Abstract
Previously, it was reported that various cortical and subcortical structures display high-frequency local field potential (LFP) oscillations in the 110- to 160-Hz range (HFOs), distinct from sharp-wave ripples. In the present study, we characterize HFOs in the extended amygdala. Rats were implanted with tetrode bundles in the bed nucleus of the stria terminalis (BNST), central amygdala (CeA), as well as adjacent regions (pallidum, caudate-putamen, and lateral septum). At all recorded sites, HFO power showed a systematic dependence on behavioral state: highest during quiet wakefulness, intermediate during paradoxical sleep, and lowest during active waking or slow-wave sleep. CO2 asphyxiation as well as anesthesia with isoflurane or urethane abolished HFOs. HFOs stood out relative to all other fast-frequency LFP components because they were highly coherent between distant sites of the extended amygdala, ipsi- and contralaterally. HFOs affected neuronal firing in two ways: firing rate could vary as a function of HFO power (rate modulation) or HFOs could entrain firing on a cycle-to-cycle basis (phase modulation). The incidence of phase-modulated neurons was about twice higher in BNST and CeA (20–40%) than in adjacent regions (≤8%). Among BNST and CeA neurons, many more were phase-modulated than rate-modulated, although about half of the latter were also phase-modulated. Overall, these results indicate that HFOs entrain the activity of a high proportion of neurons in the extended amygdala. A major challenge for future studies will be to identify the mechanisms supporting the high coherence of HFOs within and across hemispheres.
Keywords: bed nucleus of the stria terminalis, amygdala, fast oscillation, gamma
even in the absence of sensory stimulation, neuronal networks generate rhythmic population events, measurable in local field potentials (LFPs) as oscillations of various frequencies (Buzsáki 2006). Rhythms of different frequencies predominate depending on the brain region, and these oscillations vary markedly as a function of the subject's behavioral state. Understanding these oscillations is important because neuronal events underlying cognitive activity are embedded in these endogenous population rhythms.
Recently, a novel form of oscillatory activity (110–160 Hz) termed high-frequency oscillations (HFOs) was observed in the neocortex, hippocampus (reviewed in Tort et al. 2013), and many subcortical structures (reviewed in Olszewski et al. 2013). Although the HFO frequency band overlaps with that of sharp-wave ripples (Buzsáki et al. 1992; Ylinen et al. 1995), the two oscillations are undoubtedly distinct because they predominate in different behavioral states. Indeed, HFOs were initially identified in studies of phase-amplitude coupling between oscillations of different frequencies, revealing a modulation of HFO amplitude as a function of theta phase (Tort et al. 2008). Subsequently, HFOs were demonstrated using power density analysis and observed in unfiltered LFPs (Scheffzük et al. 2011; Scheffer-Teixeira et al. 2012) with a phase reversal between superficial and deep hippocampal layers (Tort et al. 2008).
Although little is known about the cellular mechanisms generating HFOs, the available evidence suggests that unlike gamma-oscillations, HFOs are not dependent on rhythmic interactions between glutamatergic and GABAergic neurons (Fisahn et al. 1998; Whittington et al. 2000). Indeed, hippocampal HFOs resist blockade of AMPA/kainate receptors but are abolished by GABAA receptor antagonists (Jackson et al. 2011). Moreover, in many subcortical structures (basal ganglia, septum, and thalamus), HFO amplitude increases markedly following systemic administration of NMDA receptor antagonists (Hunt et al. 2011; Hunt and Kasicki 2013). In the case of nucleus accumbens at least, HFOs are generated locally and/or in a nearby structure because they disappear or are largely reduced following local infusions of tetrodotoxin or muscimol (Olszewski et al. 2013). In contrast, TTX infusions in the frontal or parietal cortices as well as in the caudate nucleus had no effect on the power of HFOs recorded locally at these sites (Olszewski et al. 2013), suggesting that they are volume-conducted from a distant source.
Although studying the relationship between unit activity and HFOs might yield useful insights into the underlying network interactions, this question has received little attention so far. In part, this results from the difficulty of distinguishing genuine entrainment of unit firing by HFOs from spurious correlations resulting from spectral leakage of spike waveforms into LFPs (Scheffer-Teixeira et al. 2013). In the present study, we circumvent this confound by taking advantage of the high spatial coherence of HFOs. Because HFOs depend on interactions between GABAergic neurons (Jackson et al. 2011), we focused on structures of the extended amygdala, particularly the bed nucleus of the stria terminalis (BNST) and central amygdala (CeA), where GABAergic neurons prevail (Poulin et al. 2009; Swanson and Petrovich 1998).
MATERIALS AND METHODS
Procedures were approved by the Institutional Animal Care and Use Committee of Rutgers University in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). Our subjects were male Lewis rats (310–360 g; Charles River Laboratories, Wilmington, MA) maintained on a 12:12-h light-dark cycle. They were housed individually with ad libitum access to food and water. Before the experiments, they were habituated to the animal facility and handling for 1 wk.
Surgery.
Eleven rats were anesthetized with a mixture of isoflurane and O2 and administered atropine sulfate (0.05 mg/kg im) to aid breathing. In aseptic conditions, rats were mounted in a stereotaxic apparatus with nonpuncture ear bars. A local anesthetic (subcutaneous bupivacaine) was injected in the region of the scalp to be incised. Fifteen minutes later, the scalp was incised, and a craniotomy was performed above the regions of interest. Then, movable bundles of four tetrodes (nichrome microwires, 20-μm inner diameter, impedance 100–300 kΩ following gold plating) were stereotaxically aimed to the BNST and, in a subset of rats, CeA and immediately adjacent components of the extended amygdala (Heimer et al. 2008). Tetrode tip positions within a bundle were staggered to facilitate histological reconstructions. The reference electrode was a stainless steel screw anchored over the cerebellum. In two rats, we also compared HFOs referenced to the cerebellum vs. the bone overlying the olfactory bulbs. However, the same results were obtained with the two references in terms of HFO power, coherence, and unit entrainment.
Data acquisition and analysis.
Rats were allowed 1 wk to recover from the surgery and then acclimated to handling for 2 additional days. In each rat, spontaneous neuronal activity was then recorded during prolonged (≥3 h) daily recording sessions so that sufficient data could be obtained during different states of vigilance. Behavior was recorded by an overhead video camera. During the recording sessions, rats were placed in a dimly lit (20 lx), standard plastic rat cage with bedding at the bottom. Electrodes were not moved during the experiments unless all units were lost overnight across all tetrodes within a bundle. In such rare cases (<5%), the tetrode bundle was lowered 60 μm, and recordings resumed the following day.
Different behavioral states of vigilance were identified using a combination of spectral analyses of BNST LFPs and behavioral observations. Long epochs of spontaneous LFP activity were segmented in 2-s windows, and frequency distributions of LFP power in different frequency bands were computed. Active waking could be distinguished from all other states because it was associated with a broad-band increase in the power of high frequencies (200–240 Hz). This broad-band increase at high frequencies likely reflects electromyographic activity. After eliminating active waking, we could easily distinguish slow-wave sleep (SWS) from quiet waking and rapid-eye-movement (REM) sleep because total power at frequencies <20 Hz was distributed bimodally among the three states: epochs of high power at low frequencies corresponded to periods of SWS. We then defined quiet waking as movement-free periods that followed active waking, whereas REM sleep was defined as epochs that followed SWS and were characterized by a high ratio of theta (6–10 Hz) to delta (1–5 Hz) power. These inferences were repeatedly confirmed by correlating LFP-based state scoring with offline analyses of the rats' behavior, recorded on video. Invariably, as the broad-band 200- to 240-Hz activity diminished and the rats transitioned from active to quiet waking, there was a sharp and sustained drop in movement. In contrast, analysis of behavior during electrophysiologically identified REM sleep epochs revealed punctual movements (muscle twitches).
The signals were sampled at 40 kHz and stored on a hard drive. For spike extraction, the data were first high-pass filtered using a median-based filter and then thresholded to extract spikes. Next, we ran principal component analysis on the spikes, and the first three components were clustered using KlustaKwik (http://klustakwik.sourceforge.net). Spike clusters were then refined manually using Klusters (Hazan et al. 2006). The reliability of cluster separation was verified by inspecting auto- and cross-correlograms. Units with unstable spike shapes during a given recording session were excluded from the analyses.
For LFP analyses, we first downsampled the data to 1,250 Hz with sinc interpolation. Analyses were performed in MATLAB. Spectral and phase analyses were done using Chronux (http://chronux.org) and the Circular Statistics Toolbox for MATLAB (Berens 2009), respectively. For spectral LFP analyses, the magnitude of each frequency in a given behavioral state was expressed as the deviation from the power law fit to the power spectrum of the data obtained during the entire recording period. When examining time-dependent changes in the spectral composition of LFPs, we used nonoverlapping windows of 2 s and multitaper spectral estimates with five tapers and a time-bandwidth product of three. Band-passed LFP signals were obtained using forward and reverse filtering with a fifth-order Butterworth filter over the frequency range indicated, and LFP phase and envelope were obtained using the Hilbert transform of the band-passed signal.
For phase analyses of unit firing, the reference LFP was obtained using a microwire from a different tetrode from the one used to record the unit of interest, and we performed a Rayleigh test with a significance threshold of P < 0.01. The lack of spike waveform in the reference LFP excludes the possibility that spectral leakage of the spike waveform is responsible for significant unit-HFO relationships. To assess whether high-amplitude HFO spindles were associated with significant firing rate modulations, we first computed spike counts in windows of ±1 s centered on HFO spindles of high amplitude (≥2 SD of averaged peak values). To assess significance, we performed a Kolmogorov-Smirnov (K-S) test comparing the actual distribution of spike counts with a uniform distribution for each cell independently. The significance threshold we used was P ≤ 0.01. In addition to performing the K-S test, we excluded spurious positive results due to baseline modulations in firing rate using a binomial test comparing spike counts between the 2 500- to 1,000-ms intervals flanking HFOs (with the null hypothesis that total spike counts in these 2 intervals are equal). For unit analyses, we ignored neurons that fired <200 spikes in the epoch under consideration.
Histology.
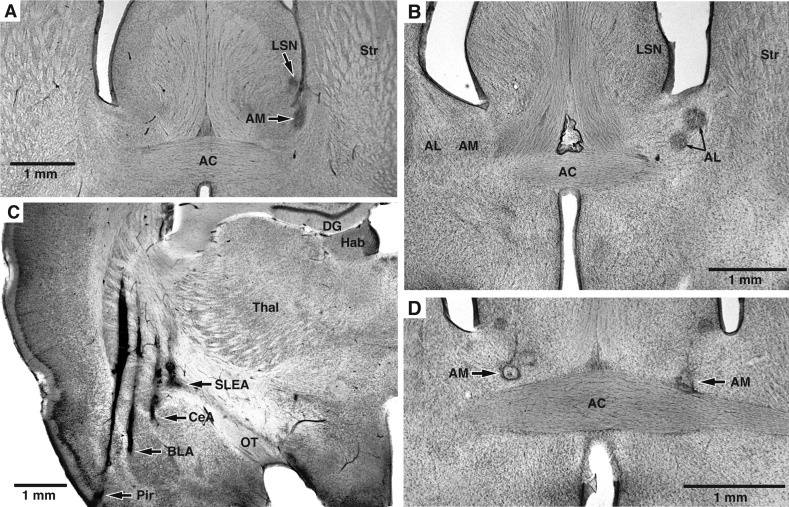
At the end of the experiments, the animals were deeply anesthetized, and recording sites were marked with small electrolytic lesions (20 μA between a tetrode channel and the animals' tail for 15 s). One day later, the rats were perfused-fixed through the heart, their brains extracted and cut on a vibrating microtome, and the sections counterstained with cresyl violet, as shown in Fig. 1.
Fig. 1.
Histological identification of recording sites. A–D: coronal sections counterstained with cresyl violet. Recording sites are marked with small electrolytic lesions (arrows). A: electrolytic lesions in the lateral septal nucleus (LSN) and bed nucleus of the stria terminalis (BNST) medial site (AM). B: 2 recording sites in BNST lateral site (AL). C: recording sites in the piriform (Pir) cortex, basolateral complex of the amygdala (BLA), central amygdala (CeA), and sublenticular extended amygdala (SLEA). Too few BLA and Pir recordings were obtained for meaningful analyses. Data obtained in CeA and SLEA were pooled since they are thought to constitute an anatomic entity. D: 2 recording sites in the left and right BNST-AM. AC, anterior commissure; DG, dentate gyrus; Hab, habenula; OT, optic tract; Str, striatum; Thal, thalamus.
RESULTS
All rats included in this study (n = 11) had tetrode bundles implanted in the anterior part of BNST, many bilaterally (n = 6). BNST recording sites were subdivided in lateral (BNST-AL) and medial (BNST-AM) sites depending on whether the electrodes were positioned laterally or medially to the intra-BNST component of the stria terminalis. In a subset of rats (n = 4), we also aimed tetrode bundles to CeA. In some cases, tetrode bundles did not reach their intended targets because of tetrode bending. As a result, we also obtained data about HFOs in a number of additional structures, including the lateral septum (5 rats), striatum (4 rats), and globus pallidus (2 rats). Figure 1 shows examples of histologically identified recording sites.
Relationship between HFO amplitudes and behavioral states of vigilance.
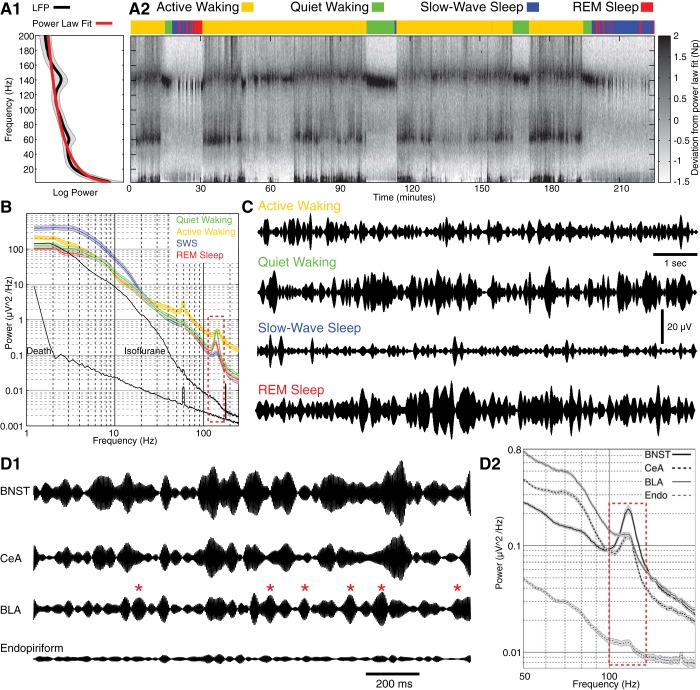
To study the dependence of HFO amplitudes on the states of vigilance, we conducted LFP power analyses (Fig. 2A1) on prolonged (≥3 h) epochs of spontaneous activity (Fig. 2A2) during which the rats cycled through different behavioral states multiple times. The magnitude of each frequency in a given 2-s window (nonoverlapping) was expressed as the deviation from the power law fit to the power spectrum of the data obtained during the entire recording period (Fig. 2A1; black, averaged data; red, fit). At all recording sites, we observed consistent variations in HFO amplitudes depending on the rats' behavioral state.
Fig. 2.
State-dependent changes in the spectral composition of local field potentials (LFPs) recorded in BNST. A1: mean spectral power across frequencies for the entire session (black) is fit by a power law (red). A2: deviations from power law fit (grayscale) for different frequencies (y-axis) as a function of time (x-axis). The behavioral state of the rat is color coded (see key at top). B: average ± SE power spectra in different behavioral states. A dashed, red rectangle marks the high-frequency oscillation (HFO) frequency band. For active waking, quiet waking, slow-wave sleep (SWS), and rapid-eye-movement (REM) sleep, the data shown are the averages ± SE of the power spectra of 32 BNST LFPs recorded in 7 rats. For death and anesthesia, we show the average of 9 LFP recordings obtained from 3 rats. C: examples of LFPs recorded in BNST in different behavioral states (band-pass filtered 125–150 Hz). D1: simultaneously recorded LFPs in BNST, CeA, BLA, and endopiriform nucleus (Endo) during quiet waking. LFPs were band-pass filtered at the HFO frequency (125–150 Hz). Note near complete lack of HFOs in Endo and generally lower HFO amplitude in BLA relative to CeA and BNST. Red asterisks mark BLA HFO spindles that had no counterparts in CeA or BNST. D2: power spectra of the LFPs shown in D1. Dashed, red rectangle marks the HFO frequency band and immediately adjacent regions. To compute these power spectra, a long epoch of quiet waking was segmented in 2-s windows, and the data were averaged across windows. Shading represents SE.
Figure 2, A2 and C, illustrates this point using LFPs recorded in BNST-AM. Although there were marked moment-to-moment variations in HFO amplitudes, they were generally higher during quiet waking (green) and REM sleep (red). In contrast, HFO amplitudes were generally lower during SWS (blue) and active waking (yellow). This is also shown in Fig. 2B, where we plot the average (line) ± SE (shading) of the power spectra of 32 BNST LFPs recorded in 7 rats in active (yellow) and quiet (green) waking as well as in SWS (blue) and REM sleep (red). Note that for this analysis, HFO amplitude was defined as the difference between the power spectrum peak and the linear fit to flanking frequencies.
An ANOVA on HFO amplitudes revealed a main effect of behavioral state (F = 149, P < 0.0001). Bonferroni post hoc t-tests (P < 0.01) revealed that the ranking of states by HFO power was quiet waking > REM > active waking ≈ SWS with no significant difference between the latter two states. However, it should be emphasized that active waking and SWS were not devoid of HFOs. Although HFO amplitudes were generally lower in these two states, high-amplitude HFO spindles also occurred (Fig. 2C).
In a few rats, we also examined the impact of isoflurane (0.2% inhaled; n = 3) or urethane (1.8 g/kg ip; n = 2) anesthesia on HFO amplitudes. In addition, to examine the possibility that HFOs constitute an electronic artifact independent of neuronal activity, we also recorded LFPs after CO2 asphyxiation (n = 2). As shown in Fig. 2B (black lines), no HFO peak could be detected under anesthesia or after death.
Importantly, not all recording sites displayed HFOs. For instance, Fig. 2D illustrates examples of simultaneously recorded LFPs in the endopiriform nucleus and basolateral amygdala (BLA) as well as in BNST and CeA, all in the same hemisphere. HFOs were nearly absent from the endopiriform nucleus (Fig. 2D1) and of consistently lower amplitude in the BLA relative to those seen in BNST and CeA (Fig. 2D1). Moreover, whereas moment-to-moment variations HFO amplitudes in BNST and CeA generally coincided (see below), those seen in BLA showed much more independence (red asterisks mark BLA HFO spindles with no counterparts in BNST and CeA). The lower power of HFOs in the endopiriform nucleus and BLA relative to BNST and CeA is also documented in the power spectra depicted in Fig. 2D2 (t-tests, P < 0.0001). Together, these results indicate that HFOs are not electronic artifacts of our recording equipment.
HFO coherence within and across hemispheres.
Previously, it was observed that LFP coherence decreases with distance and that this effect is more pronounced for high-frequency components (Collins et al. 1999, 2001; Steriade et al. 1993, 1996). Although it was reported that the coherence of fast rhythms between distant cortical sites could be high during some stimulation paradigms (Desmedt and Tomberg 1994; Gray et al. 1989), this is generally not the case in spontaneous conditions (Bullock and McClune 1989; Murthy and Fetz 1992; Steriade et al. 1996).
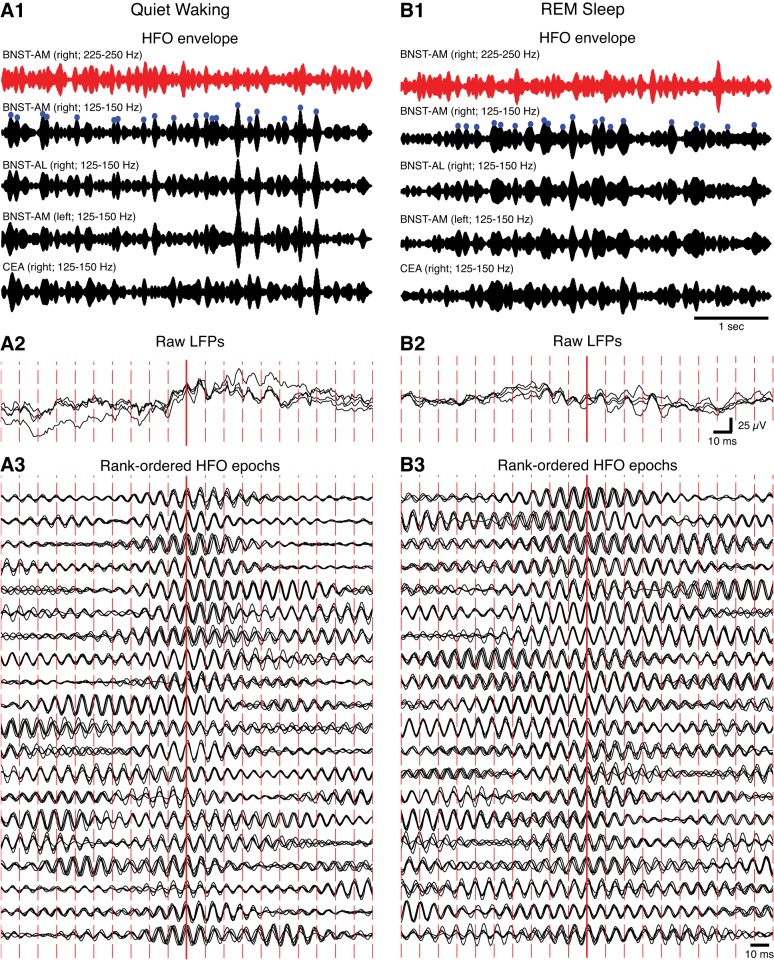
In contrast, spontaneous HFOs were highly coherent in the extended amygdala, within and across hemispheres. Indeed, in all rats, superimposition of LFPs recorded in the same or different hemispheres during waking or sleep states revealed that HFOs occurred nearly synchronously. For instance, Fig. 3 shows band-pass filtered LFPs (black, 125–150 Hz; red, 225–250 Hz) recorded in different subregions of the right BNST and CeA as well as in the left BNST during quiet waking (Fig. 3A) and REM sleep (Fig. 3B). The 20 HFO spindles with the highest amplitude seen in the right BNST-AM (panels 1; blue circles) are shown with an expanded time base in panels 3, superimposed on LFPs from the other ipsi- and contralateral recording sites. Note the remarkable cycle-to-cycle synchrony of high-amplitude HFOs within and across hemispheres for quiet waking (Fig. 3A) and REM sleep (Fig. 3B). Similar results were obtained in active waking and SWS.
Fig. 3.
Synchrony of HFOs in BNST and the amygdala during different behavioral states. A: quiet waking. B: REM sleep. 1: Band-pass filtered LFPs from BNST and the CeA (red traces, 225–250 Hz; black traces, 125–150 Hz) shown with a slow time base. Blue circles mark the 20 largest HFO envelope peaks, which are depicted in panels 3 with an expanded time base. Note that high-amplitude HFO spindles (black) were not associated with parallel increases in the power of the 225- to 250-Hz band (red). Thus HFOs do not reflect a nonspecific increase in the power of all high-frequency components. To facilitate within-state comparisons, all signals are normalized by their SD. 2: Raw LFPs with a fast time base. They correspond to the HFO epoch with the highest peak in panel 1. 3: Twenty HFO envelopes marked by blue circles in panels 1 rank-ordered by amplitude (highest to lowest from top to bottom). The signals in each row are normalized by the maximal value found across the 4 traces.
To characterize further this phenomenon, we compared the coherence of HFOs to that of other high-frequency LFP components. In particular, in all cases of tetrodes in the CeA of 1 hemisphere and BNST bilaterally, we compared coherence between all available pairs of recording sites located in different parts of BNST ipsilaterally (24 pairs of recording sites in 6 rats), BNST bilaterally (36 pairs of recording sites in 6 rats), or between BNST and CeA ipsilaterally (16 pairs of recording sites in 4 rats; Fig. 4). Because movement artifacts occurring during active waking yielded artificially high coherence values in all frequencies (yellow period in Fig. 4), we focused on behavioral states associated with immobility: quiet waking, SWS, and REM sleep.
Fig. 4.
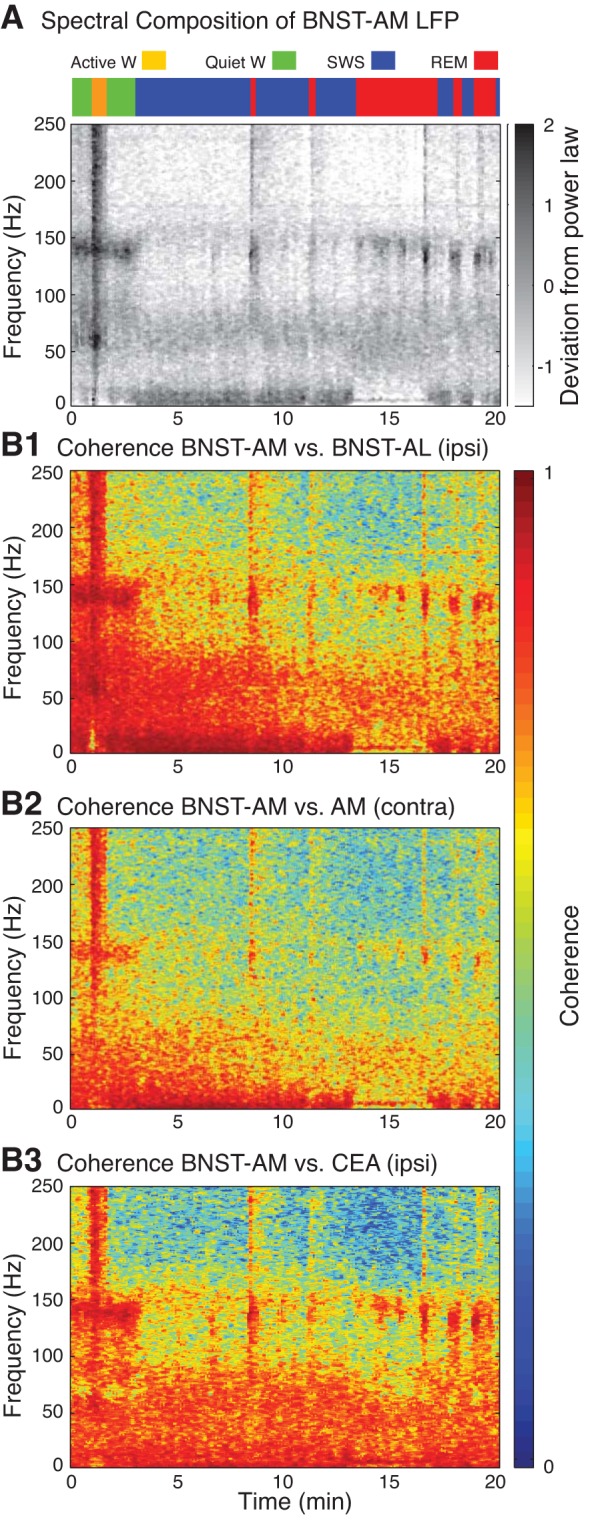
HFOs are highly coherent ipsilaterally (ipsi) but less so across hemispheres (contra). A: state-dependent changes in spectral composition of LFPs recorded in BNST-AM. W, waking. B: time and frequency-dependent changes in coherence between LFPs recorded in BNST-AL and AM ipsilaterally (B1), BNST-AM in both hemispheres (B2), as well as in BNST-AM and CeA (B3).
An ANOVA on HFO coherence values revealed a significant dependence on recording configuration in the two behavioral states associated with high-amplitude HFOs: quiet waking (Fig. 5A) and REM sleep (Fig. 5B; Fs ≥ 37.8, Ps < 0.0001). Bonferroni-corrected post hoc t-tests revealed that ipsilateral intra-BNST HFO coherence was the highest (P < 0.0001), as expected given the short distance between these recording sites (0.4–0.6 mm). Although distance between bilateral BNST recording sites was shorter (2–3 mm) than between ipsilateral BNST-CeA tetrode pairs (3.5–4.5 mm), HFO coherence across hemispheres was significantly lower (P < 0.0001). Of particular interest, for all recording configurations except cases where electrodes were within 0.6 mm of each other in BNST ipsilaterally, the coherence of HFOs was significantly higher than that of high (75–100 Hz) and low gamma (30–55 Hz, Ps < 0.0001) as well as for the 100- to 125- and 150- to 175-Hz frequency bands (Ps < 0.0001). Overall, these results indicate that HFOs stand out, relative to all other fast LFP frequency components, for their remarkably high coherence.
Fig. 5.
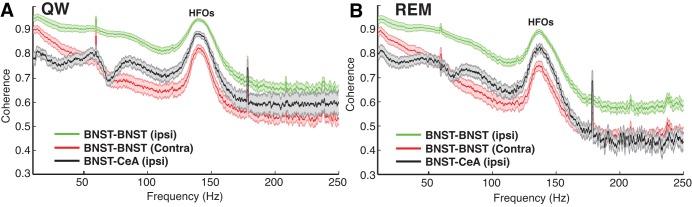
LFP coherence as a function of frequency. A: quiet waking (QW). B: REM sleep. Group analyses of LFP coherence for 3 recording configurations depicted with different colors. Green: when the 2 electrodes were positioned in BNST of the same hemisphere. Red: when the 2 electrodes were positioned in BNST-AM of different hemispheres. Black: with 1 electrode in BNST-AM and the other in CeA ipsilaterally. Colored shading represents SE.
Entrainment of unit firing by HFOs.
A possible explanation for the high HFO coherence is volume conduction. Indeed, one could conceive of a situation where a densely interconnected group of neurons would generate open fields that are instantaneously conducted over large distances. This phenomenon would generate spurious LFP correlations, with no actual HFO-related firing or synaptic activity. However, if this were the case, one would not expect to find entrainment of unit activity by HFOs at many of the sites displaying them.
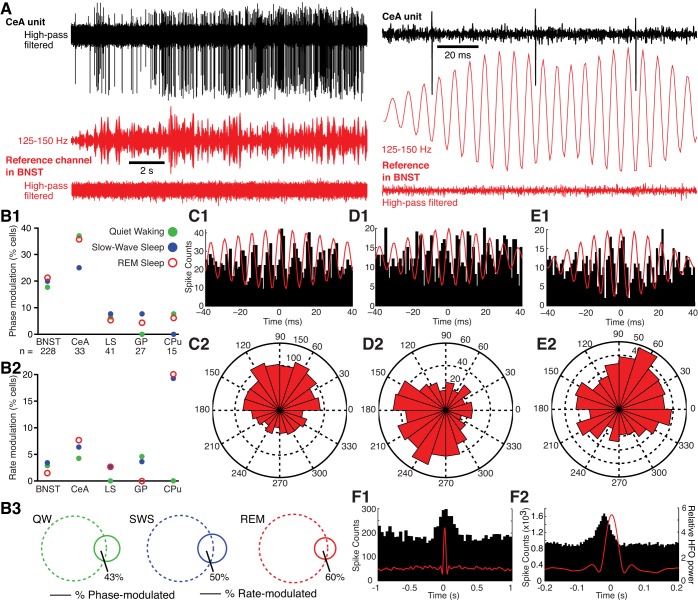
One problem when testing this prediction however, is spectral leakage of spike waveforms into LFPs (Scheffer-Teixeira et al. 2013). Indeed, spike shapes can leak into several LFP bands, causing spurious unit-field correlations. To circumvent this difficulty, for all available units, we used spikeless reference LFPs (Fig. 6A, red; downsampled to 1,250 Hz) from a different tetrode than the one used to record the unit of interest (Fig. 6A, black) and performed Rayleigh tests (significance threshold of P < 0.01). The lack of spike waveforms in the reference LFP excludes the possibility that spectral leakage of the spike shape into the LFP is responsible for significant unit-HFO relationships.
Fig. 6.
A: approach used to study entrainment of unit activity by HFOs. The band-passed (125–150 Hz) LFP recorded by a BNST reference channel (red) devoid of unit activity was used to assess the timing of unit activity in a different channel (CeA; black). From top to bottom: CeA unit activity (high-pass filtered), BNST reference signal band-pass filtered (125–150 Hz; middle), and same signal high-pass filtered (bottom), revealing lack of unit activity. Left, slow time base; right, expanded time base. Note that the gain on reference HFOs was doubled from left to right of A to facilitate inspection of HFO-spike phase relationships. B: incidence of units with significant phase and rate modulation by HFOs in different brain structures and behavioral states. B1: phase modulation. B2: rate modulation. B3: Venn diagrams showing overlap between cells (BNST and CeA combined) with significant phase (dashed lines) and/or rate (continuous lines) modulation by HFOs in QW (left), SWS (middle), and REM sleep (right). Numbers adjacent to areas of overlap indicate proportion of rate-modulated units that are also phase-modulated. LS, lateral septum; GP, globus pallidus; CPu, caudate-putamen. C–E: examples of units that were significantly phase-modulated by HFOs. C1, D1, and E1: peri-HFO histogram of neuronal discharges. To compute these histograms, HFO spindles of high amplitude (top half of the overall distribution) were detected and centered on the positive cycle with the highest amplitude. Unit activity was then referenced to the corresponding HFO spindle. C2, D2, and E2: rose plots of the cells shown in B1, C1, and D1, respectively. Unit in B was recorded in CeA during QW. Unit in C was recorded in BNST in REM sleep. Unit in D was recorded in BNST during QW. F: examples of cells recorded in BNST (F1) or CeA (F2) with significant firing rate modulation by HFOs. To construct these histograms, we computed spike counts (left y-axis; 20-ms bins) in windows (±1 s, x-axis) centered on high-amplitude HFO spindles (≥2 SD of averaged peak values). Right y-axis represents HFO power. To assess entrainment, we referenced unit activity to HFOs from a different tetrode from the 1 used to record the unit and performed a Rayleigh test with a significance threshold of P < 0.01. To assess dependence of firing rate on HFO power, we computed spike counts in windows of ±1 s centered on high-amplitude HFO spindles (≥2 SD of averaged peak values). Then, to assess significance, we performed a Kolmogorov-Smirnov test comparing the actual distribution of spike counts to a uniform distribution for each cell independently (significance threshold of P ≤ 0.01). In addition, we excluded spurious positive results due to baseline modulations in firing rate using a binomial test comparing spike counts between the 2 500- to 1,000-ms intervals flanking HFOs.
This analysis revealed that the incidence of units with significant HFO entrainment varied markedly depending on the recording sites (Fig. 6B1). Indeed, as many as 18–39% of BNST and CeA cells exhibited significant firing phase modulation by HFOs (Fig. 6, C and D), indicating that HFOs are not volume-conducted to the extended amygdala. In contrast, in the lateral septum, globus pallidus, and caudate-putamen, a significantly lower proportion of cells were entrained by HFOs (≤8%, χ2-test, P = 0.016, units in BNST and CeA vs. all other sites). The higher incidence of phase-modulated neurons in BNST and CeA relative to the other three recording sites was observed in all behavioral states (see colored symbols in Fig. 6B1). Although there were cell-to-cell variations in the preferred HFO firing phase of BNST and CeA neurons (compare Fig. 6, C–E), most discharged preferentially during the negative phase of HFOs, irrespective of the behavioral state (Fig. 7).
Fig. 7.
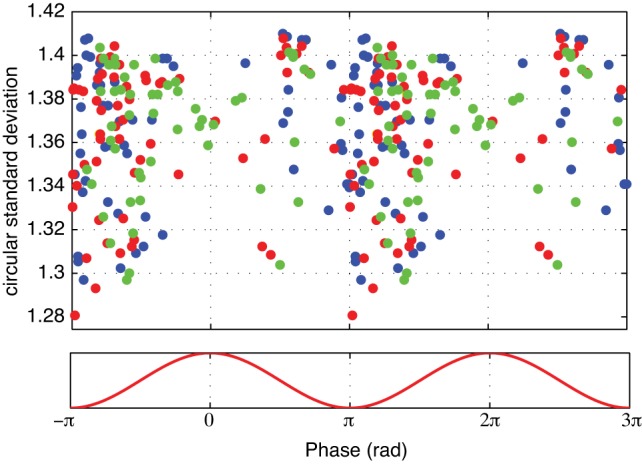
Preferred firing phase of significantly modulated BNST and CeA units. Colored symbols indicate behavioral state of epoch analyzed (red, REM; blue, SWS; green, QW). Y-axis indicates depth of modulation. Data are replicated over 2 cycles for clarity.
It should be noted that a significant entrainment of spiking by HFOs does not imply that units fired at the HFO frequency, even transiently. In fact, the majority of BNST and CeA cells with significant phase modulation had low firing rates, with 60, 68, and 79% of them firing <5 Hz in quiet waking, REM sleep, and SWS, respectively. However, the occasional spikes they generated tended to cluster at a consistent phase of the HFOs.
As mentioned above, relatively few neurons in the lateral septum, globus pallidus, and caudate-putamen showed a significant firing phase modulation by HFOs. Yet, a proportion displayed a significant firing rate modulation as a function of HFO power (Fig. 6B2). To assess this, we computed spike counts in windows of ±1 s centered on high-amplitude HFO spindles (≥2 SD of averaged peak values). Then, to assess significance, we performed a K-S test comparing the actual distribution of spike counts to a uniform distribution for each cell independently (significance threshold of P ≤ 0.01). In addition, we excluded spurious positive results due to baseline modulations in firing rate using a binomial test comparing spike counts between the two 500- to 1,000-ms intervals flanking HFOs.
Using this approach, we observed that at all recording sites and in most behavioral states, a small proportion of neurons showed significant firing rate modulations in relation to HFOs (Fig. 6B2). As shown in the representative examples of Fig. 6F, the vast majority of these cells (∼90%) showed increases in firing rates in relation to HFOs with the remaining 10% showing either inhibitory or biphasic (excitation followed by inhibition) modulations. Importantly, the incidence of phase-modulated cells among BNST and CeA neurons was much higher (≥18%) than that of rate-modulated cells (≤7%; compare Fig. 6, B1 and B2; note different y-axes), and only about half of the latter were also phase-modulated (range 43–60%; Fig. 6B3). The highest incidence of rate-modulated cells was observed among striatal units in REM sleep and SWS (Fig. 6B2), even though very few of these cells showed a significant phase modulation by HFOs (Fig. 6B1).
DISCUSSION
The present study was undertaken to characterize HFOs in structures of the extended amygdala, particularly, BNST, CeA, and immediately adjacent structures. We focused on the extended amygdala because work in other brain regions indicated that HFOs arise from interactions between GABAergic neurons (Jackson et al. 2011), the main cell type found in BNST and CeA (Poulin et al. 2009; Swanson and Petrovich 1998). Our experiments revealed that HFOs are prominent in the extended amygdala, show systematic variations in amplitude as a function of behavioral states, are coherent within and across hemispheres, and entrain unit activity in a high proportion of neurons. The significance of these findings is considered below.
HFOs vary in a state-dependent manner and are highly coherent within and across hemispheres.
In addition to the entrainment of extended amygdala neurons by HFOs (see below), several observations suggest that HFOs are not artifacts of our recording or filtering methods. First, periods of high-amplitude HFOs could be observed in the raw LFPs throughout the sleep-waking cycle. Second, HFOs were abolished by common anesthetics (isoflurane and urethane) and could not be detected after CO2 asphyxiation. Third, HFO amplitudes varied as a function of the animals' behavioral states. Indeed, they were significantly more pronounced during quiet wakefulness and REM sleep than during active waking or SWS.
An unusual property of HFOs relative to all other fast-frequency LFP components was their high coherence. This is remarkable because LFP coherence generally decreases with distance, and this reduction is particularly steep for high-frequency components (Collins et al. 1999, 2001; Steriade et al. 1993, 1996). In contrast, HFO coherence was high within and between ipsilateral structures of the extended amygdala, whereas that of other high-frequency components was generally lower. Interestingly, for contralateral recording sites, HFO coherence, although still very high, was significantly lower than between ipsilateral but more distant recording sites in the extended amygdala, again arguing against volume conduction.
HFOs are generated locally within the extended amygdala.
Previously, HFOs were observed in a variety of cortical and subcortical structures (Olszewski et al. 2013; Tort et al. 2013). In some cases, such as the hippocampus (Tort et al. 2013) and nucleus accumbens (Olszewski et al. 2013), HFOs appear to be generated locally. However, in others, such as the caudate nucleus as well as the frontal and parietal cortices, local tetrodotoxin or muscimol infusions did not affect HFO power (Olszewski et al. 2013), suggesting that they are volume-conducted from a distant source.
Consistent with this, we found that the incidence of neurons for which the firing was modulated by the phase of HFOs varied markedly depending on the recording sites. In all behavioral states, it was highest in the extended amygdala (BNST ≥ 18%; CeA ≥ 25%) and lowest in the lateral septum, globus pallidus, and caudate-putamen (≤8%). These findings support the view that the HFOs seen in the extended amygdala are not volume-conducted from a distant source but are generated locally. The scarcity of significantly entrained units in the globus pallidus and caudate-putamen is consistent with earlier findings suggesting that the HFOs seen in the caudate nucleus are not generated locally (Olszewski et al. 2013).
Origin of HFOs.
It was reported that hippocampal HFOs resist blockade of AMPA/kainate receptors but are abolished by GABAA receptor antagonists (Jackson et al. 2011). Therefore, these results suggest that HFOs are not dependent on rhythmic interactions between glutamatergic and GABAergic neurons, in contrast with gamma-oscillations (Fisahn et al. 1998; Whittington et al. 2000). Given their high frequency and independence from fast glutamatergic transmission, the high HFO coherence between distant recording sites raises a major explanatory challenge: what mechanisms could support the long-range synchronization of HFOs? Unfortunately, the study of HFOs is just beginning, and we are reduced to conjectures.
One possibility, put forward by Traub and colleagues (2011), is that HFOs arise from electrical coupling between axons. Although computational modeling supports this possibility (for instance, see Maex and De Schutter 2007; Traub et al. 2008), there is little if any electron microscopic evidence of gap junctions between axons in the hippocampus, amygdala, or BNST. A related possibility is that ephaptic coupling among unmyelinated axons is sufficient to synchronize the firing of many closely spaced axons. Ephaptic interactions are maximized when the extracellular conductance is low (Barr and Plonsey 1992), a condition met in tightly packed unmyelinated bundles of axons such as found in the olfactory nerve, stria terminalis, and anterior commissure (Blinder et al. 2003; Lamantia and Rakic 1990). A computational modeling study of the olfactory nerve provided support for this possibility (Bokil et al. 2001). Furthermore, actual demonstration of such ephaptic interactions was obtained in crab by Katz and Schmitt (1940, 1942). Of particular relevance to HFOs is the fact that ephaptic interactions can minimize differences in conduction velocity between neighboring axons with slightly different condition speeds (reviewed in Debanne et al. 2011). By synchronizing activity in bundles of unmyelinated axons, this effect might contribute to the high coherence of HFOs. However, this hypothetical model awaits experimental scrutiny.
GRANTS
This work was supported by R01 Grants MH-098738 and MH-083710 from the National Institute of Mental Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.H. and D.P. conception and design of research; D.H. performed experiments; D.H. and D.P. analyzed data; D.H. and D.P. interpreted results of experiments; D.H. and D.P. prepared figures; D.P. drafted manuscript; D.H. edited and revised manuscript; D.H. and D.P. approved final version of manuscript.
REFERENCES
- Barr RC, Plonsey R. Electrophysiological interaction through the interstitial space between adjacent unmyelinated parallel fibers. Biophys J 61: 1164–1175, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens P. CircStat: A MATLAB Toolbox for Circular Statistics. J Stat Softw 31: 2009 [Google Scholar]
- Blinder KJ, Pumplin DW, Paul DL, Keller A. Intercellular interactions in the mammalian olfactory nerve. J Comp Neurol 466: 230–239, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokil H, Laaris N, Blinder K, Ennis M, Keller A. Ephaptic interactions in the mammalian olfactory system. J Neurosci 21: RC173, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock TH, McClune MC. Lateral coherence of the electrocorticogram: a measure of brain synchrony. Electroencephalogr Clin Neurophysiol 73: 479–498, 1989 [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Rhythms of the Brain. New York: Oxford Univ. Press, 2006 [Google Scholar]
- Buzsáki G, Horváth Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science 256: 1025–1027, 1992 [DOI] [PubMed] [Google Scholar]
- Collins DR, Lang EJ, Paré D. Spontaneous activity of the perirhinal cortex in behaving cats. Neuroscience 89: 1025–1039, 1999 [DOI] [PubMed] [Google Scholar]
- Collins DR, Pelletier JG, Paré D. Slow and fast (gamma) neuronal oscillations in the perirhinal cortex and lateral amygdala. J Neurophysiol 85: 1661–1672, 2001 [DOI] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev 91: 555–602, 2011 [DOI] [PubMed] [Google Scholar]
- Desmedt JE, Tomberg C. Transient phase-locking of 40 Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci Lett 168: 126–129, 1994 [DOI] [PubMed] [Google Scholar]
- Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature 9: 186–189, 1998 [DOI] [PubMed] [Google Scholar]
- Gray CM, Konig P, Engel AK, Singer W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338: 334–337, 1989 [DOI] [PubMed] [Google Scholar]
- Hazan L, Zugaro M, Buzsáki G. Klusters, NeuroScope, NDManager: a free software suite for neurophysiological data processing and visualization. J Neurosci Methods 155: 207–216, 2006 [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW, Trimble M, Zahm DS. The anatomy of the basal forebrain. In: Anatomy of Neuropsychiatry. Amsterdam: Elsevier, 2008, chapt. 3, p. 27–67 [Google Scholar]
- Hunt MJ, Falinska M, Łeski S, Wójcik DK, Kasicki S. Differential effects produced by ketamine on oscillatory activity recorded in the rat hippocampus, dorsal striatum and nucleus accumbens. J Psychopharmacol 25: 808–821, 2011 [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Kasicki S. A systematic review of the effects of NMDA receptor antagonists on oscillatory activity recorded in vivo. J Psychopharmacol 27: 972–986, 2013 [DOI] [PubMed] [Google Scholar]
- Jackson J, Goutagny R, Williams S. Fast and slow γ rhythms are intrinsically and independently generated in the subiculum. J Neurosci 31: 12104–12117, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Schmitt OH. A note on interaction between nerve fibers. J Physiol 100: 369–371, 1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Schmitt OH. Electric interaction between two adjacent nerve fibres. J Physiol 97: 471–488, 1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol 29: 520–537, 1990 [DOI] [PubMed] [Google Scholar]
- Maex R, De Schutter E. Mechanism of spontaneous and self-sustained oscillations in networks connected through axo-axonal gap junctions. Eur J Neurosci 25: 3347–3358, 2007 [DOI] [PubMed] [Google Scholar]
- Murthy VN, Fetz EE. Coherent 25–35 Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA 89: 5670–5674, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewski M, Dolowa W, Matulewicz P, Kasicki S, Hunt MJ. NMDA receptor antagonist-enhanced high frequency oscillations: are they generated broadly or regionally specific? Eur Neuropsychopharmacol 23: 1795–1805, 2013 [DOI] [PubMed] [Google Scholar]
- Poulin JF, Arbour D, Laforest S, Drolet G. Neuroanatomical characterization of endogenous opioids in the bed nucleus of the stria terminalis. Prog Neuropsychopharmacol Biol Psychiatry 33: 1356–1365, 2009 [DOI] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Caixeta FV, Souza BC, Ribeiro S, Tort AB. Theta phase modulates multiple layer-specific oscillations in the CA1 region. Cereb Cortex 22: 2404–2414, 2012 [DOI] [PubMed] [Google Scholar]
- Scheffer-Teixeira R, Belchior H, Leão RN, Ribeiro S, Tort AB. On high-frequency field oscillations (>100 Hz) and the spectral leakage of spiking activity. J Neurosci 33: 1535–1539, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffzük C, Kukushka VI, Vyssotski AL, Draguhn A, Tort AB, Brankačk J. Selective coupling between theta phase and neocortical fast gamma oscillations during REM-sleep in mice. PLoS One 6: e28489, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Contreras D. Synchronization of fast (30–40 Hz) spontaneous cortical rhythms during brain activation. J Neurosci 16: 392–417, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A novel slow (<1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci 13: 3252–3265, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci 21: 323–331, 1998 [DOI] [PubMed] [Google Scholar]
- Tort AB, Kramer MA, Thorn C, Gibson DJ, Kubota Y, Graybiel AM, Kopell NJ. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci USA 105: 20517–20522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort AB, Scheffer-Teixeira R, Souza BC, Draguhn A, Brankačk J. Theta-associated high-frequency oscillations (110–160Hz) in the hippocampus and neocortex. Prog Neurobiol 100: 1–14, 2013 [DOI] [PubMed] [Google Scholar]
- Traub RD, Cunningham MO, Whittington MA. Chemical synaptic and gap junctional interactions between principal neurons: partners in epileptogenesis. Neural Netw 24: 515–525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, Middleton SJ, Knöpfel T, Whittington MA. Model of very fast (> 75 Hz) network oscillations generated by electrical coupling between the proximal axons of cerebellar Purkinje cells. Eur J Neurosci 28: 1603–1616, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol 38: 315–336, 2000 [DOI] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nádasdy Z, Jandó G, Szabó I, Sik A, Buzsáki G. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci 15: 30–46, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]