinic acetylcholine receptors on dopamine neurons [74] (not shown). (3) In contrast, both AMPH- and cocaine-like DAT-Is suppress firing by elevating extracellular dopamine that activates somatodendritic D2 dopamine autoreceptors [4]. (4) At dopamine terminals, nicotine and opiates up-regulate vesicular dopamine release. Nicotine mobilizes the reserve pool of dopamine vesicles to the readily releasable pool [89] and shares with opiates the ability to increase the amplitude of phasic relative to tonic dopamine signals [90,91]. (5) Cocaine inhibits dopamine uptake by blocking DAT [92]. (6) As a DAT substrate, AMPH enters the dopamine terminal to deplete vesicular dopamine stores and promote DAT-mediated reverse dopamine transport [5]. B. New Model. The new model of drug action on dopamine neurons extends the old model described in A. above by reclassifying DATIs. Actions proposed for other abused drugs and for DAT-is inhibiting dopamine uptake are thus not changed in the new model and appear shaded. The new classification of DAT-Is is only briefly described here. Details and supporting references are found in text. (1) Cocaine and AMPH directly and indirectly activate burst firing by dopamine neurons by enhancing noradrenergic input. Cocaine increases burst firing by (2) enhancing glutamatergic input via presynaptic D1 dopamine receptors and (3) inhibiting GABAergic input. (4) By acting as DAT substrates, AMPH and its analog methamphetamine directly depolarize dopamine neurons. (5) AMPH and cocaine up-regulate vesicular dopamine release. (6) AMPH-induced dopamine efflux is modest, suggesting that this action potential-independent mechanism is not the primary AMPH target for activating dopamine signaling.
Text Box 3. Generation of burst firing: nicotine and ethanol.
Nicotine and ethanol, which unlike the cocaine- and AMPH-like psychostimulants do not inhibit dopamine uptake, have been extensively investigated for their ability to generate burst firing by dopamine neurons. Indeed, pharmacological activation of burst firing is essential for nicotine [76,112] and ethanol [77] to exert their reinforcing properties. Nicotine activates dopamine cell bodies via nAChRs directly [74,76,112] and indirectly via glutamatergic [75] and GABAergic [76] inputs, resulting in an overall facilitation of burst firing. Similar to nicotine, ethanol elicits burst firing by activating nAChRs on dopamine cell bodies [113], although this occurs indirectly via facilitation of presynaptic cholinergic input. Ethanol also increases burst firing by elevating excitatory glutamatergic drive [77] via actions on presynaptic D1 dopamine receptors [78], and decreasing inhibitory GABAergic input [77] via actions on presynaptic opioid receptors [78]. A number of brain regions provide afferent control of dopamine neurons to regulate drug seeking and taking [110,114,115]. Well-established excitatory inputs originate from the lateral dorsal tegementum and pedunculopontine nucleus, which contribute both glutamatergic and cholinergic input, and the medial prefrontal cortex and lateral hypothalamus, which predominantly contribute glutamatergic input. Critical GABAergic inputs arise from the ventral pallidum, lateral habenula, bed nucleus of the stria terminalis, and rostromedial tegmental nucleus and from local interneurons. It should be noted, however, that the number of afferent regions regulating dopamine neurons appears to be much greater than previously thought [116]. Moreover, midbrain dopamine neurons are quite heterogeneous in terms of firing rate, autoregulatory control, and projection target [117,118]. Functional heterogeneity is additionally apparent in that anatomically distinct populations of dopamine neurons appear to encode either rewarding stimuli, aversive stimuli, or both [100,100,119]. It therefore appears that for abused drugs to reinforce behavior by generating burst firing of dopamine neurons, these substances must selectively activate sub-populations of dopamine neurons – specifically, the reward-encoding versus aversion-encoding neurons. While this appears to be the case at least for cocaine [100], the neural mechanisms that mediate this selective activation remain to be determined.
While consistent with elevated brain dopamine levels as a shared action of abused drugs, the traditional view of drug action does not account for the effects of addictive DAT-Is on phasic dopamine signaling. For example, cocaine augments the frequency, amplitude, and duration of dopamine transients [10,30,31,40]. Inhibition of uptake should mediate increased transient duration. However, it is difficult to reconcile the autoreceptor-mediated suppression of dopamine-cell firing with robust increases in transient frequency [30,31,40]. Moreover, increases in transient amplitude suggest actions besides inhibition of dopamine uptake [28,32]. An even more prominent discrepancy exists for AMPH. This psychostimulant should disrupt phasic dopamine signaling by depleting vesicular dopamine stores and impairing action potential-dependent exocytotic dopamine release according to its historic mechanism. Yet, genetic disruption of norepinephrine synthesis supports AMPH-induced afferent activation of dopamine neurons [41], and recent work with FSCV demonstrates that AMPH acts like cocaine and robustly increases both the frequency and amplitude of dopamine transients (Figure 1D) [42]. While bringing AMPH into the fold further supports hijacking of reward circuits by hyper-activating dopamine transients as a unifying hypothesis of abused drugs, it is clear that established psychostimulant pharmacology is inconsistent with this hypothesis. It is thus important to revisit the traditional view of drug action from the perspective of phasic dopamine signaling. As we describe in the next section, new evidence calls for a reclassification of addictive DAT-Is that is congruent with activation of dopamine transients (Figure 2B; Table 1).
Table 1. Reclassifying addictive DAT-Is: actions on dopamine neurons in addition to inhibiting dopamine uptake.
Actions of abused drugs on dopamine neurons: new view
a. Abused drugs generate burst firing by dopamine neurons
We argue here that, similar to other abused drugs, addictive DAT-Is generate burst firing by dopamine neurons. This postulate is supported by recent evidence demonstrating that cocaine activates burst firing by dopamine neurons in awake but suppresses firing in anesthetized animals [43]. Thus, suppression of dopamine-cell firing does not appear to be the dominate action of addictive DAT-Is in awake animals, indicating that other drug effects overcome inhibition by somatodendritic autoreceptors. In excellent agreement, several addictive DAT-Is, including cocaine, methylphenidate, AMPH and methamphetamine, robustly enhance bursting firing by dopamine neurons in anesthetized animals when administered in the presence of raclopride to block dopamine autoreceptors [44,45]. Co-administration of cocaine and raclopride also increases the frequency of dopamine transients in anesthetized animals [46], further linking these extracellular phasic signals to burst firing.
Diverse mechanisms potentially underlie the activation of burst firing by addictive DAT-Is. For example, cocaine and AMPH increase noradrenergic input, which activates dopamine neurons directly [47] or indirectly via glutamatergic afferents [44]. Additionally, cocaine-induced elevations in extracellular dopamine acting on D1 dopamine receptors may depolarize dopamine neurons directly [48], or indirectly by exciting glutamatergic [49] or inhibiting GABAergic [50] inputs. As DAT substrates, AMPH and methamphetamine could depolarize dopamine neurons directly during uptake [51,52]. Regardless of the cellular mechanism, cocaine and AMPH generating burst firing by dopamine neurons is consistent with these psychostimulants increasing the frequency of dopamine transients.
b. Sub-classes of abused drugs, including addictive DAT-Is, up-regulate vesicular dopamine release
We argue here that, similar to opiates and nicotine, addictive DAT-Is up-regulate vesicular dopamine release. This mechanism is consistent with these psychostimulants increasing the amplitude of dopamine transients and could also increase apparent transient frequency by raising transient amplitude above detection thresholds. Our postulate is supported by a large body of evidence encompassing several addictive DAT-Is, although cocaine is perhaps the best studied. Indeed, cocaine has been found to up-regulate vesicular dopamine release in several preparations, including brain-slice [53,54], anesthetized [55-57], and awake [58]. Up-regulation of dopamine release has more recently been extended to methylphenidate, a cocaine-like DAT-I [59], and surprisingly, even AMPH [42,55,56,60]. It should be emphasized that the evidence for up-regulated dopamine release by addictive DAT-Is is typically based on studies using a single dose administered non-contingently. Thus, this line of inquiry should be extended to repetitive dosing paradigms such as self-administration, especially for AMPH and methamphetamine, which have been demonstrated in brain slices to deplete vesicular dopamine stores in a dose-dependent fashion [61]. Further complicating this endeavor, however, is that extended access self-administration of methamphetamine is associated with a neurotoxic loss of markers for dopamine neurons [62].
Diverse mechanisms also potentially mediate the up-regulation of vesicular dopamine release by addictive DAT-Is. Cocaine and methylphenidate may mobilize the reserve dopamine pool through actions on synaptic proteins [54,57,59] and enhance both vesicular dopamine uptake and trafficking [63-67]. AMPH may similarly promote mobilization of the reserve pool [60] and vesicular trafficking [66] but also up-regulate vesicular dopamine release by distinct mechanisms. These include: (1) elevation of cytosolic dopamine levels by enhancing dopamine synthesis and inhibiting dopamine degradation [60], and selectively depleting the reserve pool [56]; (2) liberation of intracellular Ca2+ stores [68]; (3) enhancing presynaptic membrane excitability as a DAT substrate [51]. Similar mechanisms may apply to AMPH analogs, such as methamphetamine, which also enhances membrane excitability [52] and alters vesicular dopamine trafficking [64].
c. Inhibition of dopamine uptake is not the defining mechanism for addictive DAT-Is to activate phasic dopamine signaling
Cocaine augmenting the frequency, amplitude, and duration of dopamine transients was originally attributed to this psychostimulant inhibiting dopamine uptake [10,30,31,40]. In contrast, we argue here that inhibition of dopamine uptake is not the defining action for addictive DAT-Is to activate phasic dopamine signaling. This postulate is based on two lines of reasoning. First, as discussed above, addictive DAT-Is increase frequency and amplitude of dopamine transients by actions independent of inhibiting dopamine uptake [28,32]. In this regard, addictive DAT-Is resemble nicotine and ethanol, which increase both the frequency and amplitude of dopamine transients, but do not inhibit dopamine uptake or prolong transient duration [26,27]. Second, while inhibiting dopamine uptake prolongs transient duration due to the slowed extracellular clearance of dopamine, transient amplitude is relatively insensitive to uptake inhibition (Box 2). Thus, uptake inhibition may not necessarily lead to an increase in transient amplitude and, by virtue of surpassing detection thresholds, apparent frequency. In excellent agreement, the CB1 cannabinoid receptor antagonist, rimonabant, prevents the cocaine-induced increase in transient amplitude and frequency without altering the increase in transient duration due to uptake inhibition [26]. Inhibiting dopamine uptake is further questioned as a defining action by the demonstration that several DAT-Is with high affinity for DAT do not exhibit reinforcing properties [69,70].
Abused drugs augment extant dopamine transients and elicit dopamine transients de novo
The new view of drug mechanism proposed herein identifies two shared actions of abused drugs. This first common action is augmenting extant dopamine transients. These “ongoing” transients are evoked by natural rewards and their predictive cues or occur spontaneously. All three functional targets of abused drugs should contribute to the augmentation of extant dopamine transients. For example, up-regulation of vesicular dopamine release and inhibition of dopamine uptake would increase the amplitude and prolong the duration of dopamine transients, respectively. In addition, because ethanol and cannabinoids increase transient amplitude without up-regulating vesicular dopamine release or inhibiting dopamine uptake [25-27,71], drug-induced alterations in intra-burst properties (e.g., increase in number or frequency of action potentials) would also increase amplitude. Because larger dopamine transients evoked by food-predicting cues enhance the ability of these cues to promote food seeking [19], abused drugs augmenting extant dopamine transients should similarly drive ongoing appetitive behavior. Indeed, low-dose AMPH increases the amplitude and duration of dopamine transients evoked by cues predicting food reward [42] and enhances cue-driven food seeking [72]. It is interesting to speculate that these actions may also contribute to the efficacy of addictive DAT-Is as cognitive enhancers (see Outstanding Questions).
The second common action of abused drugs is eliciting dopamine transients de novo. As opposed to modifying extant transients, this drug action creates new transients. Considerable evidence supports the conclusion that, of the three functional targets, only generating burst firing by dopamine neurons elicits dopamine transients de novo. For example, genetic disruption of the NMDA receptor impairs both burst firing and dopamine transients [21], and selective optogenetic stimulation of dopamine neurons with burst patterns evokes transient-like signals [22]. Moreover, pharmacologically disrupting burst firing prevents the ability of cocaine, nicotine, ethanol, and cannabinoids to increase the frequency of dopamine transients [25,26,28,73]. Finally, ethanol and cannabinoids augment burst firing and dopamine transients without up-regulating dopamine release or inhibiting dopamine uptake [25-27,71,74-78]. Once elicited, other actions of abused drugs would enhance these now “extant” dopamine transients as described above, thereby producing an even more exaggerated drug response. Indeed, hyper-activation of dopamine transients by high-dose AMPH is so intense that it produces an effective pharmacological “deafferentation”, decoupling previously acquired cue-food reward associations and abolishing ongoing appetitive behavior [42].
Generating burst firing by dopamine neurons is the keystone action of abused drugs
We now bring forward and integrate key ideas developed in preceding sections to argue that generating burst firing is the keystone action of abused drugs. To begin, dopamine transients arise from burst firing by dopamine neurons, and are necessary and sufficient for predictive cues to form cue-reward associations and to promote reward seeking during appetitive behavior. To hijack this process, abused drugs must act robustly on dopamine neurons. For cues to promote drug seeking, abused drugs must also act similarly to natural rewards and elicit a dopamine transient that can transfer to the predictive cue. However, unlike natural rewards that are processed by sensory systems and afferent input to generate burst firing and elicit dopamine transients, abused drugs act centrally to activate dopamine neurons. Moreover, their effects are ultimately mediated by three functional targets on dopamine neurons: firing of action potentials, vesicular dopamine release, and dopamine uptake. Nevertheless, to mimic natural rewards and provide a dopamine transient for transferring to the predictive cue, abused drugs must elicit dopamine transients de novo. Of available functional targets on dopamine neurons, only burst firing achieves this essential outcome.
The theoretical argument that generating burst firing by dopamine neurons is the keystone action of abused drugs is thus surprisingly straightforward. This action also appears to meet principal empirical criteria to be deemed essential:
Necessary – all abused drugs generate burst firing by dopamine neurons.
Sufficient – ethanol and cannabinoids generate burst firing by dopamine neurons but do not up-regulate dopamine release or inhibit dopamine uptake.
Robust – abused drugs intensely increase burst firing by dopamine neurons and the frequency of dopamine transients. Additional effects of abused drugs to increase transient amplitude by up-regulating vesicular dopamine release and prolonging transient duration by inhibiting dopamine uptake are similarly robust and would further contribute to the augmentation of newly elicited dopamine transients. The robust activation of dopamine transients is thus consistent with a higher reward magnitude conferred to abused drugs compared to natural rewards and should result in cue-evoked transients with correspondingly greater amplitude [7,13,14]. Exaggerated cue-evoked dopamine transients would in turn increase the relative value ascribed to drug-associated cues and may mediate the powerful ability of conditioned stimuli to reinstate drug seeking and taking [39].
Reliable – generating burst firing by dopamine neurons faithfully elicits dopamine transients de novo. In contrast, up-regulating vesicular dopamine release and inhibiting dopamine uptake, while robust, are not reliable because these actions modify extant dopamine transients, which must be elicited independently. The reliable activation of dopamine transients even after the establishment of drug predicting cues as conditioned stimuli would be interpreted as a persistent positive prediction error that when coupled to robust activation, may act to “hyper-reinforce” behaviors preceding drug delivery [37,38].
The well-established ability of abused drugs to elicit long-term potentiation at excitatory glutamatergic synapses on dopamine neurons [79,80] may serve to enhance their ability to generate burst firing and thereby increase both the robustness and reliability by which dopamine transients are elicited.
Summary and Conclusions
On the basis of reclassifying addictive DAT-Is with an emphasis on phasic dopamine signaling, we have argued that generating burst firing of dopamine neurons is the keystone action of abused drugs. The essential outcome of this action is eliciting dopamine transients de novo. Reclassifying DAT-Is thus reconciles dopamine theories of appetitive behavior with a mechanistic understanding of how abused drugs hijack reward circuits, leading to an overlearning of cues predicting drug availability. Identifying this keystone action of abused drugs also targets burst firing by dopamine neurons as a potential therapeutic intervention. In support of this strategy, the CB1 cannabinoid receptor antagonist, rimonabant, which suppresses drug- and cue-evoked activation of dopamine transients via disrupting burst firing [26,81], shows promise in treating drug abuse [82,83]. We readily acknowledge substantive caveats in our argument. In particular, activation of dopamine transients has not been confirmed for all abused drugs, and particularly attention should be directed at other DAT-Is besides cocaine and AMPH, and the opiates, which can act independently of dopamine signaling [84]. Moreover, generating burst firing by dopamine neurons has also not been confirmed in awake animals for all abused drugs, and there is critical need for establishing this mechanism for the addictive DAT-Is. This is a not a simple task, however, because of difficulties with in vivo identification of dopamine units [85]. Therefore, FSCV and refined electrophysiological approaches will be instrumental in the future for further characterizing actions of abused drugs on dopamine neurons.
Outstanding Questions.
What mechanisms mediate clinical efficacy of DAT-Is?
DAT-Is are prescribed as cognitive enhancers for attention deficit hyperactivity disorder, traumatic brain injury, and drug abuse [120-123]. Whether activation of phasic dopamine signaling, as is described herein for cocaine and AMPH, contributes to clinical efficacy of DAT-Is is not known. The ability of AMPH to enhance associative learning [72] and augment dopamine transients [42] supports this possibility.
What is the role of tonic dopamine signaling in the actions of addictive DAT-Is?
Addictive DAT-Is robustly increase extracellular dopamine levels measured by microdialysis [1]. These results have been interpreted to reflect enhanced tonic dopamine signaling and could be mediated by addictive DAT-Is acting on vesicular dopamine release and dopamine uptake similar to phasic dopamine signaling and uniquely enhancing tonic firing by dopamine neurons. However, probe implantation damage limits quantifying these drug-induced increases [124], and other mechanisms besides tonic firing by dopamine neurons, such as glutamatergic input and drug-induced dopamine transients, may prominently contribute to basal dopamine levels [42,125,126].
Does DAT function as a dopamine “receptor” in drug reinforcement?
DAT-Is can induce conformational changes in DAT that are capable of triggering distinct downstream signaling events via a number of DAT-interacting proteins [70,127]. Similar to a transmembrane receptor, these actions may promote alterations in scaffolding proteins and intracellular second messenger pathways. It is not known whether actions of addictive DAT-Is other than inhibiting dopamine uptake, such as up-regulating vesicular dopamine release and activating burst firing of dopamine neurons, are mediated by DAT functioning as a transmembrane receptor.
What is the relationship between dopamine transients and synaptic plasticity?
Pulsatile changes in extracellular dopamine, such as the dynamics exhibited by dopamine transients, are thought to be critical for synaptic plasticity mediated by D1 dopamine receptors during reward learning [16,17]. However, precise relationships between attributes (e.g., frequency, amplitude, duration, and pattern) of dopamine transients and synaptic plasticity and between drug-induced activation of these phasic dopamine signals and enhanced synaptic plasticity have not been established.
What is the significance of addictive DAT-Is uniquely activating dopamine signaling?
Of all classes of abused drugs, only addictive DAT-Is activate phasic dopamine signaling by acting on all three functional drug targets of dopamine neurons: burst firing, vesicular dopamine release, and dopamine uptake. Moreover, DAT substrates, such as AMPH and methamphetamine, uniquely increase tonic dopamine signaling via action potential-independent dopamine efflux. However, it is not known what this unique activation of dopamine signaling confers to drug reinforcement.
How do subpopulations of dopamine neurons respond to acute drug exposure?
Figure 2 presents a summary of drug actions on dopamine neurons. However, in recent years it has become increasingly clear that midbrain dopamine neurons are a heterogeneous group of cells [128,129] that show diversity in terms of their electrophysiological properties and behavioral functions depending on their respective afferent inputs [9] and projection targets [117,118]. How abused drugs differentially affect these subpopulations of dopamine neurons, and how cell-specific actions support their acute and long-term behavioral effects remains to be elucidated.
Highlights.
Reward-related events/behaviors elicit phasic increases in dopamine (DA transients)
Abused drugs pharmacologically evoke DA transients and thus mimic natural rewards.
We propose a reclassification of addictive DA transporter inhibitors (DAT-Is)
Reclassification based on their ability to elicit DA transients via DA cell bursting
This bursting could be basis for initial reinforcement of drug-seeking/-taking
Acknowledgements
The authors gratefully thank the National Institute of Drug Abuse (Grants DA021770 and DA024036 to P.A.G. and DA025634 to M.F.R.) and National Science Foundation (Grant DBI0754615 to P.A.G.) for funding their research on amphetamine.
Glossary
- Dopamine transporter (DAT)
DAT is a plasma membrane protein that is a member of the SLC6 gene family of Na+/Cl--dependent transporters and terminates dopamine signaling by clearing extracellular dopamine released by exocytosis [92]. Uptake via DAT also contributes to recycling dopamine, as newly re-uptaken dopamine is repackaged into vesicles by the vesicular monoamine transporter for re-release. DAT-Is target DAT and have been traditionally thought to exert their effects on drug reinforcement by inhibiting dopamine uptake.
- Psychostimulant
The psychostimulants are a class of drugs that increase psychomotor activity and exhibit antidepressant effects, and act by increasing monoamine (e.g., dopamine, norepinephrine, and serotonin) levels in the brain [120]. Popular psychostimulants, such as cocaine, amphetamine, methamphetamine, and ecstasy, produce feelings of euphoria, relief from fatigue, improved performance on some simple tasks, and anorexia.
- Drug reinforcement
In general, reinforcement (positive or negative) refers to an increase in behavior directed toward a particular outcome [130]. Just like natural rewards, abused drugs promote positive reinforcement. Drug reinforcement is demonstrated by the paradigm of drug self-administration, in which delivery of abused drugs contingent on an instrumental response (e.g., lever pressing or nose poking) increases the likelihood that the response is made.
- Conditioned Stimulus
A conditioned stimulus (e.g., a tone or light) is predictively and temporally associated with an unconditioned stimulus (e.g., natural reward or abused drug). While initially neutral and evoking no innate response, after associative learning the conditioned stimulus evokes a conditioned response similar to that evoked by the unconditioned stimulus. Drug-conditioned stimuli come to elicit approach behavior and support the maintenance of drug taking. They also can act as conditioned reinforcers driving instrumental responding [20] and are critical for the reinstatement of drug seeking and taking even after long periods of abstinence [6].
- Fast-scan cyclic voltammetry (FSCV)
FSCV is an electrochemical, microsensor-based approach established for temporally, spatially, and chemically resolving neurochemicals in situ [10]. The typical microsensor is the carbon-fiber microelectrode (CFM). For dopamine measurements using FSCV in awake animals, the potential of the CFM is linearly scanned from −0.4 to 1.3 V and back at regular 10-Hz intervals. FSCV is so named, because the potential sweep is cyclical and made at high rates (e.g., 400 V/s). Dopamine is oxidized to dopamine-o-quinone at ~+0.65 V during the positive sweep, which is reduced back to dopamine at ~-0.2 V during the negative sweep. The relationship between applied potential and measured current, called a voltammogram, serves as a chemical signature to identify the detected species. Chemical specificity of FSCV is improved by chemometrics called principle component regression.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mogenson GJ, et al. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 1980;14:69–97. doi: 10.1016/0301-0082(80)90018-0. [DOI] [PubMed] [Google Scholar]
- 3.Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat. Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- 4.Luscher C, Ungless MA. The mechanistic classification of addictive drugs. PLoS. Med. 2006;3:e437. doi: 10.1371/journal.pmed.0030437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sulzer D. How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron. 2011;69:628–649. doi: 10.1016/j.neuron.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hyman SE, et al. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev. Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 7.Willuhn I, et al. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr. Top. Behav. Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dreyer JK, et al. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammel S, et al. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson DL, et al. Monitoring rapid chemical communication in the brain. Chem. Rev. 2008;108:2554–2584. doi: 10.1021/cr068081q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 12.Fiorillo CD, et al. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- 13.Tobler PN, et al. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- 14.Gan JO, et al. Dissociable cost and benefit encoding of future rewards by mesolimbic dopamine. Nat. Neurosci. 2010;13:25–27. doi: 10.1038/nn.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wanat MJ, et al. Delays conferred by escalating costs modulate dopamine release to rewards but not their predictors. J Neurosci. 2010;30:12020–12027. doi: 10.1523/JNEUROSCI.2691-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds JN, et al. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 17.Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–69. doi: 10.1016/j.tins.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Fiorillo CD. Two dimensions of value: dopamine neurons represent reward but not aversiveness. Science. 2013;341:546–549. doi: 10.1126/science.1238699. [DOI] [PubMed] [Google Scholar]
- 19.Flagel SB, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- 21.Zweifel LS, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. U. S. A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witten IB, et al. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wall VZ, et al. A behavioral genetics approach to understanding D1 receptor involvement in phasic dopamine signaling. Mol. Cell Neurosci. 2011;46:21–31. doi: 10.1016/j.mcn.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheer JF, et al. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheer JF, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson DL, et al. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin. Exp. Res. 2009;33:1187–1196. doi: 10.1111/j.1530-0277.2009.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aragona BJ, et al. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci. 2008;28:8821–8831. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robinson DL, et al. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuber GD, et al. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 31.Stuber GD, et al. Rapid dopamine signaling in the nucleus accumbens during contingent and noncontingent cocaine administration. Neuropsychopharmacology. 2005;30:853–863. doi: 10.1038/sj.npp.1300619. [DOI] [PubMed] [Google Scholar]
- 32.Porter-Stransky KA, et al. Cocaine must enter the brain to evoke unconditioned dopamine release within the nucleus accumbens shell. Neurosci Lett. 2011;504:13–17. doi: 10.1016/j.neulet.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Wise RA, Kiyatkin EA. Differentiating the rapid actions of cocaine. Nat. Rev. Neurosci. 2011;12:479–484. doi: 10.1038/nrn3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips PE, et al. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- 35.Aragona BJ, et al. Regional specificity in the real-time development of phasic dopamine transmission patterns during acquisition of a cue-cocaine association in rats. Eur. J Neurosci. 2009;30:1889–1899. doi: 10.1111/j.1460-9568.2009.07027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown HD, et al. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur. J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schultz W. Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron. 2011;69:603–617. doi: 10.1016/j.neuron.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Redish AD. Addiction as a computational process gone awry. Science. 2004;306:1944–1947. doi: 10.1126/science.1102384. [DOI] [PubMed] [Google Scholar]
- 39.Ciccocioppo R, et al. Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat. Neurosci. 2004;7:495–496. doi: 10.1038/nn1219. [DOI] [PubMed] [Google Scholar]
- 40.Wightman RM, et al. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur. J. Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- 41.Schank JR, et al. Dopamine beta-hydroxylase knockout mice have alterations in dopamine signaling and are hypersensitive to cocaine. Neuropsychopharmacology. 2006;31:2221–2230. doi: 10.1038/sj.npp.1301000. [DOI] [PubMed] [Google Scholar]
- 42.Daberkow DP, et al. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koulchitsky S, et al. Differential effects of cocaine on dopamine neuron firing in awake and anesthetized rats. Neuropsychopharmacology. 2012;37:1559–1571. doi: 10.1038/npp.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi WX, et al. Psychostimulants induce low-frequency oscillations in the firing activity of dopamine neurons. Neuropsychopharmacology. 2004;29:2160–2167. doi: 10.1038/sj.npp.1300534. [DOI] [PubMed] [Google Scholar]
- 45.Shi WX, et al. Dual effects of D-amphetamine on dopamine neurons mediated by dopamine and nondopamine receptors. J. Neurosci. 2000;20:3504–3511. doi: 10.1523/JNEUROSCI.20-09-03504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park J, et al. In vivo voltammetric monitoring of catecholamine release in subterritories of the nucleus accumbens shell. Neuroscience. 2010;169:132–142. doi: 10.1016/j.neuroscience.2010.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paladini CA, et al. Amphetamine selectively blocks inhibitory glutamate transmission in dopamine neurons. Nat. Neurosci. 2001;4:275–281. doi: 10.1038/85124. [DOI] [PubMed] [Google Scholar]
- 48.Brown MT, et al. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS. One. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–5388. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bocklisch C, et al. Cocaine Disinhibits Dopamine Neurons by Potentiation of GABA Transmission in the Ventral Tegmental Area. Science. 2013;341:1521–1525. doi: 10.1126/science.1237059. [DOI] [PubMed] [Google Scholar]
- 51.Ingram SL, et al. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat. Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 52.Branch SY, Beckstead MJ. Methamphetamine produces bidirectional, concentration-dependent effects on dopamine neuron excitability and dopamine-mediated synaptic currents. J. Neurophysiol. 2012;108:802–809. doi: 10.1152/jn.00094.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jones SR, et al. Different effects of cocaine and nomifensine on dopamine uptake in the caudate-putamen and nucleus accumbens. J. Pharmacol. Exp. Ther. 1995;274:396–403. [PubMed] [Google Scholar]
- 54.Kile BM, et al. Synapsins differentially control dopamine and serotonin release. J Neurosci. 2010;30:9762–9770. doi: 10.1523/JNEUROSCI.2071-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramsson ES, et al. High doses of amphetamine augment, rather than disrupt, exocytotic dopamine release in the dorsal and ventral striatum of the anesthetized rat. J Neurochem. 2011;119:1162–1172. doi: 10.1111/j.1471-4159.2011.07407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Covey DP, et al. Amphetamine elicits opposing actions on readily releasable and reserve pools for dopamine. PLoS. One. 2013 doi: 10.1371/journal.pone.0060763. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Venton BJ, et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J. Neurosci. 2006;26:3206–3209. doi: 10.1523/JNEUROSCI.4901-04.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oleson EB, et al. Real-time voltammetric detection of cocaine-induced dopamine changes in the striatum of freely moving mice. Neurosci. Lett. 2009;467:144–146. doi: 10.1016/j.neulet.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chadchankar H, et al. Methylphenidate modifies overflow and presynaptic compartmentalization of dopamine via an alpha-synuclein-dependent mechanism. J. Pharmacol. Exp. Ther. 2012;341:484–492. doi: 10.1124/jpet.111.189225. [DOI] [PubMed] [Google Scholar]
- 60.Avelar AJ, et al. Amphetamine augments vesicular dopamine release in the dorsal and ventral striatum through different mechanisms. J Neurochem. 2013 doi: 10.1111/jnc.12197. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.John CE, Jones SR. Voltammetric characterization of the effect of monoamine uptake inhibitors and releasers on dopamine and serotonin uptake in mouse caudate-putamen and substantia nigra slices. Neuropharmacology. 2007;52:1596–1605. doi: 10.1016/j.neuropharm.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krasnova IN, et al. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS. One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brown JM, et al. Cocaine-induced increases in vesicular dopamine uptake: role of dopamine receptors. J Pharmacol. Exp. Ther. 2001;298:1150–1153. [PubMed] [Google Scholar]
- 64.Sandoval V, et al. Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci. 2002;22:8705–8710. doi: 10.1523/JNEUROSCI.22-19-08705.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Volz TJ, et al. Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. J Pharmacol. Exp. Ther. 2007;323:738–745. doi: 10.1124/jpet.107.126888. [DOI] [PubMed] [Google Scholar]
- 66.Riddle EL, et al. Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur. J Pharmacol. 2007;571:25–28. doi: 10.1016/j.ejphar.2007.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Volz TJ, et al. Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and dopamine D2 receptors. J Pharmacol. Exp. Ther. 2008;327:161–167. doi: 10.1124/jpet.108.139386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mundorf ML, et al. Amine weak bases disrupt vesicular storage and promote exocytosis in chromaffin cells. J Neurochem. 1999;73:2397–2405. doi: 10.1046/j.1471-4159.1999.0732397.x. [DOI] [PubMed] [Google Scholar]
- 69.Rothman RB, et al. Dopamine transport inhibitors based on GBR12909 and benztropine as potential medications to treat cocaine addiction. Biochem. Pharmacol. 2008;75:2–16. doi: 10.1016/j.bcp.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmitt KC, et al. Non-classical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators and partial substrates. J. Pharmacol. Exp. Ther. 2013 doi: 10.1124/jpet.111.191056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones SR, et al. Effect of moderate ethanol dose on dopamine uptake in rat nucleus accumbens in vivo. Synapse. 2006;60:251–255. doi: 10.1002/syn.20294. [DOI] [PubMed] [Google Scholar]
- 72.Holden JM, Peoples LL. Effects of acute amphetamine exposure on two kinds of Pavlovian approach behavior. Behav. Brain Res. 2010;208:270–273. doi: 10.1016/j.bbr.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sombers LA, et al. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maskos U, et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. Nature. 2005;436:103–107. doi: 10.1038/nature03694. [DOI] [PubMed] [Google Scholar]
- 75.Mansvelder HD, et al. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 76.Tolu S, et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry. 2013;18:382–393. doi: 10.1038/mp.2012.83. [DOI] [PubMed] [Google Scholar]
- 77.Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int. Rev. Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao C, et al. Ethanol facilitates glutamatergic transmission to dopamine neurons in the ventral tegmental area. Neuropsychopharmacology. 2009;34:307–318. doi: 10.1038/npp.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ungless MA, et al. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 80.Saal D, et al. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 81.Oleson EB, et al. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–373. doi: 10.1016/j.neuron.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb. Perspect. Med. 2012:2. doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Badiani A, et al. Opiate versus psychostimulant addiction: the differences do matter. Nat. Rev. Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35:422–430. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schultz W, et al. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 87.Clark JJ, et al. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat. Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Melis M, et al. Different mechanisms for dopaminergic excitation induced by opiates and cannabinoids in the rat midbrain. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2000;24:993–1006. doi: 10.1016/s0278-5846(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 89.Turner TJ. Nicotine enhancement of dopamine release by a calcium-dependent increase in the size of the readily releasable pool of synaptic vesicles. J Neurosci. 2004;24:11328–11336. doi: 10.1523/JNEUROSCI.1559-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- 91.Britt JP, McGehee DS. Presynaptic opioid and nicotinic receptor modulation of dopamine overflow in the nucleus accumbens. J Neurosci. 2008;28:1672–1681. doi: 10.1523/JNEUROSCI.4275-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Torres GE, et al. Plasma membrane monoamine transporters: structure, regulation and function. Nat. Rev. Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 93.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Everitt BJ, et al. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond B Biol. Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nestler EJ. Is there a common molecular pathway for addiction? Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 97.Pontieri FE, et al. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 99.Di CG, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007;7:69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 100.Lammel S, et al. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr. Opin. Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 102.Luscher C. Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb. Perspect. Med. 2013;3:a012013. doi: 10.1101/cshperspect.a012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 104.Haber SN, et al. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–2382. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Willuhn I, et al. Hierarchical recruitment of phasic dopamine signaling in the striatum during the progression of cocaine use. Proc. Natl. Acad. Sci. U. S. A. 2012;109:20703–20708. doi: 10.1073/pnas.1213460109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine-dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–441. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 107.Ito R, Hayen A. Opposing roles of nucleus accumbens core and shell dopamine in the modulation of limbic information processing. J Neurosci. 2011;31:6001–6007. doi: 10.1523/JNEUROSCI.6588-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saunders BT, et al. Cue-evoked cocaine “craving”: role of dopamine in the accumbens core. J Neurosci. 2013;33:13989–14000. doi: 10.1523/JNEUROSCI.0450-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 110.Ikemoto S, Bonci A. Neurocircuitry of drug reward. Neuropharmacology. 2014;76(Pt B):329–341. doi: 10.1016/j.neuropharm.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Howard CD, et al. Methamphetamine-induced neurotoxicity disrupts naturally occurring phasic dopamine signaling. Eur. J Neurosci. 2013;38:2078–2088. doi: 10.1111/ejn.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pons S, et al. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu L, et al. Nicotinic acetylcholine receptors containing the alpha6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons. Biochem. Pharmacol. 2013;86:1194–1200. doi: 10.1016/j.bcp.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stuber GD, et al. Optogenetic modulation of neural circuits that underlie reward seeking. Biol. Psychiatry. 2012;71:1061–1067. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jentsch JD, Pennington ZT. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology. 2014;76(Pt B):479–486. doi: 10.1016/j.neuropharm.2013.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Watabe-Uchida M, et al. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 117.Margolis EB, et al. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 119.Volman SF, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–17576. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem. Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 121.Sofuoglu M, et al. Pharmacological treatment of comorbid PTSD and substance use disorder: Recent progress. Addict. Behav. 2013;39(2):428–33. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Berridge CW, Devilbiss DM. Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2011;69:e101–e111. doi: 10.1016/j.biopsych.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bales JW, et al. Persistent cognitive dysfunction after traumatic brain injury: A dopamine hypothesis. Neurosci. Biobehav. Rev. 2009;33:981–1003. doi: 10.1016/j.neubiorev.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Borland LM, et al. Voltammetric study of extracellular dopamine near microdialysis probes acutely implanted in the striatum of the anesthetized rat. J Neurosci Methods. 2005;146:149–158. doi: 10.1016/j.jneumeth.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 125.Kulagina NV, et al. Glutamate regulates the spontaneous and evoked release of dopamine in the rat striatum. Neuroscience. 2001;102:121–128. doi: 10.1016/s0306-4522(00)00480-2. [DOI] [PubMed] [Google Scholar]
- 126.Owesson-White CA, et al. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Eriksen J, et al. Regulation of dopamine transporter function by protein-protein interactions: new discoveries and methodological challenges. J Neurochem. 2010;113:27–41. doi: 10.1111/j.1471-4159.2010.06599.x. [DOI] [PubMed] [Google Scholar]
- 128.Roeper J. Dissecting the diversity of midbrain dopamine neurons. Trends Neurosci. 2013;36:336–342. doi: 10.1016/j.tins.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 129.Bromberg-Martin ES, et al. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology. (Bethesda.) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]