Abstract
INTRODUCTION
Total sacrectomy for recurrent rectal cancer is controversial. However, recent publications suggest encouraging outcomes with high sacral resections. We present the first case report describing technical aspects, potential pitfalls and treatment of complications associated with total sacrectomy performed as a treatment of recurrent rectal cancer.
PRESENTATION OF CASE
A fifty-three year old man was previously treated at another institution with a low anterior resection (LAR) followed by chemo-radiation and left liver tri-segmentectomy for metastatic rectal cancer. Three years following the LAR, the patient developed a recurrence at the site of colorectal anastomosis, manifesting clinically as a contained perforation, forming a recto-cutaneous fistula through the sacrum. Abdomino-perineal resection (APR) and complete sacrectomy were performed using an anterior–posterior approach with posterior spinal instrumented fusion and pelvic fixation using iliac crest bone graft. Left sided vertical rectus abdominis muscle flap and right sided gracilis muscle flap were used for hardware coverage and to fill the pelvic defect. One year after the resection, the patient remains disease free and has regained the ability to move his lower limbs against gravity.
DISCUSSION
The case described in this report features some formidable challenges due to the previous surgeries for metastatic disease, and the presence of a recto-sacral cutaneous fistula. An approach with careful surgical planning including considerationof peri-operative embolization is vital for a successful outcome of the operation. A high degree of suspicion for pseudo-aneurysms formation due infection or dislodgement of metallic coils is necessary in the postoperative phase.
CONCLUSION
Total sacrectomy for the treatment of recurrent rectal cancer with acceptable short-term outcomes is possible.A detailed explanation to the patient of the possible complications and expectations including the concept of a very high chancefor recurrence is paramount prior to proceeding with such a surgery.
Keywords: Colorectal, Rectal cancer, Recurrence, Sacrectomy
1. Report
1.1. Background
Traditionally, cancer rectal surgeries requiring S1 and S2 nerve root resections have been considered inoperable. This is mostly due to perceived low possibility for cure and high morbidity rates.1,2 However, recent reports suggest encouraging outcomes with high sacral resections.3,4 A nine patient series from the Mayo Clinic, which included one resection at L5–S1 level, was recently published suggesting that high sacrectomy can be performed with acceptable results. The reported median survival was 31 months. All deaths were due to metastatic disease.3
Although complete sacrectomy for recurrent rectal cancer is often rejected by colorectal surgeons, it is part of an extended skill set for spinal and reconstructive surgeons that deal with primary malignant tumors such as chordoma, chondrosarcoma, and plasmacytoma.5 A review of surgical management of these lesions has been published by a team from Stanford University Medical Center.6
This is the first case report which describes technical details, potential pitfalls and treatment of complications of total sacrectomy when performed as a treatment for recurrent rectal cancer.
2. Presentation of case
A fifty-three year old man was initially treated at another institution with LAR for a T3N0M0 rectal cancer located 5 cm from the anal verge. Because of obstructive symptoms, it was decided to proceed with a resection up-front without neo-adjuvant treatment. Subsequently, the patient developed an anastomotic leak, which required a diverting loop ileostomy. Following recovery, the patient was treated with adjuvant chemo-radiation therapy, after which, the diverting ileostomy was reversed. A year after the initial diagnosis, the patient was found to have extensive left-sided liver metastases which were treated with chemotherapy, and left-sided liver tri-segmentectomy. As a consequence of all these surgeries, the patient developed a large incisional hernia extending from the site of the Kocher incision to the site of prior ileostomy. The hernia was repaired laparoscopically with a prosthetic mesh. Three years following the initial diagnosis, the patient was referred to our center with an anastomotic recurrence. This recurrence presented as a retroperitoneal perforation forming a recto-cutaneous fistula through the sacrum with extensive adjacent soft tissue infection and necrosis (Fig. 1). The patient's quality of life was poor.
Fig. 1.

Sagital CT scan view of recurrent rectal cancer which presented as a retroperitoneal perforation forming a recto-cutaneous fistula through the sacrum with extensive adjacent soft tissue infection and necrosis.
The diagnosis and treatment options were reviewed in a multidisciplinary setting and with the patient. Both operative and non-operative options were discussed. An APR with total sacrectomy was offered. With respect to the proposed surgery, the patient was made aware of inevitable loss of bowel, bladder and sexual functions. Other risks discussed included significant blood loss, permanent lower extremity neurological injury, lower body mobility impairment, cerebrospinal fluid leak, persistent infection, hardware failure, structural non-union, tumor recurrence, and death. The surgical team involved colorectal, spinal, and plastic surgery consultants.
APR with complete sacrectomy was performed using an anterior–posterior approach with posterior spinal instrumented fusion and pelvic fixation using iliac crest bone graft. Left-sided vertical rectus abdominis and right-sided gracilis muscle flaps were used for closure of the perineal and pelvic defects.
2.1. Operative details
2.1.1. Rectal mobilization
In supine position, after insertion of ureteric stents, a generous laparotomy extending from the xiphoid process to the pubic symphysis was performed. A dilated descending colon was observed, proximal to the disease complex, due to the stenosing nature of the recurrent pelvic lesion. The left colon was dissected off the retro-peritoneum and the splenic flexure was mobilized. Dissection of the rectum was started anteriorly until the seminal vesicles were reached and then continued down to the levator ani. The lateral rectal space contained purulent fluid related to the perforation. This fluid was promptly aspirated. Once the dissection of the anterior and lateral side of the rectum was completed from the abdominal approach, the operation continued through the perineal approach. The dissection of the anus was carried around the external sphincter cephalad reconnecting with the abdominal dissection. The rectum was then divided above the sacral promontory.
After opening of the posterior peritoneum, the aorta and the vena cava were dissected off the spine distal to the paramesenteric bursa. The dissection continued with the isolation of the common iliac artery and common iliac vein on the right side and ligation of the right internal iliac vessels. Pre-surgery radiation therapy and extensive inflammation triggered by the anastomotic leak made the dissection of the left iliac vessels very challenging since they were completely adherent to the pelvis. It was possible to ligate and divide the left internal iliac artery but it was not feasible to isolate the left common iliac vein and safely ligate the left internal iliac vein. The decision was made to continue the mobilization of the remainder of the pre-sacral soft tissue to free up the sacrum as much as possible anteriorly. The team elected to carry out the surgery in two steps and perform embolization of the left iliac vein and coiling of the internal iliac branches under radiological guidance, before completing the sacrectomy, to reduce bleeding during the posterior phase of the operation.
The plastic surgery team was called in and a left-sided vertical rectus abdominis myocutaneous flap was raised. The origin of the deep inferior epigastric artery was dissected. Although the patient had a previous incisional hernia repair done laparoscopically, the tacks used to anchor the mesh to the abdominal wall were easily dissected off the muscle. Marking sutures were used in order to maintain orientation of the flap at all times.
2.1.2. Sacral resection – anterior approach
The psoas muscles lateral to the sacrum were incised and the lumbosacral plexus was visualized bilaterally. The L5 and S1 nerve roots were identified and the S1 nerve roots were sacrificed bilaterally by transecting them sharply with a scalpel after tying them off with a #2 silk. The sacroiliac joints were identified bilaterally first with intraoperative fluoroscopic imaging and subsequently by direct visualization after continuation of the psoas mobilization down to bone. The L5–S1 disk space was exposed just above the sacral promontory at the bifurcation of the aorta and vena cava. A discectomy at the L5–S1 disk space was completed. The annulus fibrosus was incised anteriorly with a disk knife. The nucleus pulposus was then evacuated with pituitary rongeurs, and finally, the annulus fibrosus and posterior longitudinal ligament were incised to expose the thecal sac. Assisted by both direct visualization and intraoperative fluoroscopic imaging, an osteotome was used to create a longitudinal osteotomy (anterior to posterior) 5 mm lateral to the sacroiliac joints bilaterally. While protecting the L5 nerve roots, the psoas muscle incisions were then continued in a horizontal fashion above the sacral ala to communicate with the L5–S1 discectomy.
The colostomy was fashioned on the right side of the abdomen since the left side of the abdominal wall had been raised to make the vertical rectus abdominis myocutaneous flap. The flap was then put inside the abdomen in its anatomical orientation through a small inferior fascial incision. The abdomen was closed and the patient was then turned prone.
2.1.3. Posterior approach
Based on intraoperative fluoroscopic imaging, a posterior midline incision was made from L3 to the distal sacrum, down through skin and subcutaneous tissues to the lumbosacral fascia. The spinous processes were identified and the erector spinae muscles were elevated off the posterior spinal elements laterally to the transverse processes. The sacrum was exposed posteriorly from midline, out laterally past the sacroiliac joints to the previous osteotomy cuts. In addition, all soft tissue was removed from the sacrum.
A decompression was then carried out at L5. This consisted of a wide laminectomy using a Kerrison rongeur and a facetectomy bilaterally to visualize the thecal sac and exiting L5 nerve roots, respectively. The thecal sac was tied off just below the exiting L5 nerve roots with #2 silk and transected with a scalpel. The L5–S1 disk space was identified and the discectomy was completed so that there was now continuity with the anterior transaction. Cartilage was then removed with curettes from the inferior endplate of the L5 vertebral body.
Following this, an iliac crest bone graft was harvested using a standard bone harvesting technique from the posterior superior iliac spine bilaterally utilizing the same midline skin incision but through separate facial incisions laterally.
Attention was next shifted to spino-pelvic reconstruction and stabilization. Synthes Matrix Spine System instrumentation was employed. 5.5 mm diameter bilateral pedicle screws were placed from L3–L5 and 8 mm × 80 mm iliac bolts were secured into the iliac wings. Radiographic guidance was used throughout this part to confirm acceptable placement of instrumentation. A titanium rod was contoured and fixed to the pedicle screws and iliac bolts. A cross-link between both rods was placed for rotational stability. The wound was irrigated and hemostasis was achieved using combination of bone wax, FloSeal and surgical gauze. The posterior wound was left open, covered with a temporary dressing. The patient was transferred to the Intensive Care Unit (ICU) with borderline hemodynamic parameters, where he was stabilized. There was more than 4000 ml of estimated blood loss.
2.1.4. Completion of anterior dissection
Following 48 h hemodynamic stabilization in the ICU, the left iliac vein was embolized and its internal branches were coiled to better control sacral venous bleeding.
The midline incision was reopened and irrigated. The left iliac vein and its internal branches were positively identified, dissected off the sacrum, and ligated. The distal nerve roots S2–S5, sacro-tuberous and sacro-spinus ligaments at the sacral insertion were transected together with soft tissue to communicate with the posterior incision.
Due to the size of the pelvic defect and concerns about the viability of the vertical rectus abdominis myocutaneous flap, a decision was made to also raise a right-sided gracilis myocutaneous flap for improved coverage. The flap was tunneled to the pelvis. The abdomen and leg incisions were then closed.
2.1.5. Sacral resection completion
The patient was repositioned prone and the packing was removed. Irrigation and debridement was carried out with 9 L of jet-lavage (Fig. 2). The sacrum was then removed from the pelvis en bloc together with the rectum and the specimen was sent to pathology (Fig. 3). The stabilization of the spine to the pelvis was completed by placing two large Synthes SynMesh cylindrical cages (Fig. 4), which were impacted into the inferior endplate of L5 and secured to the ilium bilaterally with large fragment cortical screws. Previously harvested iliac crest bone graft was mixed with 1 g vancomycin and placed within the cage to facilitate fusion.
Fig. 2.
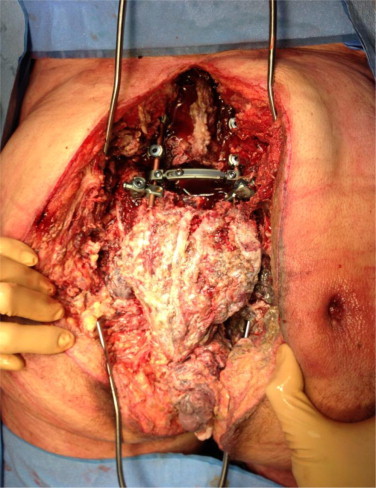
Intraoperative view of the surgical specimen in situ just prior to its removal from the body.
Fig. 3.

Resected specimen.
Fig. 4.
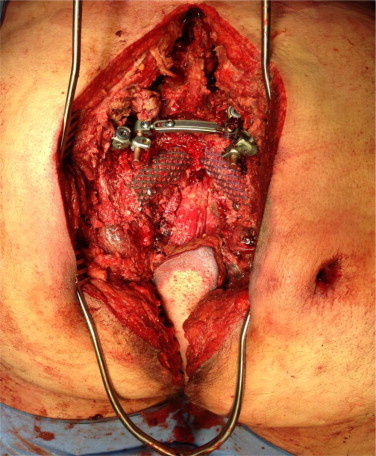
The stabilization of the spine to the pelvis with Synthes SynMesh cylindrical cages.
2.1.6. Soft tissue coverage
After inclusion of the cutaneous fistulous tract with the final specimen and debridement of infected gluteal tissue, it was determined that a local advancement gluteal cutaneous flap would be sufficient for skin coverage. The vertical rectus abdominis myocutaneous flap was therefore converted to a muscular flap. The rectus muscle flap was then suture-anchored together with the gracilis muscle flap to cover the hardware and fill the cavity.
There was excellent tissue coverage of the area of the retroperitoneum. Jackson Pratt drains were left in place and skin closure was then performed using a simple advancement gluteal flap after copious irrigation.
2.2. Post-operative course
The patient's initial recovery course was rather uneventful with the patient tolerating full diet at day seven. Bed rest to allow complete healing of the spine was ordered for four weeks. Five weeks after the operation the patient suddenly became hemodynamically labile and blood started pouring out of the perineal wound. While he was being actively resuscitated he had a cardiac arrest, which lasted for nine minutes during which cardiopulmonary resuscitation was performed to re-establish cardiac activity. An emergency CT-angiography was performed which showed a ruptured pseudo-aneurysm of the right iliac artery. The right iliac artery was stented in the angio suite with subsequent arrest of the bleeding. Because of the compressive symptoms caused by the large collection of blood in the abdomen, the patient was taken the next day to the operating room for evacuation of the hematoma.
Forty-five days after this event, while doing some physiotherapy, the patient was found to have blood leaking from the wound in the perineum. Because of the high index of suspicion for another bleeding event an urgent angiogram was performed. The angiogram showed a ruptured pseudo-aneurysm from the previously clipped left internal iliac artery. The artery was stented and the bleeding stopped immediately. Due to the prompt intervention, the patient never became unstable and didn’t require any vasopressors.
One year after the resection, the patient remains disease free. Fig. 5 depicts the surgical site at one year post operation. The patient's bladder dysfunction is managed by an indwelling catheter. There is no activity in his feet, necessitating the use of bilateral ankle braces. He ambulates primarily by wheelchair although his lower leg strength has progressed to the point where he is able to bend his knees and lift his legs at the hips against gravity, which allows him to assist with transfers.
Fig. 5.

Perineum one year post-op.
3. Discussion
Total or near total sacrectomy is a well-established approach for primary sacral tumors.5,6 The feasibility of this approach for recurrent rectal cancer has been suggested in several studies.1–4 Although in these case series the majority of the patients treated for recurrent rectal cancer underwent near total sacrectomy, it has been suggested that, in carefully selected cases, a total sacrectomy might have some benefits in terms of prolonging survival, especially when negative margins are achieved.3,7
The case described in this report features some formidable challenges. These included previous treatments and surgeries for primary as well as liver metastatic disease. Together with the presence of a recto sacral cutaneous fistula, these challenges undermined the curative intent and decreased the chance to obtain an R0 resection.
The decision to offer a surgical option to the patient took into account several factors including: the young age of the patient, the poor quality of life caused by the recto-sacral cutaneous fistula, the patient's otherwise good health, as well as the midline rather than lateral spread of the malignant fistula which increased the chance of performing a successful negative margin resection. The patient's thorough understanding of the expected neurological impairment and his awareness that the chance of obtaining a cure was very low were considered essential prior to proceeding with the surgery.
Preoperative embolization of the internal iliac vessels should be considered in cases where a difficult dissection is expected, although the increased venous pressure of the pelvic collaterals following the embolization might increase the blood loss during the dissection of the rectum. It is therefore suggested that the rectal dissection be performed prior to ligation of the internal iliac vessels.
A high degree of vigilance for pseudo-aneurysm formation and rupture, secondary to infection and dislodgment of metallic coils, is required during the postoperative phase. Any drop in hemoglobin during the recovery phase, should be considered secondary to a pseudo-aneurysm rupture until proven otherwise. In the event of a pseudo-aneurysm rupture, angiographic intervention seems to be a safe and effective way to control the hemorrhage.
4. Conclusion
Sacrectomy as a treatment for recurrent rectal cancer with acceptable short-term outcomes is possible. A staged, multidisciplinary approach with careful surgical planning including consideration of peri-operative embolization is vital for a successful outcome of the operation. A detailed explanation to the patient of the possible complications and expectations including the concept of a very high chance for recurrence, especially in the setting of previously treated metastatic disease, is paramount prior to proceeding with such a surgery.
Conflict of interest
The authors report that there are no conflicts of interest.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Author contributions
(1) George Melich, MD: drafting of article, revision of article critically for important intellectual content; (2) Michael Weber, MD: revision of article critically for important intellectual content; (3) Barry Stein: revision of article critically for important intellectual content; (4) Vincenzo Minutolo, MD: revision of article critically for important intellectual content; (5) Manuel Arena, MD: revision of article critically for important intellectual content; (6) Goffredo O. Arena: drafting of article, revision of article critically for important intellectual content, final approval of the version to be submitted.
References
- 1.Büchler M.W., Heald R.J., Ulrich B., Weitz J. Rectal cancer treatment. In: Weitz J., editor. Recent results in cancer research. Springer; Berlin: 2005. [Google Scholar]
- 2.Niamh M.H., Myles R.J. Surgical management of locally recurrent rectal cancer. Int J Surg Oncol. 2012;2012:1–6. doi: 10.1155/2012/464380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dozois E.J., Privitera A., Holubar D.S., Aldrete J.F., Sim F.H., Rose P.S., Walsh M.F., Bower T.C., Leibovich B.C., Nelson H., Larson D.W. High sacrectomy for locally recurrent rectal cancer: can long-term survival be achieved? J Surg Oncol. 2011;103(2):105–109. doi: 10.1002/jso.21774. [DOI] [PubMed] [Google Scholar]
- 4.Ohta K., Ikeda M., Kagawa Y., Ohtsuka M., Takemasa I., Mizushima T., Yamamoto H., Mor M. Two cases of curative resection for locally recurrent rectal cancer with high-level sacrectomy after preoperative chemoradiation therapy (CRT) Gan To Kagaku Ryoho. 2011;38(12):1992–1994. [PubMed] [Google Scholar]
- 5.Bridwell K.H. Management of tumors at the lumbosacral junction. In: Margulies J.Y., Floman Y., Farcy J.P.C., Neuwirth M.G., editors. Lumbosacral and spinopelvic fixation. Lippincott-Raven; Philadelphia: 1996. pp. 109–122. [Google Scholar]
- 6.Zhang H.Y., Thongtrangan I., Balabhadra R.S.V., Murovic J.A., Kim D.H. Surgical techniques for total sacrectomy and spinopelvic reconstruction. Neurosurg Focus. 2003;15(2) doi: 10.3171/foc.2003.15.2.5. [Article 5] [DOI] [PubMed] [Google Scholar]
- 7.Wanebo H.J., Begossi G., Varker K.A. MD surgical management of pelvic malignancy: role of extended abdominoperineal resection/exenteration/abdominal sacral resection. Surg Oncol Clin N Am. 2005;14:197–224. doi: 10.1016/j.soc.2004.12.001. [DOI] [PubMed] [Google Scholar]


