Abstract
The mechanisms which contribute to the time-dependent recovery of resting ventilation and the ventilatory CO2 chemoreflex after carotid body denervation (CBD) are poorly understood. Herein we tested the hypothesis that there are time-dependent changes in the expression of specific AMPA, NMDA, and/or neurokinin-1 (NK1R) receptors within respiratory-related brain stem nuclei acutely or chronically after CBD in adult goats. Brain stem tissues were collected acutely (5 days) or chronically (30 days) after sham or bilateral CBD, immunostained with antibodies targeting AMPA (GluA1 or GluA2), NMDA (GluN1), or NK-1 receptors, and optical density (OD) compared. Physiological measurement confirmed categorization of each group and showed ventilatory effects consistent with bilateral CBD (Miller et al. J Appl Physiol 115: 1088–1098, 2013). Acutely after CBD, GluA1 OD was unchanged or slightly increased, but GluA2 and GluN1 OD were reduced 15–30% within the nucleus tractus solitarius (NTS) and in other medullary respiratory nuclei. Chronically after CBD, GluA1 was reduced (P < 0.05) within the caudal NTS and in other nuclei, but there was significant recovery of GluA2 and GluN1 OD. NK1 OD was not significantly different from control after CBD. We conclude that the initial decrease in GluA2 and GluN1 after CBD likely contributes to hypoventilation and the reduced CO2 chemoreflex. The partial recovery of ventilation and the CO2 chemoreflex after CBD parallel a time-dependent return of these receptors to near control levels but likely depend upon additional initiating and maintenance factors for neuroplasticity.
Keywords: carotid body, plasticity, neurochemical receptor
the neural network that controls ventilation in mammals requires feedback from peripheral (carotid body) and central chemoreceptors to maintain blood gas homeostasis. Carotid body denervation (CBD) in humans (2, 9, 23) and other mammals (4, 5, 11, 33, 35) results in severe hypoventilation and a decreased ventilatory CO2 chemoreflex. Despite these major effects of CBD, eupneic ventilation returns to near normal in most species studied to date, albeit more completely and with different time requirements in different species (2, 4, 9, 11, 33). Our ongoing studies in awake goats aim to better understand the brain stem mechanisms that account for the hypoventilation and/or attenuated CO2 sensitivity immediately after CBD and to understand the fundamental processes that govern the time-dependent restoration of ventilatory function after CBD.
We recently found chronically increased inhibitory and decreased excitatory neuromodulator levels within the ventral medullary column (VMC) up to 30 days after CBD (25). Furthermore, there was an apparently reduced capacity of medullary raphé neurons to produce and synaptically regulate the excitatory neuromodulator 5-HT after CBD (25). This increased inhibition and decreased excitation of the respiratory network likely contributes to the hypoventilation and reduced ventilatory CO2 chemoreflex after CBD. Moreover, these data suggest that intact carotid afferents contribute to ventilatory control in part via driving the output of central neurochemical levels within the VMC. However, these findings do not reveal mechanisms that contribute to the restoration of ventilatory function after CBD.
Currently, the specific mechanisms which underlie time-dependent recovery of ventilation after CBD are poorly understood. There are data supporting respiratory plasticity within the brain stem after CBD, but the exact mechanism is unknown (3, 11). Plasticity within structures rostral to the brain stem have demonstrated that the recovery of neuronal activity following lesioning and/or altered neuronal afferent input is associated with changes in postsynaptic excitability and/or synaptic strength. These changes can occur via activity-dependent alterations in postsynaptic glutamate receptor expression, specifically involving the ionotropic (AMPA; GluA1-A4) and/or metabotropic (NMDA; GluN1) glutamate receptor subunits (7, 20, 31, 36, 39–41) and potentially several other neurochemical receptors important to neural respiratory control such as the substance P receptor (neurokinin-1; NK-1) (10, 30, 43). Elucidation of whether similar changes occur within the brain stem respiratory network after experimental perturbations such as CBD is important because of the potential application to disease-induced or traumatic brain injury to the respiratory network. Accordingly, the major objective of the current study was to test the hypotheses that there are time-dependent changes in GluA1, GluA2, and/or GluN1 containing glutamate receptors, and/or NK1 receptors at sites that contribute to respiratory control, and furthermore to determine if observed receptor changes coincide with the recovery of ventilation with time after CBD.
METHODS
Data were obtained from 29 female adult goats weighing 42.5 ± 8.3 kg [22 goats were also part of our previous study (25)]. The goats were housed in a chamber with a fixed ambient temperature and photoperiod. The goats were given access to feed and water ad libitum except during study and 24-h fasting periods prior to surgeries. All aspects of the study were reviewed and approved by the Medical College of Wisconsin Animal Care and Use Committee.
Experimental Design
Eight goats (naive) were euthanized upon arrival to the laboratory for histological control purposes. Six naive goats were used for immunohistochemical controls. Two naive goats were utilized for Western blot analysis. Twenty-one goats underwent surgery for subcutaneous elevation and subsequent catheterization of the carotid arteries. After 2 wk of recovery, 10 of these goats underwent a second surgery for bilateral microtubule (MT) implantation into the ventral medullary column (VMC) targeting the pre-Bötzinger complex (preBötC) for microdialysis and injection studies (25). These MT-implanted goats were allowed two additional weeks of recovery before baseline studies were initiated.
Baseline studies were completed prior to CBD surgery. Following CBD surgery in the first two goats, ventilatory support was required immediately following surgery; thus a chronic tracheostomy was created. In all subsequent goats, a tracheostomy was performed during CBD surgery. Studies began 24–48 h post-CBD/tracheostomy surgery. In total, bilateral CBD surgery was performed on 17 of the 21 experimental goats. Eleven bilateral CBD goats were euthanized 30 days post-CBD, and six were euthanized 5 days post-CBD. Sham-CBD surgery was performed on the remaining 4 of the 21 experimental goats. One sham-CBD goat was euthanized 5 days post-sham surgery, and three were euthanized 30 days post-sham. After euthanasia, the head was perfused and fixed, and the medullary tissue was harvested.
Surgical Procedures
Instrumentation surgery.
The anesthesia procedure is standard for all surgeries in this laboratory. The goats were induced with ketamine, intubated, and ventilated with 2% isoflurane in 100% O2. Sterile conditions were maintained for all procedures. For the initial surgery, the carotid arteries were separated from the vagus nerve, elevated superficially to the muscle, and skin sutured. For analgesic purposes, flunixin meglumine (1 mg/kg im) was administered once before surgery followed by buprenorphine hydrochloride (0.005 mg/kg im, BID) for 2 days postoperatively. To prevent infection, the goats received ceftiofur sodium (2 mg/kg, QD) for 1 wk.
Craniotomy surgery.
Implantation of stainless steel MTs into the VMC was performed as previously described (16, 29). The data obtained as a result of implantation of MTs into the VMC have been previously published (25). Briefly, under anesthesia, a rotary drill was used to remove bone rostral to the foramen magnum until the medulla was exposed. A patch of dura mater was removed to locate obex. Obex was then used as a reference point for placement of MTs in or near the target site, the preBötC. After implantation, the MTs were secured to the surrounding bone and the craniotomy was sealed with dental acrylic. Experienced laboratory personnel monitored the goats continuously for 24 h after MT implantation surgery. For analgesic purposes, flunixin meglumine (1 mg/kg im) was administered once before surgery followed by buprenorphine hydrochloride (0.005 mg/kg im, BID) for 2 days postoperatively. Brain edema was controlled with dexamethasone injections (0.4 mg/kg and decreasing by 0.05 mg/kg each day, iv, TID) for 1 wk. Infection was prevented with chloramphenicol injections (20 mg/kg iv, TID) for 3 days postsurgery and with daily injections of ceftiofur sodium (2 mg/kg) and gentamycin (3 mg/kg) until euthanized.
CBD surgery.
CBD or sham-CBD surgery was performed as previously described (11, 25, 33). Briefly, under anesthesia, a ventral midline incision was made and the carotid bifurcations were exposed. The carotid sinus nerve is located proximal to the bifurcation. Once identified, two sutures were placed around the nerve and a small segment was excised. This procedure was performed bilaterally. For sham-CBD, the carotid bifurcation was exposed, but the nerves were not sectioned. After completion, the ventral midline incision was sutured closed.
Tracheostomy.
During CBD surgery, a small midline incision was made caudal to the larynx. A segment of cartilage from the trachea was then removed creating a 1 cm opening. The surrounding skin was then sutured to the stoma. Upon recovery, the goats were fitted with an endotracheal tube. All goats were monitored continuously for 24 h following surgery. For analgesia, flunixin meglumine (1 mg/kg im, BID) was administered for 2 days postoperatively.
Physiological Studies
As previously described (25, 29, 44), during experiments an airtight mask with a two-way breathing valve was secured to the goats' snout or endotracheal tube (tracheostomized goats). A pneumotach and computerized data acquisition software were used to measure inspired ventilation (V̇i) and a spirometer was used to measure expired ventilation (V̇e). Arterial blood was sampled via a chronically placed catheter in the elevated carotid artery to measure pH, arterial Pco2 (PaCO2), and arterial Po2 (PaO2) (Rapid Lab 248, Bayer Health Care). Peripheral chemoresponsiveness was assessed pre- and post-CBD to confirm CBD. V̇i was measured during 30 min of room air breathing followed by exposure to hypoxia for 5 min. PaO2 was decreased to ∼30 mmHg, which in intact goats required a fraction of inspired oxygen (FiO2) of 0.10 (N2 balance) and post-CBD required an FiO2 of 0.12 (N2 balance). Ventilation, resting blood gases, and the CO2 chemoreflex were measured 3–5 times pre-CBD and every 1–3 days post-CBD until the animal was euthanized. The CO2 chemoreflex was expressed as ΔV̇e/ΔPaCO2 when progressing from room air breathing to 3%, 5%, and 7% inspired CO2 in room air. Arterial blood was sampled twice during room air breathing and once during each of the last 2 min of each level of increased inspired CO2.
Histological Studies
Upon completion of physiological studies and in histological naive goats, the goats were anesthetized with a ketamine/xylazine mixture (to facilitate isolation of circulation to the head) and were then euthanized with B-euthanasia. For goats used for immunohistochemistry, the medullary tissue was harvested after the head was flushed (10% sucrose in PBS) and fixed (4% paraformaldehyde in PBS). The tissue was cryoprotected in increasing concentrations of sucrose (20% and 30%) for at least 72 h each and was then frozen and serially sectioned (25 μm) in the transverse plane from obex to the pontomedullary junction (∼10 mm). Each section was adhered to electrostatically-treated slides and was either Nissl-stained for identification of gross anatomical structures (data not shown) or immunostained for GluA1, GluA2, GluN1, or NK1R. For immunostaining, CBD and sham-CBD goat tissue were processed with tissue of a naive goat in an adjacent chamber, or CBD and sham CBD tissue were processed simultaneously.
Immunostaining protocol.
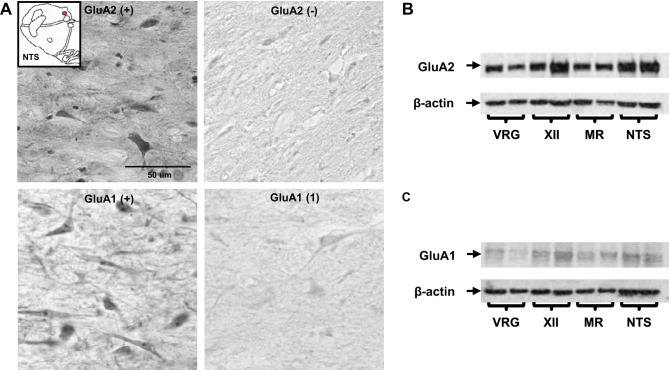
After a 2-h drying period, the tissue underwent heat antigen retrieval (DAKO) (12 min at 92°C) and was rinsed in PBS for 1 h. The tissue was then washed in a 0.4% Triton X-100 in PBS solution and blocked with 5% normal horse serum, 5% normal goat serum, and 1% bovine serum albumin in 0.1% Triton X-100 PBS for 1 h. The tissue was incubated with the primary antibody (GluA1 1:2,000, Millipore; GluA2 1:2,000, Abcam; GluN1 1:1,000, Santa Cruz Biotechnology; NK1R 1:4,000, Millipore) with 1.25% normal horse serum and 1.25% normal goat serum in 0.1% Triton X-100 PBS overnight followed by rinsing with 0.1% Triton X-100 PBS. The tissue was then incubated with a biotinylated anti-rabbit secondary antibody (1:500 in 0.1% Triton X-100 PBS) for 30 min, rinsed with PBS, and incubated with ABC reagents (Vector Labs) for 30 min. After a final rinse with PBS, the tissue was exposed to 3,3′-diaminobenzidine (∼2 min). The tissue was then dehydrated, cleared, and coverslipped. Tissue was stained at 400-μm intervals. A negative control was performed to established specificity for each immunostain by omitting the primary antibody incubation step (Fig. 1A).
Fig. 1.
The specificity of AMPA receptor immunolabeling was established by negative controls and Western blotting. A, left: example images (40X, 0.75 NA) from the nucleus tractus solitarius (NTS) of a naive goat immunostained with GluA1 and GluA2. A, right: corresponding negative controls in which the primary antibody was omitted during incubation. B and C: Western blot bands for GluA2 and GluA1 from tissue within the ventral respiratory group (VRG), hypoglossal motor nucleus (XII), medullary raphé (MR), and NTS. Both the GluA1 and GluA2 bands were present at the expected molecular weight for these proteins, with few or no additional bands detected. Note in B and C that the β-actin band is comparable between GluA1 and GluA2; however, the GluA1 band is qualitatively less dense than GluA2, suggesting inherently lower GluA1 expression in control (naive) goats.
Western blot protocol.
Western blot analysis was performed on brain tissue from two naive goats (n = 2) to confirm specificity of the GluA1 and GluA2 primary antibodies used for immunostaining. The goats were euthanized and the brain tissue was flash frozen (methylbutane with dry ice). The tissue was maintained at −80°C until 2–2.5 mm coronal slices and 0.9 mm tissue punches of the nucleus tractus solitarius (NTS), hyoglossal motor nucleus (XII), ventral respiratory group (VRG), and medullary raphe (MR) were made at −20°C. Tissue was then homogenized via sonication in Laemmli buffer (250 mM Tris·Cl, pH 6.8, 40% glycol, 8% SDS, 8% β-mercaptoethanol) at a concentration of 50 mg/ml. Criterion precast gels were used, wells washed with ddH20, and then submerged in running buffer (10% SDS-Page in ddH20). Twenty microliters of sample was added to each well. Electrophoresis was performed at 80 V for 10 min and then at 100 V for 1 h. Transfer of protein onto nitrocellulose membrane ran for 1 h at 100 V in transfer buffer [20% 5 × transfer buffer (Bio-Rad), 20% methanol, 60% ddH20]. The membrane was removed and washed (2 × 2 min) with TBS-T. Then, membranes were added to the block solution (5% BSA in TBS-T) for 1 h followed by 3 × 7 min TBS-T washes. Primary antibodies were added (see Immunostaining protocol for primary antibody information and concentrations) and incubated overnight at 4°C. Nitrocellulose membranes were washed 4 × 7 min with TBS-T and then secondary antibody was added (1:3,000 in 3% milk) for 1 h at room temperature. Blots were developed in a dark room after exposure to ECL or ECL plus solution (GE Healthcare). Film was on membrane for 30 s during the development process. For both GluA1 and GluA2, Western blot bands were present at the expected molecular weight with few if any additional bands indicating specificity of the antibodies used for quantification of immunostaining (Fig. 1B).
Immunostain densitometric quantification.
GluA1, GluA2, GluN1, and NK1R optical densitometric (OD) measurement of horseradish peroxidase reaction products were quantified using a Nikon E-600 20× objective lens (0.50 NA). White light was filtered through optical glass type B440 (Hoya Optics) and conditions calibrated to an OD standard (Edmund Industrial Optics). We examined known sites of respiratory neurons including the nucleus tractus solitarius (NTS), dorsal motor nucleus of the vagus (DMNV), hypoglossal motor nucleus (XII), medullary raphé (MR), facial nucleus (FN), and a region ventral to the FN, the retrotrapezoid nucleus (RTN). We also examined the cuneate nucleus (CN), which is outside of the respiratory network.
Immunostaining was performed by an investigator with knowledge of CBD and control status, but quantification was performed by technical staff blinded to the origin of the tissue. The OD of neurons within each site was measured using a custom-written program in MATLAB (Natick, MA) (Fig. 2A). Image processing comprised two steps. The first step, which was automated, objectively applied edge detection (6) and then backfilled the resulting borders to form predicted objects within the image. The second step presented the user with an image displaying an overlay with the predicted objects on the original image (Fig. 2B). The user was then able to objectively select objects deemed relevant for OD measurement (neurons and often dendritic processes; Fig. 2C). Typically there were 60–100 selected objects within the NTS and 40–50 objects at all other sites. The program recorded, grouped, and then averaged all pixel values (OD) from the selected objects as one compiled meta-object, providing a single OD measurement for each image. Other metrics obtained from analysis of the meta-object include standard deviation of pixel OD, maximum pixel OD, and minimum pixel OD (data not shown). This analysis was performed on images acquired every 400 μm at each nuclei.
Fig. 2.
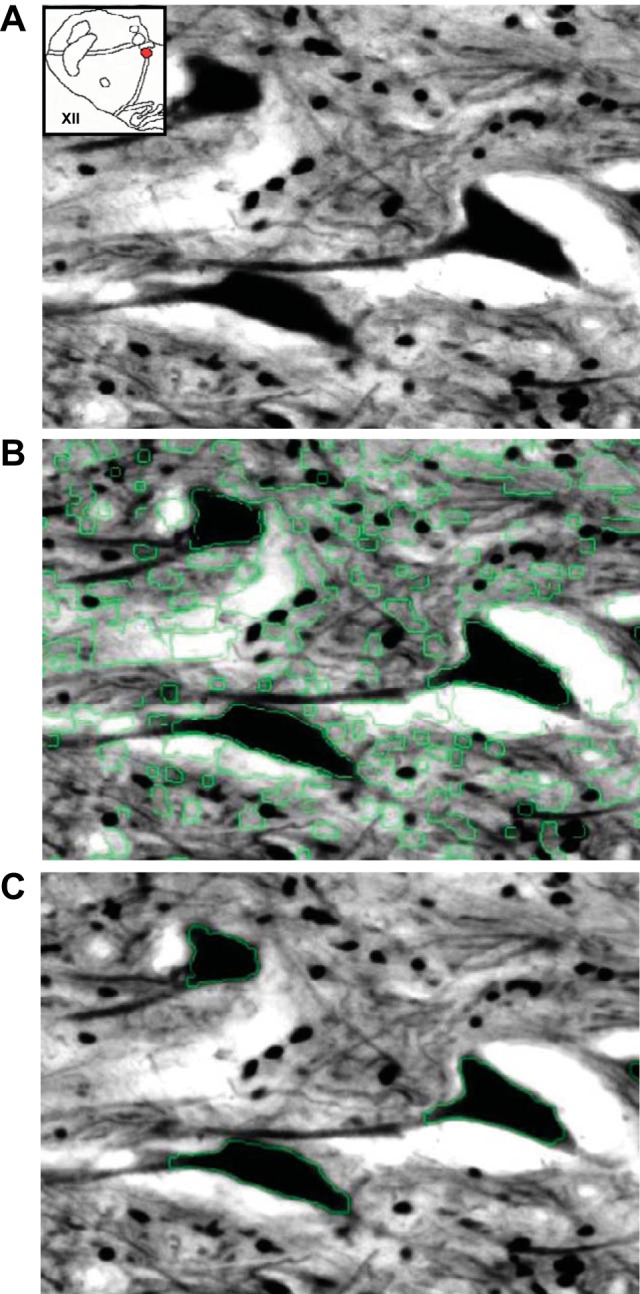
We used Matlab software for optical densitometric quantification of neuronal cell bodies. A: a raw, light-calibrated image of GluA2 immunostained XII neurons from a naive goat. B: the Matlab-processed image mask with the detected edges shown in green, including the edges of neuronal cell bodies. C: the final processed image after user selection of neuronal cell bodies for OD measurement. These images were contrast enhanced to highlight detected edges.
Data and Statistical Analysis
Hypoxic response studies.
V̇i was calculated and binned into 1-min periods. The hypoxic response was determined by averaging V̇i during the last 5 min of room air breathing and V̇i during the 5 min of hypoxia. These values were then subjected to a one-way ANOVA with repeated measures to compare V̇i during each minute of room air and hypoxic gas breathing. This analysis was performed before and after CBD surgery on the raw values for V̇i; however, data are expressed as percent of mean V̇i during the last 5 min of room air breathing. The threshold for significance for this analysis and all subsequent statistical analyses was set to P < 0.05.
Blood gases and CO2 sensitivity.
Measured ventilatory variables were binned post-CBD as follows: peak hypoventilation (3–5 days post-CBD); nadir of CO2 sensitivity (8–10 days post-CBD); transient recovery of CO2 sensitivity (15 days post-CBD); and 20–30 days post-CBD. These values and the pre-CBD values were subjected to a one-way ANOVA with repeated measures to establish effects of CBD [data reported previously (25)].
Immunostain OD analysis.
GluA1, GluA2, GluN1, and NK1R OD was quantified and binned every 400 μm. The average raw OD (immunoreactivity) values of all caudal and rostral NTS sections are presented for naive (n = 5 or 6) and sham (n = 2) CBD goats in Fig. 3. There were insufficient sham CBD goats for meaningful statistical comparison with naive goats, but as shown for the NTS (Fig. 3), the OD values for the sham goats were within the range of values for naive goats for all nuclei. Overall, the OD values from sham CBD goats were 92–105% of the naive goats. As stated previously, sections from CBD goats were immunostained with sections from naive and in a few cases with sham CBD goats. The OD values from the CBD goats were normalized to the values of the naive or sham CBD goats. We believe the close agreement in raw OD values (Fig. 3) between naive and sham CBD goats indicates that we obtained a valid representation of the effects of CBD on immunoreactivity for each variable studied. Furthermore, combining sham CBD and naive goats does not misrepresent the data.
Fig. 3.
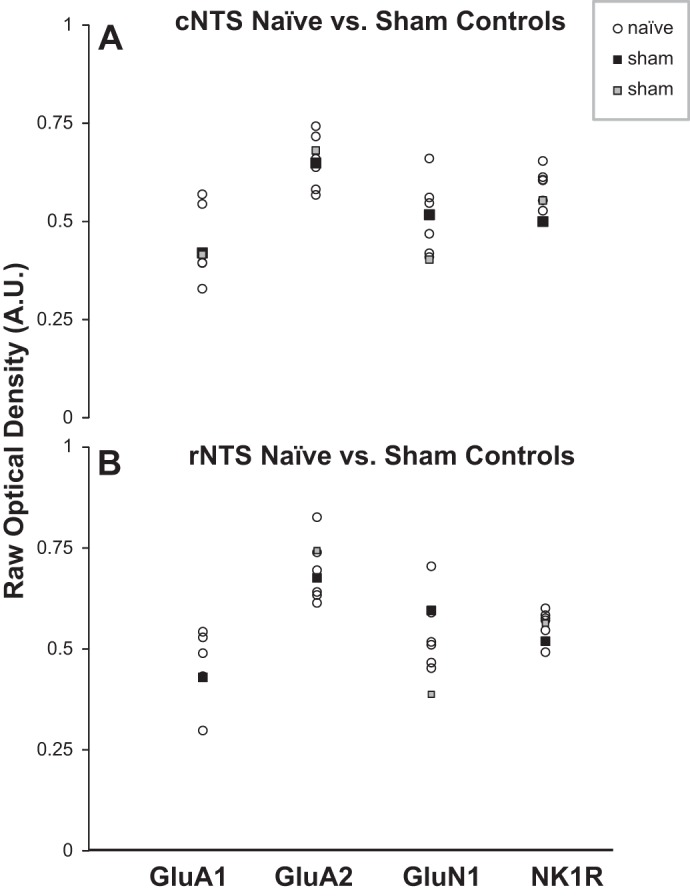
Raw optical density measured values for tissue from the caudal (cNTS; A) and rostral NTS (rNTS; B) of 2 sham-carotid body denervated (sham-CBD) goats (black and gray squares) were generally within the range of naive, unoperated goats (n = 5 or 6) for each protein. This close agreement was also observed in the other nuclei. As stated in methods, tissue from CBD goats was always immunostained together with tissue from a naive or a sham-CBD goat. The raw values for the CBD goats were normalized to the values of the simultaneously immunostained naive or sham-CBD goats. The data indicate that those normalized to sham-CBD goats did not cause a misrepresentation of the CBD effects. Note that data from 2 sham-CBD goats are presented here, whereas for physiological data, data from 3 goats are presented (Fig. 4). The difference reflects use of tissue from 1 sham-CBD goat in a previous publication (25).
Goats were categorized naive/sham-CBD, acute-CBD (euthanized at 5 days post-CBD), and chronic CBD (euthanized at 30 days post-CBD). A two-way ANOVA with repeated measures (distance from obex and condition) was used to compare normalized OD values of chronic and acute CBD goats with naive/sham-CBD goats at each rostrocaudal distance. For some nuclei, there was significant (P < 0.05) interaction between distance and condition. Accordingly, for each anatomic site, the individual values were evenly categorized as “rostral” or “caudal” and then averaged for each goat. A one-way ANOVA was used to compare average rostral and caudal OD values of chronic and acute CBD goats with naive/sham-CBD goats.
RESULTS
Effects of CBD on Ventilatory Parameters and the CO2 Chemoreflex
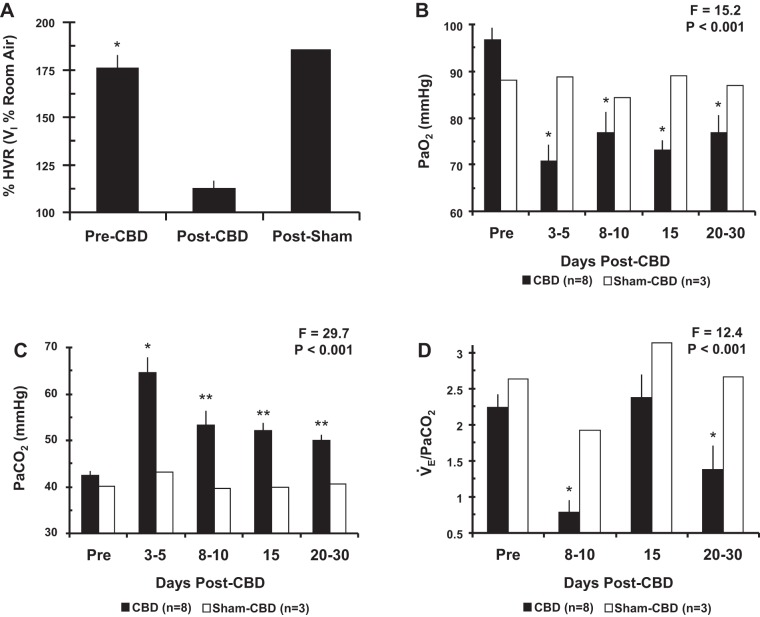
The physiological data reported herein have been presented previously (25) but are shown here for completeness. CBD was confirmed by absence of a significant hypoxic ventilatory response (HVR) after CBD (Fig. 4A). In total eight goats in which tissues were collected and quantified (see below) 30 days post-CBD were considered bilateral CBD, and three were partially denervated (hypoxic response attenuated; data not shown). The HVR was unaffected in the goats that underwent sham-CBD.
Fig. 4.
Carotid body denervation results in a loss of the ventilatory response to hypoxia (A), arterial hypoxemia (B), arterial hypercapnia (C), and a reduced CO2 chemoreflex (D). On the y-axis in A is plotted V̇i during acute hypoxia expressed as a percentage of room air breathing (±SE) pre-CBD (n = 7), post-CBD (n = 7), and post sham-CBD (n = 3). On the y-axis in B, C, and D is plotted eupneic arterial Po2 (PaO2; mmHg ± SE), eupneic arterial Pco2 (PaCO2; mmHg ± SE), and the CO2 chemoreflex (ΔV̇e/ΔPaCO2 ± SE), respectively. Filled bars represent bilateral CBD goats (n = 8) and open bars represent sham-CBD goats (n = 3). Note that data from 3 sham-CBD goats are presented for physiologic data, but there are only 2 sham-CBD goats for proteins shown in Fig. 3. The difference reflects use of tissue from 1 sham CBD goat in a previous publication (25). The F and P values are from one-way ANOVA on data obtained from the CBD goats. In A, *P < 0.05 vs. room air breathing. In B–D, *P < 0.05 vs. pre-CBD, **P < 0.05 vs. peak hypoventilation (1-way repeated-measures ANOVA).
Before bilateral CBD, resting PaCO2 (42.3 ± 1.0 mmHg) and PaO2 (96.6 ± 2.7 mmHg) (Fig. 4, B and C) were within the normal range for goats during eupnea (11, 44). Bilateral CBD resulted in severe hypoventilation as shown by the marked increase in eupneic PaCO2 (22.3 ± 3.4 mmHg above baseline; P < 0.001), which peaked 3–5 days post-CBD. Consistent with hypoventilation, PaO2 also decreased after bilateral CBD (P < 0.05). In most goats by 8–15 days post-CBD, resting PaCO2 had partially but significantly (P < 0.05) recovered.
The ventilatory CO2 chemoreflex (ΔV̇e/ΔPaCO2 = 2.24 ± 0.18) (Fig. 4D) was within the normal range for goats before CBD (11, 43). By 8–10 days post-CBD a nadir in CO2 sensitivity occurred (34.8 ± 7.8% of pre-CBD; P < 0.05). A transient recovery (105.8 ± 14.2% of pre-CBD) was observed by 15 days post-CBD. Thereafter, a secondary reduction in CO2 sensitivity (61.2 ± 14.7% of pre-CBD; P < 0.05) was observed. This temporal pattern of the ventilatory chemoreflex has been previously observed in CBD goats (11, 33).
In sham CBD goats, resting blood gases and the CO2 chemoreflex were unaffected (Fig. 4). Partial-CBD goats exhibited a transient but mild hypoventilation (4 mmHg increase in PaCO2) and a 50% decrease in CO2 sensitivity (data not shown).
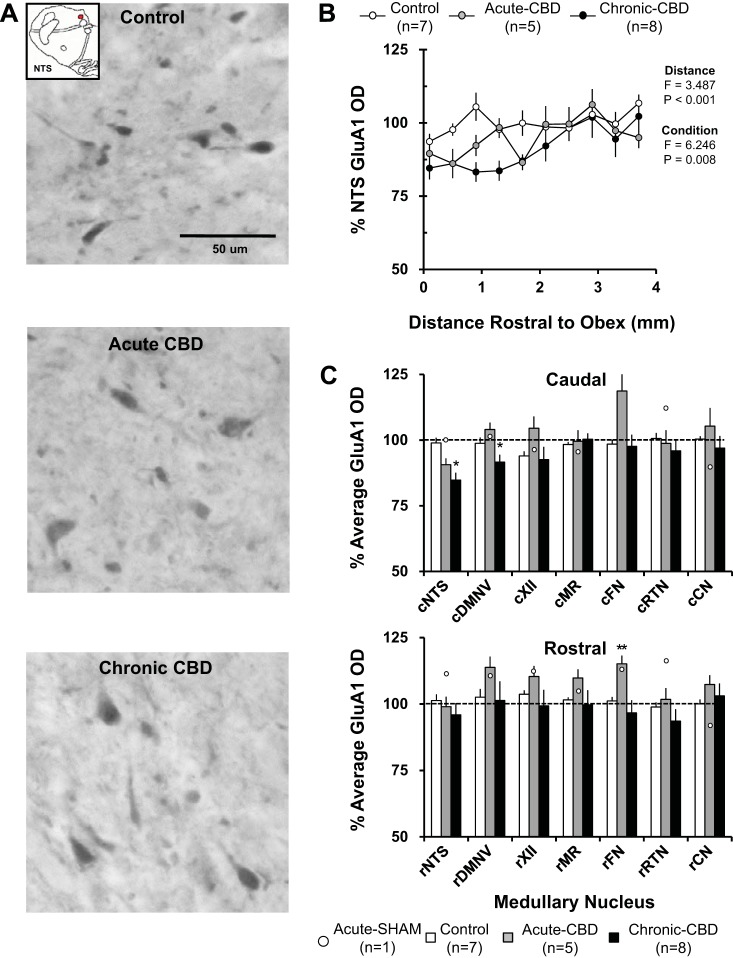
GluA1-ir Quantification in Medullary Nuclei
Both acutely and chronically after CBD, the immunoreactivity (ir) for GluA1 in the NTS (Fig. 5B), DMV, XII, MR, and CN was significantly (P < 0.05) greater in the rostral than in the caudal portion of the nuclei. Acutely after CBD (during peak hypoventilation), average GluA1 immunoreactivity was significantly increased compared with naive/sham-CBD goats to 115 ± 3.1% (P = 0.047) within the rostral FN (Fig. 5C). GluA1 OD was not significantly affected at all other rostral and caudal sites, but there was a tendency for small increases in the caudal/rostral DMNV, XII, CN, and rostral MR. GluA1 OD for the sham-CBD goat euthanized 5 days postsurgery was within the range of naive goats (Fig. 5C).
Fig. 5.
GluA1 immunoreactivity demonstrated site-specific effects both acutely and chronically after CBD. A: GluA1-ir neurons within the NTS (20X, 0.50 NA) of a naive goat (top panel), a goat euthanized during hypoventilation after CBD (acute CBD-5 days; middle panel), and a goat euthanized after partial recovery of resting PaCO2 (chronic CBD-30 days; bottom panel). Note there were minimal qualitative decreases in GluA1 immunoreactivity in the acute CBD goat and chronic CBD goat. B: GluA1 optical density quantification within the NTS. On the y-axis is plotted normalized GluA1 OD ± SE, and on the x-axis is plotted distance rostral from obex (mm) in naive/sham-CBD goats, acute-CBD goats, and chronic CBD goats. The F and P values are from the 2-way ANOVA (distance and condition as factors) on these data. C: average normalized GluA1 OD ± SE in 6 different respiratory nuclei and one nonrespiratory nucleus, the cuneate nucleus, in naive/sham-CBD goats, acute CBD goats, and chronic-CBD goats. C, top panel, shows GluA1 OD from the caudal (c) division of each nucleus, and C, bottom panel, shows the rostral (r) division. The open symbols represent GluA1 OD from the individual sham-CBD goat euthanized at 5 days post-sham surgery. *P < 0.05 vs. naive/sham-CBD, **P < 0.05 vs. naive/sham-CBD and chronic CBD. DMNV, dorsal motor nucleus of the vagus nerve; XII, hypoglossal motor nucleus; MR, medullary raphé; FN, facial motor nucleus; RTN, retrotrapezoid nucleus (region ventral to the FN); CN, cuneate nucleus.
After partial recovery of resting PaCO2 (30 days post-CBD), GluA1 immunoreactivity compared with control was significantly reduced to 84.8 ± 2.5% (P = 0.002) within the caudal NTS and 91.6 ± 2.7% (P = 0.016) within the caudal DMNV (Fig. 5C). At all other sites, GluA1 OD was not significantly affected. GluA1 OD was unchanged from naive/sham-CBD goats at all sites in partial-CBD goats 30 days post-CBD (data not shown).
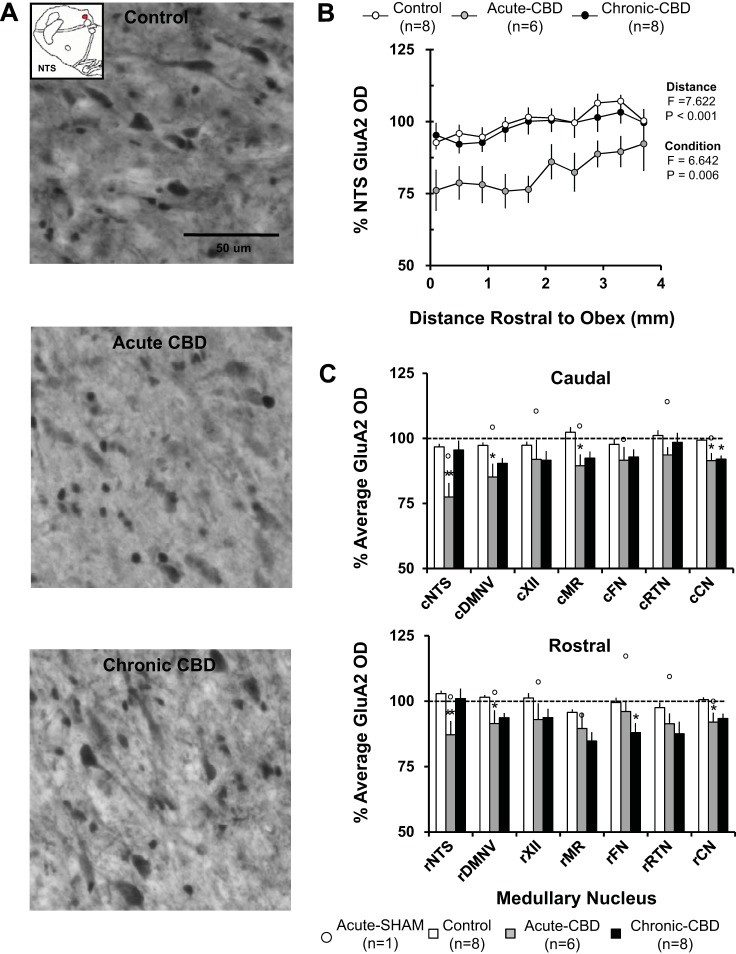
GluA2-ir Quantification in Medullary Nuclei
Acutely (during peak hypoventilation), after CBD, the immunoreactivity for GluA2 in the NTS (Fig. 6B), DMV, MR, and CN was significantly (P > 0.05) greater in the rostral than the caudal portion of the nuclei. Acutely after CBD, average GluA2 immunoreactivity was reduced compared with naive/sham-CBD goats to 71.5 ± 4.9% (P = 0.004) within the caudal NTS and to 87.1 ± 5.2% (P = 0.019) within the rostral NTS. GluA2 OD was also significantly reduced to 85.1 ± 5.1% (P = 0.023) within the caudal DMNV, 91.4 ± 5.1% (P = 0.05) within the rostral DMNV, 89.4 ± 4.3% (P = 0.018) within the caudal MR, 91.4 ± 2.9% (P = 0.008) within the caudal CN, and 92.0 ± 3.5% (P = 0.035) within the rostral CN (Fig. 6C). GluA2 OD was not affected at all other sites measured acutely after CBD. GluA2 OD for the sham-CBD goat euthanized 5 days postsurgery was within the range of naive goats (Fig. 6C).
Fig. 6.
There was decreased GluA2 immunoreactivity in acute CBD goats with partial recovery 30 days post-CBD. A: GluA2-ir neurons within the NTS (20X, 0.50 NA) of a naive goat (top panel), a goat euthanized during hypoventilation after CBD (acute CBD-5 days; middle panel), and a goat euthanized after partial recovery of resting PaCO2 (chronic CBD-30 days; bottom panel). Note the qualitative decrease in GluA2 expression in the acute CBD goats and return of GluA2 expression in the chronic CBD goat. B: GluA2 optical density quantification within the NTS. On the y-axis is plotted normalized GluA2 OD ± SE, and on the x-axis is plotted distance rostral from obex (mm) in control/sham-CBD goats, acute-CBD goats, and chronic CBD goats. The F and P values are from the 2-way ANOVA (distance and condition as factors) on these data. C: average normalized GluA2 OD ± SE in 6 different respiratory nuclei and 1 nonrespiratory nucleus, the CN, in control/sham-CBD goats, acute CBD goats, and chronic-CBD goats. C, top panel, shows GluA2 OD from the caudal (c) division of each nucleus, and C, bottom panel, shows the rostral (r) division. The open symbols represent GluA2 OD from the individual sham-CBD goat euthanized at 5 days post-sham surgery. *P < 0.05 vs. control/sham-CBD, **P < 0.05 vs. naive/sham-CBD and chronic CBD.
After partial recovery of resting PaCO2 (30 days post-CBD), there was no rostral-caudal difference in GluA2 immunoreactivity in the NTS (Fig. 6B) or any other nuclei. At 30 days post-CBD, there was near-complete recovery within the caudal and rostral NTS (P > 0.05 vs. control/sham-CBD), and a partial but significant recovery within the caudal and rostral DMNV, caudal MR, and rostral CN (Fig. 6C). GluA2 OD was significantly reduced within the rostral FN to 88.0 ± 3.5% (P = 0.044 vs. naive/sham-CBD) and remained significantly decreased to 93.3 ± 1.8% (P = .012) with the caudal CN 30 days post-CBD. GluA2 OD was unchanged from naive/sham-CBD goats at all sites in partial-CBD goats at 30 days post-CBD (data not shown).
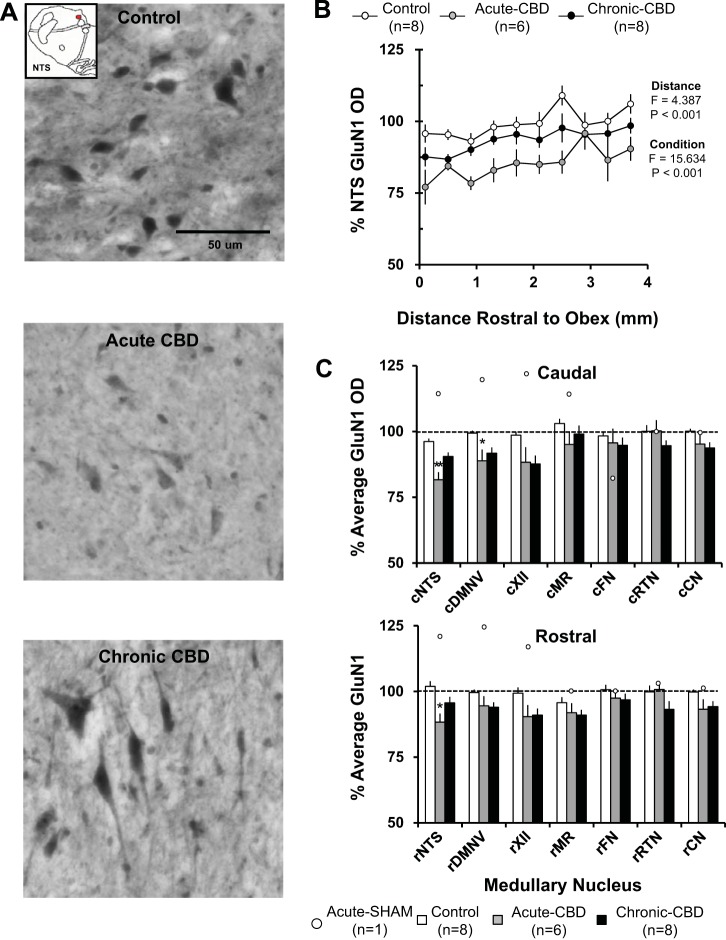
GluN1-ir Quantification in Medullary Nuclei
Both acutely and chronically after CBD, the immunoreactivity for GluN1 in the NTS (Fig. 7B), DMV, XII, MR, and CN was significantly (P < 0.05) greater in the rostral than in the caudal portion of the nuclei. Acutely after CBD (during peak hypoventilation), average GluN1 immunoreactivity was reduced compared with naive/sham-CBD goats to 81.6 ± 2.8% (P < 0.001) within the caudal NTS and to 88.3 ± 3.2% (P = 0.003) within the rostral NTS. GluN1 immunoreactivity was also significantly reduced to 88.8 ± 4.2% (P = 0.019) within the caudal DMNV (Fig. 7C). GluN1 OD was not affected at other sites measured. In the goat euthanized 5 days post-sham surgery, GluN1 OD tended to be higher than in the naive goats in several nuclei (Fig. 7C).
Fig. 7.
There was decreased GluN1 immunoreactivity in acute CBD goats with partial recovery by 30 days post-CBD. A: GluN1-ir neurons within the NTS (20X, 0.50 NA) of a naive goat (top panel), a goat euthanized during hypoventilation after CBD (acute CBD-5 days; middle panel), and a goat euthanized after partial recovery of resting PaCO2 (chronic CBD-30 days; bottom panel). Note the qualitative decrease in GluN1 expression in the acute CBD goats and return of GluN1 expression in the chronic CBD goat. B: GluN1 optical density quantification within the NTS. On the y-axis is plotted normalized GluN1 OD ± SE, and on the x-axis is plotted distance rostral from obex (mm) in control/sham-CBD goats, acute-CBD goats, and chronic CBD goats. The F and P values are from the two-way ANOVA (distance and condition) on these data. C: average normalized GluN1 OD ± SE in 6 different respiratory nuclei and 1 nonrespiratory nucleus, the CN, in naive/sham-CBD goats, acute CBD goats, and chronic-CBD goats. C, top panel, shows GluN1 OD from the caudal (c) division of each nucleus, and C, bottom panel, shows the rostral (r) division. The open symbols represent GluN1 OD from the individual sham-CBD goat euthanized at 5 days post-sham surgery. *P < 0.05 vs. control/sham-CBD, **P < 0.05 vs. control/sham-CB, and chronic CBD.
After partial recovery of resting PaCO2 (30 days post-CBD), there was a partial but significant recovery in the caudal NTS in GluN1 immunoreactivity, and within the rostral NTS and caudal DMNV (P > 0.05 vs. naive/sham-CBD) (Fig. 7C). GluN1 immunoreactivity was unchanged from naive/sham-CBD goats at all sites in partial-CBD goats 30 days post-CBD (data not shown).
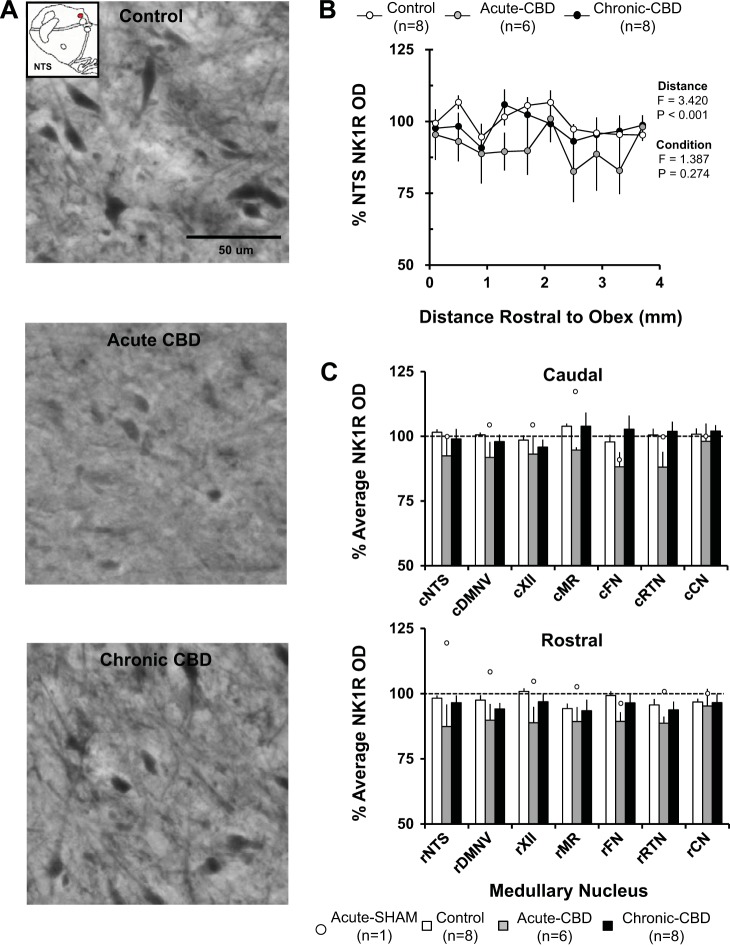
NK1R-ir Quantification in Medullary Nuclei
There were no rostral-caudal differences (P > 0.05) in the NTS (Fig. 8B) or any other nucleus in NK1R-r immunoreactivity acutely or chronically after CBD. In contrast to the glutamate receptor subunits, CBD did not significantly (P > 0.05) affect NK1R immunoreactivity acutely after CBD (Fig. 8C). NK1R immunoreactivity in the goat euthanized 5 days post-sham surgery was within the range of naive goats (Fig. 8C). In partial-CBD goats 30 days post-CBD (data not shown), NK1R-ir did not differ from naive goats.
Fig. 8.
NK1R immunoreactivity was nonsignificantly decreased in acute CBD goats with partial recovery by 30 days post-CBD. A: NK1R-ir neurons within the NTS (20X, 0.50 NA) of a naive goat (top panel), a goat euthanized during hypoventilation after CBD (acute CBD-5 days; middle panel), and a goat euthanized after partial recovery of resting PaCO2 (chronic CBD-30 days; bottom panel). Note the small qualitative decrease in NK1R expression in the acute CBD goats and return of NK1R expression in the chronic CBD goat. B: NK1R optical density quantification within the NTS. On the y-axis is plotted normalized NK1R OD ± SE, and on the x-axis is plotted distance rostral from obex (mm) in naive/sham-CBD goats, acute-CBD goats, and chronic CBD goats. The F and P values are from the 2-way ANOVA (distance and condition) on these data. C: average normalized NK1R OD ± SE in 6 different respiratory nuclei and 1 nonrespiratory nucleus, the CN, in control/sham-CBD goats, acute CBD goats, and chronic-CBD goats. C, top panel, shows NK1R OD from the caudal (c) division of each nucleus, and C, bottom panel, shows the rostral (r) division. The open symbols represent NK1R OD from the individual sham-CBD goat euthanized at 5 days post-sham surgery.
DISCUSSION
The major findings of the present study are the time-dependent changes in excitatory neurotransmitter and neuromodulator receptor immunoreactivity within the respiratory network following CBD. The data demonstrate that acutely after CBD there are decreases in GluA2 and GluN1, but increases or no change in GluA1 or NK1R immunoreactivity in multiple brain stem nuclei. At 30 days post-CBD there was a near-complete normalization of immunoreactivity of GluA2 and GluN1 receptors, which coincided with a time point when resting PaCO2 had partially but significantly (P < 0.05) recovered to near pre-CBD levels. Thus glutamatergic signaling and/or key molecules associated with established mechanisms of neuroplasticity significantly change within the brain stem respiratory network after CBD and therefore may contribute to the functional respiratory neuroplasticity following CBD in adult goats.
Caveats and Limitations
There are potential limitations in the use of immunohistochemical labeling techniques to measure changes in protein levels. For example, although the specificity of the GluA1 and GluA2 primary antibodies is supported by Western blots demonstrating bands at the predicted molecular weights (Fig. 1), broad expression of these receptors throughout the central nervous system makes it difficult to establish a negative control within the brain. Furthermore, it is not possible to knock out these (or any other) genes in goats to provide unequivocal control tissues. Additionally, the receptors that are of greatest physiological significance are those located at the synapse. The immunostaining approach utilized cannot establish changes in insertion of receptors in the postsynaptic membrane. However, the somatodendritic staining pattern we observed in goat medullary tissues was nearly identical to previous reports (19, 21, 37). Another potential limitation is in the quantitation of CBD-induced changes, which required normalization of raw OD measurements from tissue of CBD goats to tissue from naive or sham CBD goats immunostained simultaneously. We acknowledge there is the potential of misrepresentation/error in this quantitation process. However, given data shown in Fig. 3 (and for reasons discussed in methods), we are confident that we have obtained a valid representation of the effects of CBD on the proteins studied. Another potential limitation is that we quantified changes in proteins in a limited number of sites within the ventilatory control network; thus important changes may have occurred at sites which we did not study (for example NTS caudal to obex, pontine respiratory neurons, etc.). Finally, it was recently demonstrated that some types of respiratory plasticity involve changes in phosphorylated AMPA receptor expression, whereas total AMPA receptor expression is unchanged (32). Our present methods used primary antibodies that do not distinguish between phosphorylated and unphosphorylated glutamate receptors, and thus our present data cannot make this distinction.
Acute Effects of CBD on Brain Stem Protein Expression and Neurochemicals
A major acute effect of bilateral CBD in adult goats is hypoventilation and an attenuation of the ventilatory CO2 chemoreflex. These changes are associated with reduced excitatory neurochemicals (glutamine and dopamine) and increased inhibitory neurochemicals (GABA and glycine) within or near the pre-Botzinger complex as measured by microdialysis (25). In addition, there is a reduction in 1) the number of tryptophan hydroxylase (TPH)-expressing midline raphé neurons, and 2) the serotonin transport protein (SERT) density within respiratory nuclei, which would presumably reduce the capacity for the biosynthesis and recycling of synaptic 5-HT. These data were consistent with the hypothesis that the CBD-induced effects on ventilation could be due to “shifts in the balance between excitatory and inhibitory neurochemicals within the respiratory network” (25).
The ventilatory effects of CBD are transient, suggesting neuroplasticity within the respiratory network, and the changes in neurochemicals and proteins listed above did not recover over the first 30 days following CBD. Thus it is unlikely that 5-HT, a major initiator of several forms of brain stem/spinal cord neuroplasticity, would play a prominent role in the observed ventilatory recovery. Thus we tested the hypothesis herein that other neurochemical receptors, thought to participate and/or drive other forms of neuroplasticity, may display time-dependent changes in expression consistent with a role in initiating or maintaining the observed respiratory neuroplasticity. Synaptic scaling, a well-established form of “homeostatic plasticity” in the hippocampus and cortex, involves the upregulation or downregulation of postsynaptic receptors to stabilize neuronal activity. Synaptic scaling in vitro is known to occur within hours of a change in synaptic input (39, 40). If after CBD, synaptic scaling occurred within the NTS, there would have been an upregulation of excitatory receptors to counter the reduced presynaptic activity to thereby maintain postsynaptic excitability/output, and this increase in receptors should occur rapidly. Furthermore, scaling within the NTS should also prevent changes at other presumably downstream sites within the respiratory network. Given that 1) CBD leads to hypoventilation and a reduced CO2 chemoreflex for multiple weeks and 2) the reductions in excitatory AMPA receptor expression occur acutely after CBD at multiple sites, the present data are not consistent with the strict concept of homeostatic synaptic scaling.
Nevertheless, the putative mechanisms driving respiratory neuroplasticity after CBD appear to be regulated by and/or include a mechanism similar to scaling. Synaptic scaling involves the exchange of specific ionotropic glutamate receptors at the postsynaptic membrane to enable/disable calcium permeability. In adult animals, the insertion of GluA1/3 containing AMPA receptors into the synaptic membrane increases calcium permeability, a key regulator of this form of neuroplasticity (20, 39–41). Conversely, the insertion of GluA2 subunit-containing AMPA receptors reduces calcium permeability via a charged arginine residue at the “Q/R” site within the pore lining region (8, 37). Thus it may be predicted that in the case of a reduction in presynaptic activity there would be an acute increase in calcium-permeable AMPA receptors and a subsequent reduction in GluA2-containing AMPA receptors. For example, tetrodotoxin, high K+, and AMPA blockers increase the expression of calcium-permeable AMPA receptors in slice or cultured cerebellar granule (15) and stellate cells (22), and also in cells from the hippocampus (14, 38) and visual cortex (1). Further, after the initiating event, GluA2 might then return to normal (or perhaps greater) levels during the maintenance phase of recovery.
Our data fit this proposed shift as the relative ratio of calcium-permeable AMPA receptors are increased relative to calcium-impermeable AMPA receptors in brain stem tissues collected from goats 5 days post-CBD. Additionally, GluN1 subunit-containing NMDA receptors followed the same expression pattern as GluA2 in these brain stem nuclei. Given that NMDA receptors are known to increase calcium permeability, these data suggest that any increase in calcium permeability acutely after CBD is likely specific to shifts in AMPA receptors. Indeed, recent studies suggest that some types of synaptic plasticity are specifically regulated by NMDAR-independent calcium entry (17, 18, 24). However, this point remains contentious as others found that during activity-dependent changes in glutamate receptor expression, synaptic currents from AMPA receptors and NMDA receptors remain proportional (34, 42). In summary, the time-dependent decrease and return of AMPA and NMDA glutamate receptor expression may represent a form of plasticity which is regulated by NMDAR-independent calcium entry.
Direct and Indirect Effects of CBD
The physiological effects of CBD include chronic hypercapnia, mild hypoxemia, and a loss of tonic carotid body drive to breathe. Each of these conditions may alter receptor expression independently and contribute to shifts in excitatory and inhibitory mechanisms. Previous studies have found that acute hypoxia and CBD can alter brain stem glutamate metabolism (13) and glutamate release under anesthesia (12, 27). Our current studies suggest that the absence of carotid afferents transiently decreases medullary glutamate receptor expression levels. It is likely that all of these effects on the glutamatergic system contribute to the uniform reduction in ventilation under multiple conditions after CBD (33). Indeed, there may be a direct contribution of chronic hypercapnia and/or hypoxia on neurochemical receptor expression after CBD as we found clear effects in the CN, which is a non-respiratory-related nucleus. However, the changes in glutamate receptor immunoreactivity were not correlated with the severity of hypoventilation post-CBD (25). Alternatively, it may be that glutamate receptor expression is not regulated in a site-specific manner.
The transient return of the CO2 chemoreflex by 15 days after CBD in this and previous studies (11, 33) suggests multiple mechanisms contributing to ventilatory recovery after CBD, i.e., separate mechanisms of neuroplasticity for eupneic PaCO2 and the CO2 chemoreflex. Additionally, the incomplete recovery of eupneic PaCO2 and the decreased CO2 chemoreflex by 30 days post-CBD also suggests that the central respiratory network in goats have a limited or slowly developing capacity to express plasticity after CBD. Similarly, humans chronically retain CO2 after CBD despite near-complete recovery of the CO2 chemoreflex, but this recovery requires months to years (2, 9, 23). In contrast, rats reach near-total recovery of resting PaCO2 within 3 wk after CBD (28). Moreover, the CO2 chemoreflex is not attenuated after CBD in rats (28). It appears therefore that goats are a more appropriate model than rats to obtain insights into the respiratory neuroplasticity that occurs in humans after CBD and likely other perturbations of the respiratory control network.
Studies beyond 30 days after CBD may provide insight into other compensatory mechanisms after CBD. In goats, recovery of breathing 4 mo after thoracic dorsal rhizotomy was associated with increased 5-HT innervation within the thoracic dorsal motor neurons innervating the intercostal muscles (26). Also in goats, recovery from CBD induced hypoventilation 40 days or more after CBD was associated with increased responses to ibotenic acid injections in the raphé nucleus (11). These two findings suggest that long-term plasticity after peripheral sensory denervation may involve both serotonergic and glutamatergic mechanisms. Furthermore, these findings also warrant the study of spinal plasticity and/or plasticity within respiratory sites outside of the medulla after CBD.
Significance of Findings
The findings herein are significant for several reasons. First, the data are unique in that there is a paucity of previous data on CNS mechanisms of neuroplasticity associated with peripheral deafferentation. Second, the changes in glutamate receptor expression tend to follow aspects of known forms/mechanisms of neuroplasticity within the hippocampus and cortex; thus, the considerable understanding that has been obtained regarding plasticity in these higher centers may be useful in further studies on neuroplasticity in brain stem nuclei. Third, the suggestion that plasticity after loss of carotid afferents may have been affected by hypercapnia and hypoxia suggests that during clinical conditions in which hypoventilation occurs, secondary factors likely affect brain stem neurochemicals and/or the capacity for neuroplasticity. Fourth, as stated above, the goat appears to be an appropriate model for respiratory neuroplasticity in humans after perturbations of the respiratory control network; thus, the present study provides a basis for future studies aimed at obtaining insights into disease- and/or traumatic injury-induced perturbation of the respiratory control network.
We conclude that there are multiple shifts in both excitatory and inhibitory neurochemicals, and glutamate receptor expression profiles, within multiple brain stem nuclei after CBD in adult goats. The initial depression, and subsequent increase to near-control levels, observed in the expression of multiple excitatory receptors after CBD suggests that the mechanisms of central neuroplasticity are complex and multiphasic but likely include shifts in the expression of AMPA and NMDA receptors. Finally, compared with humans, the similar findings of hypoventilation and reduced CO2 chemoreflex after CBD in goats suggests our model provides a viable approach to further study plasticity within the respiratory system in vivo.
GRANTS
Funding for this study was provided by National Heart, Lung, and Blood Institute Grants HL-25739, HL-112996, and HL-007852 and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.R.M., M.R.H., and H.V.F. conception and design of research; J.R.M., S.E.N., C.M., S.J.O., L.G.P., and A.o.D. performed experiments; J.R.M., S.E.N., S.J.O., J.D.B., A.o.D., and H.V.F. analyzed data; J.R.M., M.R.H., and H.V.F. interpreted results of experiments; J.R.M., S.E.N., S.J.O., and A.o.D. prepared figures; J.R.M. drafted manuscript; J.R.M., S.E.N., S.J.O., M.R.H., and H.V.F. edited and revised manuscript; J.R.M. and H.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
All experiments were performed at The Medical College of Wisconsin, Milwaukee, WI.
REFERENCES
- 1.Bai X, Wong-Riley MT. Neuronal activity regulates protein and gene expressions of GluR2 in postnatal rat visual cortical neurons in culture. J Neurocytol 32: 71–78, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol 46: 843–853, 1979 [DOI] [PubMed] [Google Scholar]
- 3.Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J Appl Physiol 49: 964–970, 1980 [DOI] [PubMed] [Google Scholar]
- 4.Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J Appl Physiol 40: 184–190, 1976 [DOI] [PubMed] [Google Scholar]
- 5.Bouverot P, Collin R, Favier R, Flandrois R, Sebert P. Carotid chemoreceptor function in ventilatory and circulatory O2 convection of exercising dogs at low and high altitude. Respir Physiol 43: 147–167, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Canny J. A computational approach to edge detection. IEEE Transact Pattern Analysis Machine Intell 8: 679–698, 1986 [PubMed] [Google Scholar]
- 7.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5: 952–962, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opinion Neurobiol 16: 288–297, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med 4: e239, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges MR, Opansky C, Qian B, Davis S, Bonis JM, Krause K, Pan LG, Forster HV. Carotid body denervation alters ventilatory responses to ibotenic acid injections or focal acidosis in the medullary raphe. J Appl Physiol 98: 1234–1242, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Hoop B, Beagle JL, Maher TJ, Kazemi H. Brainstem amino acid neurotransmitters and hypoxic ventilatory response. Respir Physiol 118: 117–129, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Hoop B, Masjedi MR, Shih VE, Kazemi H. Brain glutamate metabolism during hypoxia and peripheral chemodenervation. J Appl Physiol 69: 147–154, 1990 [DOI] [PubMed] [Google Scholar]
- 14.Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci 7: 244–253, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol 486: 297–303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Botzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science 285: 1411–1414, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron 33: 921–933, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Lin LH, Taktakishvili OM, Talman WT. Colocalization of neurokinin-1, N-methyl-d-aspartate, and AMPA receptors on neurons of the rat nucleus tractus solitarii. Neuroscience 154: 690–700, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA 95: 7097–7102, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Wong-Riley MT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J Appl Physiol 98: 1442–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Liu SJ, Cull-Candy SG. Activity-dependent change in AMPA receptor properties in cerebellar stellate cells. J Neurosci 22: 3881–3889, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lugliani R, Whipp BJ, Seard C, Wasserman K. Effect of bilateral carotid-body resection on ventilatory control at rest and during exercise in man. N Engl J Med 285: 1105–1111, 1971 [DOI] [PubMed] [Google Scholar]
- 24.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature 394: 683–687, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Miller JR, Neumueller S, Muere C, Olesiak S, Pan L, Hodges MR, Forster HV. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J Appl Physiol 115: 1088–1098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell GS, Bach KB, Martin PA, Foley KT, Olson EB, Brownfield MS, Miletic V, Behan M, McGuirk S, Sloan HE. Increased spinal monoamine concentrations after chronic thoracic dorsal rhizotomy in goats. J Appl Physiol 89: 1266–1274, 2000 [DOI] [PubMed] [Google Scholar]
- 27.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol 478: 55–66, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J Physiol 590: 3335–3347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Botzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol 114: 694–704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101: 1596–1606, 2006 [DOI] [PubMed] [Google Scholar]
- 31.O'Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron 21: 1067–1078, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Pamenter M, Carr A, Go A, Fu Z, Reid S, Powell F. Glutamate receptors in the nucleus tractus solitarius contribute to ventilatory acclimatization to hypoxia in rat. J Physiol 592: 1839–1856, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan LG, Forster HV, Martino P, Strecker PJ, Beales J, Serra A, Lowry TF, Forster MM, Forster AL. Important role of carotid afferents in control of breathing. J Appl Physiol 85: 1299–1306, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci 28: 229–238, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Rodman JR, Curran AK, Henderson KS, Dempsey JA, Smith CA. Carotid body denervation in dogs: eupnea and the ventilatory response to hyperoxic hypercapnia. J Appl Physiol 91: 328–335, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Tanaka H, Grooms SY, Bennett MV, Zukin RS. The AMPAR subunit GluR2: still front and center-stage. Brain Res 886: 190–207, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47: 725–737, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harbor Perspect Biol 4: a005736, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- 41.Wang G, Gilbert J, Man HY. AMPA receptor trafficking in homeostatic synaptic plasticity: functional molecules and signaling cascades. Neural Plast 2012: 825364, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron 26: 659–670, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Botzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol 97: 1620–1628, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Botzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629–1636, 2004 [DOI] [PubMed] [Google Scholar]