Abstract
We studied the effects of alemtuzumab on T-regulatory cells (Tregs) during alloactivation, first by differences in depletion of various naive versus alloactivated cell subsets in peripheral blood of healthy volunteers, then by adding serial concentrations to human leukocyte antigen (HLA)–DR–matched and –mismatched responding and stimulating cells in mixed lymphocyte reaction (MLR). Lymphoproliferation inhibition and the development of proliferating carboxyfluorescein succinimidyl ester (CFSE)–diluted CD4+CD25highCD127−FOXP3+ (phenotypic) Tregs by flow cytometry were measured. Also, the ability of alemtuzumab-treated versus nontreated MLR generated CD4+CD127− cells to allospecifically inhibit MLRs and recruit additional responding Tregs was tested. We found a more pronounced refractoriness of alloactivated versus naive CD4+CD25high cells to alemtuzumab induced lymphodepletion. Alemtuzumab dose dependently inhibited lymphoproliferation while amplifying percentages of MLR-generated Tregs. This was somewhat augmented by human complement addition. CD127−CD4+ cells immunoselected after 7 days from alemtuzumab-treated MLRs allospecifically inhibited lymphoproliferation and recruited additional Tregs in fresh MLR-responding cells, similar to modulators derived from MLRs without drug addition (media). Addition of tacrolimus and sirolimus to alemtuzumab further inhibited MLR proliferation. However, Treg percentages were markedly higher with sirolimus. These results support the notion that alemtuzumab induces immunoregulation in naïve T cells undergoing alloactivation absent presensitization, especially used in conjunction with maintenance SRL.
Keywords: Mixed lymphocyte reaction, Regulatory T cells, FOXP3, Alemtuzumab, mTOR inhibition
1. Introduction
Induction therapy with alemtuzumab (AL) the humanized monoclonal antibody to the pan-lymphocyte CD52 molecule is selectively practiced in solid organ transplantation primarily to deplete naive T cells [1]. This may decrease the rate of acute rejection and facilitate lower doses of maintenance agents, such as calcineurin inhibitors (CNI; tacrolimus [TAC]) or mTOR inhibitors (sirolimus [SRL]). Thereby, minimal immunosuppression (IS) therapy (prope tolerance) might eventually occur [2–5]. Related to this is the proposed development of immunoregulation [6]. We have noted such a profile in human leukocyte antigen (HLA)–identical renal transplant recipients totally withdrawn from IS when given AL among other agents, all proposed to induce immunoregulation [7]. Mechanisms of AL-induced immunoregulation might be assessed by ex vivo immunophenotyping and functional assays, testing recipients treated with AL undergoing alloactivation after organ transplantation. Although treatment with heterologous polyclonal antibodies, such as thymoglobulin has been reported to have similar effects, the mechanisms are more complex because of multiple epitope binding [8]. We therefore questioned whether, during in vitro alloimmune responses i.e., mixed lymphocyte reactions (MLR), AL would influence the development of CD4+ PBMC with a regulatory CD4+CD127−CD25highFOXP3+ phenotype.
In the present report, we have tested whether these phenotypic T regulatory cells (Tregs) would be as functionally regulatory in vitro [2,9] as those we have described in the absence of AL [10]. In addition, although we and others have reported clinical and ex vivo data on maintenance IS agents favoring higher Treg generation with SRL versus calcineurin inhibitors [11,12], it was questioned what the additional influence of AL would be with these maintenance agents in vitro on this Treg subset. Consequently, we now report on (1) a proposed mechanism of the regulatory effects in MLR unique to AL, and (2) the contrasting effects in MLR of AL in combination with TAC versus SRL. Although some of these effects have been proposed previously, the detection methods described in this report might serve to clarify how the agent exerts a prolonged immunoregulatory effect.
2. Subjects and methods
2.1. Human subjects and HLA typing
Peripheral blood mononuclear cells (PBMC) were obtained from healthy volunteers who were human leukocyte antigen (HLA) typed by the Northwestern HLA laboratory using molecular methods (reverse sequence specific oligonucleotide probe hybridization), and selected as HLA 2 DR matched or mismatched. In some volunteers, panel reactive antibody testing by Luminex was performed to assess for nonreactivity. The research was conducted in human subjects with institutional review board approval after obtaining written consent. Some volunteers were tested in laboratories at the University of Miami with approval similar to that of the institutional review board and written consent.
2.2. Assessment of susceptibility to AL treatment
Bulk cultures of 20 × 106 responding PBMC stimulated with 20 × 106 HLA-mismatched allogeneic x-irradiated stimulating cells (3000 rad) in normal AB serum containing culture medium (NAB-CM) consisting of RPMI 1640 with 15% heat-inactivated normal human AB serum (Gemini Bio-Products, Sacramento, CA), 2 mmol/l l-glutamine, 10 mmol/l HEPES, and 100 U/ml penicillin–streptomycin (all from Mediatech, Manassas, VA) were prepared in 40-ml volumes in T75 flasks. After 7 days, these were harvested, washed, and counted. One million of these cultured cells versus freshly obtained unstimulated control PBMC from the same subjects were incubated without or with 20 μg/ml AL in 15% NAB-CM media with or without rabbit complement (One Lambda, Canoga Park, CA) for 24 hours. Cells surviving treatment were counted by Trypan Blue dye exclusion and then tested in four-color flow cytometry in multiple tubes to quantitate cell subsets as described elsewhere [13,14] and below.
2.2.1. Flow cytometry in MLR responses
The antibodies used were as follows: (1) fluorescein isothiocyanate (FITC)–conjugated anti-CD45, CD4, CD52, TcR-αβ, and CD80; (2) phycoerythrin (PE)–conjugated anti-CD45RA, CD8, CD154, and CD86; (3) electron coupled dye (ECD) conjugated anti-CD45RO, CD3, and CD14; and (4) peridinin chlorophyll protein (Percp)–conjugated anti-CD19, CD25, HLA-DR, CD16, CD56, and CD83 (all antibodies were obtained from either Beckman-Coulter, Miami, FL, or Becton-Dickinson, San Jose, CA, with the exception of anti-CD52 which was from Serotec, Raleigh, NC). The negative population(s) were established with isotype controls on the lymphocyte–monocyte gate. The data were analyzed using the “prism” gating for the four fluorochromes, and the values were expressed as percentage positive cells or were calculated as absolute number of cells.
2.3. Treg-MLR
2.3.1. Materials, media, phytohemagglutinin, and MLR culture conditions
These were as previously described [10].
2.3.2. Immunosuppression agent additives
The soluble formulation of AL (Campath1H; 30 mg/ml; Genzyme, Cambridge, MA;) was diluted in NAB-CM to 10 or 100 μg/ml, and then five- or 10-fold descending serial dilutions were used in each experiment. In MLRs testing the effects of TAC or SRL, 5–10 ng/ml (i.e., clinically therapeutic trough levels) of these agents, either alone or in combination with varying concentrations of AL were added at the culture outset. These were also compared to cultures in which the same volume of complete media (no drug) was added.
2.3.3. MLR Treg generation with and without complement
These assays were performed with serum obtained from AB blood group male volunteer donors who did not have panel reactive antibodies by Luminex platform testing. In these MLRs, the PBMC of these individuals were used as responders in 15% autologous serum as the medium supplement. The sera were maintained at ~4°C and were either not “decomplemented” or heat inactivated at 56°C.
2.3.4. Assessment of proliferation
This was measured using both CFSE labeling and flow cytometry (as below) for cell counts and by standard 3H-TdR incorporation assays. For the latter, 1 × 105 responding PBMC from healthy volunteer (A) were cultured with 1 × 105 irradiated (3000 rad) stimulator cells from HLA-2 DR matched (Bx) or mismatched (Ix) laboratory volunteers in 96-well U-bottomed culture plates in NAB-CM in triplicate, as previously described [10]. On days 5, 7, and 9, 1 μCi 3H-TdR was added to each well and, after 18 hours were processed using a Tomtec cell harvester (Hamden, CT). Radioactive incorporation was measured using a Packard-Beta counter (Meriden, CT). The SI was calculated using the following formula:
Inhibition was calculated using the following formula:
2.3.5. MLR flow cytometry, MLR inhibition and Treg recruitment
Flow cytometry for MLR Tregs was carried out as follows: 5 × 105 CFSE labeled responding PBMC from healthy volunteer (A) were cultured with 5 × 105 irradiated PKH26 labeled stimulator cells from HLA-2 DR matched (Bx) or mismatched (Ix) laboratory volunteers in 48-well culture plates. On days 5, 7, and 9, flowcytometric analyses were performed for the surface markers CD4-ECD, CD25-PC7, and CD127-PE (all from Beckman-Coulter, Miami, FL) and for intracellular FOXP3-PC5 (eBiosciences, San Diego, CA) as previously described [10,15]. Viable lymphocytes were gated followed by CFSE bright and dim cells which were negative for both CD127-PE and PKH26 (thus gating out CD127+ responders and any residual stimulators). This was followed by gating for CD4+ cells. CFSE dilution in these CD4+ responders assessed the extent of proliferation, i.e., nonproliferating (CFSE high) or proliferating (CFSE low) cells. The expression of CD25 and FOXP3 were analyzed in the nonproliferating and proliferating populations. The percentages of Tregs were defined by flow cytometry staining as CD4+CD25highCD127−FOXP3+ [10].
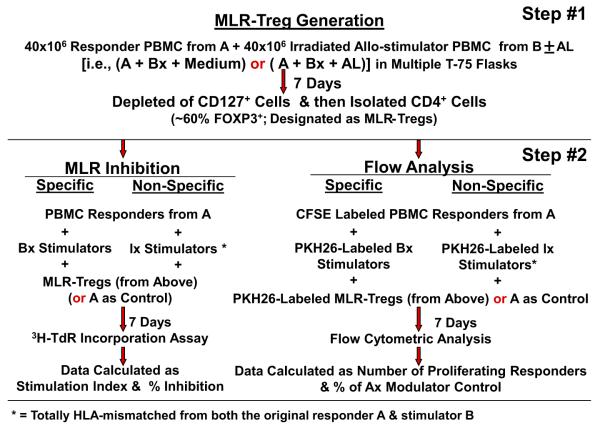
MLR Treg inhibition and in vitro recruitment were examined via bulk culture with AL versus media controls and immunoselection. These methods, also previously described [10], are summarized by a flow diagram (Fig. 1). Bulk cultures of responder PBMC from volunteer A were stimulated with 2 DR-matched x-irradiated cells from volunteer B either in the presence of no drugs (A+Bx+ Medium) or 1 μg/ml AL (A+Bx+AL) for 7 days. CD127+ cells were then depleted using CD127 microbeads, and CD4+ cells were enriched using CD4− microbeads following the manufacturer’s instructions (Miltenyi Biotech, Auburn, CA). The expression of CD25 and FOXP3 in these purified CD127−CD4+ cells was assessed by flow cytometry as above. The FOXP3+ percentage of the cells selected in this manner was 50% ± 5%. These were then used as modulator cells in read out MLR Treg recruitment/inhibition assays as described below. For the MLR inhibition readout, standard 3H thymidine incorporation assays were performed with 1 × 105 each of the responders and stimulators in the presence of indicated numbers of third component modulator CD127−CD4+ cells selected from the above-mentioned bulk cultures or by using fresh A-PBMC as control modulators. The stimulation indexes (SI) were calculated as above and percentage inhibition was calculated using the following formula:
Fig. 1.
Flow diagram (see Subjects and methods).
For the recruitment readout, in parallel, 5 × 105 CFSE labeled responding fresh PBMC from the original healthy volunteer A were cultured with 5 × 105 irradiated PKH26-labeled stimulator cells from the original healthy volunteer (Bx) versus an indifferent nonspecific HLA-mismatched volunteer (Ix), in the presence of the indicated numbers of PKH26-labeled third component CD127−CD4+ modulators. On days 5, 7, and 9, flow-cytometric analyses were performed using the scheme described above (including the gating out of the PKH26 positive modulators and residual stimulators as well as the CD127+ responders). Data were calculated as percentage increase in CD4+CD25highFOXP3+ responder cell generation using the following formula:
To avoid variability between the magnitudes of responses of individual MLRs, in most experiments the percent change in inhibition and in in vitro recruitment from baseline (no drug) using fresh A modulator controls was also calculated.
2.4. Statistical methods
Descriptive statistics (mean, standard deviation) and graphical methods were used to record the SI and percent Tregs in each MLR. Comparisons between the peak SI and percent Treg, including the percent difference between the MLR with and without the various IS agent dilutions, were performed using the Student t tests and Wilcoxon signed rank tests. p values of ≤ 0.05 were considered statistically significant. Analyses were performed using SAS 9.2 statistical software (SAS Institute, Cary, NC).
3. Results
3.1. Cells that survived AL treatment
To detect and compare the effects of AL in vitro on the survival of alloactivated versus nonactivated cells, first freshly obtained (nonalloactivated) PBMC were treated for 24 hours with therapeutic range concentrations of AL (20 μg/ml) in the presence of complement. It was observed that only 6.6% ± 0.7% of the total number of cells survived the treatment overall (vs 100% with no AL treatment, i.e., medium controls). This is depicted for non-alloactivated cells in Table 1A, top of left columns (Total). Among the surviving cells were CD4+CD25high, CD16/56+ (NK and NK-T), and CD14+, CD83+ or CD80/86+ (antigen presenting) cells (Table 1, upper portion in remaining left columns [Surviving Subsets]). These non-alloactivated cells that survived were able to mount only marginal proliferative responses to an allogeneic and mitogenic stimulus (Table 1B, lower left columns).
Table 1.
Differential effect of alemtuzumab (AL) on the survival and functions of fresh vs activated PBMC (n = 5)
| Fresh PBMCa |
Alloactivated PBMCa |
|||
|---|---|---|---|---|
| No alemtuzumab | Alemtuzumab treated | No alemtuzumab | Alemtuzumab treated | |
| A: Mean ± SD absolute numbers (or percentages) of cells that survive AL treatment, if 1 × 106 were treated | ||||
| Survival (total) | 100% | 6.6 ± 0.7%c | 100% | 17.6 ± 3%c |
| Surviving subsets | ||||
| CD45+ | 503,193 ± 36,010 | 14,982 ± 3325 (3.0 ± 0.7%)c | 348,583 ± 68,648 | 55,853 ± 11,306 (16 ± 2%)c |
| CD45+ 19+ | 43,396 ± 9965 | 300 ± 58 (0.7 ± 0.3%)c | 14,596 ± 9687 | 2314 ± 1840 (17 ± 5%)c |
| CD3+ | 360,768 ± 11,522 | 1337 ± 548 (0.4 ± 0.1%)c | 263,547 ± 26,476 | 34,174 ± 7269 (13 ± 4%)c |
| CD4+ 25high | 8761 ± 1495 | 287 ± 134 (3.2 ± 1.2%)c | 19,101 ± 11,071 | 5302 ± 2751 (29 ± 11%)c |
| CD3+ CD16/56+ | 4659 ± 2942 | 96 ± 60 (2.1 ± 0.5%)c | 10,720 ± 3693 | 2366 ± 1129 (22 ± 11%)c |
| CD8+ CD16/56+ | 47,607 ± 27,666 | 3462 ± 1172 (8.7 ± 4.7%)c | 25,107 ± 24,729 | 6749 ± 6688 (29 ± 8%)c |
| CD83+ | 21,180 ± 13,949 | 2390 ± 2241 (9.7 ± 4.1%)c | 6959 ± 4005 | 1383 ± 1001 (19 ± 5%)c |
| CD14+ | 2229 ± 1558 | 205 ± 89 (12 ± 6.9%)c | 7964 ± 4023 | 1233 ± 934 (17 ± 8%)c |
| CD80+ 86+ | 3325 ± 556 | 435 ± 19 (13 ± 1.9%)c | 17,400 ± 7333 | 5290 ± 2154 (32 ± 10%)c |
| CD3+ 52high b | 197,934 ± 31,441 | 15 ± 15 (0.0 ± 0.0%)c | 51,609 ± 17,517 | 654 ± 342 (1.5 ± 1.3%)c |
| CD3+ 52med b | 147,664 ± 30,445 | 77 ± 83 (0.0 ± 0.0%)c | 148,877 ± 11,518 | 4086 ± 1955 (2.7 ± 1.3%)c |
| CD3+ 52low b | 32,138 ± 15,871 | 254 ± 227 (0.8 ± 0.5%)c | 53,259 ± 27,152 | 19,071 ± 5971 (45 ± 25%)c |
| B: Proliferative responses to PHA and to allostimulation by cells that survive AL treatment (SI; mean ± SD) | ||||
| PHA (3-day) | 71.2 ± 42.2 | 4.3 ± 2.1** | 26.1 ± 24.3 | 12.1 ± 10.6* |
| MLR (3-day)d | 2.1 ± 0.6 | 1.7 ± 0.8 | 14.8 ± 11.6 | 13.3 ± 13.0 |
| MLR (7-day)d | 38.4 ± 19.0 | 3.2 ± 1.5** | 6.3 ± 8.0 | 1.9 ± 0.9 |
Cells were cultured with allogeneic stimulators for 7 days. Then they were either treated or not treated with AL for 24 hours before analyses (right columns). These were compared with fresh cells from the same donor simultaneously treated or not treated with AL for 24 hours.
CD52 is the target receptor for AL.
Percentage of the total or specific subset of cells that survived AL treatment (100% is the comparison to which the AL treated cells were analyzed, i.e., a normalized untreated denominator).
Against the same stimulator as the one used for producing alloactivated PBMC; *p < 0.05 and **p < 0.001 vs respective “No AL” combination
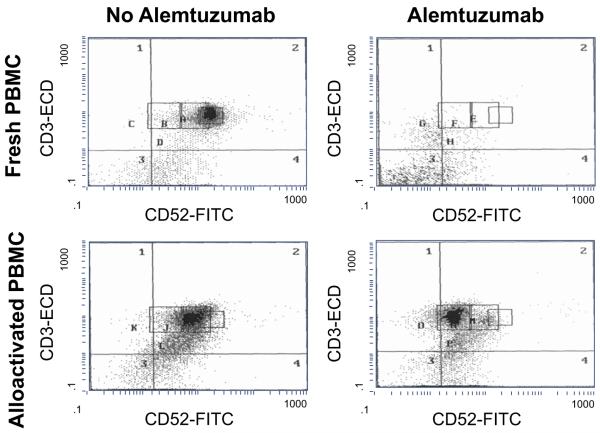
To contrast effects of AL on alloactivated cells, such cells already stimulated in 7 day MLR were treated for 24 hours with AL in the presence of complement, as depicted in Table 1, upper portion, right columns (in parallel with the similar experiments on fresh (naive) cells described above). Compared to fresh PBMC, higher percentages of alloactivated PBMC survived AL treatment, i.e., 17.6% alloactivated versus 6.6% fresh unactivated (Table 1, upper portion, total). As in fresh non-alloactivated PBMC, the surviving cell subsets included CD4+CD25high, CD16/56+, and CD80/86+ cells that were refractory to AL treatment, but these subsets persisted in even higher percentages (p < 0.01). Analysis of the density of the receptor for AL (CD52) on CD3+ T cells indicated that allostimulation decreased CD52 expression and that the cells that survived AL treatment were those with lower CD52 density (Fig. 2, Table 1, upper portion). Of note is that, unlike naive cells, the alloactivated PBMC were able to respond to both a phytohemagglutinin mitogenic stimulus as well as specific allogeneic restimulation in the 3-day primed lymphocyte reaction (Table 1B, lower portion). These results suggested that, in contrast to naive T cells, alloactivated T cells are relatively resistant to AL mediated lymphodepletion retaining their capacity to mount a secondary immune response (p < 0.01).
Fig. 2.
Survival after alemtuzumab treatment of CD52+ cell subsets in fresh versus allostimulated PBMC. PBMC from a healthy volunteer were stimulated with irradiated allogeneic PBMC from another volunteer in 15% NAB-RPMI culture medium for 7 days. After washing to remove cellular debris, they were either treated or not treated with AL in the presence of 15% complement containing medium for another 24 hours at 37°C in 5% CO2. As controls, freshly obtained non-alloactivated PBMC from the same volunteer were also treated or not treated with AL. Flow cytometry was then performed with fluorochrome-labeled-monoclonal antibodies. The figure represents one of the five separate experiments with similar findings in that cells that survive AL treatment are those with low CD52 expression.
3.2. AL in the MLR
Our previous work had demonstrated that Tregs capable of potent allospecific inhibition can be generated during MLRs [10], mediated by the recruitment of additional Tregs in vitro. It was now questioned whether AL would increase the percentages of CD4+CD25highFOXP3+CD127− cells generated during allostimulation, and whether these cells would have functional regulatory characteristics.
3.2.1. AL inhibits lymphoproliferation and generates Tregs in MLR
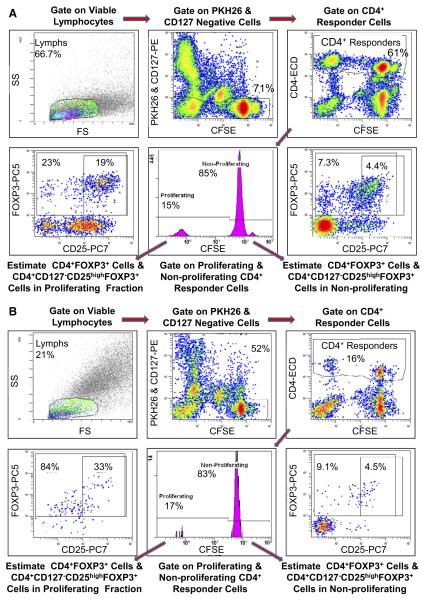
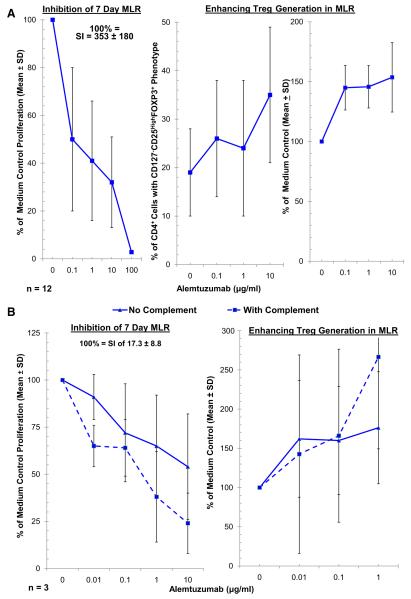
Increasing concentrations of AL (0, 0.1, 1, 10, 100 μg/ml) were tested in MLRs using PBMC of healthy volunteers. Figure 3 depicts flow cytometry density plots of a typical experiment. Compared to Fig. 3A in which no drug but only media was added, Fig. 3B shows that a significant increase in the percentage Tregs occurred in the proliferating (CFSE dim) fraction with AL addition, despite a marked decrease in overall cell numbers. This could be demonstrated more quantitatively in graphic form in Fig. 4 the means and SDs for 12 separate experiments, that is 12 sets of responder versus 2 DR matched and mismatched stimulators. Figure 4A (left panel) demonstrates a stepwise inhibition in lymphoproliferation as measured by SI comparing media controls with increasing AL concentrations (p < 0.05, n = 12, i.e., A+Bx and A+Ix combinations). Figure 4A (middle and right panels) demonstrates a significant increase in % Treg that is markedly above the baseline medium control in AL-spiked MLRs (p < 0.05). This is more clearly indicated when percent change from media (no AL added) controls is calculated (right panel). This is rather than using percentages of Tregs generated from individual experiments because of the variability in individual proliferative responses (middle panel). The use of serum containing human complement to more closely simulate in vivo conditions appeared to slightly increase lymphoproliferative inhibition, especially at the highest AL concentration (Fig. 4B, left panel; p < 0.05). However, Treg generation appeared relatively unaffected, although again with some amplification at the highest AL concentration (Fig. 4B, right panel; p = 0.038). In these and the subsequent experiments only 2 DR matched combinations were tested.
Fig. 3.
Assessment of lymphoproliferative inhibitory effects and Treg enhancing effects of AL in MLR. (A) Scheme of flow analysis in medium control (representative 7-day experiment shown): 5 × 105 CFSE labeled responding PBMC from healthy volunteer A were cultured with 5 × 105 PKH26 labeled stimulator cells from an HLA- 2 DR matched laboratory volunteer B and flow-cytometric analysis was performed on day 7. Viable lymphocytes were gated followed by CFSE bright and dim cells which were negative for both CD127-phycoerythrin (PE) and PKH26 (thus gating out CD127+ responders and any residual stimulators). This was followed by gating for CD4+ cells. These CD4+ responders were then further gated to either nonproliferating (CFSE high) or proliferating (CFSE low) cells. The cells in these two populations were analyzed by dot plots for various subsets, including CD25+ and FOXP3+ cells. (B) Representative 7-day experiment with Alemtuzumab: Experimental scheme similar to that in in Fig. 3A, but in the presence of 1 μg/ml AL. Note that when compared to the medium control, fewer CD4+ cells survived the AL treatment confirming the results shown in Table 1 and Fig. 2. However, the total CD4+CD127−FOXP3+ cells (84%) and the CD4+CD127−CD25highFOXP3+ Tregs (33%) were the predominant populations in these proliferating cells (bottom left). Since only the CFSE diluted proliferating fraction demonstrated differences under various culture conditions, the results from only these are shown in subsequent experiments. Also, despite the relatively few cells remaining after AL addition, dot plots in each of the 12 such MLRs (as in Fig. 3) showed equivalent patterns of enhanced CD25highFOXP3+ expressing cells, comparing the lower left points on the bottom of the Fig. B with the lower left points on the top (A) (p < 0.05).
Fig. 4.
Cumulative assessment of AL lymphoproliferative inhibitory and Treg expansion in MLR. (A) Means and SDs of 12 experiments in which 5 ×105 CFSE labeled responding PBMC from healthy volunteer A were cultured with 5 × 105 PKH26 labeled x-irradiated stimulator cells from HLA-2 DR matched and mismatched healthy volunteers (Bx or Ix, respectively) in the presence of the indicated concentrations of AL. On days 5, 7, and 9 flow-cytometric analysis using the scheme shown in Fig. 3 was performed. In parallel, standard 3H thymidine incorporation assays were performed with 1 × 105 PBMC each of the responders and stimulators. The results from peak responses on day 7 are depicted. AL inhibited the proliferation in 3H-thymidine incorporation assay (left panel), but conversely induced a higher percentage of CD4+CD127−CD25highFOXP3+ Tregs (middle and right panels) in a dose dependent manner (p < 0.05, n = 12). To minimize the variations between experiments, the data for Tregs were also calculated as percentage of medium control (right panel). In subsequent experiments the data are shown in this manner (as percentage of the media control). (B) Effect of AL in the presence of complement. Since AL mediates much of leukocyte depletion through complement mediated lysis in vivo, MLRs were also performed in presence of complement. Both 3H-TdR incorporation assays and flow-cytometric analyses were performed as described in Fig. 4A, but with 15% serum autologous to the responder (male healthy donors with AB blood group), instead of commercial (heat inactivated/“decomplemented”) normal AB serum supplements. In the cultures with “no complement” the autologous serum samples were heat inactivated at 56°C, and “with complement” were maintained at 4°C after the initial serum preparation to preserve the complement components. The presence of complement had an increasing effect on the inhibition of proliferation by AL in the highest concentration (left), and appeared to increase the percentages of Tregs in the highest concentration (right), i.e., 1 μg/ml (p = 0.038).
3.2.2. CD127−CD4+ cells derived from MLRs with or without AL significantly regulate reactivity in fresh MLR readouts by allospecific lymphoproliferative inhibition and in vitro recruitment
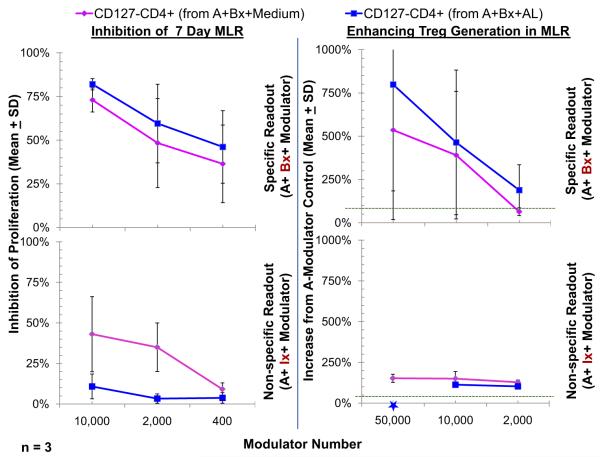
Since we previously demonstrated that CD4+CD127− cells derived from 7-day MLRs caused immunoregulatory effects in fresh allospecific MLR readouts [10] it was questioned whether these effects would be more or less marked with cells from MLRs spiked with AL. For this, CD127−CD25+ cells were immunoselected from bulk 7-day MLRs of responder cells from individual A stimulated with irradiated PBMC from individual B cultured with or without AL. As depicted in Fig. 5 (top left), when added as third component modulators, such immunoselected CD127−CD25+ cells inhibited the lymphoproliferation in fresh MLR readouts of A-PBMC stimulated with irradiated Bx-PBMC in a dose dependent manner (Bx being the original stimulator). This occurred equivalently with modulator CD127−CD25+ cells from cultures with or without AL, each inhibitory effect being equivalently more profound than with fresh control A modulators, the 100% baseline shown in the figure (see Subjects and methods). Such increased lymphoproliferative inhibition in the read out MLRs was also accompanied by the generation of additional CD4+CD127−CD25highFOXP3+ cells (Tregs) in the proliferating A responder cells over that generated using control fresh A PBMC modulators, i.e., the latter, as mentioned being the 100% baseline depicted in Fig. 5 top right panel (p < 0.02 at 50,000 and 10,000 modulator cells). This in vitro recruitment of more responding cells to become Tregs was only marginally more pronounced (statistically not significant) in the read out cultures in which the 3rd component modulator CD127−CD25+ cells were generated in the presence of AL (Fig. 5, top right). However, if the readouts were performed using A responders but nonspecific HLA-mismatched third-party (indifferent) stimulators (Ix), the in vitro inhibition/recruitment was much less marked (Fig. 5, bottom) and differed significantly from the allospecific in vitro inhibition/recruitment seen with the original stimulators (Bx) (p < 0.05). Taken together, these results indicated an antigen specific amplification of immunoregulation in vitro by modulator cells taken from bulk MLRs that was equivalent with or without AL spiking. Speculatively, the magnitude of the effect of immunoselected modulator cells from AL spiked MLRs over that of modulator cells from non-AL spiked MLR media controls may have been dampened by the possible persistence of small numbers of contaminating effector cells also refractory to AL in the immunoselected third component modulators, as previously depicted in Table 1 and Fig. 2. That is, the refractoriness of both alloactivated effector and regulatory cells to AL in MLR may have resulted in a competitive effect between them to undermine the overall enhanced in vitro inhibition/recruitment, over media controls that might have occurred testing AL. This enhanced in vitro inhibition/recruitment had been described in our previous reports testing SRL [15].
Fig. 5.
Assessment of CD4+ CD25highFOXP3+ in vitro inhibitory and recruitment functions by Tregs generated in MLR with or without the presence of AL. Bulk cultures of responder PBMC from volunteer A were stimulated with 2 DR-matched x-irradiated cells from volunteer B either in the presence of no drugs (A+Bx+Medium) or 1 μg/ml AL (A+Bx+AL) for 7 days. Then, after depleting CD127+ cells, the CD4+ cells were enriched as described in the methods. The enriched cells contained 50% ± 5% FOXP3+ cells. (Left) MLR Inhibition: Standard 3H thymidine incorporation assays were performed with 1 × 105 each of the responders and stimulators in presence of indicated numbers of CD127−CD4+ cells from the above bulk cultures with versus without AL, contrasted with control fresh A-PBMC modulators. Note that there appeared to be no greater effect of CD127−CD4+ cells from “A+Bx+AL” cultures in inhibiting 7-day 3H-TdR incorporation (not statistically significant) over that of CD127−CD4+ cells immunoselected from “A+Bx+Medium” cultures (p > 0.2). Nonetheless, there was a much less marked inhibition if the stimulator cells came from a nonspecific donor (Ix) (p < 0.05). (Right) Treg recruitment: In parallel, 5 × 105 CFSE labeled responding fresh PBMC from healthy volunteer A were cultured with 5 × 105 PKH26-labeled stimulator cells from the original healthy volunteer B in the presence of the indicated numbers of PKH26-labeled CD127−CD25+ modulators. On days 5, 7, and 9 flow-cytometric analysis using the scheme shown in Fig. 3 was performed (including the gating out of the PKH26 positive modulators and residual stimulators as well as the CD127+ responders). Data are calculated as percentage increase in CD4+CD25highFOXP3+ cell generation with the indicated modulators, over that observed with the control fresh A-PBMC modulators (100% shown by dotted line). As previously reported CD127−CD4+ cells from “A+Bx+Medium” cultures enhanced Treg generation [10,15]. Of note in the upper panel was no significant increase in CD127−CD4+ cells from “A+Bx+AL” cultures to generate higher Tregs than the CD127−CD4+ cells from “A+Bx+Medium” cultures (p > 0.2). Also, as indicated in the lower panel, using an HLA-mismatched indifferent nonspecific stimulator (Ix) virtually completely abrogated the in vitro recruitment effect (p = 0.02). *Cultures in which not enough third component modulator cells were available for testing.
3.3. Direct effects of AL on MLRs with or without addition of TAC or SRL
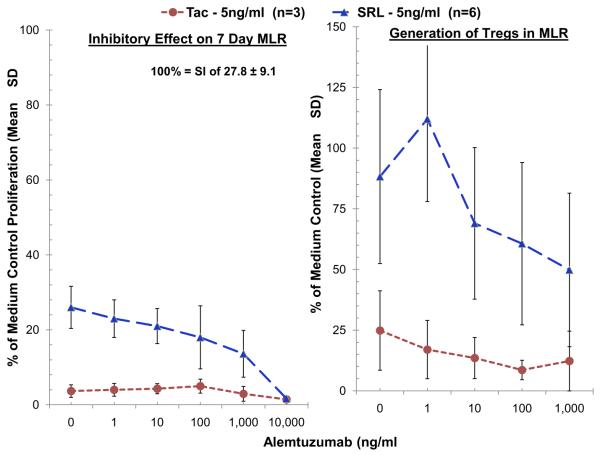
Because clinical AL administration (at one or two doses) is accompanied by the use of maintenance immunosuppressive drugs TAC or SRL, and because AL has a prolonged pharmacokinetic decay similar to that of human IgG, we tested AL in decreasing concentrations (from those seen clinically) in combination with therapeutic trough levels of TAC versus SRL. As SRL had shown significantly more regulatory effect than TAC when the two agents were tested singly [15], we tested them here in a 2:1 comparison, i.e., six AL versus three TAC experiments to more clearly demonstrate SRL effects when used together with AL. Similar to our previous report in which titrations of both TAC and SRL were performed [15], clinically therapeutic trough concentrations of TAC (5 ng/ml) markedly inhibited proliferation but also dampened Treg generation in MLR when used in combination with titrated concentrations of AL (Fig. 6, left panel). This was in contrast to more residual Tregs seen when clinically therapeutic trough concentrations of SRL (5 ng/ml) and descending AL concentrations were used in combination (Fig. 6, right panel, p < 0.05). Interestingly, in a few experiments, sub-therapeutic concentrations of AL (primarily 1 ng/ml) were shown to amplify the Treg percentages seen with therapeutic concentrations of SRL (5 ng/ml) over those of SRL alone. This could be noted with SRL in the right panel of Figure 6 (n = 6), not seen with the tighter overall values inhibiting Treg generation seen with TAC (n = 3).
Fig. 6.
Effect of tacrolimus and sirolimus on AL mediated lymphoproliferative inhibition and Treg generation in MLR. Because Alemtuzumab in single doses is used clinically in conjunction with Tacrolimus and Sirolimus, MLRs were also performed with AL in presence of combinations of these maintenance drugs. Both 3H-TdR uptake assays and flow-cytometric analysis were performed, the latter as described in Fig. 3, with serial dilutions of AL as indicated on the horizontal axis and with fixed concentrations of the maintenance agents (5 ng/ml-trough therapeutic concentrations) (TAC – n = 3, SRL – n = 6). Both TAC and SRL by themselves inhibited lymphoproliferation as previously described, reflected by the points in the left panel. There were also different effects on Treg percentages favoring SRL (right panel) (p < 0.05). Increasing AL concentrations had an appreciable difference on the differing Treg effects of the maintenance agents and significantly favored SRL over TAC.
4. Discussion
Our study supports the immunoregulatory effects of AL [6]. Using reproducible alloactivation assays, this would include both inhibition of lymphoproliferation and the in vitro expansion/recruitment of Treg percentages in MLR, i.e., during alloactivation [10]. Although it must be stated that the irradiated stimulating cells in these in vitro assays would also be affected by AL because of the binding capacity of the antibody on any cell expressing the CD52 epitope, this should not detract from the present observations of the generation of phenotypic responder Tregs by the agent. Therefore, the in vitro experimental design might be considered more closely analogous with the clinical environment in which these agents are most commonly used, in that the allogeneic stimulus of the organ transplant is presented virtually simultaneously with the administration of the antibody induction agent. This is the novelty of this set of experiments. This is opposed to testing the in vitro effects on naive T cells. As such, AL had a profound effect on alloimmune lymphoproliferation inhibition. However, at the same time, the percentages of Tregs induced with AL superseded those induced in control cultures without AL, i.e., media only, as also reported previously [10] (Figs. 3 and 4), where inhibition might have been expected because of AL lymphodepletion. This somewhat paradoxical effect is speculated to be due to depletion by AL of the predominantly naive CD4+ population expressing high membrane density CD52 epitopes compared with relative preservation of CD4+ CD25high cells with low membrane density CD52 (Fig. 2). In support of this, we tested effects on T cell subsets by adding AL to MLR assays after 7 days in culture. Although significant lymphodepletion occurred with AL after 24 hours’ incubation, the remaining fraction of T cells from the MLRs (mainly CD3+52+Low) included high percentages of alloactivated CD4+CD25+ cells and CD8+CD56+ NK cells (Table 1) [16]. Taken together these results indicated that already activated cells were relatively resistant to AL mediated lymphodepletion and retained their ability to mount a secondary immune response. Extrapolation to the clinic would suggest that AL may not deplete activated T cells sufficiently and therefore may not be as effective for sensitized recipients. In fact, Pearl et al. have observed that immunocompetent memory-like cells are dominant in patients after AL-mediated T-cell depletion [17]. However, AL might conversely be preferential in generating regulatory T cells in nonsensitized individuals at the time of transplantation, as observed here in vitro.
Clinically, data are supportive of the expansion of Tregs in organ transplant recipients in vivo after treatment with AL. In renal recipients, Bloom et al. reported a rise in Tregs and the ratio of Treg/Teff from pre-to post-transplantation with AL (with SRL maintenance) but not with Basiliximab [18]. In addition, AL + SRL monotherapy patients retained intact immune responses to third party alloantigen but appeared to be hyporesponsive to donor antigen [19]. Moreover, a significant increase in Treg percentages in the peripheral blood of AL-treated renal transplant recipients persisted only if CNI therapy was withdrawn [9]. As have others [20], we have also demonstrated inhibitory effects of TAC on Treg generation in contrast to SRL, both in vitro and in vivo [12,15]. The long-term persistence of these effects in AL treated patients, however, still needs to be evaluated.
The CD52 antigen recognized by AL is abundantly expressed on lymphocytes and has been previously shown to act as a costimulatory molecule providing a strong signal to activate T cells[21]. To date, the clinical application of AL in human disease has depended upon robust lymphodepletion induced by both complement dependent cytotoxicity and antibody dependent cell mediated cytolysis. Our present study provides evidence that a non-depletional, immunomodulatory effect of AL may include the induction of regulatory T cells. In this regard, Watanabe et al. have recently reported that costimulation of naive T cells with anti-CD3 and AL induces a CD4+CD25+ regulatory T-cell population from CD4+CD25− cells which is antigen specific and is contact dependent for suppression [6]. In contrast to our study, the CD52-induced Tregs described by Watanabe et al. were Foxp3 negative.
It is important to stress that, in this study, we were not interested in generating functional Tregs in AL spiked cultures by in vitro cytokine addition, or resting them after a period of alloactivation and testing such a manufactured product. Such experiments might well enhance Treg generation, i.e., amplified by AL, but would not more closely reflect the alloactivation environment occurring in vivo (i.e., clinical transplantation). Nonetheless, when compared with functional regulatory effects we described in previous reports, using third component modulator cells, expressive of FOXP3, there was an equivalent regulatory effect on the readout, i.e., with or without AL spiking of the primary bulk cultures. This is consistent with the notion that such allo-primed cells at least had equivalent regulatory function with or without the agent, i.e., no negative overall counter-regulatory effect of AL due to the increased presence of AL spared effector cells. Such a negative effect on Treg generation might have been expected were AL to have preferentially spared alloactivated effector cell function.
In considering transplant tolerance/IS withdrawal protocols, this report provides support for the use of AL in previously nonprimed recipients to eliminate potential effector T cell populations and expand Tregs. That is, it would be expected that a marked reduction in naive T cells would occur because of such antibody therapy. Although effector T cells are also AL refractory, in the absence of presensitization, these should not play a predominant role. This is not discounting the notion that there would be at least some memory/effector cells present even in naive recipients (putative heterologous immunity) that immunoregulation by AL would need to supersede. Therefore, in selected clinically nonprimed renal transplant recipients taking part in a tolerance trial [7], it might be expected that with the overall AL reduced cell numbers, the relative increased Treg percentages in the CD4+ population circulating through the periphery and graft would serve to regulate alloimmune responses. Speculatively, this allospecific immunoregulation might be more pronounced in protocols using AL and SRL rather than TAC as temporary maintenance therapy, before IS withdrawal.
Acknowledgments
This work was in part supported by NIH grant 2R01DK25243-25A2 and a VA Merit Review Award.
References
- [1].Newell KA, Cendales LC, Kirk AD. Finding the right job for the tool: Alemtuzumab and its role in renal transplantation. Am J Transplant. 2008;8:1363–4. doi: 10.1111/j.1600-6143.2008.02291.x. [DOI] [PubMed] [Google Scholar]
- [2].Morales J, Bono MR, Fierro A, Iñiguez R, Zehnder C, Rosemblatt, et al. Alemtuzumab induction in kidney transplantation: clinical results and impact on T-regulatory cells. Transplant Proc. 2008;40:3223–8. doi: 10.1016/j.transproceed.2008.03.066. [DOI] [PubMed] [Google Scholar]
- [3].Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth, et al. Prope tolerance, perioperative Campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–2. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- [4].Tzakis AG, Tryphonopoulos P, Kato T, Nishida S, Levi DM, Madariaga JR, et al. Preliminary experience with alemtuzumab (Campath-1H) and low-dose tacrolimus immunosuppression in adult liver transplantation. Transplantation. 2004;77:1209–14. doi: 10.1097/01.tp.0000116562.15920.43. [DOI] [PubMed] [Google Scholar]
- [5].Calne R. “Prope” tolerance: induction, lymphocyte depletion with minimal maintenance. Transplantation. 2005;80:6–7. doi: 10.1097/01.tp.0000164351.72220.1a. [DOI] [PubMed] [Google Scholar]
- [6].Watanabe T, Masuyama J-i, Sohma Y, Inazawa H, Horie K, et al. Kojima Ket al: CD52 is a novel costimulatory molecule for induction of CD4+ regulatory T cells. Clin Immunol. 2006;120:247–59. doi: 10.1016/j.clim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- [7].Miller J, Leventhal J, Friedewald J, Levitsky J, charette j, Huang X, et al. enhanced immunoregulatory profiles in HLA identical renal transplant recipients given donor Hematopoetic stem cells alemtuzumab and sirolimus followed by immunosuppression withdrawal. Am J Transplant. 2011;11:92. [Google Scholar]
- [8].Feng X, Kajigaya S, Solomou EE, Keyvanfar K, Xu X, Raghavachari, et al. Rabbit ATG but not horse ATG promotes expansion of functional CD4+ CD25highFOXP3+ regulatory T cells in vitro. Blood. 2008;111:3675–83. doi: 10.1182/blood-2008-01-130146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells. Am J Transplant. 2008;8:1529–36. doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Levitsky J, Miller J, Leventhal J, Huang X, Flaa C, et al. The human “Treg MLR”: immune monitoring for FOXP3+ T regulatory cell generation. Transplantation. 2009;88:1303–11. doi: 10.1097/TP.0b013e3181bbee98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7:1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Levitsky J, Miller J, Wang E, Rosen A, Flaa C, Abecassis, et al. Immunoregulatory profiles in liver transplant recipients on different immunosuppressive agents. Hum Immunol. 2009;70:146–50. doi: 10.1016/j.humimm.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mathew JM, Fuller L, Carreno M, Garcia-Morales R, Burke GW, 3rd, Ricordi, et al. Involvement of multiple subpopulations of human bone marrow cells in the regulation of allogeneic cellular immune responses. Transplantation. 2000;70:1752–60. doi: 10.1097/00007890-200012270-00015. [DOI] [PubMed] [Google Scholar]
- [14].Mathew JM, Garcia-Morales R, Fuller L, Rosen A, Ciancio G, Burke GW, et al. Donor bone marrow-derived chimeric cells present in renal transplant recipients infused with donor marrow. I. Potent regulators of recipient antidonor immune responses. Transplantation. 2000;70:1675–82. doi: 10.1097/00007890-200012270-00003. [DOI] [PubMed] [Google Scholar]
- [15].Levitsky J, Gallon L, Miller J, Tambur AR, Leventhal J, Flaa, et al. Allospecific regulatory effects of sirolimus and tacrolimus in the human mixed lymphocyte reaction. Transplantation. 2011;91:199–206. doi: 10.1097/TP.0b013e318200e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mathew JM, Vallone T, De Faria W, Kato T, Carreno M. Blomberg B Relative resistance of activated T cells and NK cells to alemtuzumab (Campath-1H) used in clinical transplantation. Hum Immunol. 2004;65:5. [Google Scholar]
- [17].Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–74. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- [18].Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8:793–802. doi: 10.1111/j.1600-6143.2007.02134.x. [DOI] [PubMed] [Google Scholar]
- [19].Bloom DD, Hu H, Fechner JH, Knechtle SJ. T-lymphocyte alloresponses of Campath-1H-treated kidney transplant patients. Transplantation. 2006;81:81–7. doi: 10.1097/01.tp.0000191940.13473.59. [DOI] [PubMed] [Google Scholar]
- [20].Knechtle SJ, Pirsch JD, Fechner HJ, Jr., Becker BN, Friedl A, Colvin RB, et al. Campath-1H induction plus rapamycin monotherapy for renal transplantation: results of a pilot study. Am J Transplant. 2003;3:722–30. doi: 10.1034/j.1600-6143.2003.00120.x. [DOI] [PubMed] [Google Scholar]
- [21].Rowan WC, Hale G, Tite JP, Brett SJ. Cross-linking of the CAMPATH-1 antigen (CD52) triggers activation of normal human T lymphocytes. Int Immunol. 1995;7:69––77. doi: 10.1093/intimm/7.1.69. [DOI] [PubMed] [Google Scholar]