Abstract
After vestibular labyrinth injury, behavioral deficits partially recover through the process of vestibular compensation. The present study was performed to improve our understanding of the physiology of the macaque vestibular system in the compensated state (>7 wk) after unilateral labyrinthectomy (UL). Three groups of vestibular nucleus neurons were included: pre-UL control neurons, neurons ipsilateral to the lesion, and neurons contralateral to the lesion. The firing responses of neurons sensitive to linear acceleration in the horizontal plane were recorded during sinusoidal horizontal translation directed along six different orientations (30° apart) at 0.5 Hz and 0.2 g peak acceleration (196 cm/s2). This data defined the vector of best response for each neuron in the horizontal plane, along which sensitivity, symmetry, detection threshold, and variability of firing were determined. Additionally, the responses of the same cells to translation over a series of frequencies (0.25–5.0 Hz) either in the interaural or naso-occipital orientation were obtained to define the frequency response characteristics in each group. We found a decrease in sensitivity, increase in threshold, and alteration in orientation of best responses in the vestibular nuclei after UL. Additionally, the phase relationship of the best neural response to translational stimulation changed with UL. The symmetry of individual neuron responses in the excitatory and inhibitory directions was unchanged by UL. Bilateral central utricular neurons still demonstrated two-dimension tuning after UL, consistent with spatio-temporal convergence from a single vestibular end-organ. These neuronal data correlate with known behavioral deficits after unilateral vestibular compromise.
Keywords: vestibular nuclei, vestibular compensation, plasticity, utricle, spatio-temporal convergence
behavioral deficits following unilateral vestibular compromise improve over time. Acute deficits following unilateral insult to the vestibular labyrinth, which are apparent when the animal is stationary, reliably resolve with time. Recovery of these so-called static signs and symptoms (spontaneous nystagmus, head nystagmus, head tilt, acute vertigo) have been shown to correspond to recovery of resting neuronal activity in the ipsilesional vestibular nucleus (Precht et al. 1966; Smith and Curthoys 1988, 1989). In contrast, recovery of vestibular functions in response to passive motion, termed dynamic vestibular compensation, is incomplete, and the degree of recovery that is seen is highly dependent upon the magnitude, frequency, and modality (rotation or translation) of the stimulation (Allum et al. 1988; Angelaki et al. 2000; Broussard et al. 1999; Crane and Demer 1998; Fetter and Zee 1988; Lasker et al. 2000; Maioli et al. 1983; Newlands et al. 2005; Sadeghi et al. 2006; Ushio et al. 2011). In addition to recovery of static and dynamic compensation, use of other sensory signals to replace missing vestibular signals, known as sensory substitution, correlate with functional improvement.
Most of the literature on dynamic vestibular compensation pertains to rotational stimuli that impact reflexes related to semicircular canal function. There is very little written about compensation of reflexes related to translation. Data documenting eye movement responses to translation in primates have shown severe deficits in the translational vestibulo-ocular reflex (trVOR) after unilateral labyrinthectomy (UL) (Angelaki et al. 2000; Lempert et al. 1998). In particular, acutely following UL, the eye kinematics for near target viewing during lateral and fore-aft translation are disrupted, the gain of the trVOR response during lateral translation is decreased (particularly with acceleration to the side of injury), and the eye movement response to pure horizontal translation acquires a vertical component (Angelaki et al. 2000). With time, there is incomplete improvement of trVOR gain (Angelaki et al. 1999, 2000; Lempert et al. 1998) and of appropriate kinematics to lateral motion (Angelaki et al. 2000). The eye kinematics required for maintaining fixation on near targets in frontal eyed animals during fore-aft movement are disrupted after UL in macaques and do not recover appropriately (Angelaki et al. 2000). The one study of perception of translation after UL, performed in rhesus macaques, similarly demonstrated that perception of straight ahead is biased toward the side of the UL (Liu et al. 2013).
Neural correlates of rotational vestibulo-ocular reflex (VOR) deficits and compensation have been found in the vestibular nuclei (Newlands and Perachio, 1990a, 1990b; Newlands and Wei 2013; Sadeghi et al. 2011). To date, neural correlates of compensation of deficits from ablation of the otolith organs have been studied primarily in anesthetized animals (Chan 1997; Chan et al. 1999; Hoshino and Pompeiano 1977; Lacour et al. 1985; Xerri et al. 1983), although two recent studies have investigated aspects of central recovery of otolith pathways in alert macaques (Liu et al. 2013; Thomassen et al. 2012). These studies have noted that differences in neural responses to linear acceleration after UL are found in the bilateral vestibular nuclei. The threshold for activation of central vestibular neurons to rotation is elevated compared with normal, but translational sensitivity in three-dimensional space is not significantly different from neurons in normal animals (Thomassen et al. 2012).
Otolith afferents have been shown to be cosine tuned, meaning that these neurons have maximum sensitivity in one orientation and at that sensitivity is reduced with the cosine of the angle of the direction of stimulation to the direction of maximum sensitivity (Angelaki and Dickman 2000; Dickman et al. 1991; Fernandez et al. 1972; Fernández and Goldberg 1976b; Loe et al. 1973; Purcell et al. 2003; Si et al. 1997). For cosine tuned neurons, there is no response for translation perpendicular to the direction of maximum sensitivity, and, as such, these neurons are considered as being one-dimensional. In contrast, the spatial response properties of central vestibular neurons to translation differ widely between being narrowly tuned in the horizontal plane (afferent like) and being more broadly tuned and responsive in all directions in the horizontal plane (Angelaki et al. 1993; Angelaki and Dickman 2000; Bush et al. 1993, Chen-Huang and Peterson 2006; Dickman and Angelaki 2002; Kaufman et al. 2000; Schor 1974; Schor and Miller 1982, Schor et al. 1984, 1985; Yakushin et al. 2006). This broad tuning is attributed to spatio-temporal convergence (STC) in the vestibular nuclei, which is characterized both by a non-zero sensitivity in all directions and variant phase relationships with changing orientation of translation. STC can theoretically arise from the convergence of two cosine tuned utricular afferents with different spatial and temporal characteristics onto a central vestibular neuron, although other forms of convergence could also result in two-dimensional responses (Angelaki 1991, 1992; Angelaki et al. 1993; Bush et al. 1993; Hess and Angelaki 1993; Schor and Angelaki 1992). While theoretically STC properties centrally can arise from one labyrinth, the presence of STC after UL has not been directly tested. Whether UL compromises the properties of STC is also unknown.
The goal of the present study was to compare central vestibular responses after UL with labyrinth-intact animals. As with the recent study of rotational responses after UL from our laboratory (Newlands and Wei 2013b), the present study is not intended to document the change in responses over time. Instead, we seek to describe the compensated state. Our hypothesis was that responses of vestibular neurons to translation would mirror those of the vestibular reflexes in that the individual neurons would display asymmetry in their responses with decreased sensitivity toward the side of the compromised labyrinth, and that the spatial organization of the population of neurons after UL would differ from that in labyrinth-intact animals. We found that, in compensated animals after UL, there was a decrease in sensitivity to translation. The overall distribution of vectors of maximum sensitivity was disrupted spatially, primarily due to a decrease in the number of neurons in the contralesional vestibular nucleus responsive to ipsilesional translation and temporally, as evidenced by a change in the distribution of phases of neuronal responses.
METHODS
Animal preparation.
Data were collected from two juvenile rhesus macaque monkeys (Macaca mulatta), one male (M1) and one female (M2) weighing 5.5 and 6.2 kg, respectively. The initial surgical procedures, including placement of recording chambers and eye coils, were detailed in a previous report (Newlands et al. 2009). In short, we initially implanted a dental acrylic head cap for head immobilization with a stereotaxically-placed recording chamber aimed at the rostral vestibular nuclei. In a later surgery, unilateral or bilateral 3-turn 15.5-mm subconjunctival eye coils were attached to the sclera with 7–0 prolene sutures.
After these procedures, control data were collected. Subsequently, these animals underwent left UL with removal of the entire membranous labyrinth under isoflurane anesthesia in sterile conditions, as described previously (Newlands and Wei 2013a). The animals were treated with postoperative antibiotics for 7 days and intramuscular buprenorphine (0.01 mg/kg) for pain control twice a day for 3 days. All surgical procedures and data collection were performed according to institutional, American Physiological Society and National Institutes of Health guidelines and under a protocol approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch.
Experimental setup and neural recordings.
The experimental apparatus and techniques used to perform these experiments were described in detail previously (Newlands et al. 2009; Newlands and Wei 2013a, 2013b). In short, animals were placed into primate chairs on a computer-controlled vestibular stimulation apparatus, which allowed the animal to be rotated in yaw or pitch, or to be translated horizontally in any direction. Their heads were restrained with the nose pitched 20° down. Control of rotation and translation was accomplished using custom-written software (LabVIEW, National Instruments, Austin, TX). Laser targets were either controlled using an x–y mirror galvanometer or presented head-fixed for VOR suppression using lasers mounted on the primate chair. Eye movements were recorded with a two-field magnetic search coil system (C-N-C Engineering Systems, Seattle, WA). Animals were trained to fixated targets for juice rewards prior to neural recordings.
Extracellular recordings were made of rostral vestibular nuclei neurons using stereotaxically placed epoxy-coated tungsten microelectrodes (2 -10 MΩ impedance, Fredrich-Haer, Bowdointam, ME). The electrodes were advanced through a 26-gauge cannula starting 5–7 mm above the vestibular nuclei. Recordings were concentrated in the rostral vestibular nuclei. Passes were limited to 0–6 mm posterior and 0–6 mm lateral to the abducens nucleus. Histological confirmation of recording sites was available for both monkeys, showing that these passes were in the middle to rostral end of the vestibular nuclear complex.
The search stimulus was horizontal interaural translation 0.5 Hz, ±0.2 g with a head-fixed target. Once a neuron was isolated which responded to horizontal translation, it was further tested for eye movement sensitivity during saccades to targets (spaced every 5°, ±20° to the left and right of straight ahead) and smooth pursuit of a target moving in the horizontal plane (0.2 Hz at ±23° or 0.5 Hz at ±10°). Based on the sensitivity of neuron firing to eye movements, the neurons were classified using standard definitions. Cells without eye movement sensitivity were classified as non-eye movement (NEM) neurons, but are the same group of cells also referred to as vestibular only in many studies (Scudder and Fuchs 1992). Cells sensitive to eye position or velocity were less commonly encountered during these experiments and, consequently, were not included in our analysis. Translation-sensitive neurons were then recorded in response to a series of translations in the horizontal plane. The orientation was defined as acceleration toward the right ear as 0°, acceleration toward the nose as 90°, acceleration toward the left ear as 180°, and acceleration toward the occiput as 270°. For most of the neurons, sensitivity to yaw rotation was also documented. For all of these translation trials, at least 40 s of data were collected (≥20 cycles). For translation-sensitive neurons, data was collected at 0.5 Hz and 0.2 g at each of six orientations (0°: interaural; 30°, 60°, 90°: naso-occipital; 120°; and 150°). We then collected data with acceleration along the interaural and naso-occipital directions at 0.25, 0.5, 1.0, and 2.0; all at 0.2 g and 5.0 Hz at 0.1 g. Equipment limitations prevented collecting 5.0 Hz data at 0.2 g.
In these experiments, data were collected from two distinct time periods. The first was prior to UL. During this time, the vestibular nuclei were explored and mapped, and normal neuronal responses to our protocols were recorded. After the UL procedure, the animals were allowed to recover prior to resumption of recording. The time that passed before starting recordings after UL was chosen to be at least 6 wk after UL, well after the time when other studies have noted a plateau for most measures of compensation (Fetter and Zee 1988; Sadeghi et al. 2010, 2011). Both animals had negligible nystagmus in the dark pre-UL. One day after UL, the spontaneous nystagmus rate was 27.8°/s in M1 and 37.5°/s in M2. In M1, nystagmus in the dark was reduced to 11°/s by day 27 postlesion and around 9°/s from day 60 postlesion onward. In M2, nystagmus in the dark was reduced to 11.3°/s on postlesion day 28 and was negligible on postlesion day 72. This degree of nystagmus in the dark was consistent with the findings of Fetter and Zee (1988) in this species. Neither monkey had an obvious behavior deficit during normal activities by the time recordings were resumed. Recordings continued from day 51 to day 136 after UL in monkey M1 and from day 71 to day 177 after UL in monkey M2. During the recording period, 57 electrode penetrations were made in M1 (30 in the ipsilesional vestibular nuclei and 27 at the contralesional vestibular nuclei), and 46 electrode penetrations were made in M2 (24 ipsilesion and 22 contralesion).
Data analysis.
All of the details of the data analysis have been previously reported (Newlands et al. 2009; Newlands and Wei 2013a, 2013b). All analysis was done offline. In short, the neural data were filtered (100–10,000 Hz), recorded and stored to disk at 40 kHz. Offline, the neural data were converted into spike trains using both feature analysis and time-amplitude window discrimination to discriminate the time of spike occurrence. The spike train was then converted to instantaneous firing rate (defined as the 1/interspike interval between the spike preceding and follow that time point) for comparison with eye and head position, velocity and acceleration traces, all sampled at a rate of 8 kHz. Calculation of sensitivity and phase of the neuronal response to sinusoidal translations began by averaging both the instantaneous firing rate and the stimulus (linear acceleration) over all of the cycles collected and by fitting each to a sine wave using a Levenberg-Marquardt least-squares algorithm. The sensitivity (neuronal gain) to the horizontal translation stimulus was determined by comparing the amplitude of the response peak to that of the stimulus. The phase of the neuron response to horizontal translation was determined by comparing the timing of the peak of the sinusoidal fit to the stimulus (head acceleration) relative to the peak of the sinusoidal fit to the instantaneous firing rate response.
For translation-sensitive neurons, several metrics were calculated. These included the orientation in the horizontal plane that elicited the maximal response (orientation vector or the direction of the vector Smax), the sensitivity (gain) to translation along the orientation vector (Smax), the orientation of the vector orthogonal to Smax (Smin), the sensitivity (gain) to translation orthogonal to the orientation vector (Smin), and the ratio of Smin/Smax (tuning ratio). This was done by characterizing the spatial tuning of each neuron through fitting the Angelaki two-dimensional spatio-temporal model for both sensitivity and phase (Angelaki 1991; Angelaki et al. 1992, 1993). Briefly, the sensitivity and phase of a neuron's response to any given direction in the horizontal plane is defined by two orthogonal movement response vectors that are in spatial and temporal quadrature (they differ spatially and temporally by 90°). The vector with the largest magnitude is Smax and is oriented in the direction of maximum sensitivity in the plane of stimulation, and the vector Smin is oriented 90° from Smax and in the direction of least sensitivity (Angelaki 1991; Angelaki et al. 1992). The sinusoidal fit to the data from six head orientations in the present experiment were fitted simultaneously with the following equations to the sensitivity and phase:
| (1)) |
| (2)) |
| (3)) |
where S(α) and φ(α) [−180° < φ(α) < 180°] are neuronal sensitivity and phase for any head orientation α, respectively; and Sx/Sy and βx/βy are sensitivities and phases computed (not directly measured) during interaural and naso-occipital motions, respectively. There are two alternatives for vector direction of Smax and Smin, respectively. These two alternatives are 180° apart. The vector direction of Smin is defined as the one of two alternative Smin which temporally leads Smax by 90°. The neuronal response phase of Smax was expressed between −90° and 90°. If the calculated phase is between 90° and 270°, then Smax is redefined by 180° such that the response phase falls between −90° and 90°. Custom-written Matlab scripts (The MathWorks, Natick, MA) were used to estimate parameters in Eqs. 1 and 2 for each orientation through minimization of the least squares error of the sensitivity and phase simultaneously. The goodness of fit was calculated through the variance-accounted-for (VAF; VAF = 1 − var[(model − data)/var(data)]. Neurons with VAF value >80% were included in the present report. The estimated parameters Sx, Sy, βx, and βy were used to reconstruct tuning curve and ellipse for each neuron, as demonstrated in Fig. 1B.
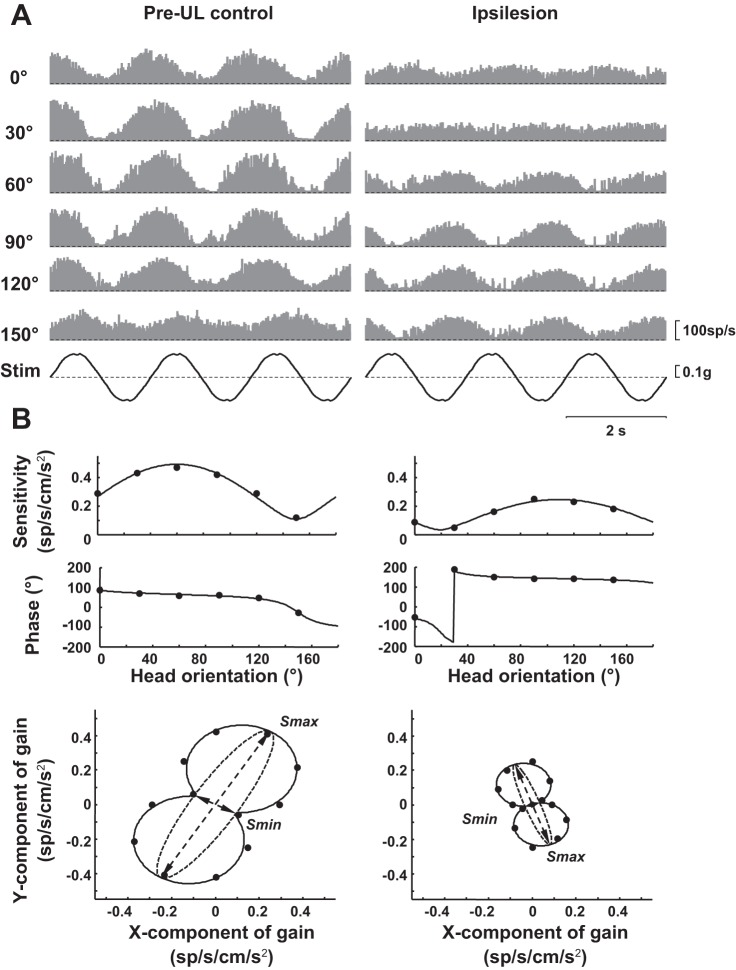
Fig. 1.
Representative examples of 2 neurons included in this study. A: binned firing rate histograms (bin size 20 ms) demonstrating the response of each neuron at 6 different orientations of the head relative to the direction of translation. Stim, acceleration of the sled, upward is to the right. All trials are at 0.5 Hz with peak acceleration at 0.2 g (196 cm/s2). B: the process of fitting the data in A to generate a vector with the direction of maximum sensitivity (orientation vector, Smax), sensitivity along the orientation vector (Smax) and sensitivity orthogonal to the orientation vector (Smin). In both the top and bottom, the points represent the values from fitting the instantaneous firing rate using a least squares algorithm at each of the 6 head orientations, and the line represents the fit using the method of Angelaki (Angelaki 1991; Angelaki et al. 1992; 1993). For the bottom row, polar coordinates have been converted to Cartesian coordinates and the 12 data points by using each trial to represent both two orientations 180° apart (for example, the 0° and 180° responses are of the same magnitude by 180° out of phase). UL, unilateral labyrinthectomy; sp, spikes.
Further analysis was performed on the neuronal response to the direction of translation closest to Smax. These trials were used to determine the phase of the response along the best orientation. The symmetry of the response along the orientation closest to Smax was determined comparing the response during the excitatory half-cycle to the inhibitory half-cycle using independent left-right x–y plot fits after correcting for the phase relationship between the stimulus and response (Newlands and Wei 2013a, 2013b). The threshold of the responses in both directions and the variability of firing at the best orientation were calculated using the technique of Jamali, Cullen and colleagues (Jamali et al. 2009, 2013, 2014; Sadeghi et al. 2007). For this analysis, the spike train was passed through a Kaiser window filter with a cut-off frequency of 0.6 Hz (0.1 Hz above the 0.5-Hz stimulus frequency; Cherif et al. 2008). Kaiser window filtered neural responses were plotted against stimulus velocity after adjusting for the phase relationship between the stimulus and response. We calculated the mean and standard deviation of the distribution of firing rates within bins of translational acceleration 10°/s2 in size (Jamali et al. 2013). The reported response variability is the standard deviation of a neuron's firing distribution at zero velocity. For the calculation of detection thresholds, we calculated the degree of overlap between the firing rate distributions at each acceleration bin with that at zero velocity as d′ from signal detection theory (Green and Swets 1966), as described previously (Jamali et al. 2009, 2013, 2014). The detection threshold is defined for each direction for a given trial as the acceleration at which d′ = 1.
Analysis is based on the division of the data into three groups. Neurons recorded before UL are labeled as “pre-UL control.” Neurons recorded after UL on the left are labeled “ipsilesion,” whereas neurons on the right recorded after the left UL are labeled “contralesion.” All statistical comparisons utilized a threshold of P < 0.05 for significance (SPSS 14.0 for windows). Nonparametric ordinal tests were used for analysis between the groups. A circular statistic (Kuiper test) was used to compare nonordinal phase data utilizing algorithms executed in Matlab. To determine whether a neuron responded to the sinusoidal stimulus for a particular trial, the Rayleigh coefficient was calculated, and significant correlation with P < 0.05 was required (Mardia 1972).
RESULTS
Data are reported here from a total of 285 NEM neurons recorded from 2 animals, either before labyrinthectomy (96 neurons) and at least 51 days after UL, either in the ipsilesional (82 neurons) or contralesional (107 neurons) mid- to rostral vestibular nuclei. Of the 285 translational-sensitive neurons in this study, 265 were tested for rotation in the yaw plane, and 101 (38%) of these neurons were sensitive to both translational and rotational movement. The distribution of canal-otolith convergent neurons is included in Table 1. The proportion of neurons with canal-otolith convergence did not differ significantly based on group (χ2, P = 0.43).
Table 1.
Properties of recorded neurons
| Group | n | Smax, spikes·s−1·°−1·s−1 | Tuning Ratio | Clockwise,% | Excited by Ipsilateral Acceleration, % | R-T Convergent, % | Excitatory Detection Threshold, cm/s2 | Inhibitory Detection Threshold, cm/s2 | Response Variability, spikes/s |
|---|---|---|---|---|---|---|---|---|---|
| Pre-UL | 96 | 0.19 (0.12) | 0.18 (0.15) | 51 | 30 (31) | 31/87 (35) | 55 (38) | 56 (36) | 7.5 (5.3) |
| Ipsilesion | 82 | 0.16 (0.09) | 0.21 (0.18) | 54 | 32 (39) | 34/77 (44) | 66 (42) | 69 (41) | 6.6 (3.8) |
| Contralesion | 107 | 0.14 (0.10) | 0.18 (0.16) | 56 | 59 (55) | 36/101 (36) | 85 (46) | 81 (37) | 8.4 (4.8) |
Values are means (SD); n, no. of responses. Smax, amplitude in the direction of maximum sensitivity; R-T, rotational translational; UL, unilateral labyrinthectomy.
Figure 1A shows typical examples of two translation-sensitive neurons. The left column example shows the responses of one pre-UL control neuron in the left vestibular nucleus in animal M1 to translation over six orientations with the frequency at 0.5 Hz. Similarly, the response of an ipsilesion neuron in the same animal after left UL is shown in the right column of Fig. 1A.
Neuronal responses such as those demonstrated in Fig. 1A were further analyzed to determine the Smax, Smin, and the tuning ratio (magnitude Smin/magnitude Smax) (Angelaki et al. 1992, 1993). Figure 1B demonstrates the result of the analysis of the two sample neurons from Fig. 1A. The cell on the left has the Smax direction calculated at 60° with a response phase 64° (lead). The Smin vector is located at −30° with a phase lead of 154° (this is equivalent to a phase lag of 26° at 150° orientation). In this case, the magnitude Smax is 0.49 spikes·s−1·cm−1·s−2, and the magnitude Smin is 0.11 spikes·s−1·cm−1·s−2 for a tuning ratio of 0.22. The right column similarly shows analysis for an ipsilesion neuron with the Smax direction of −71° with a response phase −35°. The Smin is oriented at −161° with a phase lead of 55°. For this neuron, the magnitude Smax is 0.25 spikes·s−1·cm−1·s−2, magnitude Smin is 0.04 spikes·s−1·cm−1·s−2, and the tuning ratio is 0.14 (Fig. 1A, right column). In this study, data were collected at all head orientations for each neuron, or the neuron was not included in the analysis. Cells were included in the analysis with at least responses from four of these orientations meeting Rayleigh criteria for significantly responding to the sinusoidal stimulus.
The response of each neuron could be described as being clockwise (CW) or counterclockwise (CCW), depending on whether Smin is spatially oriented 90° CW or CCW to Smax (Angelaki et al. 1993). The pre-UL normal neuron on the left in Fig. 1 is CW (Smax at 60° and Smin at −30°). The ipsilesion neuron on the right in Fig. 1 is also CW (Smax oriented at −71° and Smin oriented at −161°). Overall, 54% of neurons were CW. Table 1 shows the proportion of CW neurons in each group. These differences are not statistically significant (χ2, P = 0.80).
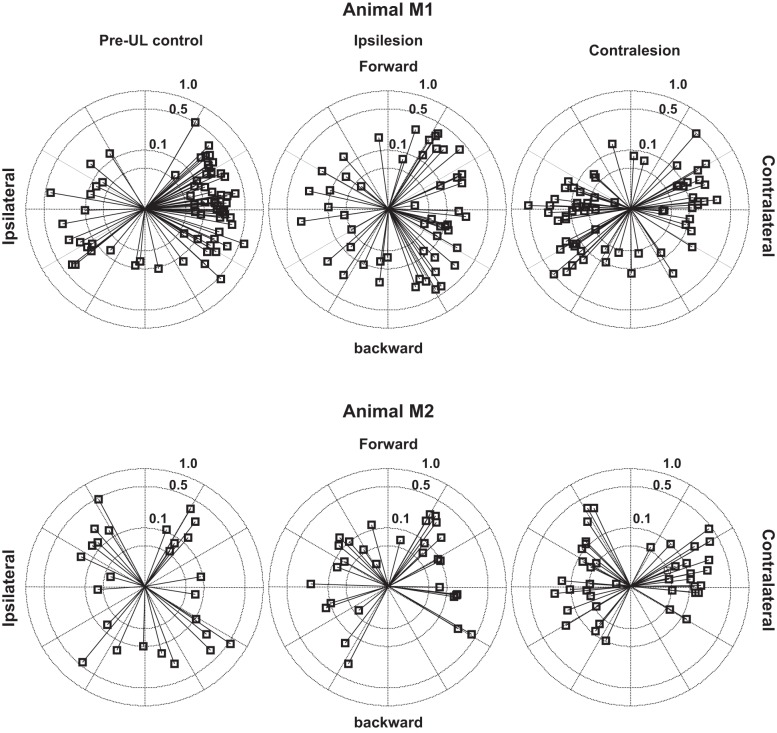
The Smax for each of our units before and after UL are demonstrated in Fig. 2. In this figure, each of three groups (pre-UL control, ipsilesion, and contralesion) is represented separately for each animal. The Smax are adjusted based on the side of the brain stem from which they were recorded, such that the reference for sensitivity in this figure is ipsilateral or contralateral acceleration, rather than the left or the right. Overall, 121 of 285 neurons tested had orientation vectors responding to ipsilateral translation, and 164 were oriented to respond to contralateral translation. For the purpose of evaluation of the effect of lesion on response properties of central vestibular neurons, data from both monkeys are considered together. Table 1 lists the proportion of translation-sensitive neurons that were oriented toward ipsilateral acceleration. While most neurons in pre-UL control animals had orientation vectors favoring linear acceleration contralateral to the side of the neuron, after UL the majority of contralesion neurons had orientation vectors aligned to ipsilateral acceleration. The distribution of orientation vectors between ipsilateral and contralateral acceleration was significantly different between the pre-UL control, ipsilesion, and contralesion neuronal populations (χ2, P = 0.002). For all cells recorded after UL (both ipsilesion and contralesion), only 43% are excited by acceleration toward the side of the UL (ipsilateral for the ipsilesion group and contralateral for the contralesion group), while 57% are excited by acceleration toward the intact labyrinth (contralateral for the ipsilesion group and ipsilateral for the contralesion group).
Fig. 2.
Polar plots representing the vectors of maximum sensitivity of all recorded units, distributed by group (pre-UL, ipsilesion, and contralesion) and animal. The radius is on a log scale. The orientations of the vectors represent the best response for each cell relative to direction of acceleration sensitivity.
The mean Smax for all of the data in this study was 0.16 ± 0.11 (SD) spikes·s−1·cm−1·s−2. The Smax for each group is reported in Table 1. There was a difference in Smax by group (Kruskal-Wallis test, P < 0.001). These differences are only significant between the pre-UL control and contralesion groups (Mann-Whitney U-test, P < 0.001), but not for pre-UL control and ipsilesion (Mann-Whitney U-test, P = 0.06) or ipsilesion and contralesion (Mann-Whitney U-test, P = 0.053).
Figure 3A presents the response from each neuron after aligning all of the Smin vectors at 90°. Each of the three groups of neurons is represented individually (pre-UL control, ipsilesion, and contralesion). In Fig. 3B, the tuning ratios for neurons in each of the three groups and each animal are represented. For our population of neurons, the mean tuning ratio is 0.19 ± 0.16 (SD). The tuning ratios for each group are shown in Table 1. There is no difference in tuning ratio by groups (Kruskal-Wallis test, P = 0.18).
Fig. 3.
A: plots of the individual responses for each neuron at each orientation grouped by animal and experimental group. In this plot, the responses of each neuron are aligned such that the peak response is at 0° and the minimum response is at 90°. These plots demonstrate the heterogeneity in the magnitude of Smax (the sensitivity at 0 and 180°) and Smin (the magnitude at 90°). The lines connect points from the same neuron, but do not represented the data fit. B: the distribution of tuning ratios of neurons by animal and experimental group.
An analysis was performed to evaluate whether UL affected the balance of excitatory and inhibitory sensitivity in individual neurons. In response to sinusoidal stimuli, vestibular neurons generally demonstrate excitation in roughly one-half of the cycle and inhibition in the other one-half of the cycle. For these translation-sensitive neurons, we separately calculated an excitatory and an inhibitory sensitivity as we have done previously for rotationally sensitive neurons (Newlands et al. 2009, Newlands and Wei 2013a, 2013b). We fit the excitatory and inhibitory half-cycles separately for every trial for each neuron. Using the trials closest to the Smax direction of the neuron (for example, in Fig. 1 using the 60° orientation for the pre-UL control neuron and 120° for the ipsilesion neuron), we found that the mean sensitivity to translation in the excitatory direction for our population of 285 neurons was 0.176 ± 0.121 spikes·s−1·cm−1·s−2, and the mean sensitivity to translation in the inhibitory direction was 0.160 ± 0.126 spikes·s−1·cm−1·s−2. For each trial, we then calculated a symmetry index, [(excitatory sensitivity − inhibitory sensitivity)/whole cycle sensitivity]. For the entire population, the mean symmetry index was 0.15 ± 0.82. We examined whether the symmetry of the response varied by group. The symmetry index was not affected by group or whether the neuron's orientation vector was toward ipsilesional or contralesional acceleration (Kruskal-Wallis test, P = 0.27). The excitatory sensitivity did change depending upon which group (Kruskal-Wallis, P < 0.001), such that excitatory sensitivity is greater in pre-UL controls than contralesion neurons (Mann-Whitney U-test, P < 0.001) and greater for ipsilesion than contralesion neurons (Mann-Whitney U-test, P = 0.02). These findings for the excitatory sensitivity are similar to what we found for Smax, as would be expected. These data suggest that the reduction in sensitivity of translational neurons after UL on the contralesional side is not solely due to changes in either the inhibitory or excitatory aspects of the response.
The phase relationship between firing rate and linear motion for utricular afferents is such that the phase leads acceleration (Angelaki and Dickman 2000; Fernández and Goldberg 1976c; Purcell et al. 2003). In contrast, studies of the dynamic properties of central vestibular neurons have noted a wider range of phase relationships between the stimulus and response and preponderance of neurons that lag linear acceleration by 30–110° at 0.5 Hz (Angelaki and Dickman 2000; Tomlinson et al. 1996). Figure 4 demonstrates the neuronal phase of the response to translation at the maximum sensitivity vector for our sample by group and by animal. The phases of the responses to translation are not evenly distributed in the pre-UL control group (Kolmogorov-Smirnov test, P < 0.01), rather the majority of neurons having lag, re: acceleration between 35 and 75°. After UL, in both the ipsilesion and the contralesion groups, the phases of responses are widely distributed, and there is no significant deviation from a uniform distribution (Kolmogorov-Smirnov test, P > 0.06). Comparing the distributions of phases in the three groups, there is a significant difference between the pre-UL control group and both the ipsilesion (Kuiper test, P < 0.001) and contralesion groups (Kuiper test, P < 0.002).
Fig. 4.
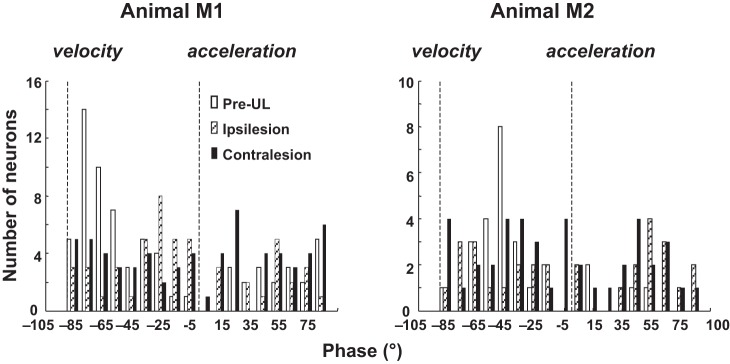
Distribution of the phases at Smax of pre-UL (open bars), ipsilesion (dotted bars), and contralesion (black solid bars) neurons during translational motion. A phase at 0° corresponds to the Smax response in phase with acceleration, and −90° phase lag corresponds to in phase with velocity.
The detection threshold and variability for translation were calculated for the orientation closest to Smax. For all neurons in study, the mean (±SD) detection threshold in the excitatory direction was 69 ± 44 cm/s2, in the inhibitory direction was 69 ± 39 cm/s2, and variability was 7.6 ± 4.8 spikes/s. The detection threshold in both directions and the variability for each group are presented in Table 1. The differences between detections thresholds in both directions were statistically significant (Kruskal-Wallis test, P < 0.001), as were differences in variability (Kruskal-Wallis test, P = 0.03). The differences were statistically significant on pairwise comparison between intact and ipsilesion groups only for inhibitory direction detection threshold (Mann-Whitney U-test, P = 0.04), between the intact and contralesion groups for excitatory and inhibitory detection threshold (Mann-Whitney U-test, P < 0.001 for both) but not variability. As threshold is related to gain (Smax) and variability (Jamali et al. 2013, 2014), the finding that only the comparison between the pre-UL control group and contralesion group demonstrates a threshold difference in both directions, no significant difference in variability, and a significant difference in Smax is consistent with the notion that the threshold difference between pre-UL control and contralesion neurons is due to the reduction in sensitivity, not increased variability.
Figure 5A shows the response of two neurons (not those in Fig. 1) over five frequencies of stimulation, all of the trials are with an orientation of 0° (interaural translation). For many of the recorded neurons, we tested the responses of the neurons to translation along the interaural axis (pre-UL control, n = 69; ipsilesion, n = 70; contralesion, n = 77), while for fewer neurons we tested the responses to translation along the naso-occipital axis (pre-UL control, n = 27; ipsilesion, n = 38; contralesion, n = 19) over five frequencies. We held the peak acceleration constant at 0.2 g at four frequencies (0.25, 0.50, 1.0, and 2.0 Hz), and at 0.1 g at 5 Hz. Plots of sensitivity vs. frequency are shown in Fig. 5B for the interaural and naso-occipital directions. The amplitude of the response depends on the sensitivity of the neuron, the tuning of the neuron and the angle of the best response vector relative to the direction of translation. Neurons demonstrate higher sensitivity at higher frequency in all three groups for interaural translation (Kruskal-Wallis test, P ≤ 0.01) and for the pre-UL control (Kruskal-Wallis test, P = 0.02), and in ipsilesion groups (Kruskal-Wallis test, P = 0.01) for naso-occipital translation, but not for the contralesion group (Kruskal-Wallis test, P = 0.25). For interaural translation, there is a difference in gain between pre-UL control, ipsilesion and contralesion at each frequency except 5 Hz (Kruskal-Wallis test: 0.25 Hz, P = 0.007; 0.50 Hz, P = 0.038; 1.0 Hz, P = 0.001; 2.0 Hz, P = 0.001; 5.0 Hz, P = 0.24). Pairwise comparisons reveal that for the 0.25- to 2-Hz frequencies, the pre-UL control group neurons have higher sensitivity in the interaural orientation than the post-UL groups, ipsilesion and contralesion (Mann Whitney U-test, P < 0.05 for all comparisons), but that there is no difference between the two sides after UL (Mann Whitney U-test, P > 0.05). In the naso-occipital orientation, we found no difference in the sensitivity by group at any of the five frequencies (Kruskal Wallis test, P ≥ 0.13 for all comparisons).
Fig. 5.
A: binned firing rate histograms (bin size 20 ms) of responses of two neurons to interaural translation (head orientation at position 0°) at 5 different frequencies and with peak acceleration held constant at 0.2 g or 0.1 g at 5 Hz. Stim, sled acceleration. B, top: log-log plot of sensitivity vs. frequency of acceleration in either the interaural direction (left plot) or naso-occipital direction (right plot). Average responses with standard error of the mean are shown. For the phase plots (bottom), the phase is re: acceleration in the excitatory direction.
DISCUSSION
The central otolith system.
The otolith system is distinct from the semicircular canal system in several fundamental ways. One of these differences is that, because hair cells in semicircular canal ampulla are all aligned in the same direction, neurons innervating the semicircular canals all respond to stimulation in a spatially consistent manner. Otolith hair cells in any utricular or saccular epithelium, in contrast, are not aligned with one another. Utricular afferents from the same end organ have spatial alignments throughout the horizontal plane (Angelaki and Dickman 2000; Fernández and Goldberg 1976b; Goldberg et al. 1990; Purcell et al. 2003; Si et al. 1997). Thus one utricular nerve includes some units excited and others inhibited by translation in any direction in the horizontal plane. So unlike the canal systems, where vestibular afferents excited by angular acceleration in one labyrinth are complemented by afferents on the other side responding to oppositely directed stimulation, in the utricular system the push and pull of complementary excitatory and inhibitory responses theoretically can arise from one end-organ, making the bilateral otolith organs potentially redundant. Therefore, after removal of one labyrinth, the animal potentially still has complete access to all the information necessary to drive vestibular reflexes and perception of motion. In a number of species, afferents responding to contralateral acceleration (or ipsilateral tilt, which are equivalent) outnumber afferents responding to ipsilateral acceleration (Fernández and Goldberg 1976a; Loe et al. 1973; Si et al. 1997).
The projections from the utricular nerve to the vestibular nuclei have been examined by single-unit and multiunit anatomical techniques (Imagawa et al. 1995; Naito et al. 1995; Newlands et al. 2002, 2003; Newlands and Perachio 2003). In particular, utricular afferents in the rhesus macaque project heavily to the rostral medial vestibular nuclei (Newlands et al. 2003). There is a robust utricular related commissural system from neurons in the medial, inferior and lateral vestibular nuclei (Bai et al. 2002). Otolith responses can be found after UL on the lesion side, even after removal of the cerebellum, confirming that the brain stem commissural fibers carry linear acceleration information (Hoshino and Pompiano 1977). This brain stem commissural system likely drives the ipsilesional responses we report in this study, although longer loop pathways, including those through the cerebellum, may contribute.
Comparison to other studies of central otolith neurons.
The response properties of central vestibular neurons to translation in the horizontal plane with the labyrinth intact have been examined in a number of species, including rats (Angelaki et al. 1993; Bush et al. 1993), pigeons (McArthur et al. 2011), cats (Benson et al. 1970), squirrel monkeys (Chen-Huang and Peterson 2006, 2010), and rhesus monkeys (Angelaki and Dickman 2000; Dickman and Angelaki 2002; Zhou et al. 2006). Zhou et al. (2006) found 42% of translation-sensitive vestibular nucleus neurons also responded to rotation, which is similar to our 35%. In contrast, Dickman and Angelaki (2002) describe 86 of 136 translation-sensitive cells being canal-otolith convergent, although roughly one-half of these were convergent with the vertical canals, such that ∼31% would be convergent with the horizontal canals, similar to the present data. In decerebrate cats, by stimulation of individual branches of the labyrinth, vertical canal afferents convergent with utricular afferents on single central vestibular neurons were found twice as commonly as canal-otolith convergence between horizontal canal and utricular afferents of the same neurons (Zakir et al. 2000; Zhang et al. 2001). In the present study, neither pitch nor tilt responses were examined.
Zhou et al. (2006) found both low-sensitivity, low-frequency “tilt” neurons and higher sensitivity neurons with high-pass characteristics in the rostral vestibular nuclei of rhesus macaques. In our study, all neurons were characterized at 0.5 Hz. Because we applied a strict statistical requirement to characterize neurons as responsive, we may have excluded some lower sensitivity neurons. None of our pre-UL control neurons had sensitivity below the 25 spikes·s−1·g−1 (0.025 spikes·s−1·cm−1·s−2) threshold Zhou et al. (2006) used to define low-sensitivity units. In total, our neurons have higher sensitivity for lateral translation than the high-sensitivity neurons in that study, suggesting that the high-sensitivity units are the population we studied.
Jamali et al. (2014) recently described the relationship between neuronal detection thresholds during rotational compensation and concluded that higher thresholds seen in contralesional neurons after recovery from UL were a result of higher variability, despite near normal sensitivity to rotation. In this study, we did not find a significant change in variability between pre-UL controls and either the ipsilesional or contralesional neurons. We attribute the elevated thresholds (compared with normal animals) for neurons contralateral to the UL to reduced sensitivity (Smax), not to increased variability. Of course, our study differs from that of Jamali et al. in that we selected for translational-sensitive neurons, although many were rotationally sensitive as well. Our population was drawn only after compensation: we did not follow sensitivity, threshold, or variability over time, and we did sample both sides of the brain stem after UL. Understanding how unilateral lesions impact thresholds is still an area of interest where more studies need to be performed.
Spatio-temporal response properties after UL.
Angelaki and Dickman (2000) noted that STC was found in translationally sensitive vestibular nucleus neurons without semicircular canal inputs. Their study confirmed the theoretical postulate that STC could be created from purely utricular inputs, although it is not clear what role commissural cross-utricular signals play in the creation of central vestibular STC. What has not previously been shown is whether STC can arise from an individual utricle. Convergence of afferents from both sides of the striola from the ipsilateral utricle and commissural input from the contralateral utricle is found on single second-order neurons (Uchino et al. 1999). A characteristic of STC is not only the creation of two-dimension tuning, but also phase relationships between the signal and response, such that, along the orientation vector, the neuron responds in phase with head velocity rather than acceleration at lower frequencies (≤0.5 Hz) (Angelaki and Dickman 2000; Tomlinson et al. 1996). In this study, we have shown STC with similar tuning ratios before surgery (with both labyrinths intact) and bilaterally after UL. These data suggest that signals from a single utricular end-organ may be enough to maintain STC properties, even for neurons in the vestibular nuclei contralateral to the intact labyrinth (ipsilesion neurons). However, we also noted that, unlike before UL, where response associated with Smax for most neurons lagged peak acceleration by 35–75° at 0.5 Hz, after UL the phases were evenly distributed. These data suggest that, while STC can be seen with afferent input available from only one utricle, in normal animals, input from both utricles probably shape normal responses.
Bilateral distribution of translation-sensitive neurons after UL.
Studies of vestibular compensation for yaw rotation have noted that, even in compensated animals, there is a large asymmetry in the prevalence of type I neurons between the ipsilesion and contralesion side (Smith and Curthoys 1988; Maioli et al. 1983; Newlands and Wei 2013b). In our recent study, we estimated that type I neurons were reduced 71% after UL in compensated animals, based on the ratio of type I to type II neurons. For translation-sensitive neurons, we note a smaller discrepancy. Although it is a very imprecise estimate of cell density, we can get an idea for the prevalence of target cells based on the ratio of electrode penetrations to cells that were fully characterized and included in this study. On the lesion side, we made 54 passes to collect the 82 cells we included in this study (1.52 cells per pass) compared with making 47 passes on the intact side and collecting 107 units (2.27 cells per pass). This yield suggests a potential 33% reduction in the density of cells on the side of lesion, but clearly this is a very rough estimate. Additionally, since we have no understanding of whether the cells recorded before and after UL or on either side after UL project similarly to other areas of the brain, the meaning of the relative paucity of units ipsilateral to the UL is not clear. However, a reduction in the number of translation-responsive cells ipsilateral to the UL is not surprising, given that, as with rotationally sensitive neurons, these cells lost their primary excitatory drive (the ipsilateral vestibular nerve) and are modulated by inhibitory commissures (Bai et al. 2002). Combining the 33% reduction in the number of ipsilesional neurons with the finding that only 39% of ipsilesion and 45% of contralesion neurons are sensitive to motion toward the side of the UL (Table 1) suggests that roughly 42% of neurons bilaterally respond to ipsilesional motion and roughly 58% respond to contralesional motion.
Correlation with behavior.
Despite the theoretic availability of appropriate spatial information concerning linear acceleration from just one labyrinth, UL results in behavioral deficits related to both semicircular canal and otolith function. There is a robust literature on static deficits of gravity detection after UL. In particular, clinical tests, such as the subjective visual vertical, are believed to result from imbalance in otolith tone (Böhmer et al. 1996; Min et al. 2007; Tarnutzer et al. 2009; Vibert et al. 1999). However, there is less information on dynamic behavioral deficits, including reflex and perceptual measures during linear translations.
An interesting phenomenon after UL is inappropriate eye movements in response to naso-occipital translation in rhesus monkeys. Normally, during forward (nasal) movement, with a target straight ahead, the right eye moves to the left and the left eye to the right for vergence that is kinematically appropriate for the movement. After UL, Angelaki et al. (2000) noted kinematically inappropriate horizontal eye responses to naso-occipital movement, such that, for straight-ahead near targets, the both eyes moved to away from the side of the UL, as if the target were eccentric toward the intact side. In fact, this same inappropriate eye movement was present for targets presented to the lesion side. Inappropriate tuning of eye kinematics for naso-occipital translations remained for at least 3 mo after UL. These data were felt to be consistent with a shifting of the zero-sensitivity point toward the side of the lesion. Recent efforts aimed at understanding perceptual deficits after UL have noted deficits for a choice discrimination task where animals were asked to report their perceived heading direction during translation near the naso-occipital axis. UL caused a bias in perception toward the side of lesion. This finding is consistent with the trVOR data discussed above (Angelaki et al. 2000). These authors attributed this bias in perception and a reduction in the thresholds of individual neurons in detecting head direction to changes in the tuning slope of individual neurons to heading directions around straight ahead. In that study, in a smaller sample of neurons, no significant differences were found in the distribution of heading preferences in the control group vs. a population of neurons in the contralesion nucleus.
Angelaki et al. (2000) noted that the compensatory eye movement velocity during interaural translations was reduced, more so for ipsilesional translation and in a more pronounced way during near-target viewing. Unlike the deficits with naso-occipital movement, the interaural translation deficits improved over time. Asymmetric responses to interaural translation have also been observed to transient linear motion in darkness in humans 1 wk after UL (Lempert et al. 1998). These data are consistent with a deficit in the spatial organization of the vestibular nucleus. In addition to this spatial disruption of neuronal responses, the temporal disruption of neuronal responses, evidenced by the change in phase distributions after UL (Fig. 4) may further compromise appropriate trVOR, other vestibular reflexes and vestibular perception.
In net, we found that a smaller portion of neurons in the bilateral nuclei responded to ipsilesional translation after UL. We do not know if any NEM neurons we recorded were involved directly in the trVOR. Anatomically, central utricular pathways are poorly understood, so we can only speculate that the NEM neurons studied here might be involved in modulation of the trVOR. In the horizontal plane, the main ipsilateral excitatory connection between the utricle and the eye movement motor nuclei appears to be to the ipsilateral abducens nucleus. Presumably, if this were the primary driver of the trVOR, then UL should compromise trVOR for contralesional linear motion greater than for ipsilesional motion, because most primary afferents in primates respond to contralateral acceleration, which is equivalent to ipsilateral tilt (Angelaki and Dickman 2000; Fernández and Goldberg 1976a). The fact that the opposite occurs suggests that the organization of the trVOR is far more complex. Evidence strongly suggests that NEM neurons are not directly involved in the rotational VOR (Scudder and Fuchs 1992). Neurons involved in the rotational VOR, particularly eye-head velocity cells, have been implicated in the trVOR (Chen-Huang and McCrea 1999). However, both neuron types involved in the direct rotational VOR pathway, type I position vestibular pause neurons and contralateral (type II) eye-head velocity neurons, do not respond to translation when eye movements are suppressed (Meng et al. 2005). NEM cells, through projections to the cerebellar nodulus and uvula, which carry translational signals but not signals related to linear acceleration due to gravity (Angelaki and Yakusheva 2009), and the cerebellar flocculus/ventral paraflocculus (Cheron et al. 1996), which may be required in the modulation of the trVOR with gaze angle and viewing distance (Angelaki et al. 2001; Meng and Angelaki 2006), may be very important in generating the trVOR. Our discovery of a shift after UL to more neurons being sensitive to contralesional acceleration sensitivity is consistent with finding in the trVOR of weaker responses to ipsilesional acceleration after UL, as described by Lempert et al. (1998) and Angelaki et al. (2000).
In summary, we found that UL affected a number of characteristics of central vestibular neurons, even after greater than 7 wk of recovery. These characteristics included the orientation of best response, the sensitivity to translation, the phases of responses, and possibly the number of neurons responding to translation. Understanding of how these neuronal parameters translate into the behavioral and reflex deficits found after UL will require a better characterization of deficits following UL and fuller understanding of central otolith pathways mediating behavior responses to translation.
GRANTS
This work was supported by National Institute of Deafness and Other Communications Disorders Grants R01 DC-006429 and K08 DC-000182.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: S.D.N. conception and design of research; S.D.N. and M.W. interpreted results of experiments; S.D.N. drafted manuscript; S.D.N. and M.W. edited and revised manuscript; S.D.N., N.L., and M.W. approved final version of manuscript; N.L. performed experiments; N.L. and M.W. analyzed data; M.W. prepared figures.
ACKNOWLEDGMENTS
The authors thank Dan Schneider and Rong Chu for technical assistance, Dora Angelaki and J. David Dickman for use of MatLab script to calculate spatio-temporal convergence metrics, and Adrian Perachio for helpful suggestions.
Current addresses: S. D. Newlands and M. Wei, Department of Otolaryngology, University of Rochester Medical Center, 601 Elmwood Ave., Box 629, Rochester, NY 14618; N. Lin, Department of Basic Science, Bastyr University California, 4106 Sorrento Valley Blvd., San Diego, CA 92121.
REFERENCES
- Allum JH, Yamane M, Pfaltz CR. Long-term modifications of vertical and horizontal vestibulo-ocular reflex dynamics in man. I. After acute unilateral peripheral vestibular paralysis. Acta Otolaryngol (Stockh) 105: 328–337, 1988 [DOI] [PubMed] [Google Scholar]
- Angelaki DE. Dynamic polarization vector of spatially tuned neurons. IEEE Trans Biomed Eng 38: 1053–1060, 1991 [DOI] [PubMed] [Google Scholar]
- Angelaki DE. Spatiotemporal convergence (STC) in otolith neurons. Biol Cybern 67: 83–96, 1992 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Bush GA, Perachio AA. A model for the characterization of the spatial properties in vestibular neurons. Biol Cybern 66: 231–240, 1992 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Bush GA, Perachio AA. Two-dimensional coding of linear acceleration in vestibular nuclei neurons. J Neurosci 13: 1403–1417, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, Dickman JD. Spatiotemporal processing of linear acceleration: primary afferent and central vestibular neuron responses. J Neurophysiol 84: 2113–2132, 2000 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Green AM, Dickman JD. Differential sensorimotor processing of vestibulo-ocular signals during rotation and translation. J Neurosci 21: 3968–3985, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelaki DE, McHenry MQ, Newlands SD, Dickman JD. Functional organization of primate translational vestibulo-ocular reflexes and effect of unilateral labyrinthectomy. Ann N Y Acad Sci 871: 136–147, 1999 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Newlands SD, Dickman JD. Primate translational vestibuloocular reflexes. IV. Changes after unilateral labyrinthectomy. J Neurophysiol 83: 3005–3018, 2000 [DOI] [PubMed] [Google Scholar]
- Angelaki DE, Yakusheva TA. How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus. Ann N Y Acad Sci U S A 1164: 19–28, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai R, Meng H, Sato H, Imagawa M, Sasaki M, Uchino Y. Properties of utricular-activated vestibular neurons that project to the contralateral vestibular nuclei in the cat. Exp Brain Res 147: 419–425, 2002 [DOI] [PubMed] [Google Scholar]
- Benson AJ, Guedry FE, Melvill Jones G. Response of semicircular canal dependent units in vestibular nuclei to rotation of a linear acceleration vector without angular acceleration. J Physiol 210: 475–494, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhmer A, Mast F, Jarchow T. Can a unilateral loss of otolithic function be clinically detected by assessment of the subjective visual vertical? Brain Res Bull 40: 423–427, 1996 [DOI] [PubMed] [Google Scholar]
- Broussard DM, Bhatia JK, Jones GE. The dynamics of the vestibulo-ocular reflex after peripheral vestibular damage. I. Frequency-dependent asymmetry. Exp Brain Res 125: 353–364, 1999 [DOI] [PubMed] [Google Scholar]
- Bush GA, Perachio AA, Angelaki DE. Encoding of head acceleration in vestibular neurons. I. Spatiotemporal response properties to linear acceleration. J Neurophysiol 69: 2039–2055, 1993 [DOI] [PubMed] [Google Scholar]
- Chan YS. The coding of head orientations in neurons of bilateral vestibular nuclei of cats after unilateral labyrinthectomy: response to off-vertical axis rotation. Exp Brain Res 114: 293–303, 1997 [DOI] [PubMed] [Google Scholar]
- Chan YS, Shum DK, Lai CH. Neuronal response sensitivity to bidirectional off-vertical axis rotations: a dimension of imbalance in the bilateral vestibular nuclei of cats after unilateral labyrinthectomy. Neuroscience 94: 831–843, 1999 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, McCrea RA. Effects of viewing distance on the responses of vestibular neurons to combined angular and linear vestibular stimulation. J Neurophysiol 81: 2538–2557, 1999 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, Peterson BW. Three-dimensional spatial-temporal convergence of otolith related signals in vestibular only neurons in squirrel monkeys. Exp Brain Res 168: 410–426, 2006 [DOI] [PubMed] [Google Scholar]
- Chen-Huang C, Peterson BW. Frequency-dependent spatiotemporal tuning properties of non-eye movement related vestibular neurons to three-dimensional tranlations in squirrel monkeys. J Neurophysiol 103: 3219–3237, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif S, Cullen KE, Galiana HL. An improved method for the estimation of firing rate dynamics using an optimal digital filter. J Neurosci Methods 173: 165–181, 2008 [DOI] [PubMed] [Google Scholar]
- Cheron G, Escudero M, Godaux E. Discharge properties of brain stem neurons projecting to the flocculus in the alert cat. I. Medical vestibular nucleus. J Neurophysiol 76: 1759–1774, 1996 [DOI] [PubMed] [Google Scholar]
- Crane BT, Demer JL. Human horizontal vestibulo-ocular reflex initiation: effects of acceleration, target distance, and unilateral deafferentation. J Neurophysiol 80: 1151–1166, 1998 [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol 88: 3518–3533, 2002 [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE, Correia MJ. Response properties of gerbil otolith afferents to small angle pitch and roll tilts. Brain Res 556: 303–310, 1991 [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. I. Response to static tilts and to long-duration centrifugal force. J Neurophysiol 39: 970–984, 1976a [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. II. Directional selectivity and force-response relations. J Neurophysiol 39: 985–995, 1976b [DOI] [PubMed] [Google Scholar]
- Fernández C, Goldberg JM. Physiology of peripheral neurons innervating otolith organs of the squirrel monkey. III. Response dynamics. J Neurophysiol 39: 996–1008, 1976c [DOI] [PubMed] [Google Scholar]
- Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol 35: 978–987, 1972 [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol 59: 370–393, 1988 [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Desmadryl G, Baird RA, Fernández C. The vestibular nerve of the chinchilla. IV. Discharge properties of utricular afferents. J Neurophysiol 63: 781–790, 1990 [DOI] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal Detection Theory and Psychophysics. New York: Wiley, 1966, p. xi, 445 [Google Scholar]
- Hess BJ, Angelaki DE. Modelling spatiotemporal properties of directionally sensitive multi-input single-output systems. Biol Cybern 69: 407–414, 1993 [PubMed] [Google Scholar]
- Hoshino K, Pompeiano O. Crossed responses of lateral vestibular neurons to macular labyrinthine stimulation. Brain Res 131: 152–157, 1977 [DOI] [PubMed] [Google Scholar]
- Imagawa M, Isu N, Sasaki M, Endo K, Ikegami H, Uchino Y. Axonal projections of utricular afferents to the vestibular nuclei and the abducens nucleus in cats. Neurosci Lett 186: 87–90, 1995 [DOI] [PubMed] [Google Scholar]
- Jamali M, Carriot J, Chacron MJ, Cullen KE. Strong correlations between sensitivity and variability give rise to constant discrimination thresholds across the otolith afferent population. J Neurosci 33: 11302–11313, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Mitchell DE, Dale A, Carriot J, Sadeghi SG, Cullen KE. Neuronal detection thresholds during vestibular compensation: contributions of response variability and sensory substitution. J Physiol 592: 1565–1580, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali M, Sadeghi SG, Cullen KE. Response of vestibular nerve afferents innervating utricle and saccule during passive and active translations. J Neurophysiol 101: 141–149, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman GD, Shinder ME, Perachio AA. Convergent properties of vestibular-related brain stem neurons in the gerbil. J Neurophysiol 83: 1958–1971, 2000 [DOI] [PubMed] [Google Scholar]
- Lacour M, Manzoni D, Pompeiano O, Xerri C. Central compensation of vestibular deficits. III. Response characteristics of lateral vestibular neurons to roll tilt after contralateral labyrinth deafferentation. J Neurophysiol 54: 988–1005, 1985 [DOI] [PubMed] [Google Scholar]
- Lasker DM, Hullar TE, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. III. Responses after labyrinthectomy. J Neurophysiol 83: 2482–2496, 2000 [DOI] [PubMed] [Google Scholar]
- Lempert T, Gianna C, Brookes G, Bronstein A, Gresty M. Horizontal otolith-ocular responses in humans after unilateral vestibular deafferentation. Exp Brain Res 118: 533–540, 1998 [DOI] [PubMed] [Google Scholar]
- Liu S, Dickman JD, Newlands SD, DeAngelis GC, Angelaki DE. Reduced choice-related activity and correlated noise accompany perceptual deficits following unilateral vestibular lesion. Proc Natl Acad Sci U S A 110: 17999–18004, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe PR, Tomko DL, Werner G. The neural signal of angular head position in primary afferent vestibular nerve axons. J Physiol 230: 29–50, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maioli C, Precht W, Ried S. Short- and long-term modifications of vestibulo-ocular response dynamics following unilateral vestibular nerve lesions in the cat. Exp Brain Res 50: 259–274, 1983 [DOI] [PubMed] [Google Scholar]
- Mardia KV. Statistics of Directional Data. New York: Academic, 1972, p. 133–135 [Google Scholar]
- McArthur KL, Zakir M, Haque A, Dickman JD. Spatial and temporal characteristics of vestibular convergence. Neuroscience 192: 361–371, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Angelaki DE. Neural correlates of the dependence of compensatory eye movements during translation on target distance and eccentricity. J Neurophysiol 95: 2530–2540, 2006 [DOI] [PubMed] [Google Scholar]
- Meng H, Green AM, Dickman JD, Angelaki DE. Pursuit–vestibular interactions in brain stem neurons during rotation and translation. J Neurophysiol 93: 3418–3433, 2005 [DOI] [PubMed] [Google Scholar]
- Min KK, Ha JS, Kim MJ, Cho CH, Cha HE, Lee JH. Clinical use of subjective visual horizontal and vertical in patients of unilateral vestibular neuritis. Otol Neurotol 28: 520–525, 2007 [DOI] [PubMed] [Google Scholar]
- Naito Y, Newman A, Lee WS, Beykirch K, Honrubia V. Projections of the individual vestibular end-organs in the brain stem of the squirrel monkey. Hear Res 87: 141–155, 1995 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Dara S, Kaufman GD. Relationship of static and dynamic mechanisms in vestibuloocular reflex compensation. Laryngoscope 115: 191–204, 2005 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Lin N, Wei M. Response linearity of alert monkey non-eye movement vestibular nucleus neurons during sinusoidal yaw rotation. J Neurophysiol 102: 1388–1397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Central projections of the vestibular nerve: a review and single fiber study in the Mongolian gerbil. Brain Res Bull 60: 475–495, 2003 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. I. Type I neurons. Exp Brain Res 82: 359–372, 1990a [DOI] [PubMed] [Google Scholar]
- Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. II. Type II neurons. Exp Brain Res 82: 373–383, 1990b [DOI] [PubMed] [Google Scholar]
- Newlands SD, Purcell IM, Kevetter GA, Perachio AA. Central projections of the utricular nerve in the gerbil. J Comp Neurol 452: 11–23, 2002 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Vrabec JT, Purcell IM, Stewart CM, Zimmerman BE, Perachio AA. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol 466: 31–47, 2003 [DOI] [PubMed] [Google Scholar]
- Newlands SD, Wei M. Tests of linearity in the responses of eye-movement-sensitive vestibular neurons to sinusoidal yaw rotation. J Neurophysiol 109: 2571–2584, 2013a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands SD, Wei M. Responses of central vestibular neurons to sinusoidal yaw rotation in compensated macaques after unilateral labyrinthectomy. J Neurophysiol 110: 1822–1836, 2013b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Precht W, Shimazu H, Markham CH. A mechanism of central compensation of vestibular function following hemilabyrinthectomy. J Neurophysiol 29: 996–1010, 1966 [DOI] [PubMed] [Google Scholar]
- Purcell IM, Newlands SD, Perachio AA. Responses of gerbil utricular afferents to translational motion. Exp Brain Res 152: 317–322, 2003 [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res 175: 471–484, 2006 [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Multimodal integration after unilateral labyrinthine lesion: single vestibular nuclei neuron responses and implications for postural compensation. J Neurophysiol 105: 661–673, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of mutltimodal intergration in the macaque vestibular system. J Neurosci 30: 10158–10168, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol 97: 1503–1514, 2007 [DOI] [PubMed] [Google Scholar]
- Schor RH. Responses of cat vestibular neurons to sinusoidal roll tilt. Exp Brain Res 20: 347–362, 1974 [DOI] [PubMed] [Google Scholar]
- Schor RH, Angelaki DE. The algebra of neural response vectors. Ann N Y Acad Sci 656: 190–204, 1992 [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD. Relationship of cat vestibular neurons to otolith-spinal reflexes. Exp Brain Res 47: 137–144, 1982 [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Timerick SJ, Tomko DL. Responses to head tilt in cat central vestibular neurons. II. Frequency dependence of neural response vectors. J Neurophysiol 53: 1444–1452, 1985 [DOI] [PubMed] [Google Scholar]
- Schor RH, Miller AD, Tomko DL. Responses to head tilt in cat central vestibular neurons. I. Direction of maximum sensitivity. J Neurophysiol 51: 136–146, 1984 [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol 68: 244–264, 1992 [DOI] [PubMed] [Google Scholar]
- Si X, Angelaki DE, Dickman JD. Response properties of pigeon otolith afferents to linear acceleration. Exp Brain Res 117: 242–250, 1997 [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Mechanisms of recovery following unilateral labyrinthectomy: a review. Brain Res Rev 14: 155–180, 1989 [DOI] [PubMed] [Google Scholar]
- Smith PF, Curthoys IS. Neuronal activity in the ipsilateral medial vestibular nucleus of the guinea pig following unilateral labyrinthectomy. Brain Res 444: 308–319, 1988 [DOI] [PubMed] [Google Scholar]
- Tarnutzer AA, Bockisch C, Straumann D, Olasagasti I. Gravity dependence of subjective visual vertical variability. J Neurophysiol 102: 1657–1671, 2009 [DOI] [PubMed] [Google Scholar]
- Thomassen JS, Liu S, Newlands SD, Dickman JD, Angelaki DE. Rotation and translation detection thresholds of vestibular nuclei neurons before and after unilateral labyrinthectomy. Program No. 470.14. In: 2012 Neuroscience Meeting Planner. New Orleans, LA: Society for Neuroscience, 2012 [Google Scholar]
- Tomlinson RD, McConville KM, Na EQ. Behavior of cells without eye movement sensitivity in the vestibular nuclei during combined rotational and translational stimuli. J Vestib Res 6: 145–158, 1996 [PubMed] [Google Scholar]
- Uchino Y, Sato H, Kushiro K, Zakir M, Imagawa M, Ogawa Y, Katsuta M, Isu N. Cross-striolar and commissural inhibition in the otolith system. Ann N Y Acad Sci 871: 162–172, 1999 [DOI] [PubMed] [Google Scholar]
- Ushio M, Minor LB, Della Santina CC, Lasker DM. Unidirectional rotations produce asymmetric changes in horizontal VOR gain before and after unilateral labyrinthectomy in macaques. Exp Brain Res 210: 651–660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert D, Häusler R, Safran AB. Subjective visual vertical in peripheral unilateral vestibular diseases. J Vestib Res 9: 145–152, 1999 [PubMed] [Google Scholar]
- Xerri C, Gianni S, Manzoni D, Pompeiano O. Central compensation of vestibular deficits. I. Response characteristics of lateral vestibular neurons to roll tilt after ipsilateral labyrinth deafferentation. J Neurophysiol 50: 428–448, 1983 [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Spatial properties of central vestibular neurons. J Neurophysiol 95: 464–478, 2006 [DOI] [PubMed] [Google Scholar]
- Zakir M, Kushiro K, Ogawa Y, Sato H, Uchino Y. Convergence patterns of the posterior semicircular canal and utricular inputs in single vestibular neurons in cats. Exp Brain Res 132: 139–148, 2000 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zakir M, Meng H, Sato H, Uchino Y. Convergence of the horizontal semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 140: 1–11, 2001 [DOI] [PubMed] [Google Scholar]
- Zhou W, Tang BF, Newlands SD, King WM. Responses of monkey vestibular-only neurons to translation and angular rotation. J Neurophysiol 96: 2915–2930, 2006 [DOI] [PubMed] [Google Scholar]