Abstract
Murine lumbar and coccygeal (tail) regions of spines are commonly used to study cellular signaling of age-related disc diseases, but the tissue-level changes of aging intervertebral discs and vertebrae of each spinal region remain unclear. Furthermore, the impact of aging lumbar and coccygeal discs on Wnt/β-catenin signaling, which is putatively involved in the catabolism of intervertebral discs, is also unclear. We compared disc/vertebrae morphology and mechanics and biochemical composition of intervertebral discs from lumbar and coccygeal regions between young (4–5 mo) and old (20–22 mo) female C57BL/6 mice. Center intervertebral disc height from both regions was greater in old discs than young discs. Compared with young, old lumbar discs had a lower early viscous coefficient (a measure of stiffness) by 40%, while conversely old coccygeal discs were stiffer by 53%. Biochemically, old mice had double the collagen content in lumbar and coccygeal discs of young discs, greater glycosaminoglycan in lumbar discs by 37%, but less glycosaminoglycan in coccygeal discs by 32%. Next, we compared Wnt activity of lumbar and coccygeal discs of 4- to 5-mo and 12- to 14-mo TOPGAL mice. Despite the disc-specific changes, aging decreased Wnt signaling in the nucleus pulposus from both spinal regions by ≥64%. Compared with young, trabecular bone volume/tissue volume and ultimate force were less in old lumbar vertebrae, but greater in old coccygeal vertebrae. Thus intervertebral discs and vertebrae age in a spinal region-dependent manner, but these differential age-related changes may be uncoupled from Wnt signaling. Overall, lumbar and coccygeal regions are not interchangeable in modeling human aging.
Keywords: aging, mouse, tail, caudal, WNT/β-catenin
lumbar and coccygeal (tail) discs of the mouse are commonly used to study changes in cellular signaling and composition with aging or aging-related disc degeneration (11, 17, 22, 25, 47), yet there is not a clear understanding of the tissue-level changes of each spinal level with aging that may in turn influence cellular signaling. Lumbar discs in mice and humans are subjected to ground-reactionary loads and endogenous loads (54), whereas coccygeal discs in mice sustain no weight-bearing forces, but, because coccygeal discs are easily accessible, they may be useful for interventional studies related to disc degeneration. Nevertheless, when normalized for geometry, discs from both regions of the spine of various species of animals are analogous to human discs in glycosaminoglycan content, collagen content, and mechanical properties (4, 14, 52).
Advanced aging often leads to degeneration of the intervertebral disc in humans, a putative cause of lower back pain. Several studies provide a descriptive account of the deleterious consequences age and degeneration have on the intervertebral disc (6, 50). In short, the demarcation between the nucleus pulposus and annulus fibrosus becomes unclear by the end of the second decade of life because of collagen fibril growth in diameter and number (36). Disc dehydration and loss of glycosaminoglycan persist with aging and degeneration, with the nucleus pulposus incurring most of the deterioration (2). In turn, the outer rims of the annulus develop radial fissures and posterior protrusions (6), which leads to mechanical ramifications (35). Furthermore, endplates thin and become disorganized with aging (6), accompanied by vertebral bone loss (21).
While aging and degeneration may similarly disrupt the composition and morphology of intervertebral discs, aging and degeneration are not synonymous and may differentially alter the functional properties of the disc. Consequent to the detrimental changes in disc composition with aging and degeneration, functional spinal units (bone-disc-bone) become compromised and are predisposed to mechanical failure as the distribution of mechanical loads shifts (62). Aging (40) and degeneration (24, 40) reduce the compressive stiffness of discs and increase the loading range of motion, potentially stemming from severe depletion of nucleus pulposi glycosaminoglycan (7). However, aging and degeneration differentially change the tensile stiffness of discs. During standing, the anterior portion of discs is subjected to tension, but degeneration reduces the tensile stiffness of this region (59). Contrarily, aging with minimal degeneration increases the axial tensile stiffness of spines during traction (43). Morphologically, severely degenerated discs collapse (6), but asymptomatic discs increase in height with aging (48, 56).
Presently, no studies have determined the changes in mechanical properties of murine lumbar or coccygeal discs with advanced aging, and there is an incomplete understanding of the associated biochemical changes. Biochemically, glycosaminoglycan content of lumbar discs appears normal by histology in aged mice (5), but there is no quantitative comparison to young mice. Mouse studies that measure the collagen fiber diameter of lumbar discs with advanced aging show conflicting data (22, 25) and so age-related changes to collagen content are unclear. In the coccygeal disc, aging in mice reduces glycosaminoglycan (17), but changes to collagen content are uncertain.
Molecularly, Wnt/β-catenin signaling serves as an anabolic pathway in bone (38), but has emerged as a putative catabolic pathway in the intervertebral disc, where transcription factor β-catenin accumulates in degenerated discs (58) and canine discs prone to age-related degeneration (55). In vitro, chemical stimulation of Wnt/β-catenin signaling in rodent disc cells triggers cellular senescence, apoptosis, and biomarkers of matrix breakdown (28, 29) and may modulate oxygen tension (26), a potent regulator of disc cell viability. In mice, Wnt activity of lumbar discs declines after birth and ostensibly disappears by 9 wk of age, but studies demonstrate conflicting results concerning nucleus pulposus activity (12, 41). Furthermore, Wnt signaling may interact with other signaling pathways in the disc to regulate each other and homeostasis. For instance, transforming growth factor-β, which may increase proteoglycan synthesis in disc cells (27), can regulate and be regulated by Wnt signaling (30). Similarly, inhibition of sonic hedgehog, which, in a growing mouse disc is involved in differentiation, increases Wnt signaling in the annulus fibrosus and endplate (10). The Wnt activity of coccygeal discs remains unknown, and the effect of advanced aging on Wnt/β-catenin activity of lumbar and coccygeal intervertebral discs remains unclear.
Therefore, we compared the structure, biomechanics, and biochemical content between young-adult (4–5 mo) and old (20–22 mo) coccygeal and lumbar discs and structure/biomechanics of vertebrae from female C57Bl/6 mice. Furthermore, we compared the Wnt activity of coccygeal and lumbar intervertebral discs between young-adult (4–5 mo) and middle-aged (12–14 mo old) TOPGAL mice (TCF/LEF Optimal Promoter/Galactosidase reporter of Wnt activity). Understanding these property changes will help establish the suitability of mouse coccygeal and lumbar levels for modeling aging of human lumbar spines.
MATERIALS AND METHODS
Experimental design.
Lumbar (L4-L5) and coccygeal motion segments (CC5-CC6) were harvested from young-adult (4–5 mo) and old (20–22 mo) female, virgin C57BL/6 mice (n = 9–12 for each group; Aged Rodent Colony, National Institute on Aging) stored at −20°C. Three- to six-month-old mice are generally categorized as “skeletally mature” and approximate 20–30 yr in humans (16). In the lumbar vertebrae, bone mineral density (BMD), transmembrane domains, and trabecular bone volume (BV)/tissue volume from C57Bl/6 plateau at 4–5 mo (8) and subsequently begin to lose bone, a characteristic of aging. With regards to aged C57Bl/6 mice, 50% survival occurs at ∼30 mo of age (61), old age begins at 18 mo, and 20–22 mo approximates 65 yr in humans (16). TOPGAL (13) mice were purchased from The Jackson Laboratory (Tg[Fos-lacZ]34Efu/J, Bar Harbor, ME) and used to indicate Wnt signaling in the spine. TOPGAL mice were bred, genotyped by PCR of tail DNA (F: TTGGAGTGACGGCAGTTATCTGGA, R: TCAACCACCGCACGATAGAGATTC), and aged to 4–5 mo (n = 10) and 12–14 mo (n = 7). Mice of at least 12 mo of age were chosen to represent aging, because significant age-related vertebral bone loss has been noted at this age (20, 60). Another set of wild-type C57Bl/6 mice age-matched to the TOPGAL mice (5 and 12 mo; n = 3–5 each; National Institute on Aging) were obtained and used to evaluate gene expression. Animals had ad libitum access to standard chow and water. The use of these mice was approved by the Animal Studies Committee (Institutional Animal Care and Use Committee) of Washington University.
Micro-computed tomography.
Excised lumbar and coccygeal motion segments were scanned by micro-computed tomography (VivaCT 40, Scanco, Brüttisellen, Switzerland) at a resolution of 21 μm (70 kV, 114 μA, 100-ms integration time) to determine intervertebral disc height and vertebral bone morphology and BMD. Lumbar spines were imaged between vertebrae L4 and L5. Coccygeal spines were imaged between CC5 and CC6. Intervertebral disc height was measured as the distance between vertebrae. Regional height (anterior, center, posterior, lateral) was determined from height maps (31, 34), where the mean value within a 0.1-mm2 contour at the respective region denoted the height. A simple measure of disc convexity was determined as the ratio of center height to the average of the anterior and posterior height (33), where low values are associated with disc degeneration (48). Trabecular volume of interest of L5 and CC6 was the mean of 30 slices of the cranial and caudal region of each vertebra. Cortical volume of interest was 30 slices, centered at the middle of L5 and CC6. Automatic contouring was used to analyze trabecular slices with a lower/upper threshold of 211/1,000 [424 mg hydroxyapatite (HA)/cm3] and cortical slices with a lower/upper threshold of 205/1,000 (406 mg HA/cm3) to segment bone from other tissue. The outcomes for trabecular bone included BV fraction (BV/tissue volume), trabecular thickness (Tb.Th) and number (Tb.N), connectivity density (Conn.D), structure model index, and volumetric BMD (vBMD). Cortical thickness (Ct.Th) of the lumbar vertebrae was of the anterior region, because a continuous posterior cortex was usually absent (20), and that of the coccygeal vertebrae was of the entire cross section.
Axial mechanical testing of discs and vertebrae.
Controlled mechanical tests were performed on L4-L5 and CC5-CC6 motion segments to approximate physiological axial loading of discs (quasi-static compression-tension and compressive creep) (7, 14, 32, 51). Before mechanical testing, the L4-L5 and CC5-CC6 bone-disc-bone segments were excised, facets and extraneous tissue removed, and hydrated in 1× PBS for 18 h at 4°C. Each superior vertebra was gripped by microvises, and the inferior vertebra was fixed with polymethylmethacrylate into a cylinder. Once secured, the sample was immersed in 1× PBS. At a frequency of 0.5 Hz, a materials testing system (Electropulse 1000, Instron, Norwood, MA) applied a load range of −0.8 N to 0.4 N for lumbar segments and −1.6 N to 0.8 N for coccygeal segments. The coccygeal discs required twice the force to reach the linear region of the force-displacement response. A lower load range for the lumbar segments was used to prevent failure of the old segments in tension (which was observed in pilot testing). After 20 compression-tension cycles, a 1-s ramp from zero to maximum compression (coccygeal: −1.6 N, lumbar: −0.8 N) was applied and maintained for 1 h while displacement was recorded. Following mechanical testing of discs, discs were separated from vertebrae, and both placed in −80°C, where the discs were designated for biochemical analysis and the inferior vertebrae for mechanical testing. For mechanical testing of vertebrae, the inferior region of the vertebrae was stabilized onto a platen fixture with cyanoacrylate. The superior platen fixture preloaded the vertebrae to a compression of 0.5 N and compressed at a ramp rate of 0.03 mm/s until the ultimate force using a materials testing machine.
Mechanical data analysis of discs.
The trilinear fit model determined the compressive, tensile, and neutral zone stiffness of the motion segment (32). Briefly, the compressive and tensile loading curves were isolated, and a sixth-order polynomial was fit to the 20th loading and unloading tension-compression cycle (Fig. 1A). The minimum derivative of the curve represented the neutral zone stiffness, and the derivative measured at 80% of the maximum load magnitude in the compressive and tensile direction constituted the compressive and tensile stiffness, respectively. Range of motion was the displacement between the compressive and tensile forces at 80% maximum. After the 1-s step load to maximum creep force (FC), 1 h of creep displacement was fit to a rheological model: d(t)/FC = 1/S1[1 − e−S1t/η1] + 1/S2[1 − e−S2t/η2], where S1,2 are the elastic coefficients and η1,2 are the viscous coefficients at 24% (early) and 76% (late) creep displacement (Fig. 1B), respectively. A custom LabVIEW (National Instruments, Austin, TX) program was used to determine ultimate force, yield force, stiffness, and energy-to-ultimate force from force-displacement curves (Fig. 2).
Fig. 1.
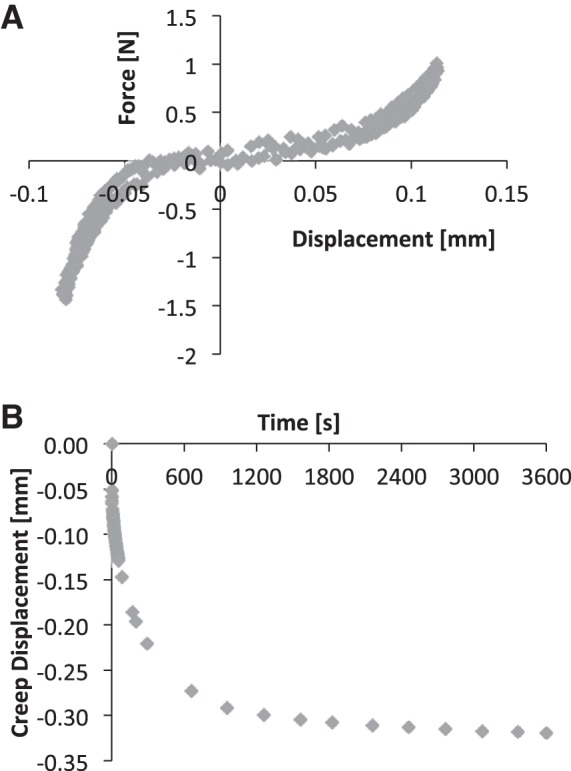
A: the 20th loading and unloading tension-compression cycle (shaded diamonds) served as the data for the polynomial fit, which was used to determine the compressive, tensile, and neutral zone stiffness of bone-disc-bone motion segments. B: following tension-compression testing, motion segments were compressed under force control for 1 h to determine creep properties.
Fig. 2.
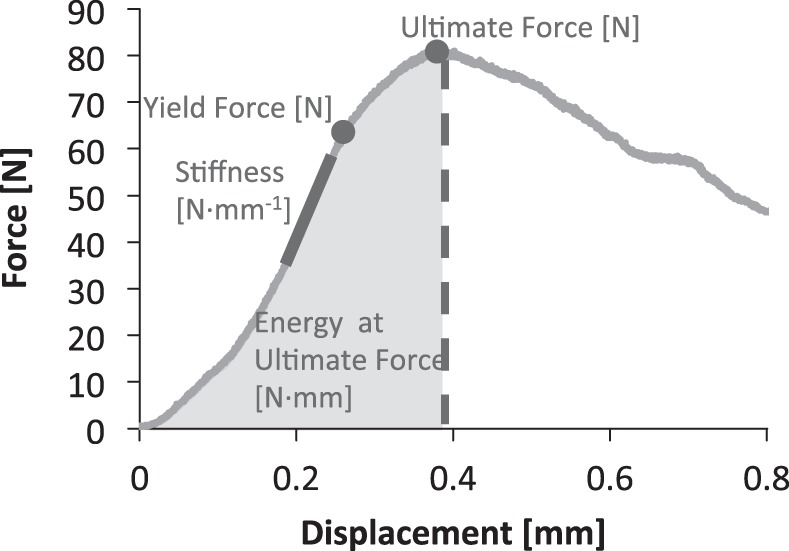
Vertebrae were compressed to failure to determine stiffness, yield force, ultimate force, and energy-to-ultimate force.
Biochemical analysis.
Once thawed, discs (annulus and nucleus) previously used for biomechanics and without endplates or bone were submerged in 1× PBS for 45 min, and wet mass was determined in duplicate. Samples were dried at 65°C for 18 h to determine the stable dry weight and weighed in duplicate. Samples were then digested in equal volumes of papain (Sigma-Aldrich, St. Louis, MO) for 18 h at 60°C (34). The content of sulfated-glycosaminoglycans (sGAG) was estimated by a 1,9-dimethylmethylene blue assay in triplicate (Sigma-Aldrich). Upon hydrolysis in HCl for 18 h at 110°C, collagen content was estimated by a hydroxyproline assay in triplicate. sGAG and collagen content were normalized to wet mass.
Histology.
Lumbar (L4-L5) and coccygeal (CC5-CC6) motion segments were dissected from young and old mice, fixed in 4% paraformaldehyde, decalcified in EDTA for 2 wk, and embedded in paraffin using routine methods. Sagittal serial sections (10 μm) were stained with Toluidine blue/Fast green to evaluate the regional proteoglycan staining of the intervertebral disc. To determine galactosidase activity, freshly harvested spines were fixed in 4% paraformaldehyde for 1 h, stained in Xgal (Invitrogen, Grand Island, NY) for 48 h, fixed overnight, decalcified in Immunocal (The Chem, Tallman, NY) for 2 days and embedded in paraffin using routine methods. Sagittal sections (10 μm) were used to visualize galactosidase cells, and serial sections were counterstained with eosin to visualize all cells. Images of sections were thresholded, and cells of the annulus fibrosus were counted using ImageJ 1.40 g (National Institutes of Health). The staining of nucleus pulposus cells was not as clear as in the annulus fibrosus. Therefore, the ratio of stained area-to-total area of the nucleus pulposus was used as an outcome.
Gene expression.
Intervertebral discs of 5- and 12-mo old C57Bl/6 mice were selected to coincide with galactosidase activity. Discs were frozen in liquid nitrogen, pulverized in a steel flask by shaking (Micro-Dismembrator, B. Braun Biotech) with a steel ball, and stabilized using TRIzol (Invitrogen, Carlsbad, CA). RNA was purified using an RNeasy Mini Kit (Qiagen, Germantown MD). Quantitative RT-PCR reactions were run (7500 Real-Time PCR System, Applied Biosystems, Foster City, CA) with SYBRgreen (Applied Biosystems) as a reporter agent. β-Catenin gene expression (F: CACCCTTCTACTATCTCCTCCA; R: TGCTATTCCACGACTAGTTCAG) was normalized to cyclophilin and analyzed using the ΔCT method (2−ΔCT).
Statistics.
Unpaired t-tests compared outcomes between young and old tissues. Paired t-tests compared outcomes between lumbar and coccygeal within an age, except for the mechanical properties of intervertebral discs, which had different testing limits. Statistical significance for all tests was considered at <5% (StatView 5.0, SAS Institute, Cary, NC).
RESULTS
Aging increases intervertebral disc height.
At both spine levels, center intervertebral disc height was greater in old mice than young mice by an average of 45% (P < 0.05) (Fig. 3; Table 1). In addition, average and lateral heights from lumbar discs were greater in old mice than young by 23% (P = 0.003) and 25% (P = 0.041), respectively. Between spinal levels in young mice, coccygeal discs had greater average height than lumbar discs by 32% (P < 0.001) and was greater at each region of the disc by 42–62% (P < 0.009). Old mice had greater coccygeal disc height than lumbar disc height by 18% (P = 0.010) and was due to greater center height by 55% (P = 0.024) and lateral height by 40% (P = 0.008). In terms of disc shape, aging did not affect the convexity of lumbar discs, but disc convexity was greater in aged coccygeal discs by 33% (P < 0.05).
Fig. 3.
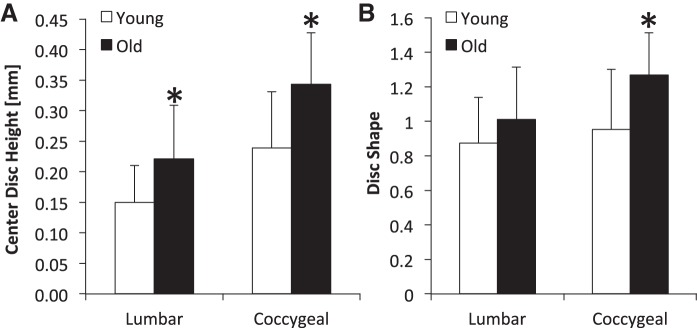
Regional height of lumbar and coccygeal intervertebral discs from young and old mice. A: both lumbar and coccygeal discs were greater with aging. B: disc shape (convexity) was greater in old coccygeal discs. Disc shape represents the ratio of center height to the mean of anterior and posterior height. Values are means ± SD. *Significant difference vs. young, P < 0.05.
Table 1.
Height and biochemical content of lumbar and coccygeal intervertebral discs from young and old mice
| Intervertebral Disc Height, mm |
Biochemical Content/Wet Mass, μg/mg |
||||||
|---|---|---|---|---|---|---|---|
| Age and Region | Average | Anterior | Center | Posterior | Lateral | Collagen | sGAG |
| Lumbar | |||||||
| Young | 0.19 ± 0.01 | 0.18 ± 0.04 | 0.15 ± 0.06 | 0.16 ± 0.03 | 0.17 ± 0.01 | 142 ± 114 | 92 ± 29 |
| Old | 0.23 ± 0.04* | 0.23 ± 0.07 | 0.22 ± 0.09* | 0.21 ± 0.10 | 0.21 ± 0.01* | 305 ± 150* | 126 ± 39* |
| Coccygeal | |||||||
| Young | 0.25 ± 0.03† | 0.25 ± 0.06† | 0.24 ± 0.09† | 0.26 ± 0.08† | 0.27 ± 0.07† | 58 ± 35† | 95 ± 60 |
| Old | 0.27 ± 0.04† | 0.27 ± 0.07 | 0.34 ± 0.08*† | 0.28 ± 0.09 | 0.30 ± 0.05† | 128 ± 73*† | 57 ± 24*† |
Values are means ± SD. sGAG, sulfated-glycosaminoglycans.
Young vs. old,
lumbar vs. coccygeal of same age: P < 0.05.
Aging decreases lumbar stiffness and increases coccygeal discs stiffness.
The axial mechanical properties of old and young spines were determined by compression-tension and creep testing. From compression-tension testing, old lumbar discs were less stiff in tension by 42% (P = 0.049) and had a greater range of motion compared with young lumbar discs by 54% (Fig. 4; Table 2). During creep testing, the early viscous coefficient η1 was less in old lumbar discs than young discs by 40% (P = 0.037). In contrast, old coccygeal discs were stiffer in tension than young coccygeal discs by 41% (P = 0.026) and were not different in range of motion. Moreover, old coccygeal discs displayed a greater early elastic coefficient (S1) and viscous coefficient η1 during creep testing than young coccygeal disc by 53 and 54% (P < 0.05), respectively. Compressive stiffness, neutral stiffness, creep deformation, and late viscous coefficient were not significantly different with age in either spinal level (P ≥ 0.068).
Fig. 4.
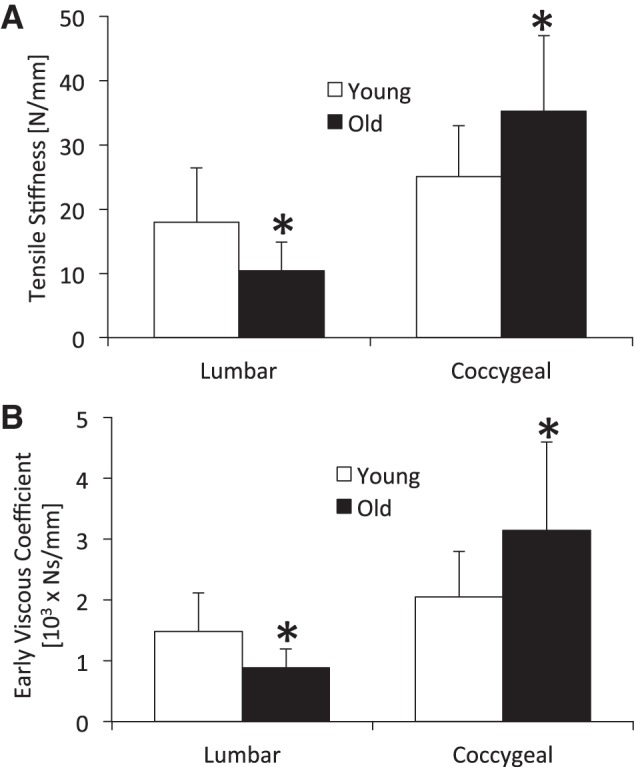
Tensile stiffness (A) and early viscous damping (B) coefficient of lumbar and coccygeal intervertebral discs from young and old mice. These mechanical properties were less in old lumbar discs and greater in old coccygeal discs than young discs. Values are means ± SD. *Significant difference vs. young, P < 0.05.
Table 2.
Structural properties of lumbar and coccygeal intervertebral discs from young and old mice
| Compression-Tension |
Creep |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age and Region | ROM, mm | STEN, N/mm | SCOM, N/mm | SNZ, N/mm | Deform, mm | S1, N/mm | S2, N/mm | η1, N·s/mm | η2, N·s/mm |
| Lumbar | |||||||||
| Young | 0.12 ± 0.05 | 18 ± 8 | 26 ± 11 | 4 ± 2 | 0.11 ± 0.03 | 26 ± 7 | 8 ± 2 | 1,483 ± 633 | 6,090 ± 2,259 |
| Old | 0.19 ± 0.05* | 10 ± 4* | 21 ± 7 | 2 ± 2 | 0.12 ± 0.04 | 29 ± 16 | 9 ± 5 | 890 ± 301* | 6,460 ± 5,165 |
| Coccygeal | |||||||||
| Young | 0.23 ± 0.07 | 25 ± 8 | 43 ± 11 | 2 ± 2 | 0.23 ± 0.03 | 30 ± 10 | 9 ± 3 | 2,047 ± 751 | 6,598 ± 2,337 |
| Old | 0.23 ± 0.05 | 35 ± 12* | 44 ± 17 | 3 ± 3 | 0.18 ± 0.07 | 45 ± 22* | 14 ± 7 | 3,150 ± 1,451* | 11,200 ± 7,975 |
Values are means ± SD. ROM, range of motion; STEN, tensile stiffness; SCOM, compression stiffness; SNZ, neutral zone stiffness; S1 and S2, elastic coefficients at early and late creep displacement, respectively; η1 and η2, viscous coefficients at early and late creep displacement, respectively.
Young vs. old, P < 0.05.
Aging increases disc collagen and differentially alters disc glycosaminoglycan between spinal regions.
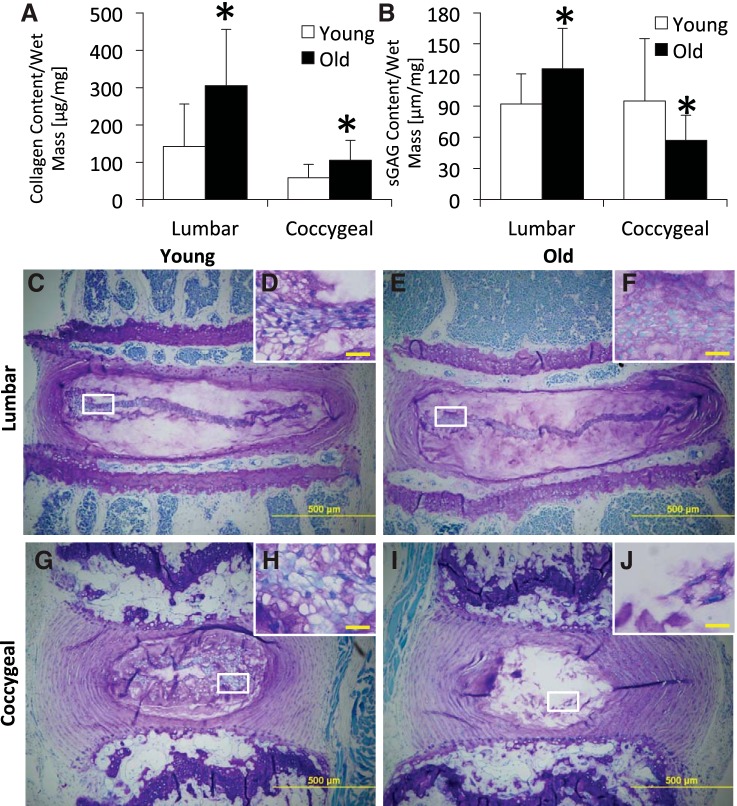
Comparing age, old lumbar and coccygeal discs had more collagen than their respective young discs by 121% (P = 0.013) and 114% (P = 0.011), respectively (Fig. 5; Table 1). Similarly, old lumbar discs had more sGAG than young lumbar discs by 37% (P = 0.033). In contrast, old coccygeal discs had less sGAG than young coccygeal discs by 32% (P = 0.048). Comparing spinal levels, young and old coccygeal discs had less collagen than young and old lumbar discs by 59% (P = 0.036) and 58% (P = 0.023), respectively. Similarly, old coccygeal discs had less sGAG than old lumbar discs by 36% (P = 0.028); sGAG content did not differ between levels in young mice (P = 0.122). The wet mass of aged (1.8 ± 0.7 mg) lumbar discs was marginally greater than that of young adults (1.6 ± 0.3 mg), but wet mass of coccygeal discs did not differ significantly with age (5 mo: 2.1 ± 0.7 mg; 22 mo: 1.9 ± 0.7 mg).
Fig. 5.
Collagen (A) and glycosaminoglycan (B) of lumbar and coccygeal intervertebral discs from young and old mice. Old lumbar and coccygeal discs had greater collagen. Old lumbar discs had greater glycosaminoglycan, but old coccygeal discs had less glycosaminoglycan. sGAG, sulfated-glycosaminoglycans. Values are means ± SD. *Significant difference vs. young, P < 0.05. Toluidine blue/Fast green stained for glycosaminoglycan of young (C and D) and old (E and F) lumbar spines and of young (G and H) and old (I and J) coccygeal spines is shown. Old lumbar discs had greater staining in the nucleus pulposus (C and E), and old coccygeal discs had less staining than young discs of the respective region (G and I). D, F, H, and J: regions of interest within the nucleus pulposus are highlighted in white boxes. D and F: in lumbar discs, aging disorganized the notochordal band and blurred its distinction from the matrix. H and J: in coccygeal discs, aged discs lost notochordal cells. Scale bars: C, E, G, I = 500 μm; D, F, H, J = 20 μm.
By histology, more Toluidine blue stain for glycosaminoglycan appeared in the nucleus pulposus of old lumbar disc than young. By contrast, there was less stain for glycosaminoglycan in the nucleus pulposus of old coccygeal disc than young. With respect to nucleus pulposus cell morphology, the lumbar notochordal band of young discs was thin and distinct, whereas the lumbar notochordal band of old discs was still thin but less distinct and more disorganized. By contrast, the notochordal cells of coccygeal discs were disorganized, clustered, and highly vacuolated in young adults, but the old coccygeal discs were highly acellular. No chondrocytic cell clusters were noted in either disc level or with aging. The fibrocartilage cells in the annulus fibrosus of both discs levels did not appear different with aging.
Aging reduces Wnt activity in discs.
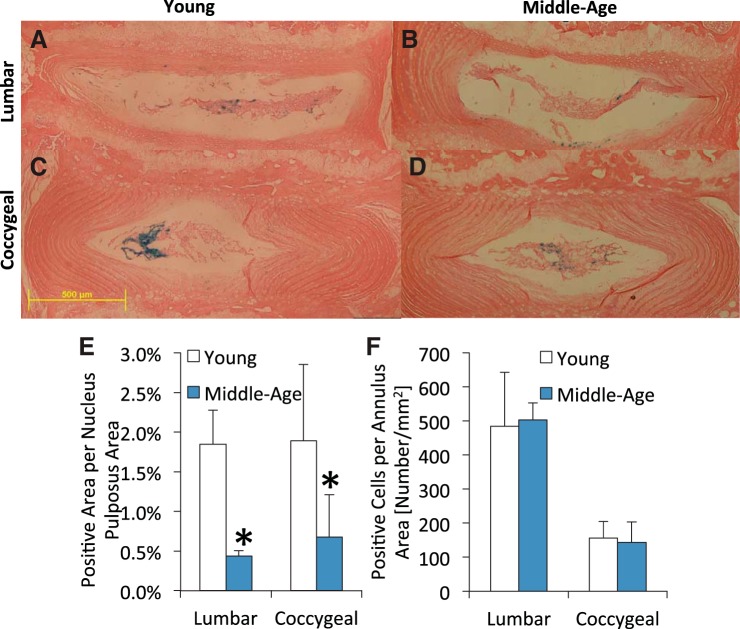
Young adult and middle-aged TOPGAL mice had Wnt/β-catenin active intervertebral discs, but age-related differences were region dependent (Fig. 6). Normalized by nucleus pulposus area, aging reduced nucleus pulposus Wnt/β-catenin activity by 76% in the lumbar and by 64% in the coccygeal discs (P < 0.05). By contrast, aging did not change Wnt/β-catenin activity in the annulus, but there were ∼70% fewer Wnt-positive cells in coccygeal discs than lumbar discs (P < 0.01). Aging from 4 to 12 mo in C57Bl/6 mice reduced β-catenin expression in lumbar intervertebral discs by 23% (P < 0.05) and in coccygeal discs by 37% (P < 0.05) (Fig. 7). Between spine levels of 4- and 12-mo mice, coccygeal intervertebral discs had less β-catenin expression than lumbar discs by 78 and 77%, respectively (P ≤ 0.015).
Fig. 6.
Histological staining of young (A and C) and middle-aged (B and D) lumbar (A and B) and coccygeal (C and D) discs from TOPGAL mice. Positive X-gal staining (blue cells) is indicative of activated Wnt signaling. Quantification of the staining for the nucleus pulposus (E) and annulus fibrosus (F) shows that aging decreased the percentage of the Wnt active cells in the nucleus pulposus in lumbar and coccygeal discs, whereas aging did not alter the number of Wnt active cells in the annulus fibrosus. Scale bar is 500 μm. Values are means ± SD. *Significant difference vs. young, P < 0.05.
Fig. 7.
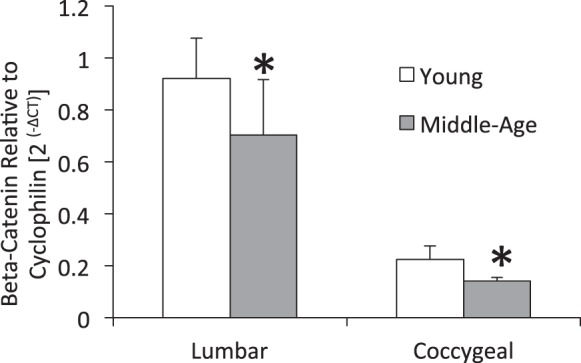
Gene expression of β-catenin of young and middle-aged lumbar and coccygeal discs. Values are means ± SD. *Significant difference vs. young, P < 0.05.
Aging differentially changes trabecular morphology and vertebral strength between lumbar and coccygeal vertebrae.
Trabecular BV fraction of old lumbar vertebrae was less than that of young lumbar vertebrae by 21% (P = 0.036) due to fewer trabeculae. Tb.N, Conn.D, and vBMD were less in old lumbar vertebrae than young by 12% (P = 0.033), 32% (P = 0.018), and 16% (P = 0.047), respectively (Fig. 8; Table 3). Tb.Th, structural model index and Ct.Th of lumbar vertebrae were not different with aging (P ≥ 0.510). By contrast, trabecular BV fraction of old coccygeal vertebrae was greater than of young coccygeal vertebrae by 49% (P < 0.001), due to more and thicker trabeculae. Tb.Th, Tb.N, and vBMD were greater in old coccygeal vertebrae than young by 28% (P < 0.001), 12% (P = 0.003), and 59% (P < 0.001), respectively, while structure model index of old coccygeal vertebrae was less. Furthermore, Ct.Th of old coccygeal vertebrae was greater than that of young coccygeal vertebrae by 31% (P < 0.001). Between spinal levels of both ages, coccygeal vertebrae had greater Ct.Th and trabecular BV fraction due to thicker trabeculae, despite fewer numbers than lumbar vertebrae.
Fig. 8.
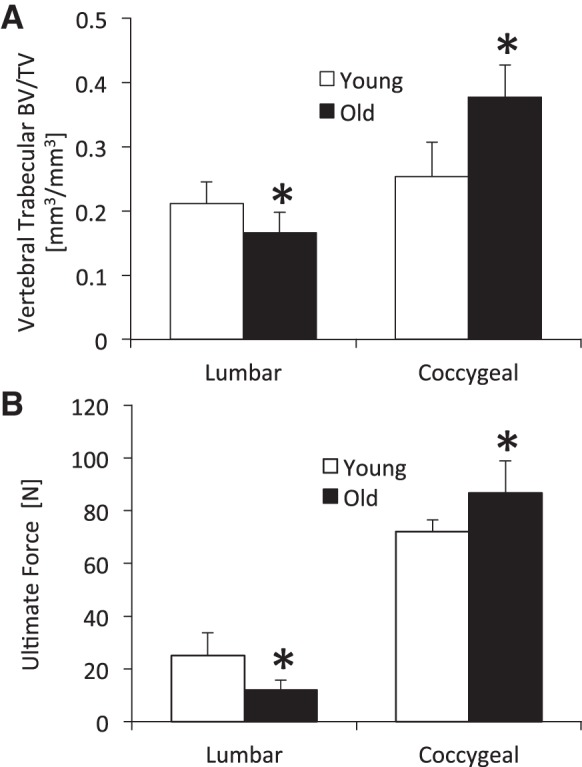
A: trabecular bone volume (BV) fraction of lumbar and coccygeal intervertebral discs from young and old mice. Trabecular BV fraction was less in old lumbar discs and greater in old coccygeal discs than the respective young disc. TV, tissue volume. B: ultimate force. Values are means ± SD. *Significant difference vs. young, P < 0.05.
Table 3.
Lumbar and coccygeal vertebra morphology of young and old mice
| Age-Level | BV/TV, % | Tb.Th, μm | Tb.N, mm−1 | Conn.D, mm−3 | SMI | vBMD, mg HA/mm3 | Cort.Th, μm |
|---|---|---|---|---|---|---|---|
| Lumbar | |||||||
| Young | 21 ± 3 | 56 ± 3 | 5.2 ± 0.5 | 154 ± 32 | 1.6 ± 0.5 | 205 ± 23 | 98 ± 4 |
| Old | 17 ± 5* | 57 ± 0.06 | 4.6 ± 0.7* | 105 ± 50* | 1.7 ± 0.6 | 171 ± 44* | 93 ± 11 |
| Coccygeal | |||||||
| Young | 25 ± 3† | 83 ± 5† | 3.5 ± 0.2† | 41 ± 8† | 1.1 ± 0.3† | 229 ± 35 | 162 ± 11† |
| Old | 38 ± 5*† | 106 ± 12*† | 3.9 ± 0.3*† | 42 ± 20† | 0.04 ± 0.7*† | 365 ± 49*† | 213 ± 31*† |
Values are means ± SD. BV/TV, bone volume/tissue volume; Tb.Th, trabecular thickness; Tb.N, trabecular number; Conn.D, connectivity density; SMI, structure model index; vBMD, volumetric bone mineral density; HA, hydroxyapatite; Cort.Th, cortical thickness.
Young vs. old of same vertebral level,
lumbar vs. coccygeal of same age: P < 0.05.
Mechanical properties of the lumbar and coccygeal vertebrae corroborated the differential changes in bone structural properties with aging. Ultimate force, stiffness, and energy-to-ultimate force were less in old lumbar vertebrae than the same in young vertebrae by 52% (P < 0.007), 33% (P = 0.0504), and 76% (P < 0.003), respectively (Table 4). By contrast, ultimate force and stiffness of old coccygeal vertebrae were greater than young by 21% (P < 0.020) and 50% (P < 0.020).
Table 4.
Lumbar and coccygeal vertebra mechanics of young and old mice
| Age-Level | Ultimate Force, N | Yield Force, N | Stiffness, N/mm | Energy at Ultimate Force, N·mm |
|---|---|---|---|---|
| Lumbar | ||||
| Young | 25.0 ± 8.7 | 9.9 ± 3.4 | 176 ± 64 | 4.9 ± 2.3 |
| Old | 12.0 ± 3.8* | 9.3 ± 3.3 | 117 ± 11* | 1.1 ± 0.6* |
| Coccygeal | ||||
| Young | 72.0 ± 4.6† | 56.2 ± 13.5† | 333 ± 83† | 14.5 ± 2.0† |
| Old | 87.7 ± 12.1*† | 42.1 ± 22.9† | 501 ± 123*† | 15.9 ± 4.1† |
Values are mean ± SD.
Young vs. old of same vertebral level,
lumbar vs. coccygeal of same age: P < 0.05.
DISCUSSION
The aim of this study was to assess the age-related changes in structure, biomechanics, biochemistry, and Wnt activity of lumbar and coccygeal intervertebral discs in mice, along with the structural and mechanical changes of the adjacent vertebrae. In the lumbar region, old murine spines had lower disc stiffness, less vertebral bone, and greater disc glycosaminoglycan than young spines. By contrast, in the coccygeal region, the age-related trends were opposite, 20- to 22-mo-old spines had greater mechanical stiffness, greater vertebral bone, and less glycosaminoglycan than 4- to 5-mo-old young spines. Thus aging differentially altered the disc glycosaminoglycan content, vertebral morphology, and disc/vertebral mechanics between the lumbar and tail regions of mice. On the other hand, both old lumbar and old coccygeal discs had greater collagen and disc height than the respective young disc, and less Wnt signaling activity than young discs, indicating some common age-related trends. Other than alterations in proteoglycans, features associated with degeneration were absent in aged discs, e.g., annular fissures, loss of distinction between nucleus pulposus and annulus fibrosus, lamellar disorganization, calcification in the disc, cleft formation, endplate sclerosis, osteophytes formation. Together, despite the negative changes in some disc properties with aging, the overall level of disc degeneration was mild in both regions of old murine spines, suggesting these changes were rather a result of remodeling, which is consistent with reduced Wnt signaling.
Strikingly, aging altered the collagen and glycosaminoglycan of old murine lumbar discs quite differently than aged human discs, yet yielded similar morphological and mechanical changes. Old lumbar discs had greater height, collagen, glycosaminoglycan, and range of motion, and less “stiffness” in compressive and tensile properties. Greater collagen, as occurs with degeneration and aging in canines (19), may reduce the mechanical properties of aged discs by inhibiting fiber reorientation during mechanical loading (23). Dissimilar to old murine lumbar discs, nondegenerate human discs lose collagen from a juvenile age (2, 53), but old and degenerated discs have elevated amounts of type I procollagen, a major component of annuli, although this latter change occurred in humans relatively older (60–80 yr) than the mice studied here (2). Unexpectedly, old lumbar intervertebral discs of C57Bl/6J mice had greater glycosaminoglycan than that of young adults and, by Toluidine staining, appeared greater in the nucleus pulposus. In another study, aging from 2 to 20 mo of discs from C57Bl/6 do not appear to lose glycosaminoglycan (5). These changes were surprising because lumbar discs in humans lose proteoglycans with aging (2, 53), but they may be specific to C57Bl/6 mice. Lumbar discs from 27-mo-old mice with a mixed background have less proteoglycans and reduced synthesis than that of young adults (57). Together, despite a difference in compositional changes with advanced age between mice and humans, a larger disc height and a reduction in compressive “stiffness” were comparable to changes that occur with human aging, degeneration, and inactivity from prolonged bed rest (33, 39, 40, 45, 48, 56).
Unlike lumbar discs, aged coccygeal discs shared some compositional and morphological changes with aging of human discs, but were mechanically dissimilar. Old coccygeal discs had greater collagen, less glycosaminoglycan, complete loss of the notochordal cells, and were stiffer than young coccygeal discs. Discs with less nuclear glycosaminoglycan, as occurs with human aging (2, 53), may not properly engage the inner annular fibers during large axial compression and overstress the outer annular fibers in the radial and axial direction (46), leading to increased stiffness from nonlinear compaction effects (3). Coccygeal discs also exhibited the greater central disc height measured in aged humans (56), and, unlike in degeneration (48), old coccygeal discs were more convex. Despite compressive property changes that were dissimilar to humans (40), aging of coccygeal discs engendered axial tensile stiffening that occurs in nondegenerate human spines (42). Overall, these results suggest that old coccygeal discs are under lower physiological stresses and, in response, incur compositional and cellular changes to the nucleus pulposus.
Similar to previous studies (18), old discs (>20 mo) from both regions of mouse spines did not appear to be degenerated. Given the putative role of Wnt signaling in disc degeneration, this result is consistent with the finding of reduced Wnt signaling in middle-aged (12-mo) discs of both regions. Both the lumbar and coccygeal discs of aged mice had less β-catenin expression than that of young adults because of decreases in the nucleus pulposus and no changes in the annulus fibrosus. Nevertheless, β-catenin expression persisted in middle-aged discs. In humans, degenerated discs present with greater expression of β-catenin (58). Conditional activation of β-catenin in mice leads to osteophyte formation and severe disc degradation by increasing biomarkers of matrix breakdown (58), but ablating Wnt signaling leads to defective lumbar disc development, establishing its necessity early in life (41). Our findings in aged lumbar discs are consistent with Kondo et al. (41), where Wnt signaling was potent in the nucleus pulposus and annulus fibrosus of 10-day-old mice and declined dramatically in the nucleus pulposus of 5-wk-old mice. We may not have noted differences in the annulus fibrosus of 4- to 5-mo-old discs because of already significant postnatal declines. Similarly, Dahia et al. (12) demonstrate an age-related decrease in Wnt signaling of lumbar discs from 1 wk to 9 wk of age, but the manner of decrease differs: cells of the annulus fibrosus expressed less Wnt signaling with aging, while Wnt signaling was absent in the nucleus pulposus at every age. Overall, the reduction of Wnt signaling in both the lumbar and coccygeal discs with advanced aging suggests that the differential changes in glycosaminoglycans and mechanical properties were possibly unrelated to Wnt signaling, and suppressed Wnt signaling may corroborate the lack of age-related disc degeneration in C57BL/6 mice.
Similar to the differential effect of aging on discs, lumbar vertebrae had less bone and decreased strength with aging, whereas old coccygeal vertebrae had greater bone and increased strength. Comparable to aging studies of vertebral bone of C57BL/6 and BALB/c mice (20, 60), Ct.Th of lumbar vertebra of C57BL/6J was not different with age, but trabecular BV fraction was less in old vertebrae because of fewer Tb.N and less Conn.D. By contrast, coccygeal vertebrae had greater Ct.Th and trabecular BV fraction due to thicker, denser, and more trabeculae. Despite use in studies of age-related diseases (37, 44), limited data exist on the morphology of aging coccygeal vertebrae of mice. Coccygeal vertebrae of BALB/c mice also demonstrate an increase in trabecular quantity and density across their life span that is uncharacteristic of human vertebrae, but a loss of lumbar bone structure and strength with advanced aging (21). Reasons for the disparity between coccygeal and lumbar vertebra in aging mice are not clear, but may relate to mechanical loading interactions. Murine coccygeal vertebrae are not weight bearing and, due to spinal level, are subjected to vastly different mechanical stresses than lumbar vertebrae, which are surrounded by muscles and other soft tissues that also incur aging-related changes. Therefore, based on the available evidence, we suggest that coccygeal vertebrae should be avoided or at least applied with caution when modeling human bone aging.
There were a few shortcomings associated with this study. The different mechanical loading limits between lumbar and coccygeal discs, necessitated because of the fragility of the old lumbar discs, precluded comparison between disc levels. However, the larger force used to test the mechanical properties of the coccygeal discs than lumbar discs may have shifted the loading curve into the higher, nonlinear region of the force/deformation curve and confounded the aging changes. Second, all of the mice used here were female, and, while it is unclear if sex influenced aging-related changes in the disc, studies show that sex affects vertebral aging in mice (60). Third, the small size of the mouse discs made separate determination of the biochemical composition of the nucleus pulposus and annulus fibrosus impractical. Instead, histology illustrated the regional effect of aging on regional proteoglycan and additionally illustrated the quality of the disc. Furthermore, neither the dimethylmethylene blue nor the hydroxyproline assays here distinguished between types of glycosaminoglycan or collagen, which may be important to determine, because different types of each change with human aging (2). Debate surrounds the use of animal models because of the presence of notochordal cells throughout adulthood that ostensibly disappear in human adults, which may elicit a different response from aging or loading-induced degeneration than human nucleus pulposus cells (1). However, notochordal cells may persist into human adulthood and what may rather be of importance to the generation of disc degeneration is the disappearance of particular notochordal cells (49). Nevertheless, the continued presence of notochordal cells in aged mouse discs (9) may protect them from aging-related degeneration (15) and posit the dissimilarity to human aging. These data offer new data on the effect of aging on the morphological and mechanical properties of lumbar and tail motion segments.
The molecular and biochemical alterations of rodent intervertebral discs and vertebrae with aging are studied extensively (11, 17, 22, 25, 47), but limited data exist on the functional consequences of such changes, which regulate the distribution of forces to the extracellular matrix. Here, we compared disc/vertebra morphology and mechanics, disc biochemical content, and disc Wnt signaling from lumbar and coccygeal regions between young adult and aged mice. Overall, the two regions displayed opposite consequences from aging, but, in accordance with other studies (17, 18), neither disc level demonstrated severe disc degeneration with aging. In bone, lumbar vertebrae exhibited changes analogous to humans, while the coccygeal discs did not. Altogether, neither spine region was ideal in duplicating human aging. Therefore, research related to disc aging must consider these age-related differences between the spinal regions when selecting murine discs and interpret findings with caution.
Conclusions.
Here, we demonstrated that murine aging 1) increases height and collagen content of lumbar and coccygeal discs; 2) stiffens lumbar discs but reduces coccygeal disc stiffness; 3) increases glycosaminoglycan of lumbar discs but decreases glycosaminoglycan of coccygeal discs, with the respective changes exhibited in the nucleus pulposus; and 4) reduces Wnt/β-catenin signaling of the nucleus pulposus of both discs. These findings demonstrate that tail and lumbar discs and vertebrae age differently and are not interchangeable for modeling human aging.
GRANTS
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases [R01 AR-047867 (M. J. Silva), P30 AR-057235 (M. J. Silva), T32 AR-060719 (N. Holguin)].
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: N.H. and M.J.S. conception and design of research; N.H., R.A., R.A.H., and B.A.B. performed experiments; N.H., R.A., R.A.H., and B.A.B. analyzed data; N.H., R.A., R.A.H., B.A.B., and M.J.S. interpreted results of experiments; N.H., R.A., R.A.H., and B.A.B. prepared figures; N.H. drafted manuscript; N.H., R.A., R.A.H., B.A.B., and M.J.S. edited and revised manuscript; N.H., R.A., R.A.H., B.A.B., and M.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Mark Johnson from University of Missouri-Kansas City for advice with LacZ staining.
REFERENCES
- 1.Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K, Melrose J, Ralphs J, Stokes I, Wilke HJ. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17: 2–19, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest 98: 996–1003, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbir A, Michalek AJ, Abbott RD, Iatridis JC. Effects of enzymatic digestion on compressive properties of rat intervertebral discs. J Biomech 43: 1067–1073, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: axial compression mechanics and glycosaminoglycan content. Spine 33: E166–E173, 2008 [DOI] [PubMed] [Google Scholar]
- 5.Blaney-Davidson EN, Vitters EL, vandenBerg WB, van der Kraan PM. Rapid decrease in Smad2/3 signaling in the annulus fibrosus co-incides with loss of lamellar structure in the aging murine intervetebral discs, while Smad1/58P stays stable until 16 months. In: ORS 2012 Meeting (Online). http://www.ors.org/Transactions/58/2175.pdf: 2012 [5 December 2013].
- 6.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine 27: 2631–2644, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Boxberger JI, Sen S, Yerramalli CS, Elliott DM. Nucleus pulposus glycosaminoglycan content is correlated with axial mechanics in rat lumbar motion segments. J Orthop Res 24: 1906–1915, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Buie HR, Moore CP, Boyd SK. Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 23: 2048–2059, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Choi KS, Cohn MJ, Harfe BD. Identification of nucleus pulposus precursor cells and notochordal remnants in the mouse: implications for disc degeneration and chordoma formation. Dev Dyn 237: 3953–3958, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahia CL, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLos One 7: e35944, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Intercellular signaling pathways active during and after growth and differentiation of the lumbar vertebral growth plate. Spine 36: 1071–1080, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Dahia CL, Mahoney EJ, Durrani AA, Wylie C. Intercellular signaling pathways active during intervertebral disc growth, differentiation, and aging. Spine (Phila Pa 1976) 34: 456–462, 2009 [DOI] [PubMed] [Google Scholar]
- 13.DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126: 4557–4568, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Elliott DM, Sarver JJ. Young investigator award winner: validation of the mouse and rat disc as mechanical models of the human lumbar disc. Spine 29: 713–722, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Erwin WM, Islam D, Inman RD, Fehlings MG, Tsui FW. Notochordal cells protect nucleus pulposus cells from degradation and apoptosis: implications for the mechanisms of intervertebral disc degeneration. Arthritis Res Ther 13: R215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flurkey K, Currer JM, Harrison DE. Mouse Models in Aging Research. San Diego, CA: Academic, 2007 [Google Scholar]
- 17.Fujita K, Ando T, Ohba T, Wako M, Sato N, Nakamura Y, Ohnuma Y, Hara Y, Kato R, Nakao A, Haro H. Age-related expression of MCP-1 and MMP-3 in mouse intervertebral disc in relation to TWEAK and TNF-alpha stimulation. J Orthop Res 30: 599–605, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Ito K, Nuka S, Hashimoto J, Takei H, Takahara M, Ogino T, Young MF, Shinomura T. Absence of biglycan accelerates the degenerative process in mouse intervertebral disc. Spine 34: E911–E917, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghosh P, Taylor TK, Braund KG, Larsen LH. The collagenous and noncollagenous protein of the canine intervertebral disc and their variation with age, spinal level and breed. Gerontology 22: 124–134, 1976 [DOI] [PubMed] [Google Scholar]
- 20.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22: 1197–1207, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Grote HJ, Amling M, Vogel M, Hahn M, Posl M, Delling G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone 16: 301–308, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Gruber HE, Sage EH, Norton HJ, Funk S, Ingram J, Hanley EN., Jr Targeted deletion of the SPARC gene accelerates disc degeneration in the aging mouse. J Histochem Cytochem 53: 1131–1138, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Guerin HA, Elliott DM. Degeneration affects the fiber reorientation of human annulus fibrosus under tensile load. J Biomech 39: 1410–1418, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Haughton VM, Lim TH, An H. Intervertebral disc appearance correlated with stiffness of lumbar spinal motion segments. AJNR Am J Neuroradiol 20: 1161–1165, 1999 [PMC free article] [PubMed] [Google Scholar]
- 25.Hickey DS, Hukins DW. Aging changes in the macromolecular organization of the intervertebral disc: an X-ray diffraction and electron microscopic study. Spine 7: 234–242, 1982 [DOI] [PubMed] [Google Scholar]
- 26.Hiyama A, Arai F, Sakai D, Yokoyama K, Mochida J. The effects of oxygen tension and antiaging factor Klotho on Wnt signaling in nucleus pulposus cells. Arthritis Res Ther 14: R105, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hiyama A, Mochida J, Omi H, Serigano K, Sakai D. Cross talk between Smad transcription factors and TNF-alpha in intervertebral disc degeneration. Biochem Biophys Res Commun 369: 679–685, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Hiyama A, Sakai D, Arai F, Nakajima D, Yokoyama K, Mochida J. Effects of a glycogen synthase kinase-3beta inhibitor (LiCl) on c-myc protein in intervertebral disc cells. J Cell Biochem 112: 2974–2986, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Hiyama A, Sakai D, Risbud MV, Tanaka M, Arai F, Abe K, Mochida J. Enhancement of intervertebral disc cell senescence by WNT/beta-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum 62: 3036–3047, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiyama A, Sakai D, Tanaka M, Arai F, Nakajima D, Abe K, Mochida J. The relationship between the Wnt/beta-catenin and TGF-beta/BMP signals in the intervertebral disc cell. J Cell Physiol 226: 1139–1148, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Holguin N, Judex S. Rat intervertebral disc health during hindlimb unloading: brief ambulation with or without vibration. Aviat Space Environ Med 81: 1078–1084, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Holguin N, Martin JT, Elliott DM, Judex S. Low-intensity vibrations partially maintain intervertebral disc mechanics and spinal muscle area during deconditioning. Spine J 13: 428–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holguin N, Muir J, Rubin C, Judex S. Short applications of very low-magnitude vibrations attenuate expansion of the intervertebral disc during extended bed rest. Spine J 9: 470–477, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Holguin N, Uzer G, Chiang FP, Rubin C, Judex S. Brief daily exposure to low-intensity vibration mitigates the degradation of the intervertebral disc in a frequency-specific manner. J Appl Physiol 111: 1846–1853, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iatridis JC, Nicoll SB, Michalek AJ, Walter BA, Gupta MS. Role of biomechanics in intervertebral disc degeneration and regenerative therapies: what needs repairing in the disc and what are promising biomaterials for its repair? Spine J 13: 243–262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iatridis JC, Weidenbaum M, Setton LA, Mow VC. Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine 21: 1174–1184, 1996 [DOI] [PubMed] [Google Scholar]
- 37.Issever AS, Walsh A, Lu Y, Burghardt A, Lotz JC, Majumdar S. Micro-computed tomography evaluation of trabecular bone structure on loaded mice tail vertebrae. Spine 28: 123–128, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Johnson ML. LRP5 and bone mass regulation: Where are we now? Bonekey Rep 1: 1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keller TS, Hansson TH, Holm SH, Pope MM, Spengler DM. In vivo creep behavior of the normal and degenerated porcine intervertebral disc: a preliminary report. J Spinal Disord 1: 267–278, 1988 [PubMed] [Google Scholar]
- 40.Keller TS, Spengler DM, Hansson TH. Mechanical behavior of the human lumbar spine. I. Creep analysis during static compressive loading. J Orthop Res 5: 467–478, 1987 [DOI] [PubMed] [Google Scholar]
- 41.Kondo N, Yuasa T, Shimono K, Tung W, Okabe T, Yasuhara R, Pacifici M, Zhang Y, Iwamoto M, Enomoto-Iwamoto M. Intervertebral disc development is regulated by Wnt/beta-catenin signaling. Spine (Phila Pa 1976) 36: E513–E518, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kurutz M. Age-sensitivity of time-related in vivo deformability of human lumbar motion segments and discs in pure centric tension. J Biomech 39: 147–157, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Kurutz M. In vivo age- and sex-related creep of human lumbar motion segments and discs in pure centric tension. J Biomech 39: 1180–1190, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lambers FM, Schulte FA, Kuhn G, Webster DJ, Muller R. Mouse tail vertebrae adapt to cyclic mechanical loading by increasing bone formation rate and decreasing bone resorption rate as shown by time-lapsed in vivo imaging of dynamic bone morphometry. Bone 49: 1340–1350, 2011 [DOI] [PubMed] [Google Scholar]
- 45.Macias BR, Cao P, Watenpaugh DE, Hargens AR. LBNP treadmill exercise maintains spine function and muscle strength in identical twins during 28-day simulated microgravity. J Appl Physiol 102: 2274–2278, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Meakin JR, Redpath TW, Hukins DW. The effect of partial removal of the nucleus pulposus from the intervertebral disc on the response of the human annulus fibrosus to compression. Clin Biomech (Bristol, Avon) 16: 121–128, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Papuga MO, Kwok E, You Z, Rubery PT, Dougherty PE, Pryhuber G, Beck CA, Hilton MJ, Awad HA, Schwarz EM. TNF is required for the induction but not the maintenance of compression-induced BME signals in murine tail vertebrae: limitations of anti-TNF therapy for degenerative disc disease. J Orthop Res 29: 1367–1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pfirrmann CW, Metzdorf A, Elfering A, Hodler J, Boos N. Effect of aging and degeneration on disc volume and shape: A quantitative study in asymptomatic volunteers. J Orthop Res 24: 1086–1094, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Risbud MV, Shapiro IM. Notochordal cells in the adult intervertebral disc: new perspective on an old question. Crit Rev Eukaryot Gene Expr 21: 29–41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine 29: 2691–2699, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Sarver JJ, Elliott DM. Mechanical differences between lumbar and tail discs in the mouse. J Orthop Res 23: 150–155, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Showalter BL, Beckstein JC, Martin JT, Beattie EE, Espinoza Orias AA, Schaer TP, Vresilovic EJ, Elliott DM. Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine 37: E900–E907, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh K, Masuda K, Thonar EJ, An HS, Cs-Szabo G. Age-related changes in the extracellular matrix of nucleus pulposus and anulus fibrosus of human intervertebral disc. Spine 34: 10–16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smit TH. The use of a quadruped as an in vivo model for the study of the spine–biomechanical considerations. Eur Spine J 11: 137–144, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smolders LA, Meij BP, Riemers FM, Licht R, Wubbolts R, Heuvel D, Grinwis GC, Vernooij HC, Hazewinkel HA, Penning LC, Tryfonidou MA. Canonical Wnt signaling in the notochordal cell is upregulated in early intervertebral disc degeneration. J Orthop Res 30: 950–957, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Twomey LT, Taylor JR. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop Relat Res 224: 97–104, 1987 [PubMed] [Google Scholar]
- 57.Vo N, Seo HY, Robinson A, Sowa G, Bentley D, Taylor L, Studer R, Usas A, Huard J, Alber S, Watkins SC, Lee J, Coehlo P, Wang D, Loppini M, Robbins PD, Niedernhofer LJ, Kang J. Accelerated aging of intervertebral discs in a mouse model of progeria. J Orthop Res 28: 1600–1607, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang M, Tang D, Shu B, Wang B, Jin H, Hao S, Dresser KA, Shen J, Im HJ, Sampson ER, Rubery PT, Zuscik MJ, Schwarz EM, O'Keefe RJ, Wang Y, Chen D. Conditional activation of beta-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum 64: 2611–2623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S, Xia Q, Passias P, Wood K, Li G. Measurement of geometric deformation of lumbar intervertebral discs under in-vivo weightbearing condition. J Biomech 42: 705–711, 2009 [DOI] [PubMed] [Google Scholar]
- 60.Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ. Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int 86: 470–483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yuan R, Tsaih SW, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8: 277–287, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao F, Pollintine P, Hole BD, Dolan P, Adams MA. Discogenic origins of spinal instability. Spine 30: 2621–2630, 2005 [DOI] [PubMed] [Google Scholar]