Abstract
We examined the hypothesis that changes in the cerebrovascular resistance index (CVRi), independent of blood pressure (BP), will influence the dynamic relationship between BP and cerebral blood flow in humans. We altered CVRi with (via controlled hyperventilation) and without [via indomethacin (INDO, 1.2 mg/kg)] changes in PaCO2. Sixteen subjects (12 men, 27 ± 7 yr) were tested on two occasions (INDO and hypocapnia) separated by >48 h. Each test incorporated seated rest (5 min), followed by squat-stand maneuvers to increase BP variability and improve assessment of the pressure-flow dynamics using linear transfer function analysis (TFA). Beat-to-beat BP, middle cerebral artery velocity (MCAv), posterior cerebral artery velocity (PCAv), and end-tidal Pco2 were monitored. Dynamic pressure-flow relations were quantified using TFA between BP and MCAv/PCAv in the very low and low frequencies through the driven squat-stand maneuvers at 0.05 and 0.10 Hz. MCAv and PCAv reductions by INDO and hypocapnia were well matched, and CVRi was comparably elevated (P < 0.001). During the squat-stand maneuvers (0.05 and 0.10 Hz), the point estimates of absolute gain were universally reduced, and phase was increased under both conditions. In addition to an absence of regional differences, our findings indicate that alterations in CVRi independent of PaCO2 can alter cerebral pressure-flow dynamics. These findings are consistent with the concept of CVRi being a key factor that should be considered in the correct interpretation of cerebral pressure-flow dynamics as indexed using TFA metrics.
Keywords: cerebral blood flow, transfer function analysis, cerebral hemodynamics, physiology, transcranial doppler, resistance, carbon dioxide
one common method to assess the dynamic relationship between arterial blood pressure (BP) and cerebral blood velocity (CBV) is through the use of transfer function analysis (TFA) (3, 39). TFA utilizes Fourier transforms to quantify the linear relationship between BP and CBV and provides information on the statistical dependence between the input (BP) and output (CBV) variables (coherence), the time lag (phase), and signal amplitude modulation (gain) (32, 39). To date, TFA has been used to examine the influence of the cerebrovascular resistance index (CVRi) on the dynamic cerebral pressure-flow relations under physiological (altered PaCO2 levels) (3, 32, 38, 39) or pharmacological interventions (e.g., via phenylephrine infusion) (15, 32, 38, 39). However, the way in which CVRi is manipulated under both of these conditions is fundamentally different, and it is unclear whether the changes in CVRi alone result in similar changes to those induced via hypocapnia.
Alterations to CVRi via PaCO2 changes occur independent of changes to arterial BP by either increasing or decreasing the diameter of downstream cerebral arteriole (15, 38). In contrast, phenylephrine increases CVRi via an entirely different mechanism (18, 38). Phenylephrine is an α1-adrenergic receptor agonist and likely does not cross the blood-brain barrier (18, 22). Instead, it acts as a systemic vasopressor to increase arterial BP, which in turn, results in an increased CVRi. How a pharmacologically induced increase in CVRi independent of alterations to PaCO2 and arterial BP may influence the dynamic cerebral pressure-flow relationship has yet to be experimentally examined.
The purpose of our study was to investigate the influence of CVRi on the cerebral pressure-flow relationships in healthy humans independent of alterations to PaCO2 and arterial BP. To address this question, we administered a clinical dose of indomethacin (INDO), a potent and reversible cyclooxygenase inhibitor that is able to cross the blood-brain barrier. INDO has been shown to lower cerebral blood flow (CBF) by 30–50% (13, 29, 36) and elevate CVRi without altering metabolic rate (13, 35), plasma catecholamines (9, 35), or PaCO2 (9, 32, 39). We also induced hypocapnia by voluntary hyperventilation to effectively match the elevations in cerebrovascular resistance that were present in the INDO trial, to confirm that the alterations in the TFA metrics were a result of the increased CVRi. The dynamic relationship between BP and CBV was quantified during exogenously driven changes in BP in the very low (0.05 Hz) and low (0.10 Hz) frequency ranges (14, 32, 39). We hypothesized that increases in CVRi, independent of alterations to PaCO2 or arterial BP, should lead to an increased phase and decreased gain metric in both the anterior and posterior regions of the brain.
MATERIALS AND METHODS
Ethical Approval
The study was approved by and complied with the standards set by the clinical ethical review board of the University of British Columbia. All volunteers provided written informed consent.
Subjects
Sixteen healthy subjects (12 men, 27.2 ± 7.2 yr, BMI 25.6 ± 2.9 kg/m2) were recruited for this study. None had a history of cardiorespiratory or cerebrovascular disease, and none were taking any form of medication. All subjects abstained from exercise, caffeine, and alcoholic beverages for a period of 12 h prior to the study. Each subject underwent familiarization of the laboratory and testing protocols before initiation of the protocols.
Experimental Conditions
The subjects were required to visit the laboratory on two occasions. The subjects underwent three experimental trials: no intervention (control), oral INDO (1.2 mg/kg), and hypocapnia. The first two trials were performed on day 1 in the following order: control measures; oral INDO administration (dose, 1.2 mg/kg body wt); 90 min rest; then INDO measures. On day 2 (minimum 2-day washout period) the subjects were instructed to hyperventilate (leading to an increase in CVRi resulting in a decrease in CBV) until the reduction in the middle cerebral artery velocity (MCAv) matched the reduction of the INDO trial. The matched level of end-tidal CO2 (PetCO2) during the hypocapnia trials was projected onto a screen, and each subject was coached on his rate and depth of breathing. This was performed to maintain a stable PetCO2 throughout each hypocapnic experimental condition (seated baseline, and both squat-stand frequencies).
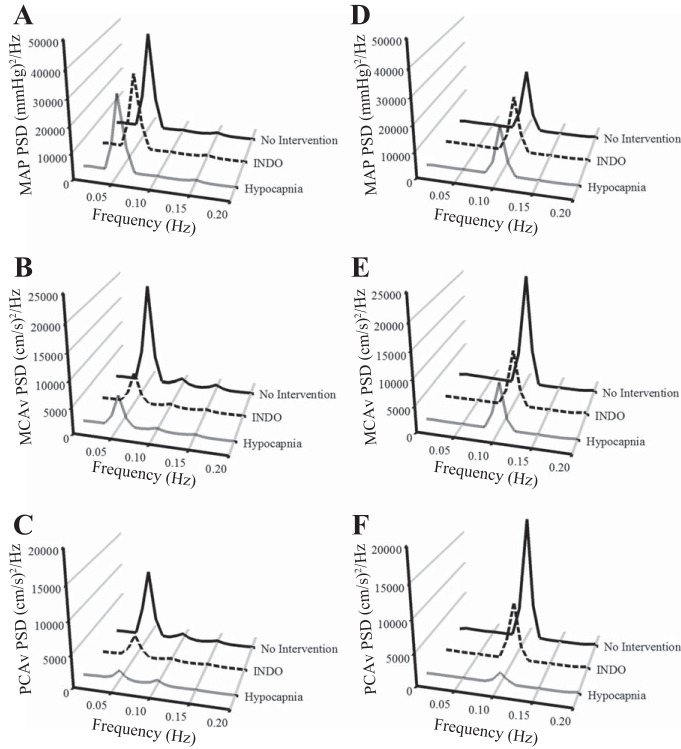
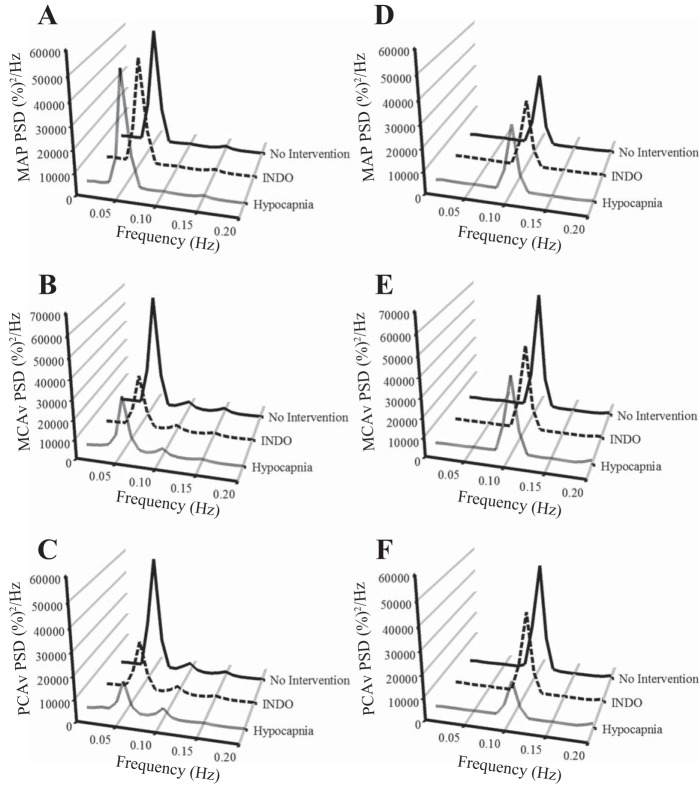
Resting spontaneous baseline data were recorded in a seated position. These data were used for the baseline measures of BP, breathing frequency, PetCO2, and CBV in the anterior (MCAv) and posterior (posterior cerebral artery velocity; PCAv) cerebral circulatory regions. To try to maximize the signal-to-noise ratio that is commonly associated with lower coherence values present in spontaneous cerebral autoregulation (CA) measures (14, 23, 26, 32), we had the subjects also perform repeated squat-stand maneuvers. The subjects mimicked the experimenter in performing these maneuvers to ensure that all subjects performed the maneuvers at a similar depth. The subjects were randomly selected to perform either a 0.05 Hz maneuver (10 s squat, 10 s stand) or 0.10 Hz maneuver (5 s squat, 5 s stand) for 5 min each or vice versa, with a 3-min rest period to return to baseline levels between trials. These data were used for the spectral analysis of the driven oscillations in BP and CBV in the MCAv and PCAv, as representative vessels of the anterior and posterior cerebral circulatory regions at the driven frequency. The driven data were selected from the point estimate at which we drove the BP (either 0.05 Hz or 0.10 Hz) because this point had the highest signal-to-noise ratio, highest power spectrum density (PSD) value, and thus the highest coherence value [refer to Fig. 1 for absolute PSD units and Fig. 2 for normalized PSD units; the variance (SD) for Figs. 1 and 2 is shown in Table 1]. The frequency at which the PSD reached peak amplitude (either 0.05 Hz or 0.10 Hz) was used as a basis for sampling the point estimates for coherence, phase and gain. The increased coherence indicates increased linearity within the BP and CBF relationship, and enables a stronger mathematical interpretation of the TFA phase and gain metrics. This practice has been used by our laboratory (28, 32) and others (8). During all trials, end tidal gases were monitored to ensure that normal breathing occurred and Valsalva-like maneuvers were avoided.
Fig. 1.
Absolute values of power spectrum densities (PSDs) for mean arterial pressure (MAP) at 0.05 Hz (A) and 0.10 Hz (D), middle cerebral artery velocity (MCAv) at 0.05 Hz (B) and 0.10 Hz (E), and posterior cerebral artery velocity (PCAv) at 0.05 Hz (C) and 0.10 Hz (F) during squat-stand maneuvers for three interventions. The frequency at which the PSD reached peak amplitude (either 0.05 Hz or 0.10 Hz) was used as a basis for sampling the point estimates for coherence, phase, and gain. At 0.05 Hz, MCAv and PCAv PSD during indomethacin and hypocapnia interventions were reduced compared with the no-intervention condition; at 0.10 Hz, MCAv PSD during indomethacin and hypocapnia interventions and PCAv PSD during the hypocapnia intervention were reduced compared with the no-intervention condition (see Table 1 for details).
Fig. 2.
Normalized values of PSDs for MAP at 0.05 Hz (A) and 0.10 Hz (D), MCAv at 0.05 Hz (B) and 0.10 Hz (E), and PCAv at 0.05 Hz (C) and 0.10 Hz (F) during squat-stand maneuvers for three interventions. The frequency at which the PSD reached peak amplitude (either 0.05 Hz or 0.10 Hz) was used as a basis for sampling the point estimates for coherence, phase, and gain. At 0.05 Hz, MCAv and PCAv PSD during indomethacin and hypocapnia interventions were reduced compared with the no-intervention condition; at 0.10 Hz, MCAv PSD during indomethacin and hypocapnia interventions and PCAv PSD during the hypocapnia intervention were reduced compared with the no-intervention condition (see Table 1 for details).
Table 1.
Absolute and normalized point estimates of the power spectrum densities of MAP, MCAv, and PCAv during the squat-stand maneuvers for the three interventions
| No Intervention | Indomethacin | Hypocapnia | |
|---|---|---|---|
| Squat-stand, 0.05 Hz | |||
| MAP power, (mmHg)2/Hz | 37,884 ± 17,586 | 29,552 ± 14,488 | 27,741 ± 12,256 |
| MAP power, %2/Hz | 51,498 ± 30,281 | 47,176 ± 44,840 | 50,710 ± 34,557 |
| MCAv power, (cm/s)2/Hz | 18,617 ± 9,625 | 5,382 ± 4,231* | 5,896 ± 4,882* |
| MCAv power, %2/Hz | 58,312 ± 27,093 | 27,218 ± 18,835* | 28,121 ± 20,516* |
| PCAv power, (cm/s)2/Hz | 10,043 ± 5,253 | 3,518 ± 2,661* | 2,244 ± 2,979* |
| PCAv power, %2/Hz | 50,626 ± 23,048 | 21,982 ± 16,680* | 14,478 ± 17,027* |
| Squat-stand, 0.10 Hz | |||
| MAP power, (mmHg)2/Hz | 24,005 ± 9,999 | 21,161 ± 12,534 | 18,916 ± 10,554 |
| MAP power, %2/Hz | 32,417 ± 22,947 | 29,684 ± 28,311 | 29,080 ± 22,642 |
| MCAv power, (cm/s)2/Hz | 21,247 ± 11,263 | 11,339 ± 7,820* | 7,860 ± 4,567* |
| MCAv power, %2/Hz | 60,262 ± 22,152 | 43,819 ± 21,599* | 39,642 ± 27,029* |
| PCAv power, (cm/s)2/Hz | 10,989 ± 5,357 | 5,651 ± 3,838 | 2,637 ± 2,098* |
| PCAv power, %2/Hz | 48,473 ± 20,166 | 36,269 ± 21,913 | 16,229 ± 13,078* |
Values are means ± SD.
MAP, mean arterial pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity.
Statistical significance was set at P < 0.05.
Significance from no intervention. †Significance from the indomethacin intervention.
Instrumentation
Subjects first received a three-lead electrocardiogram (ECG) for measurement of the R-R interval. BP was measured in the finger by photoplethysmography (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands). This method has been shown to reliably assess the dynamic changes in beat-to-beat BP that correlate well with the intraarterial recordings and can be used to characterize the dynamic relationship between BP and CBF (2, 23, 26, 37).
The middle cerebral artery (MCA) and posterior cerebral artery (PCA) were insonated by placing 2-MHz Doppler probes (Spencer Technologies, Seattle, WA) to obtain CBV in the MCA and PCA. The MCA and PCA were identified and optimized according to their signal depth, wave form, and velocities (2, 32, 37, 39). Once the MCA and PCA were identified the probes were secured and locked in place with a headband (Spencer Technologies). CVRi was calculated from mean BP/mean CBV. PetCO2 was monitored using an online gas analyzer (ML206; AD Instruments, Colorado Springs, CO) and was calibrated with a known gas concentration prior to each trial. Heart rate was calculated from the ECG, and breathing frequency was determined from the CO2 trace. All data were recorded and stored for subsequent analysis using commercially available software (LabChart version 7.1; AD Instruments).
Data Processing
All data were simultaneously sampled at 1,000 Hz via an analog-to-digital converter (Powerlab 16/30 ML880; AD Instruments). Real-time beat-to-beat mean values of BP, MCA, and PCA velocity were determined from each R-R interval. All data were processed and analyzed with custom-designed software in LabView 11 (National Instruments, Austin, TX).
Power Spectrum and TFA
Beat-to-beat BP and MCAv/PCAv signals were spline interpolated and resampled at 4 Hz for spectral and transfer function analyses on the basis of the Welch algorithm. Each 5-min recording was first subdivided into five successive windows that overlapped by 50%. Data within each window were linearly detrended and passed through a Hanning window prior to fast Fourier transform analysis. For TFA, the cross-spectrum between BP and MCAv/PCAv was determined and divided by the mean arterial pressure autospectrum to derive the transfer function coherence, gain, and phase.
The transfer function coherence, gain, and phase of the driven BP oscillations were sampled at the point estimate of the driven frequency (0.05 or 0.10 Hz). These point estimates were selected because they are in the very low frequency (VLF; 0.02–0.07 Hz) and low frequency (LF; 0.07–0.20 Hz) ranges where CA is believed to be operant (10, 32, 39).
Statistical Analysis
Statistical analyses were performed using PASW version 18.0 for Windows (PASW, Chicago, IL). The effects of trial (control, INDO, hypocapnia) on MCAv; PCAv; heart rate; breathing frequency; BP; PetCO2; CVRi; and transfer function coherence, gain (absolute and normalized), and phase were assessed using a one-way repeated measures ANOVA with a Bonferroni correction for main effects and were run for each driven condition (0.05 Hz and 0.10 Hz). Comparisons between the anterior and posterior cerebral vessels were performed using paired t-tests with Bonferroni correction. Data are presented as mean ± SD for each 5-min experimental condition, and P < 0.05 was considered statistically significant.
RESULTS
There were no differences in PetCO2 (41.4 ± 3.6 vs. 40.3 ± 2.3 mmHg); MCAv (67.7 ± 10.6 vs. 66.4 ± 10.4 cm/s); PCAv (48.8 ± 10.2 vs. 47.3 ± 9.7 cm/s); BP (84.1 ± 11.6 vs. 84.7 ± 11.3 mmHg), or heart rate (61.3 ± 9.8 vs. 63.2 ± 10.4 bpm) between baseline periods on either day 1 or day 2 of the experimental protocols.
Cerebrovascular Responses
During the seated baseline trials there was a −32% and −31% reduction (P < 0.001) in MCAv and PCAv, respectively, during the INDO intervention, and a similar −34% and −37% reduction (P < 0.001) during the hypocapnia intervention (Table 2). There were no changes in BP across either of the interventions, which in conjunction with the reduced MCAv/PCAv, resulted in an increase in CVRi of approximately +50% in both the anterior and posterior regions of the brain (P < 0.001). These alterations to CBV occurred with (hypocapnia, 25.5 mmHg) and without (INDO, 39.9 mmHg) a reduction in PetCO2. There were no significant differences between breathing frequency between the control (13.8 ± 4.4 breaths/min) and INDO (14.0 ± 3.8 breaths/min) baseline measures (P = 0.990); the hypocapnia breathing frequency (26.4 ± 6.3 breaths/min) was significantly higher than both the control intervention and INDO trials by study design (P < 0.001). Although not significantly different, heart rate trended toward a reduction in the INDO trial compared with the control intervention condition (P = 0.057). Heart rate during the hypocapnia condition was higher than during the INDO trial (P < 0.001).
Table 2.
Hemodynamic and cerebrovascular responses during squat-stand maneuvers
| No Intervention | Indomethacin | Hypocapnia | |
|---|---|---|---|
| Baseline, sitting | |||
| Mean BP, mmHg | 84.1 ± 11.6 | 85.4 ± 13.5 | 81.3 ± 9.2 |
| Mean MCAv, cm/s | 67.7 ± 10.6 | 45.5 ± 8.4* | 44.7 ± 7.1* |
| MCA CVRi, mmHg·cm−1·s−1 | 1.3 ± 0.3 | 1.9 ± 0.4* | 1.9 ± 0.3* |
| Mean PCAv, cm/s | 48.8 ± 10.2 | 33.6 ± 7.6* | 30.5 ± 9.6* |
| PCA CVRi, mmHg·cm−1·s−1 | 1.8 ± 0.5 | 2.6 ± 0.7* | 2.9 ± 0.8* |
| End tidal PCO2, mmHg | 41.4 ± 3.6 | 39.9 ± 3.7 | 25.5 ± 4.7*† |
| Breathing frequency, per min | 13.8 ± 4.4 | 14.0 ± 3.8 | 26.4 ± 6.3*† |
| Heart rate, bpm | 61.2 ± 9.8 | 52.6 ± 8.3 | 69.0 ± 12.5† |
| Squat-stand, 0.05 Hz | |||
| Mean BP, mmHg | 92.4 ± 13.0 | 91.4 ± 16.3 | 89.7 ± 11.9 |
| Mean MCAv, cm/s | 59.9 ± 12.9 | 48.9 ± 9.7* | 48.3 ± 7.5* |
| MCA CVRi, mmHg·cm−1·s−1 | 1.6 ± 0.4 | 1.9 ± 0.4* | 1.9 ± 0.3* |
| Mean PCAv, cm/s | 45.4 ± 8.2 | 35.9 ± 8.1* | 32.4 ± 7.5* |
| PCA CVRi, mmHg·cm−1·s−1 | 2.1 ± 0.4 | 2.6 ± 0.7* | 2.8 ± 0.7* |
| End tidal PCO2, mmHg | 38.9 ± 3.2 | 37.7 ± 3.5 | 25.8 ± 4.3*† |
| Breathing frequency, per min | 16.2 ± 4.5 | 17.5 ± 4.6 | 27.0 ± 7.0*† |
| Heart rate, bpm | 86.3 ± 14.2 | 78.7 ± 12.5 | 84.5 ± 11.2 |
| Squat-stand, 0.10 Hz | |||
| Mean BP, mmHg | 93.3 ± 15.4 | 91.2 ± 12.1 | 89.7 ± 11.1 |
| Mean MCAv, cm/s | 60.5 ± 13.8 | 50.0 ± 9.3* | 48.1 ± 7.4* |
| MCAv CVRi, mmHg·cm−1·s−1 | 1.6 ± 0.4 | 1.9 ± 0.3* | 1.9 ± 0.3* |
| Mean PCAv, cm/s | 44.9 ± 8.5 | 36.2 ± 7.0* | 33.1 ± 7.5* |
| PCAv CVRi, mmHg·cm−1·s−1 | 2.1 ± 0.5 | 2.6 ± 0.6* | 2.7 ± 0.6* |
| End tidal PCO2, mmHg | 39.6 ± 4.4 | 38.0 ± 4.3 | 25.5 ± 4.2*† |
| Breathing frequency, per min | 17.0 ± 4.0 | 18.1 ± 5.0 | 28.7 ± 7.8*† |
| Heart rate, bpm | 86.0 ± 12.9 | 79.0 ± 11.6 | 85.9 ± 11.2 |
Values are means ± SD.
BP, blood pressure; MCAv, middle cerebral artery velocity; PCAv, posterior cerebral artery velocity; CVRi, cerebrovascular resistance index; bpm, beats per minute.
Statistical significance was set at P < 0.05.
Significance from no intervention.
Significance from indomethacin intervention.
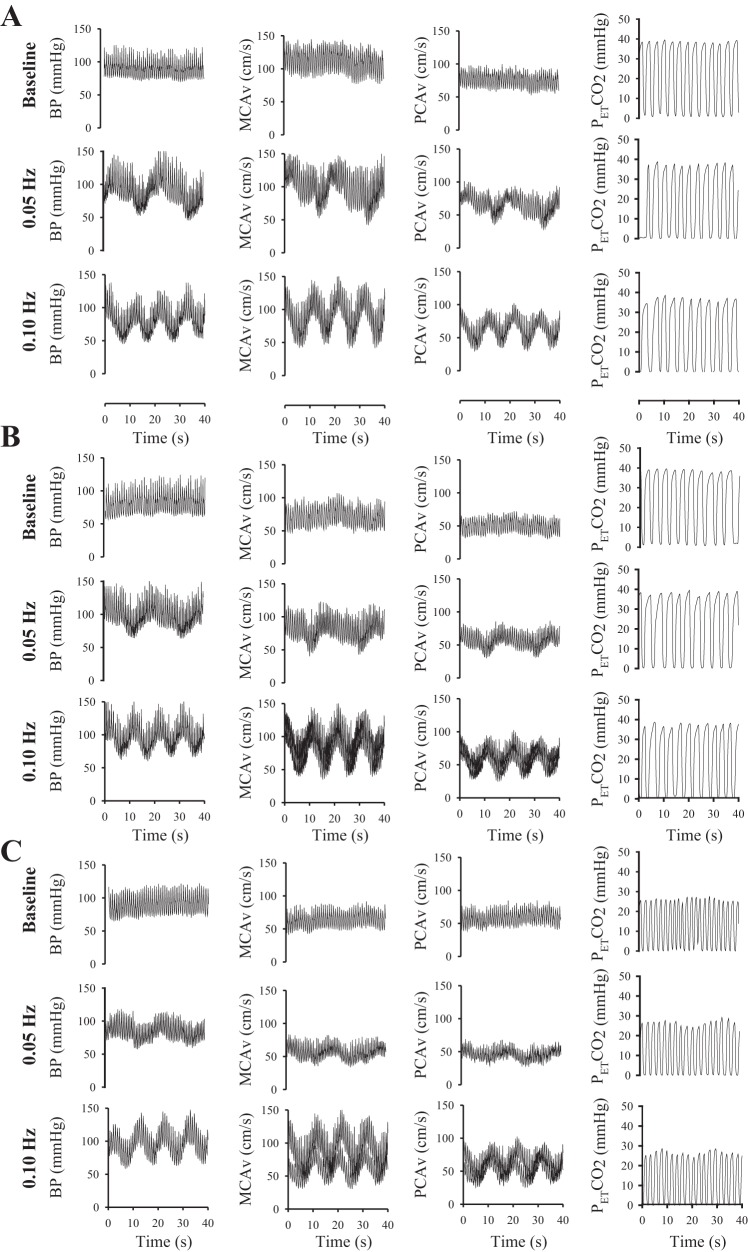
During the driven oscillations (representative trace shown in Fig. 3), compared with rest at 0.05 and 0.10 Hz, there was a reduction in both MCAv and PCAv of approximately −20% to −30% (P < 0.01) during both experimental interventions that were independent of any alteration in BP. The resulting increase in CVRi was similar in both the INDO and hypocapnia interventions (approximately +20% in MCAv and approximately +30% in PCAv; P < 0.05). There were no differences in heart rate during any of the experimental conditions at either 0.05 Hz (P > 0.22) or 0.10 Hz (P > 0.23). Breathing frequency was not different between the control and INDO trials at either 0.05 Hz (P = 0.797) or 0.10 Hz (P = 0.868). By study design, the hypocapnia trials at both 0.05 Hz and 0.10 Hz had higher breathing frequency than both the control and INDO trials (P < 0.001).
Fig. 3.
Representative individual data of the raw wave form highlighting the effects of squat-stand maneuvers on blood pressure (BP), MCAv, PCAv, and end-tidal CO2 levels (PetCO2). The data are presented for no-intervention (A), indomethacin (B), and hypocapnia (C) experimental conditions.
Transfer Function Analysis
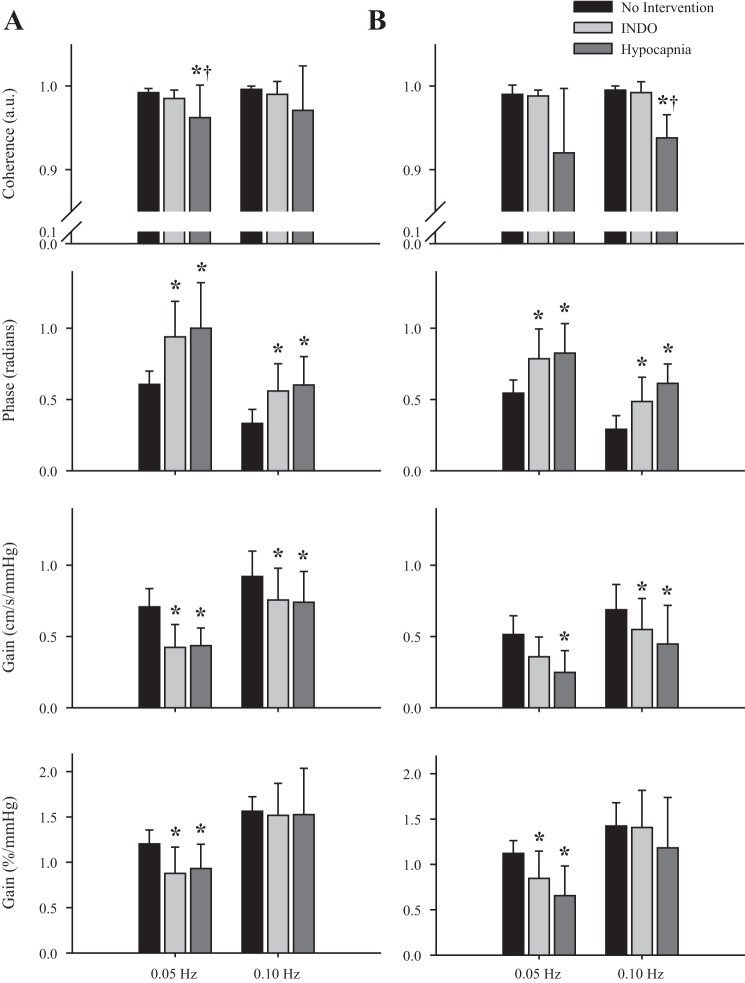
When BP was driven with the squat-stand maneuvers, coherence was increased at both frequencies (0.05 Hz and 0.10 Hz) under all conditions to >0.92 (P < 0.001). In the MCA (Fig. 4A), gain was reduced by approximately −40% at 0.05 Hz and approximately −20% at 0.10 Hz during the INDO and hypocapnia interventions, compared with baseline, respectively. These reductions were also present in the posterior circulation because the PCA (Fig. 4B) had reductions in gain of −32% INDO, −52% hypocapnia; and −20% INDO, −35% hypocapnia at 0.05 Hz and 0.10 Hz, respectively. When gain was normalized to %CBV, it was reduced in the INDO and hypocapnia interventions at 0.05 Hz (P < 0.001), but not at 0.10 Hz (P > 0.05). At the point estimate of 0.05 Hz, phase was comparable (0.60 and 0.54 radians) for the anterior and posterior regions when there was no intervention (P > 0.05). Phase within both regions increased approximately +50% with the increased CVRi during the INDO and hypocapnia interventions (P < 0.001). At 0.10 Hz, phase was once again comparable in both the anterior (0.33 radians) and posterior (0.29 radians) cerebral circulatory systems (P > 0.05). However, increased CVRi resulted in a greater phase lead in the MCA (+72% INDO, +85% hypocapnia) and PCA (+67% INDO, +111% hypocapnia; P < 0.001).
Fig. 4.
Transfer function analysis of coherence (top), phase (middle), and gain (bottom) for the group means of the MCAv-driven (A) and PCAv-driven (B) oscillations during three experimental conditions: no intervention (black), indomethacin (light gray), and hypocapnia (dark gray). *Significance from the no-intervention condition.
DISCUSSION
Using a novel approach to alter CVRi without changes to arterial BP via pharmacological (INDO) and physiological (hyperventilation) interventions, the main findings of the study were as follows: 1) increases in CVRi, independent of alterations to PetCO2, led to increased TFA phase and decreased absolute gain at 0.05 and 0.10 Hz; and 2) the anterior and posterior cerebral regions had similar cerebral pressure-flow relationships. Collectively, these findings support our hypothesis and demonstrate that increases in CVRi affect dynamic cerebral pressure-flow relations in both the anterior and posterior regions of the brain; thus, CVRi is a factor that should be considered in the correct interpretation of cerebral pressure-flow dynamics as indexed using TFA metrics.
Implications for the Assessment and Interpretation of Pressure-Flow Relationships
The findings from this study are important for understanding how alterations to CVRi affect cerebral pressure-flow dynamics. For example, the impact of PaCO2 is directly due to the changes in CVRi rather than an indirect influence of PaCO2 per se. Thus, in situations in which changes in CVRi occur naturally [e.g., CVRi increases with the development of hypertension (14, 27)] it has been suggested that the increased cerebrovascular tone associated with the increased CVRi leads to altered TFA metrics (decreased absolute gain). Also, our data are consistent with the current literature (14) showing that when mathematically interpreting TFA metrics, it is important to maximize the BP variability, thus increasing coherence and mitigating the confusion in interpreting TFA gain and phase metrics associated with lower coherence values. When input from the BP variability was enhanced with the squat-stand maneuvers (Fig. 1 and Fig. 2), we observed uniform increases in phase and reductions in gain at both 0.05 Hz and 0.10 Hz point estimates. This indicates that there appears to be an altered cerebral pressure-flow response with increased CVRi (Fig. 4). Thus applying a methodology that employs increasing the BP variability leads to an enhancement of the input signal (BP), resulting in more mathematically interpretable output values (phase, gain) (14, 31, 34). Our study also provides evidence that the anterior and posterior regions of the brain have similar pressure-flow relationships during changes in CVRi. These findings are further considered below in light of previous studies, potential mechanisms of action, and relevant methodological considerations.
Review of Previous Studies
Hypocapnia has been shown to affect the cerebral pressure-flow relationship in numerous studies under both steady-state (1, 4, 11, 17, 19, 21, 31, 34) and dynamic (1, 4, 11, 17, 19, 21) conditions. These studies took place within both healthy (1, 4, 11, 19) and clinical populations [e.g., traumatic brain injury (11, 19, 34), intracranial aneurism (31, 34), acute liver failure (17, 31), and during isoflurane anesthesia (6, 17, 24)]. The overall findings from these studies have demonstrated that cerebral vascular tone is an important protective mechanism in the regulation of CBF. In contrast to this hypocapnia literature, no studies have examined the dynamic cerebral pressure-flow response with INDO. Only two studies (6, 24) have investigated the effects of INDO via high-dose intravenous infusion on the steady-state cerebrovascular response. Both of the steady-state studies involved clinical populations [e.g., very low birth weight preterm infants (6), and patients with severe head injury (24)]. However, unlike hypocapnia, the proposed mechanisms in the two later studies were suggested to be due to the cerebral microvessels maintaining their ability to further vasodilate or constrict in response to other stimuli, despite the constrictive action of the drug. The commonality of all these studies was that during periods of either hypocapnia (PetCO2 approximately 25–35 mmHg) and during INDO treatment, there were subsequent improvements in related cerebral pressure-flow responses.
The current study is the first to examine the effects of both hypocapnia and INDO on pressure-flow relationships within the same population. To provide more interpretability to our TFA metrics, we induced large oscillations in BP, thus increasing BP variability (Figs. 1 and 2; Table 1). With the increased BP variability, coherence rose to a minimum of 0.92 during the squat-stand maneuvers (Fig. 4). Subsequently, the increased interpretability provided by the driven data within our study showed agreement with previous studies (4, 19, 38); specifically, that there were universal increases in phase lead and reductions in absolute gain (Fig. 4). There was a reduction in normalized gain at 0.05 Hz for the hypocapnia and INDO interventions, but they were not significantly different than the control intervention group at 0.10 Hz (Fig. 4). A possible explanation for the apparent discrepancy in these findings is that there appears to be a frequency-dependent normalized gain response to hypocapnia (32), with reductions occurring around 0.05 Hz, and no further alterations present with hypocapnia at 0.10 Hz. Our findings further emphasize those of Tzeng et al. (32), because these alterations to normalized gain do not appear to be specific to hypocapnia, but are linked with increased CVRi as shown by the comparable findings within the INDO intervention. This provides further confirmation that reporting TFA gain in absolute vs. normalized units can alter the interpretation of the findings (32).
The findings within the pharmacological portion of the current study were not entirely consistent with the findings by Zhang et al. (38). The conflicts between the two studies could be a result of the different mechanisms by which phenylephrine (18, 22, 38) (α1-adrenoreceptor agonist), INDO (a nonselective cyclooxygenase 1 and 2 inhibitor), and hypocapnia (cerebral vasoconstriction) increase CVRi. Phenylephrine likely does not cross the blood-brain barrier, and thereby does not directly alter the cerebral arterioles (18, 22, 30). Phenylephrine instead increases the CVRi indirectly through mechanoregulation (16) via constriction of the smooth muscle in the peripheral vasculature, which in turn, increases systemic BP. In contrast, INDO and hypocapnia both alter CVRi directly via constriction of the cerebrovascular arterioles (11, 16, 21), thus reducing CBF without altering systemic BP. It is the similar manner by which both INDO and hypocapnia influence CVRi, which make them ideal for exploring explore the mechanism(s) by which CVRi is able to influence cerebral pressure-flow dynamics.
Mechanisms by Which CVRi May Influence the Cerebral Pressure-Flow Relationship
The results from this study indicate that the changes in the pressure-flow regulation (and possibly CA) are likely related to changes in CVRi rather than the previously reported hypocapnia (11, 21, 33, 38) (Table 1). Thus we propose that the most likely cause for the alterations in TFA metrics are due to the enhanced arteriolar tone in the cerebral vasculature. These findings are broadly consistent with previous studies that have revealed TFA gain and phase parameters are determined by cerebrovascular properties such as vascular compliance and/or resistance (12, 33, 38). However, we also note that there is a possibility that both the hypocapnia and INDO interventions could cause alterations to the cerebral pressure-flow dynamics independent of the alterations in CVRi. In this case, we acknowledge that alterations to CVRi would be without an existing physiological or physical causation mechanism that would link increased CVRi to the altered TFA metrics.
Regional Differences in Cerebral Pressure-Flow Regulation
To date the vast majority of the research on the regulation between BP and CBF has focused solely on the MCA and related anterior cerebral regions, whereas there has been relatively little research on the posterior cerebral circulation. Whereas one report indicated that pressure-flow relationships in the PCA are less efficient compared with the MCA (12, 20), another revealed that alterations to the pressure-flow relationships within the PCA are likely the result of metabolic vasodilation and not an inherent difference in the autoregulatory characteristics of the posterior circulation (20, 25). Yet another study has shown that under general anesthesia the anterior and posterior regions of the of the brain have similar CO2 reactivity and static CA responses (25, 32). Our findings build upon these previous findings and are the first to reveal that similar dynamic pressure-flow relationships are present in the anterior and posterior cerebral circulatory systems (Fig. 4). This notion indicates that likely similar cerebral pressure-flow mechanisms are present in both the anterior and posterior regions, and highlights the related importance of monitoring CVRi rather than just focusing on the effects of hypocapnia when reporting TFA metrics.
Methodological Considerations
Cerebral autoregulation.
There has yet to be a firmly established gold standard for evaluation of static CA (7, 32); as such, the discussion in this paper was not focused on interpreting our TFA findings as they relate to CA. Instead, the findings were focused solely on the relationship present between BP and CBF. We chose to statistically quantify and assess this relationship via the linear analysis method of TFA. We feel that it is important to note that despite the widespread use of TFA to assess dynamic CA within the literature, it is extremely unlikely that the entire CA response can be quantified through a linear model. There will be other cocontributing factors to the CA response such as cerebrovascular compliance, downstream capacitance, intracranial pressure, and venous outflow modulation. With the view that likely nonlinear complexities can confound the spontaneous CA metrics, one may wonder why we chose to utilize TFA to assess this relationship, and our rationale is explained next.
Transfer function analysis.
Interpretation of TFA output metrics (phase and gain) are related to the overall coherence present within the analysis, because coherence represents the shared variance between the phase and gain metrics. To improve the mathematical interpretability and reliability of the TFA outputs within the VLF and LF (7, 37), we increased the input into the system (BP variability) via squat-stand maneuvers (Table 1). Squat-stand maneuvers create very large swings in BP (Fig. 1), which in turn enhance the input/output response within the cerebral pressure-flow relationship, creating a nearly linear relationship (coherence values for the driven data in this study were >0.92 a.u.). Through the application of this methodology we have increased the linearity of the cerebral pressure-flow relationship for mathematical interpretational purposes. Moreover, we view such BP challenges as a more realistic representation of daily activities (e.g., postural changes, coughing, exercise, etc.) and make our data set physiologically relevant. For the above reasons we have focused our discussion on the dynamic relationship between BP and CBF instead of CA. The data presented in this study further confirm that the interpretation of the TFA gain findings can be altered on the basis of reporting the results in absolute or normalized units (32).
Flow vs. velocity.
The main assumption of transcranial Doppler (TCD) is that the relative changes in MCAv/PCAv directly represent relative changes in the blood flow within this artery; however, at least during situations of normal BP and arterial blood gases, the majority of research suggests that TCD provides a reliable index of CBF [reviewed in (5, 37)]. We also know of no evidence to suggest that INDO may alter the diameter of the MCA/PCA. For example, previous studies using magnetic resonance imaging have demonstrated that INDO causes a similar percentage change in global CBF and MCAv (5).
Conclusion
We have demonstrated for the first time that increases in CVRi, independent of alterations to PaCO2 or systemic BP, are associated with altered cerebral pressure-flow relationship as reflected by the universally increased phase and decreased absolute gain. There are similar autoregulatory mechanisms present in the anterior and posterior cerebral circulatory systems. Collectively, these findings indicate that changes in CVRi will result in changes to the TFA metrics associated cerebral pressure-flow relationships, and as such, CVRi should be considered in the correct interpretation of TFA metrics. These finding are important for furthering our understanding of the mechanisms underlying the cerebral pressure-flow relationships in the human cerebral circulation and how these responses are commonly interpreted.
GRANTS
Support for this study was provided by a Canada Research Chair in Cerebrovascular Physiology and NSERC Discovery Grant to P.N.A.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.S., Y.C.T., and P.N.A. conception and design of research; J.D.S., B.J.M., and P.N.A. performed experiments; J.D.S., Y.C.T., and P.N.A. analyzed data; J.D.S., Y.C.T., B.J.M., and P.N.A. interpreted results of experiments; J.D.S. and P.N.A. prepared figures; J.D.S., Y.C.T., and P.N.A. drafted manuscript; J.D.S., Y.C.T., B.J.M., and P.N.A. edited and revised manuscript; J.D.S., Y.C.T., B.J.M., and P.N.A. approved final version of manuscript.
REFERENCES
- 1.Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke 20: 45–52, 1989 [DOI] [PubMed] [Google Scholar]
- 2.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg 57: 769–774, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol 296: R1473–R1495, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Birch AA, Dirnhuber MJ, Hartley-Davies R, Iannotti F, Neil-Dwyer G. Assessment of autoregulation by means of periodic changes in blood pressure. Stroke 26: 834–837, 1995 [DOI] [PubMed] [Google Scholar]
- 5.Bruhn H, Fransson P, Frahm J. Modulation of cerebral blood oxygenation by indomethacin: MRI at rest and functional brain activation. J Magn Reson Imaging 13: 325–334, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Chock VY, Ramamoorthy C, Van Meurs KP. Cerebral autoregulation in neonates with a hemodynamically significant patent ductus arteriosus. J Pediatr 160: 936–942, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol 106: 153–160, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elting JW, Aries MJ, van der Hoeven JH, Vroomen PC, Maurits NM. Reproducibility and variability of dynamic cerebral autoregulation during passive cyclic leg raising. Med Eng Phys. In press [DOI] [PubMed] [Google Scholar]
- 9.Fan JL, Burgess KR, Thomas KN, Peebles KC, Lucas SJ, Lucas RA, Cotter JD, Ainslie PN. Influence of indomethacin on the ventilatory and cerebrovascular responsiveness to hypoxia. Eur J Appl Physiol 111: 601–610, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hamner JW, Cohen MA, Mukai S, Lipsitz LA, Taylor JA. Spectral indices of human cerebral blood flow control: responses to augmented blood pressure oscillations. J Physiol 559: 965–973, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haubrich C, Steiner L, Kim DJ, Kasprowicz M, Smielewski P, Diehl RR, Pickard JD, Czosnyka M. How does moderate hypocapnia affect cerebral autoregulation in response to changes in perfusion pressure in TBI patients? Acta Neurochir Suppl (Wien) 114: 153–156, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Haubrich C, Wendt A, Diehl RR, Klotzsch C. Dynamic autoregulation testing in the posterior cerebral artery. Stroke 35: 848–852, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Hohimer AR, Richardson BS, Bissonnette JM, Machida CM. The effect of indomethacin on breathing movements and cerebral blood flow and metabolism in the fetal sheep. J Dev Physiol 7: 217–228, 1985 [PubMed] [Google Scholar]
- 14.Katsogridakis E, Bush G, Fan L, Birch AA, Simpson DM, Allen R, Potter JF, Panerai RB. Detection of impaired cerebral autoregulation improves by increasing arterial blood pressure variability. J Cereb Blood Flow Metab 33: 519–523, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavi S, Egbarya R, Lavi R, Jacob G. Role of nitric oxide in the regulation of cerebral blood flow in humans: chemoregulation versus mechanoregulation. Circulation 107: 1901–1905, 2003 [DOI] [PubMed] [Google Scholar]
- 17.McCulloch TJ, Boesel TW, Lam AM. The effect of hypocapnia on the autoregulation of cerebral blood flow during administration of isoflurane. Anesth Analg 100: 1463–1467, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Miller RD, Pardo M. Basics of Anesthesia 6th Edition. Philadelphia, PA: Elsevier, 2011 [Google Scholar]
- 19.Muller M, Bianchi O, Erulku S, Stock C, Schwerdtfeger K. Brain lesion size and phase shift as an index of cerebral autoregulation in patients with severe head injury. Acta Neurochir (Wien) 145: 643–648, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa K, Serrador JM, Larose SL, Moslehi F, Lipsitz LA, Sorond FA. Autoregulation in the posterior circulation is altered by the metabolic state of the visual cortex. Stroke 40: 2062–2067, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogoh S, Nakahara H, Ainslie PN, Miyamoto T. The effect of oxygen on dynamic cerebral autoregulation: critical role of hypocapnia. J Appl Physiol 108: 538–543, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Olesen J. The effect of intracarotid epinephrine, norepinephrine, and angiotensin on the regional cerebral blood flow in man. Neurology 22: 978–987, 1972 [DOI] [PubMed] [Google Scholar]
- 23.Omboni S, Parati G, Frattola A, Mutti E, Di Rienzo M, Castiglioni P, Mancia G. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension 22: 26–33, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Puppo C, Lopez L, Farina G, Caragna E, Moraes L, Iturralde A, Biestro A. Indomethacin and cerebral autoregulation in severe head injured patients: a transcranial Doppler study. Acta Neurochir (Wien) 149: 139–149, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Rozet I, Vavilala MS, Lindley AM, Visco E, Treggiari M, Lam AM. Cerebral autoregulation and CO2 reactivity in anterior and posterior cerebral circulation during sevoflurane anesthesia. Anesth Analg 102: 560–564, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Sammons EL, Samani NJ, Smith SM, Rathbone WE, Bentley S, Potter JF, Panerai RB. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J Appl Physiol 103: 369–375, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol 98: 151–159, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Smirl JD, Lucas SJ, Lewis NC, Dumanior GR, Smith KJ, Bakker A, Basnyat AS, Ainslie PN. Cerebral pressure-flow relationship in lowlanders and natives at high altitude. J Cereb Blood Flow Metab 34: 248–257, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St Lawrence KS, Ye FQ, Lewis BK, Weinberger DR, Frank JA, McLaughlin AC. Effects of indomethacin on cerebral blood flow at rest and during hypercapnia: an arterial spin tagging study in humans. J Magn Reson Imaging 15: 628–635, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Stewart JM, Medow MS, DelPozzi A, Messer ZR, Terilli C, Schwartz CE. Middle cerebral O2 delivery during the modified Oxford maneuver increases with sodium nitroprusside and decreases during phenylephrine. Am J Physiol Heart Circ Physiol 304: H1576–H1583, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strauss G, Hansen BA, Knudsen GM, Larsen FS. Hyperventilation restores cerebral blood flow autoregulation in patients with acute liver failure. J Hepatol 28: 199–203, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Tzeng YC, Ainslie PN, Cooke WH, Peebles KC, Willie CK, MacRae BA, Smirl JD, Horsman HM, Rickards CA. Assessment of cerebral autoregulation: the quandary of quantification. Am J Physiol Heart Circ Physiol 303: H658–H671, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Tzeng YC, Chan GS, Willie CK, Ainslie PN. Determinants of human cerebral pressure-flow velocity relationships: new insights from vascular modelling and Ca2+ channel blockade. J Physiol 589: 3263–3274, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voldby B, Enevoldsen EM, Jensen FT. Cerebrovascular reactivity in patients with ruptured intracranial aneurysms. J Neurosurg 62: 59–67, 1985 [DOI] [PubMed] [Google Scholar]
- 35.Wennmalm A, Carlsson I, Edlund A, Eriksson S, Kaijser L, Nowak J. Central and peripheral haemodynamic effects of non-steroidal anti-inflammatory drugs in man. Arch Toxicol Suppl 7: 350–359, 1984 [DOI] [PubMed] [Google Scholar]
- 36.Wennmalm A, Eriksson S, Hagenfeldt L, Law D, Patrono C, Pinca E. Effect of prostaglandin synthesis inhibitors on basal and carbon dioxide-stimulated cerebral blood flow in man. Adv Prostaglandin Thromboxane Leukot Res 12: 351–355, 1983 [PubMed] [Google Scholar]
- 37.Willie CK, Colino FL, Bailey DM, Tzeng YC, Binsted G, Jones LW, Haykowsky MJ, Bellapart J, Ogoh S, Smith KJ, Smirl JD, Day TA, Lucas SJ, Eller LK, Ainslie PN. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 196: 221–237, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Zhang R, Behbehani K, Levine BD. Dynamic pressure-flow relationship of the cerebral circulation during acute increase in arterial pressure. J Physiol 587: 2567–2577, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol Heart Circ Physiol 274: H233–H241, 1998 [DOI] [PubMed] [Google Scholar]