Abstract
The nucleus accumbens (NAcc) is a key structure of the mesolimbic dopaminergic reward system and plays an important role in mediating alcohol-seeking behaviors. Alterations in glutamatergic and GABAergic signaling were recently demonstrated in the NAcc of rats after chronic intermittent ethanol (CIE) treatment, a model of alcohol dependence. Here we studied dopamine (DA) modulation of GABAergic signaling and how this modulation might be altered by CIE treatment. We show that the tonic current (Itonic) mediated by extrasynaptic γ-aminobutyric acid type A receptors (GABAARs) of medium spiny neurons (MSNs) in the NAcc core is differentially modulated by DA at concentrations in the range of those measured in vivo (0.01–1 μM), without affecting the postsynaptic kinetics of miniature inhibitory postsynaptic currents (mIPSCs). Use of selective D1 receptor (D1R) and D2 receptor (D2R) ligands revealed that Itonic potentiation by DA (10 nM) is mediated by D1Rs while Itonic depression by DA (0.03–1 μM) is mediated by D2Rs in the same MSNs. Addition of guanosine 5′-O-(2-thiodiphosphate) (GDPβS) to the recording pipettes eliminated Itonic decrease by the selective D2R agonist quinpirole (5 nM), leaving intact the quinpirole effect on mIPSC frequency. Recordings from CIE and vehicle control (CIV) MSNs during application of D1R agonist (SKF 38393, 100 nM) or D2R agonist (quinpirole, 2 nM) revealed that SKF 38393 potentiated Itonic to the same extent, while quinpirole reduced Itonic to a similar extent, in both groups of rats. Our data suggest that the selective modulatory effects of DA on Itonic are unaltered by CIE treatment and withdrawal.
Keywords: ventral striatum, ethanol, withdrawal, neuroadaptation, tonic current
alcohol (ethanol, EtOH), consumed in moderation, has numerous beneficial effects on health (French and Zavala 2007; Gunzerath et al. 2004), yet excessive alcohol drinking can lead to alcohol dependence and loss of control over alcohol consumption, with serious detrimental health consequences (Room et al. 2005). Chronic alcohol exposure causes marked changes in reinforcement mechanisms and motivational state that are thought to contribute to the development of cravings and relapse during protracted withdrawal (Koob and Le Moal 2008). Relapse to alcohol is a critical problem in treating alcoholism, and effective treatments are yet to be found.
The nucleus accumbens (NAcc) is a key neural substrate for the rewarding actions of many drugs of abuse, including alcohol (Koob et al. 1998; Wise 2004). Enhancement of dopamine (DA) release in several brain regions, most prominently in the NAcc, is a common property of alcohol and other drugs of abuse (Di Chiara and Imperato 1985, 1988). Action potential discharge in the ventral tegmental area (VTA) DA neurons mediates DA release in the NAcc (Sombers et al. 2009); this release is highly regulated by presynaptic auto- and heteroreceptors (Zhang and Sulzer 2012). DA concentrations ([DA]) reported in the NAcc of awake rats range from low nanomolar basal levels to high submicromolar concentrations during phasic DA release (Budygin et al. 2001; Owesson-White et al. 2012; Robinson et al. 2009; Segovia and Mora 2001; Yim and Gonzales 2000). Transient increases in extracellular [DA] measured with fast scanning voltammetry techniques are more frequent after administration of drugs of abuse such as alcohol and cocaine (Robinson et al. 2009; Stuber et al. 2005) and become time-locked to cues predicting reward (Day et al. 2007; Phillips et al. 2003; Stuber et al. 2005). Indeed, the mere expectation of EtOH evokes DA release in the NAcc (Katner et al. 1996; Melendez et al. 2002; Weiss et al. 1993). Basal DA release is decreased in alcohol-dependent animals, but an EtOH challenge evokes DA release comparable to if not greater than that in nondependent control animals (Budygin et al. 2007; Diana et al. 1992, 1993; Weiss et al. 1996; Yoshimoto et al. 1996). Also, EtOH-induced DA release is greater in rats bred for alcohol preference (Bustamante et al. 2008), and alcohol cue-evoked DA release in the ventral striatum is greatest in men with a greater genetic risk for alcoholism (Oberlin et al. 2013).
Upon release, DA affects signal transmission in the NAcc via activation of various pre- and postsynaptically localized DA receptor (DAR) subtypes. The D1 class of DA receptors (D1Rs and D5Rs) is richly expressed on GABAergic medium spiny neurons (MSNs) that project to the VTA, while the D2 class of DARs (D2Rs, D3Rs, and D4Rs) are preferentially expressed on MSNs projecting to the ventral pallidum (Beaulieu and Gainetdinov 2011; Sesack and Grace 2010). In addition, there is a population of NAcc MSNs that express both D1Rs and D2Rs (Lu et al. 1998; Perreault et al. 2010, 2011). DARs are also variably expressed on GABAergic and cholinergic interneurons, as well as on glutamatergic terminals projecting to the NAcc. There is also evidence for both D1Rs and D2Rs on striatal astrocytes (Bal et al. 1994; Hosli and Hosli 1987; Miyazaki et al. 2004; Zanassi et al. 1999).
On the basis of recordings in behaving animals it has been suggested that the action potential discharge of MSNs depolarized by excitatory glutamatergic inputs is the time when NAcc neurons execute their behaviorally relevant functions (O'Donnell et al. 1999). The patterns of spike discharge are thus sculpted mainly by a combination of glutamatergic excitation, GABAergic inhibition, and modulatory influences of DA. Recently, we demonstrated alterations in the intrinsic electrical membrane properties and enhanced glutamatergic synaptic transmission of MSNs in the NAcc core of rats during protracted withdrawal from chronic intermittent ethanol (CIE) treatment, a model of alcohol dependence (Marty and Spigelman 2012). In the companion article (Liang et al. 2014), we demonstrated CIE-induced alterations in the function and expression of GABAA receptors (GABAARs). Here we studied the modulatory influence of DA and selective D1R and D2R ligands on GABAAR-mediated currents in NAcc core MSNs in CIE rats and vehicle control (CIV) rats after protracted withdrawal with the goal of obtaining a better understanding of alterations in neurotransmission within the mesolimbic reward system in alcohol dependence.
Our studies reveal that at physiologically relevant concentrations DA selectively affects tonic GABAAR currents, without effects on synaptic GABAARs. Furthermore, DA (10 nM) potentiates the tonic GABAAR current (Itonic) in MSNs, whereas higher [DA] (0.03–1 μM) depress this current. These bidirectional effects of DA on Itonic are mediated by D1Rs (potentiation) and D2Rs (depression), respectively. We also show that modulation of Itonic by selective D1R and D2R agonists is unaffected by CIE treatment and long-term withdrawal. However, because of the loss of EtOH potentiation of Itonic in CIE rats, an EtOH challenge in these rats should result in unopposed D2R-mediated depression of Itonic. Coupled with the demonstrated increases in glutamatergic excitatory neurotransmission after CIE treatment (Jeanes et al. 2011; Marty and Spigelman 2012; Szumlinski et al. 2007), we predict that EtOH-evoked DA release should produce larger increases in the “up-state” excitability of MSNs in alcohol-dependent compared with nondependent animals.
METHODS
The Institutional Animal Care and Use Committee approved all animal experiments. Male Sprague-Dawley rats (Harlan, weighing 190–220 g upon arrival) were housed in the vivarium under a 12:12-h light-dark cycle and had free access to food and water. Rats were administered a chronic intermittent EtOH (CIE) regimen: for the first 5 doses rats received 5 g/kg EtOH as a 25% (wt/vol) solution once every other day and for the following 55 doses 6 g/kg of EtOH 30% (wt/vol) once every day. The CIE control group received water (20 ml/kg). Naive rats age-matched to the CIV/CIE groups (weighing 300–350 g upon arrival) were housed under similar vivarium conditions for 1–2 mo prior to experiments.
Transverse brain slices (400 μm thick) at the level of the NAcc were obtained with standard techniques. Briefly, rats were decapitated under isoflurane anesthesia and brains were quickly removed, trimmed with a razor blade, and glued to the base of a cutting chamber (Leica VT1200S) filled with cold (∼4°C) artificial cerebrospinal fluid (ACSF) composed of (in mM) 125 NaCl, 2.5 KCl, 2 CaCl2, 2 MgCl2, 26 NaHCO3, and 10 d-glucose (Sigma). The ACSF was continuously bubbled with a 95%-5% mixture of O2 and CO2 to ensure adequate oxygenation of slices and a pH of 7.4.
Patch electrode filling solutions contained (in mM) 135 Cs-gluconate or 135 CsCl, 2 MgCl2, 1 CaCl2, 11 ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 HEPES, 2 ATP-K2, and 0.2 GTP-Na2; pH adjusted to 7.25 with CsOH. Whole cell patch-clamp recordings with CsCl- or Cs-gluconate-based pipette solutions were obtained with a Multiclamp 700B amplifier and a Digidata 1440A A/D converter (Molecular Devices, Sunnyvale, CA) from cells in the NAcc core region during perfusion (1 ml/min) with oxygenated ACSF at 34 ± 0.5°C. Most cells were putatively identified as MSNs within 30 s of membrane breakthrough by the presence of a characteristic delay in action potential generation with depolarizing current pulses (Marty and Spigelman 2012); those that did not possess these characteristics were discarded. Next, GABAAR-mediated currents were separated pharmacologically by application of TTX (0.5 μM), CNQX (10 μM), APV (40 μM), and CGP54626 (1 μM) in the ACSF. Cells were subsequently voltage-clamped at −70 mV (CsCl) or 0 mV (Cs-gluconate), and recordings began at least 10 min after membrane breakthrough to ensure adequate dialysis by intrapipette contents. Picrotoxin, DA, and various D1R and D2R ligands were applied after appropriate dilution in the ACSF. The concentrations of the DAR ligands were chosen to maximize selectivity of their actions at a given DAR subtype (Ohlstein and Berkowitz 1985; Seeman and Van Tol 1994).
The recordings were low-pass filtered (Clampfit software, Molecular Devices) at 2 kHz and analyzed with the aid of the Mini Analysis Program (Synaptosoft, Fort Lee, NJ). Miniature inhibitory postsynaptic currents (mIPSCs) were detected with threshold criteria of 5-pA amplitude and 20-fC charge transfer. Frequency of mIPSCs was determined from all automatically detected events in a given recording period. Tonic current magnitudes were obtained from the mean baseline current of a given recording period. For kinetic analysis of mIPSCs, only single events with a stable baseline, sharp rising phase, and exponential decay were chosen. Double and multiple-peak mIPSCs were excluded. The mIPSC kinetics were obtained from analysis of the averaged chosen single events (>120 events/100-s recording period) aligned with half rise time in each cell.
The investigators performing the recordings and analysis were blind to the treatment (naive, CIV, or CIE) that the rats received. All summary values are presented as means ± SE. Group differences were evaluated by unpaired Student's t-test or one-way analysis of variance (ANOVA), where appropriate. P < 0.05 was considered statistically significant.
RESULTS
Selective concentration-dependent effects of DA on tonic GABAAR current.
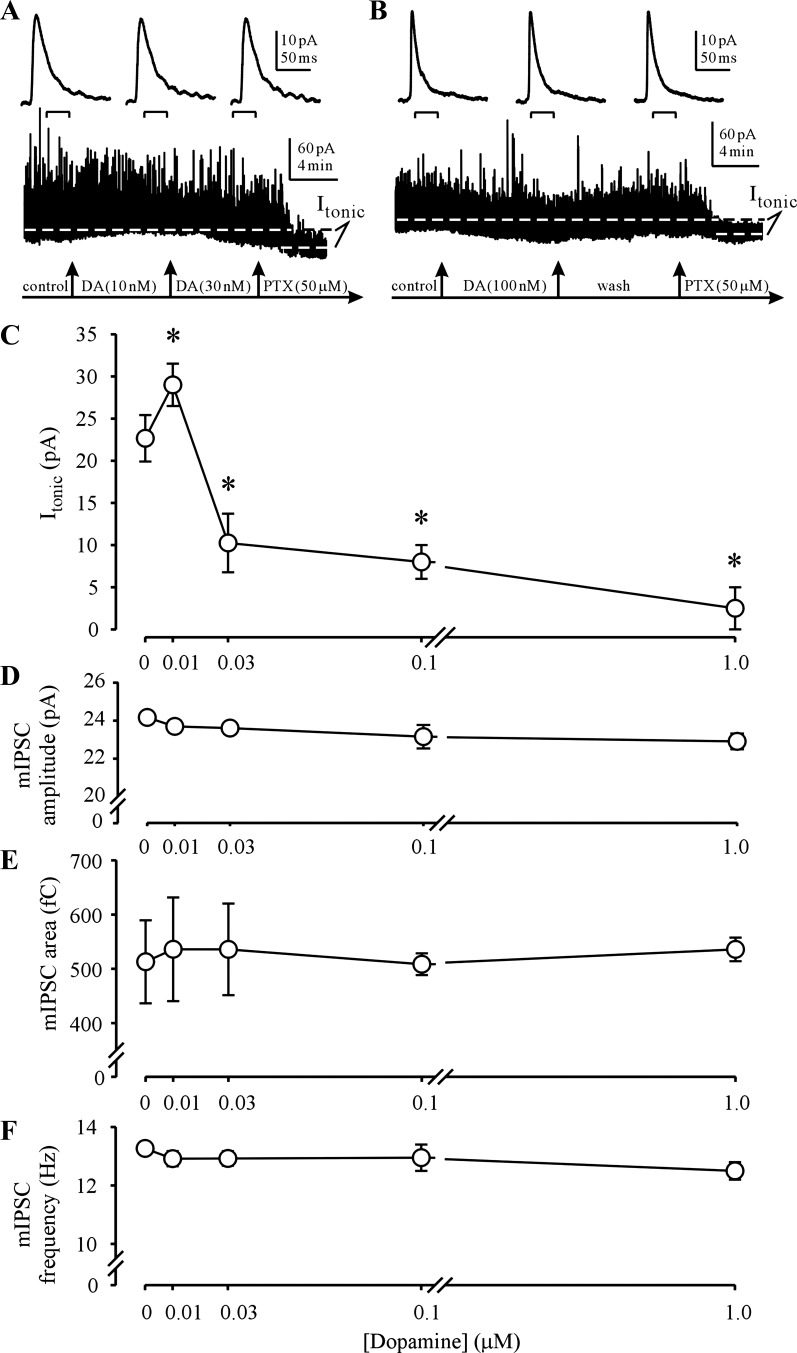
We first examined the effects of DA application on isolated GABAAR currents in NAcc MSNs from naive rats (Fig. 1). We chose to use [DA] in the range reported in the NAcc of awake rats at basal conditions and during phasic DA release (Budygin et al. 2001; Owesson-White et al. 2012; Robinson et al. 2009; Segovia and Mora 2001; Yim and Gonzales 2000). Application of DA at increasing concentrations (0.01–1 μM) had a biphasic effect on the picrotoxin (GABAAR Cl− channel blocker)-sensitive tonic current (Itonic). In all MSNs tested, DA (10 nM) produced a significant increase of Itonic, while higher [DA] (0.03–1 μM) had a significant depressant effect on Itonic (Fig. 1, A and B). In contrast, DA (0.01–1 μM) had no significant effect on the average amplitude, charge transfer (area), or frequency of mIPSCs (Fig. 1, D–F). The rise time and decay kinetic parameters of mIPSCs were similarly unaffected by DA (0.01–1 μM) application (data not shown). The effect of DA on Itonic was fully reversible upon washout, irrespective of the [DA] applied (e.g., Fig. 1B).
Fig. 1.
Concentration-dependent effects of dopamine (DA) on the tonic GABA type A receptor (GABAAR) current (Itonic). A, bottom: continuous voltage-clamp recording of pharmacologically isolated GABAAR currents in a nucleus accumbens (NAcc) core medium spiny neuron (MSN) from a naive rat. The picrotoxin (PTX, 50 μM)-sensitive Itonic is indicated by the dashed lines. Note the increase in Itonic during DA (10 nM) application and the subsequent decrease during DA (30 nM) application. Top: the miniature inhibitory postsynaptic currents (mIPSCs) averaged over the indicated 100-s recording intervals are unaffected by DA applications. B: in another MSN recording from a naive rat, application of DA (100 nM) reversibly decreases Itonic (bottom) without affecting mIPSCs (top). C: summary graph of concentration-dependent DA effects on Itonic. *P < 0.05 (n = 2–6 neurons from 2 rats, 1-way ANOVA). D–F: summary graphs of DA effects on mIPSC amplitude (D), area (E), and frequency (F).
Potentiation and depression of Itonic by DA are mediated by D1Rs and D2Rs, respectively.
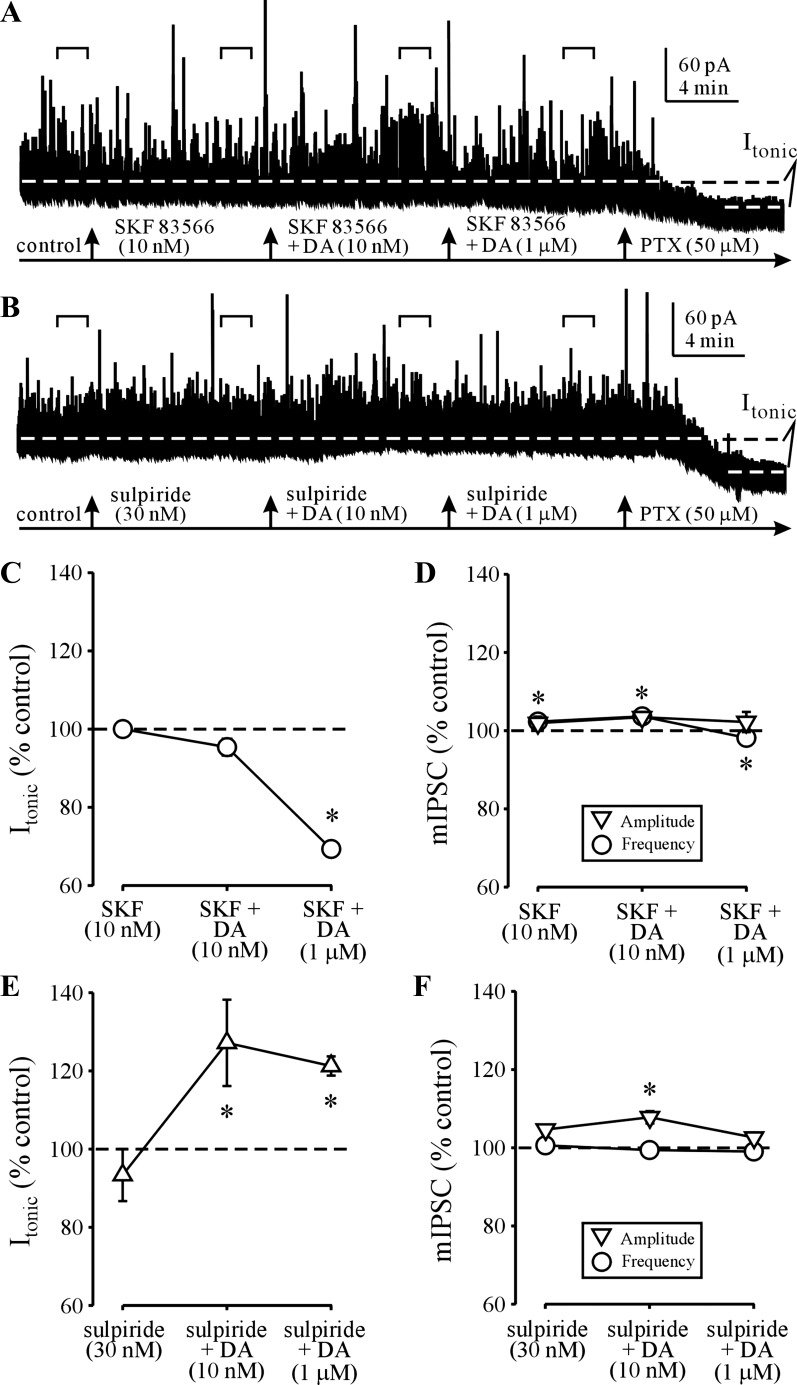
Since the effects of DA at its different receptor subtypes are determined in part by differences in the affinity for DA (Rankin et al. 2010; Seeman and Van Tol 1994; Undieh 2010), we tested for DA effects on Itonic in the presence of D1R or D2R antagonists. To that end, we recorded GABAAR current responses to DA first at 10 nM and then at 1 μM during continuous application of the selective D1R antagonist SKF 83566 at 10 nM (Ohlstein and Berkowitz 1985). SKF 83566 application alone had no discernible effects on Itonic (Fig. 2A). In the continued presence of SKF 83566, application of DA (10 nM) did not increase Itonic, whereas subsequent application of DA (1 μM) had a significant (>30%) depressant effect on Itonic (Fig. 2, A and C). These pharmacological manipulations had no effect on mIPSC amplitude or charge transfer, with only a small (<3%) effect on mIPSC frequency (Fig. 2D). These data suggested that D1Rs mediate potentiation of Itonic while D2Rs depress Itonic. To confirm this, we measured GABAAR current responses to DA first at 10 nM and then at 1 μM during continuous application of the selective D2R antagonist sulpiride at 30 nM (Seeman and Van Tol 1994). Sulpiride application alone had no discernible effects on Itonic (Fig. 2, B and E). However, in the continued presence of sulpiride, application of DA (10 nM) significantly increased Itonic by >20% (Fig. 2E). During the subsequent application of DA (1 μM), the Itonic remained significantly potentiated above baseline (Fig. 2, B and E). During these pharmacological manipulations, only the amplitude of mIPSCs during the combined sulpiride + 10 nM DA application was significantly increased (<8%), while the frequency of mIPSCs was unaffected by application of sulpiride or sulpiride-DA combinations (Fig. 2F). The charge transfer, rise time, and decay kinetic parameters of mIPSCs were similarly unaffected by sulpiride-DA application (not shown). Together, these data suggested that potentiation and depression of Itonic by DA are mediated by D1Rs and D2Rs, respectively.
Fig. 2.
D1 receptor (D1R)-mediated potentiation and D2 receptor (D2R)-mediated depression of Itonic. A: selective D1R antagonist SKF 83566 (10 nM) blocks DA (10 nM) potentiation of Itonic but not DA (1 μM) depression of Itonic. B: in the presence of the selective D2R antagonist sulpiride, DA (10 nM and 1 μM) application potentiates Itonic. Horizontal brackets indicate the 100-s recording periods used for Itonic and mIPSC analysis. C and D: summary graphs of SKF 83566 effects on DA modulation of Itonic (C) and mIPSCs (D). *P < 0.05 (n = 6 neurons from 2 rats, 1-way ANOVA vs. control). E and F: summary graphs of sulpiride effects on DA modulation of Itonic (E) and mIPSCs (F). *P < 0.05 (n = 4 or 5 neurons from 3 rats, 1-way ANOVA vs. control).
D2R-mediated effects on Itonic are blocked in recordings with intrapipette guanosine 5′-O-(2-thiodiphosphate).
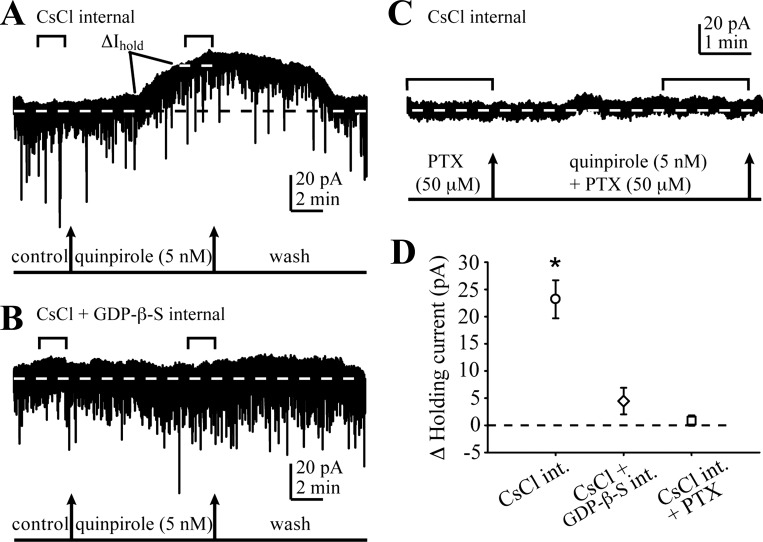
Modulation of Itonic magnitude by DA could be due to modulation of postsynaptic GABAAR function by postsynaptic DA receptors or to DA-induced changes in ambient GABA concentrations ([GABA]). To help differentiate between these two possibilities we measured responses to the application of a selective D2R agonist, quinpirole at 5 nM (Seeman and Van Tol 1994), in recordings from naive rat NAcc MSNs with patch pipettes containing a control internal solution and compared them to recordings with pipettes containing guanosine 5′-O-(2-thiodiphosphate) (GDPβS), which antagonizes G protein signaling within the recorded neurons (Schiffmann et al. 1995; Yan et al. 1997). For these recordings, we used CsCl-based intrapipette solutions and voltage-clamped the MSNs at −70 mV to demonstrate that the effects of DAR activation on Itonic are independent of the GABAAR Cl− current reversal potential. In control recordings, quinpirole (5 nM) application produced large reversible decreases in the basal holding current (Fig. 3A). Quinpirole had no significant effect on any of the postsynaptic mIPSC kinetic parameters (rise time: 90.8 ± 3.6, amplitude: 98.1 ± 10.2, decay τ1: 102.4 ± 3.1, decay τ2: 108.5 ± 13.1, charge transfer: 103.2 ± 20.3; all expressed as % of baseline). There was a trend toward decreased mIPSC frequency: 88.5 ± 7.3 of baseline, which did not reach statistical significance (paired t-test). In contrast, the quinpirole effect on the tonic holding current was almost completely abolished in recordings with GDPβS-containing patch pipettes (Fig. 3, B and D). In these recordings, quinpirole also had no significant effect on any of the postsynaptic mIPSC parameters (rise time: 112.7 ± 14.3, amplitude: 99.9 ± 11.3, decay τ1: 108.1 ± 22.3, decay τ2: 79.0 ± 39.5, charge transfer: 105.0 ± 27.1; expressed as % of baseline), but we did observe a significant (P < 0.01, paired t-test) decrease in mIPSC frequency (59.1 ± 7.4 of baseline), consistent with activation of presynaptic D2Rs (Delle Donne et al. 1997; Zhang and Sulzer 2012). We also showed that during recordings with control CsCl-based pipettes application of quinpirole (5 nM) during continuous bath application of picrotoxin had no effect on the remaining holding current (Fig. 3, C and D). Together, these data suggested that DA-mediated modulation of Itonic requires the activation of postsynaptic G protein-coupled DARs, which is separate from the D2R-mediated presynaptic effects on mIPSC frequency.
Fig. 3.
D2R-mediated decrease in Itonic is mediated by a postsynaptic G protein-dependent mechanism. A: representative recording of GABAAR currents in the NAcc MSNs from a naive rat with patch pipettes filled with a CsCl-based control internal solution. Voltage was clamped at −70 mV. Bath application of quinpirole (5 nM) produces a large, reversible decrease (ΔIhold) in the baseline holding current (dashed line) without affecting mIPSCs. Horizontal brackets indicate the 100-s recording periods used for Ihold and mIPSC analysis. B: during recording with pipettes containing guanosine 5′-O-(2-thiodiphosphate) (GDPβS), the response to quinpirole is abolished. C: when the GABAAR currents are blocked with PTX, the remaining holding current is unaffected by quinpirole. D: summary graph comparing the quinpirole effect in the 3 recording conditions. CsCl internal: n = 4; CsCl + GDPβS internal: n = 6; CsCl internal + PTX: n = 4. *P < 0.05, 1-way ANOVA. ΔHolding current = [holding current during quinpirole] − [holding current at baseline].
DA-mediated depression of Itonic is unaffected by CIE treatment.
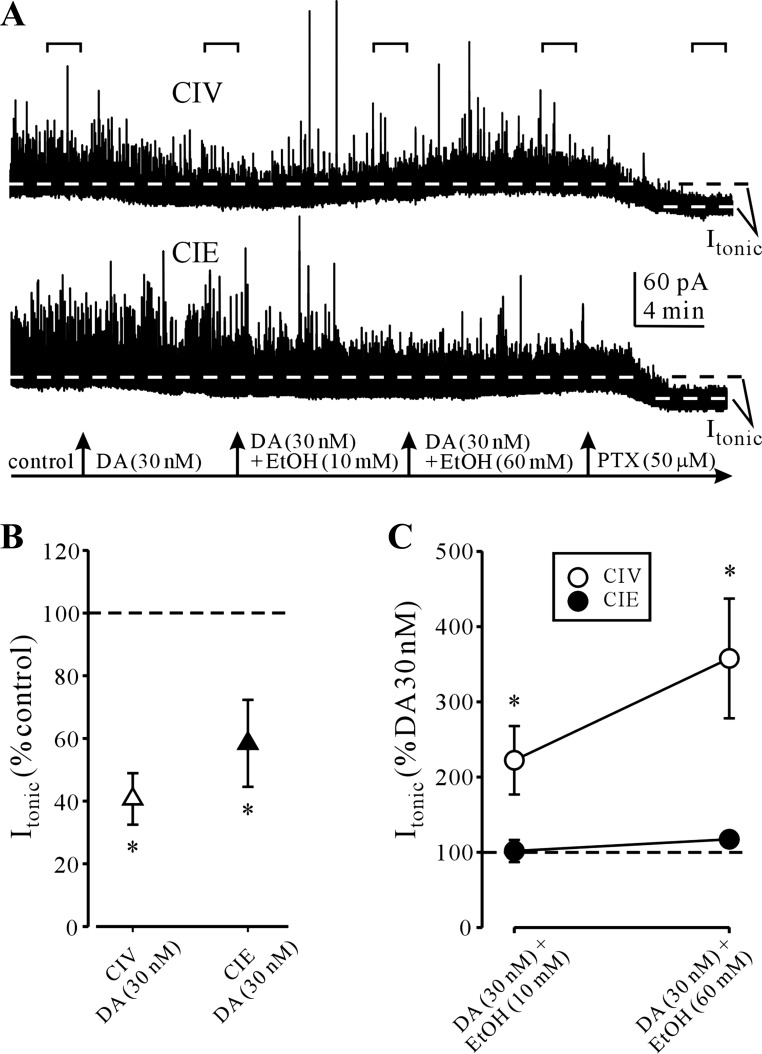
We next compared the D2R-mediated depressant effect of DA (30 nM) in NAcc MSN recordings from CIV- and CIE-treated rats. In the same recordings we also measured the effect of acute EtOH (10 and 60 mM) application during coapplication with DA (Fig. 4A). Similar to recordings from naive rats, DA (30 nM) application produced a significant depression of Itonic in the MSNs from CIV rats without significant changes in postsynaptic mIPSC kinetic parameters (rise time: 103.9 ± 3.0, amplitude: 98.8 ± 0.7, decay τ1: 111.6 ± 3.9, decay τ2: 136.5 ± 36.4, charge transfer: 103.2 ± 20.3; expressed as % of baseline). However, there was a small (92.3 ± 1.9% of baseline) but significant (P < 0.02, paired t-test) decrease in mIPSC frequency after DA application, which was not observed in the initial set of DA application recordings (cf. Fig. 1).
Fig. 4.
Alterations in DA and EtOH effects on Itonic after chronic intermittent ethanol (CIE) treatment. A: representative recordings of GABAAR currents in the NAcc MSNs from vehicle (CIV, top)- and CIE (bottom)-treated rats. DA (30 nM) application produces a visible depression of the picrotoxin-sensitive Itonic (dashed lines). Subsequent coapplication of EtOH (10 and 60 mM) with DA increases Itonic. Horizontal brackets indicate the 100-s recording periods used for Itonic and mIPSC analysis. B: summary graph compares Itonic depression by DA (30 nM) in CIV- and CIE-treated rats. Note the similar effect of DA on Itonic from the 2 groups of rats. *P < 0.05 (n = 6 neurons from 2 rats/group, t-test vs. control). C: summary graph compares Itonic potentiation by EtOH (10 and 60 mM) in the presence of DA (30 nM) in CIV- and CIE-treated rats. Note the loss of EtOH potentiation of Itonic in NAcc MSNs from CIE-treated rats. *P < 0.05 (1-way ANOVA vs. control).
During continuous application of DA, application of EtOH, first at 10 mM and then at 60 mM, resulted in significant concentration-dependent increases in Itonic during recordings from MSNs of CIV rats (Fig. 4). When comparing recordings from CIE rats, DA (30 nM) still produced a significant depression of Itonic (Fig. 4B), while EtOH (10–60 mM) no longer potentiated Itonic (Fig. 4C). In these recordings from CIE rat MSNs, DA application again had no significant effect on postsynaptic mIPSC kinetic parameters (rise time: 97.4 ± 6.0, amplitude: 99.0 ± 0.4, decay τ1: 100.6 ± 3.6, decay τ2: 112.9 ± 23.5, charge transfer: 103.2 ± 20.3; expressed as % of baseline), although we again observed a significant (P < 0.01, paired t-test) decrease (84.4 ± 2.2% of baseline) in mIPSC frequency after DA application.
CIE treatment does not alter Itonic response to selective D1R and D2R agonists.
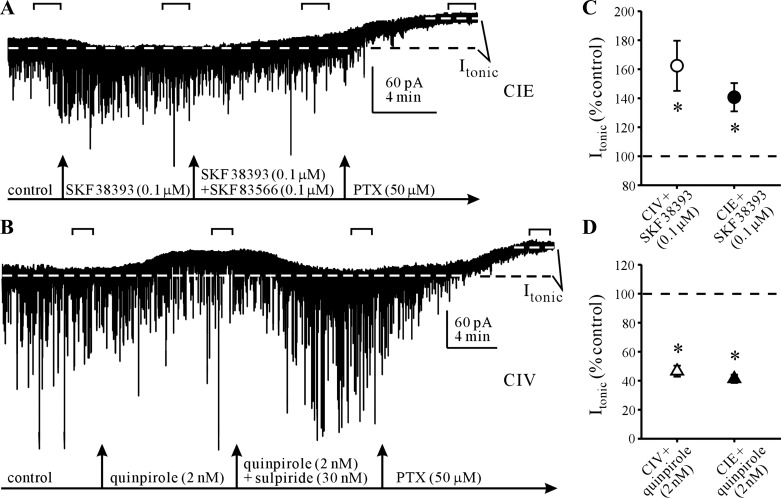
To further assess whether CIE treatment alters dopaminergic modulation of Itonic, we compared GABAAR current responses to application of the selective D1R agonist SKF 38393 at 0.1 μM or separately to application of the selective D2R agonist quinpirole at 2 nM (Seeman and Van Tol 1994). Application of SKF 38393 produced an enhancement of the tonic current in the MSNs of CIE and CIV rats (Fig. 5). Subsequent coapplication of the selective D1R antagonist SKF 83566 (0.1 μM) reversed this effect (Fig. 5A), confirming selectivity of D1R activation for Itonic potentiation. In recordings from CIV rats, SKF 38393 (0.1 μM) application had no significant effect on any mIPSC parameters (rise time: 105.2 ± 8.7, amplitude: 106.7 ± 3.5, decay τ1: 110.9 ± 12.5, decay τ2: 133.7 ± 33.9, charge transfer: 105.8 ± 7.8, frequency: 101.8 ± 1.0; expressed as % of baseline). A similar lack of effects was observed in recordings from CIE rats (rise time: 106.8 ± 12.1, amplitude: 100.5 ± 2.1, decay τ1: 135.8 ± 17.7, charge transfer: 111.8 ± 5.5, frequency: 99.2 ± 1.2; expressed as % of baseline), with the exception of mIPSC decay τ2, which was significantly (P < 0.05, paired t-test) increased (174.6 ± 23.1% of baseline).
Fig. 5.
CIE treatment does not alter D1R- and D2R-mediated modulation of Itonic. A: representative recording (CsCl-based patch electrode) of selective D1R agonist (SKF 38393, 0.1 μM) potentiation of the PTX-sensitive Itonic (dashed lines) in a NAcc MSN from a CIE-treated rat. Note that coapplication of the selective D1R antagonist SKF 83566 (0.1 μM) together with SKF 38393 reverses this potentiation. B: representative recording of selective D2R agonist (quinpirole, 2 nM) depression of Itonic in a NAcc MSN from a CIV-treated rat. Note that coapplication of the selective D2R antagonist sulpiride (30 nM) together with quinpirole reverses this depression. Horizontal brackets indicate the 100-s recording periods used for Itonic and mIPSC analysis. C: summary graph illustrates comparable potentiation of Itonic by SKF 38393 (1 μM) in CIV and CIE rats. *P < 0.05 (n = 4–6 neurons from 2–4 rats/group, t-test vs. control). D: summary graph illustrates comparable depression of Itonic by quinpirole (2 nM) in CIV and CIE rats. *P < 0.05 (n = 9–14 neurons from 4–6 rats/group, t-test vs. control).
Application of quinpirole suppressed Itonic in MSNs from CIV and CIE rats, and this suppression was reversed by coapplication of the D2R antagonist sulpiride (30 nM) (Fig. 4B). Quinpirole application in recordings from CIV rat MSNs had no significant effect on postsynaptic mIPSC kinetic parameters (rise time: 116.9 ± 7.0, amplitude: 101.5 ± 0.7, decay τ1: 100.0 ± 8.0, decay τ2: 114.4 ± 28.3, charge transfer: 107.9 ± 8.7; expressed as % of baseline), although there was a very small, but significant (P < 0.05, paired t-test), decrease in mIPSC frequency (98.6 ± 0.6 of baseline), consistent with previous observations. A mostly similar lack of effects was observed in recordings from CIE rat MSNs (rise time: 114.4 ± 7.1, amplitude: 101.3 ± 1.1, decay τ1: 111.9 ± 1.8, decay τ2: 147.3 ± 24.8, frequency: 99.2 ± 1.2; expressed as % of baseline). Surprisingly, mIPSC charge transfer was significantly (P < 0.001, paired t-test) increased (111.9 ± 1.8% of baseline), which was in contrast to the large decreases in Itonic observed in these recordings.
Importantly, comparison of D1R-mediated potentiation of Itonic between CIE and CIV rats did not reveal significant group differences (Fig. 4C). Similarly, D2R-mediated suppression of Itonic was not different between CIE and CIV rats (Fig. 4D). These data suggested that CIE treatment does not modify the modulatory effect of DA on GABAAR-mediated Itonic.
DISCUSSION
Studies utilizing microdialysis and fast scanning voltammetry techniques demonstrated that [DA] measured in the NAcc of awake rats range from low nanomolar basal levels to high submicromolar concentrations during phasic DA release (Budygin et al. 2001; Owesson-White et al. 2012; Robinson et al. 2009; Segovia and Mora 2001; Yim and Gonzales 2000). Here we demonstrate that application of DA in NAcc slices at such physiologically relevant concentrations (0.01–1 μM) preferentially modulates extrasynaptic GABAARs that mediate the tonic current, without significant effects on synaptic GABAARs. Studies in murine MSNs in the dorsal striatum have also suggested preferential modulation of Itonic by DA receptor ligands compared with synaptic currents (Janssen et al. 2009). Analogous to the hippocampus (Glykys et al. 2008; Liang et al. 2007; Scimemi et al. 2005), Itonic in NAcc MSNs is mediated by several different combinations of extrasynaptic GABAAR subunits that demonstrate considerable alterations after CIE treatment (companion article, Liang et al. 2014). These low-conductance, high-affinity extrasynaptic GABAARs are activated by ambient GABA whose sources may include spillover from the synaptic cleft during synaptic transmission (Glykys and Mody 2007; however, see Bright et al. 2011) and channel-mediated release from astrocytes (Lee et al. 2010).
The selectivity of DA actions on Itonic could result from DA modulation of extracellular [GABA]. Changes in extracellular [GABA] may result from changes in the amount of released GABA or its uptake. Presynaptic effects of DA on neuronal GABA release are well-established (Feuerstein 2008; Zhang and Sulzer 2012), and we did observe decreases in frequency of mIPSCs after DA (30 nM) or quinpirole application, without concomitant changes in postsynaptic mIPSC kinetic parameters. These data suggested that the observed DA actions on Itonic might be mediated by changes in synaptic GABA release. GABA may also be released from astrocytes via Bestrophin 1 channels (Lee et al. 2010). Striatal astrocytes were demonstrated to express both D1Rs and D2Rs (Bal et al. 1994; Hosli and Hosli 1987; Miyazaki et al. 2004; Zanassi et al. 1999). Conceivably, activation of these DARs could modulate GABA release from astrocytes. Indeed, D1R activation was shown to increase extracellular [GABA] via nonvesicular transporter-mediated GABA release in striatal slices and primary cultures (Schoffelmeer et al. 2000). However, we demonstrated that blockade of postsynaptic G protein signaling in the recorded MSNs with GDPβS abolished D2R receptor-mediated decreases in Itonic (Fig. 3), without affecting postsynaptic mIPSC kinetic parameters. Notably, during this blockade of postsynaptic G protein signaling in the recorded MSNs, mIPSC frequency could still be decreased by quinpirole application. These experiments provided concrete evidence for a postsynaptic mechanism by which extrasynaptic GABAARs are modulated by DAR activation in MSNs. A postsynaptic mechanism of action was previously demonstrated for the D3R-meditated suppression of GABAAR currents in the NAcc (Chen et al. 2006). Also, functional postsynaptic G protein-dependent cross talk between GABABRs and GABAARs that mediate Itonic has been recently demonstrated in thalamic, dentate gyrus, and cerebellar neurons (Connelly et al. 2013).
The preferential DA responsiveness of extrasynaptic GABAARs in NAcc MSNs could result from preferential removal of extracellular synaptic DA by DA transporters localized around GABAergic synapses. However, extensive cellular and subcellular studies have failed to localize DA transporter to any synaptic active zones in the striatum, suggesting that striatal DA reuptake likely occurs outside of synaptic specializations once DA diffuses from the synaptic cleft (Hersch et al. 1997). Furthermore, the fact that Itonic is also preferentially modulated by selective D1R and D2R agonists that are not subject to DA transporter-mediated removal makes this an unlikely possibility. More likely is the differential coupling of second messenger mechanisms to the unique subunit composition of extrasynaptic GABAARs versus synaptic GABAARs. In the NAcc as in other forebrain regions, extrasynaptic GABAARs include, together with the ubiquitous β subunits, various combinations of α4, α5, and δ subunits. Isoform-specific phosphorylation of individual GABAAR subunits by protein kinase A (PKA) and protein kinase C (PKC) has been shown to differentially affect activation, pharmacological responsiveness, and membrane recycling of extrasynaptic versus synaptic GABAARs (Brandon et al. 1999; Kia et al. 2011; Werner et al. 2011). More studies are needed to precisely identify the extrasynaptic GABAAR subunit combinations affected by DAR activation.
The observed selective potentiation of postsynaptic GABAARs by D1Rs and their selective depression by D2Rs is supported by previous studies in mice with genetic ablation of D1Rs or D2Rs (Centonze et al. 2003), which showed a loss of DA-dependent depression of IPSCs evoked in striatal interneurons of D2R-null mice. Differential D1R versus D2R regulation of IPSCs by DA was also demonstrated in recordings from rat prefrontal cortex neurons (Trantham-Davidson et al. 2004). It is noteworthy that while activation of both synaptic and extrasynaptic GABAARs contributes to the action potential-dependent evoked IPSCs measured in the above studies, GABAARs giving rise to action potential-independent synaptic mIPSCs were separable from the extrasynaptic GABAARs giving rise to Itonic in our recordings. Recent studies in the NAcc of mice have confirmed both D1R-mediated potentiation and D2R-mediated depression of extrasynaptic α4βδ GABAAR activity and also showed that such differential effects on MSN neuron excitability can modulate the behavioral response to psychostimulants (Maguire et al. 2014). The differential responsiveness of GABAARs to D1R or D2R activation is likely due to the activation of different intracellular signaling pathways by these DAR subtypes. The D1-class DARs (D1R and D5R) are generally known to activate the Gs/olf family of G proteins to stimulate adenylyl cyclase (AC) and PKA activity, whereas the D2-class DARs (D2R, D3R, and D4R) couple to Gi/o proteins, resulting in inhibition of AC and a decrease in PKA activity (Beaulieu and Gainetdinov 2011). Amplification of PKA signaling is mediated by the DA- and cAMP-regulated phosphoprotein (DARPP-32), which is highly expressed in MSNs, where it acts as an integrator on modulatory responses to multiple neurotransmitters, including DA (Bateup et al. 2008; Nishi et al. 1997; Svenningsson et al. 2004). Coexpression of D1Rs and D2Rs may lead to formation of D1R:D2R heterodimers that, acting through Gαq, can regulate downstream calcium signaling (Rashid et al. 2007).
Numerous studies have shown that striatal MSNs, including those in the NAcc core, form two principal subgroups that are defined by their selective expression of DAR subtypes and their projection targets (reviewed in Beaulieu and Gainetdinov 2011; Valjent et al. 2009). For example, murine NAcc MSNs projecting to the VTA selectively express D1Rs, thus resembling the striatonigral (direct pathway) MSNs, while MSNs projecting to the ventral pallidum express D2Rs and thus resemble the striatopallidal (indirect pathway) MSNs (Gangarossa et al. 2013). However, in addition to the two main subgroups there is a population of MSNs that express both D1Rs and D2Rs, with the highest incidence in NAcc (Lu et al. 1998; Perreault et al. 2010, 2011). Partial coexpression of D1R with D3R, particularly in the NAcc, has also been described (Le Moine and Bloch 1996). In our experiments, all recorded NAcc core MSNs tested with an application of a selective D1R agonist or 10 nM DA responded with Itonic enhancement (n = 14), and conversely all MSNs tested with a selective D2R agonist or 0.03–1 μM DA responded with a decrease in Itonic (n = 40). The probability that our “blind” patch recordings selectively targeted a particular subpopulation of MSNs is very small, suggesting instead that the ability of DA and selective DAR agonists to bidirectionally modulate the activity of extrasynaptic GABAARs is common to all NAcc core MSNs. This is in agreement with the immunohistochemically and functionally demonstrated coexistence of D1Rs and D2Rs in virtually all embryonic rat striatal neurons after 3 wk in culture (Aizman et al. 2000) and with the functional and single-cell RNA amplification demonstrations of D1R, D2R, and D3R coexpression in retrogradely labeled single striatonigral rat MSNs (Surmeier et al. 1992). Taken together, these data suggest that there may be important species differences in the colocalization of DAR subtypes in direct and indirect pathway NAcc MSNs between rats and mice. Furthermore, the extensive colocalization of DARPP-32 with both D1R- and D2R-positive MSNs in the rat striatum (Rajput et al. 2009) provides a plausible intracellular substrate by which bidirectional modulation of Itonic might be achieved. This conjecture would have to be thoroughly tested in future studies. It would also be of interest to determine whether there are differences in DA modulation of Itonic between rat MSNs projecting to the VTA and those projecting to the ventral pallidum.
In MSNs at their hyperpolarized resting (“down”) state, similar to hippocampal interneurons (Song et al. 2011), the GABAAR current reversal potential is depolarizing, making baseline Itonic excitatory. Indeed, excitatory synaptic activation of single action potentials in MSNs is reduced during application of L655,703, which preferentially antagonizes Itonic in MSNs (Ade et al. 2008). In contrast, when MSNs are in their depolarized “up” state of action potential discharge, the GABAAR current reversal potential is hyperpolarizing, making baseline Itonic inhibitory. Therefore, low basal levels of extracellular DA (≤10 nM) would be expected to exert an excitatory influence on MSNs at their “down” state by D1R-mediated potentiation of Itonic, but when MSNs are in their depolarized “up” state D1R-mediated potentiation of Itonic should have a hyperpolarizing inhibitory effect. Furthermore, phasic increases in DA release resulting in higher [DA] (≥30 nM) would be expected to block Itonic via D2Rs. If phasic DA release were time-locked with activation of excitatory glutamatergic inputs in the NAcc, the D2R-mediated block of Itonic would then effectively accentuate the “up” state of affected MSNs. Evidence for the synchronized release of glutamate and DA includes the demonstration that dopaminergic terminals in the NAcc (but not the dorsal striatum) corelease glutamate, such that their selective optogenetic activation results in robust glutamate-mediated excitatory postsynaptic currents (EPSCs) in NAcc (but not dorsal striatum) MSNs (Stuber et al. 2010). Other studies have shown that cholinergic interneurons also corelease glutamate and that their selective optogenetic activation not only elicits EPSCs in MSNs (Higley et al. 2011) but also elicits DA release directly from DA terminals in the NAcc, this release being dependent on glutamatergic receptor activity (Cachope et al. 2012). Also, in vivo recordings of NAcc neuron activity coupled with fast scanning voltammetry measurements showed that phasic DA selectively modulates excitatory responses of NAcc neurons during cue-evoked sucrose-seeking behavior (Cacciapaglia et al. 2011). On the basis of these studies and our present findings, we propose that during basal DA release D1R-mediated potentiation of Itonic will either increase or decrease the excitability of MSNs depending on their membrane potential. In contrast, D2R activation, which should be greatest during peak phasic DA release, will increase MSN excitability by blocking Itonic, thereby accentuating the throughput of strong glutamatergic inputs.
Neither the magnitude of Itonic nor the relative effectiveness of D1R- and D2R-mediated modulation of Itonic is altered by CIE treatment (Fig. 5 and Liang et al. 2014). The observed lack of CIE-induced changes in modulation of Itonic by selective DAR agonists is consistent with the reported absence of changes in D2Rs after a chronic EtOH diet (Tajuddin and Druse 1996). Also, in alcohol-dependent animals basal DA release is decreased, yet an EtOH challenge evokes comparable if not greater DA release than in nondependent control animals (Budygin et al. 2007; Diana et al. 1992, 1993; Weiss et al. 1996; Yoshimoto et al. 1996). However, EtOH potentiation of Itonic is lost in CIE rats (Fig. 4 and Liang et al. 2014), and therefore an EtOH challenge in alcohol-dependent rats should result in unopposed D2R-mediated block of Itonic. Coupled with the demonstrated increases in glutamatergic excitatory neurotransmission after CIE treatment (Jeanes et al. 2011; Marty and Spigelman 2012; Szumlinski et al. 2007), we predict that EtOH-evoked DA release should produce larger increases in the “up-state” excitability of MSNs in CIE rats compared with control rats. These neuroadaptive changes could contribute to the high levels of voluntary EtOH intake previously demonstrated in CIE rats (Rimondini et al. 2003). Analogous changes in the human NAcc could contribute to the development of cravings and relapse during protracted withdrawal in alcohol dependence.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA-016100 (I. Spigelman), AA-07680 (R. W. Olsen), and AA-017991 (J. Liang) and a UCLA School of Graduate Studies stipend (Y. Mulpuri).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L., V.N.M., and I.S. conception and design of research; J.L., V.N.M., Y.M., and I.S. performed experiments; J.L., V.N.M., and I.S. analyzed data; J.L., V.N.M., R.W.O., and I.S. interpreted results of experiments; J.L., V.N.M., and I.S. prepared figures; J.L., V.N.M., Y.M., R.W.O., and I.S. edited and revised manuscript; J.L., V.N.M., Y.M., R.W.O., and I.S. approved final version of manuscript; V.N.M. and I.S. drafted manuscript.
ACKNOWLEDGMENTS
We thank Dr. Thomas Otis for help with data analysis and interpretation.
REFERENCES
- Ade KK, Janssen MJ, Ortinski PI, Vicini S. Differential tonic GABA conductances in striatal medium spiny neurons. J Neurosci 28: 1185–1197, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci 3: 226–230, 2000 [DOI] [PubMed] [Google Scholar]
- Bal A, Bachelot T, Savasta M, Manier M, Verna JM, Benabid AL, Feuerstein C. Evidence for dopamine D2 receptor mRNA expression by striatal astrocytes in culture: in situ hybridization and polymerase chain reaction studies. Brain Res Mol Brain Res 23: 204–212, 1994 [DOI] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P. Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11: 932–939, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217, 2011 [DOI] [PubMed] [Google Scholar]
- Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci 19: 9228–9234, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, Mackenzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of δ-containing GABAA receptors during spillover. J Neurosci 31: 753–763, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Oleson EB, Mathews TA, Lack AK, Diaz MR, McCool BA, Jones SR. Effects of chronic alcohol exposure on dopamine uptake in rat nucleus accumbens and caudate putamen. Psychopharmacology (Berl) 193: 495–501, 2007 [DOI] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther 297: 27–34, 2001 [PubMed] [Google Scholar]
- Bustamante D, Quintanilla ME, Tampier L, Gonzalez-Lira V, Israel Y, Herrera-Marschitz M. Ethanol induces stronger dopamine release in nucleus accumbens (shell) of alcohol-preferring (bibulous) than in alcohol-avoiding (abstainer) rats. Eur J Pharmacol 591: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciapaglia F, Wightman RM, Carelli RM. Rapid dopamine signaling differentially modulates distinct microcircuits within the nucleus accumbens during sucrose-directed behavior. J Neurosci 31: 13860–13869, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang HL, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep 2: 33–41, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci 23: 6245–6254, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Kittler JT, Moss SJ, Yan Z. Dopamine D3 receptors regulate GABAA receptor function through a phospho-dependent endocytosis mechanism in nucleus accumbens. J Neurosci 26: 2513–2521, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly WM, Fyson SJ, Errington AC, McCafferty CP, Cope DW, Di GG, Crunelli V. GABAB receptors regulate extrasynaptic GABAA receptors. J Neurosci 33: 3780–3785, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10: 1020–1028, 2007 [DOI] [PubMed] [Google Scholar]
- Delle Donne KT, Sesack SR, Pickel VM. Ultrastructural immunocytochemical localization of the dopamine D2 receptor within GABAergic neurons of the rat striatum. Brain Res 746: 239–255, 1997 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Ethanol preferentially stimulates dopamine release in the nucleus accumbens of freely moving rats. Eur J Pharmacol 115: 131–132, 1985 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Gessa GL, Rossetti ZL. Lack of tolerance to ethanol-induced stimulation of mesolimbic dopamine system. Alcohol Alcohol 27: 329–333, 1992 [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci USA 90: 7966–7969, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein TJ. Presynaptic receptors for dopamine, histamine, and serotonin. Handb Exp Pharmacol 289–338, 2008 [DOI] [PubMed] [Google Scholar]
- French MT, Zavala SK. The health benefits of moderate drinking revisited: alcohol use and self-reported health status. Am J Health Promot 21: 484–491, 2007 [DOI] [PubMed] [Google Scholar]
- Gangarossa G, Espallergues J, de Kerchove d'Exaerde A, El Mestikawy S, Gerfen CR, Herve D, Girault JA, Valjent E. Distribution and compartmental organization of GABAergic medium-sized spiny neurons in the mouse nucleus accumbens. Front Neural Circuits 7: 22, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus? J Neurosci 28: 1421–1426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582: 1163–1178, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunzerath L, Faden V, Zakhari S, Warren K. National Institute on Alcohol Abuse and Alcoholism report on moderate drinking. Alcohol Clin Exp Res 28: 829–847, 2004 [DOI] [PubMed] [Google Scholar]
- Hersch SM, Yi H, Heilman CJ, Edwards RH, Levey AI. Subcellular localization and molecular topology of the dopamine transporter in the striatum and substantia nigra. J Comp Neurol 388: 211–227, 1997 [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One 6: e19155, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosli L, Hosli E. Receptors for dopamine and serotonin on astrocytes of cultured rat central nervous system. J Physiol (Paris) 82: 191–195, 1987 [PubMed] [Google Scholar]
- Janssen MJ, Ade KK, Fu Z, Vicini S. Dopamine modulation of GABA tonic conductance in striatal output neurons. J Neurosci 29: 5116–5126, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes ZM, Buske TR, Morrisett RA. In vivo chronic intermittent ethanol exposure reverses the polarity of synaptic plasticity in the nucleus accumbens shell. J Pharmacol Exp Ther 336: 155–164, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katner SN, Kerr TM, Weiss F. Ethanol anticipation enhances dopamine efflux in the nucleus accumbens of alcohol-preferring (P) but not Wistar rats. Behav Pharmacol 7: 669–674, 1996 [PubMed] [Google Scholar]
- Kia A, Ribeiro F, Nelson R, Gavrilovici C, Ferguson SS, Poulter MO. Kindling alters neurosteroid-induced modulation of phasic and tonic GABAA receptor-mediated currents: role of phosphorylation. J Neurochem 116: 1043–1056, 2011 [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci 363: 3113–3123, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron 21: 467–476, 1998 [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. Expression of the D3 dopamine receptor in peptidergic neurons of the nucleus accumbens: comparison with the D1 and D2 dopamine receptors. Neuroscience 73: 131–143, 1996 [DOI] [PubMed] [Google Scholar]
- Lee S, Yoon BE, Berglund K, Oh SJ, Park H, Shin HS, Augustine GJ, Lee CJ. Channel-mediated tonic GABA release from glia. Science 330: 790–796, 2010 [DOI] [PubMed] [Google Scholar]
- Liang J, Lindemeyer AK, Suryanarayanan A, Meyer EE, Marty VN, Ahmad SO, Shao XM, Olsen RW, Spigelman I. Plasticity of GABAA receptor-mediated neurotransmission in the nucleus accumbens of alcohol-dependent rats. J Neurophysiol (April 2, 2014). 10.1152/jn.00565.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci 27: 12367–12377, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience 82: 767–780, 1998 [DOI] [PubMed] [Google Scholar]
- Maguire EP, Macpherson T, Swinny JD, Dixon CI, Herd MB, Belelli D, Stephens DN, King SL, Lambert JJ. Tonic inhibition of accumbal spiny neurons by extrasynaptic α4βδ GABAA receptors modulates the actions of psychostimulants. J Neurosci 34: 823–838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty VN, Spigelman I. Long-lasting alterations in membrane properties, K+ currents, and glutamatergic synaptic currents of nucleus accumbens medium spiny neurons in a rat model of alcohol dependence. Front Neurosci 6: 86, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, Rodd-Henricks ZA, Engleman EA, Li TK, McBride WJ, Murphy JM. Microdialysis of dopamine in the nucleus accumbens of alcohol-preferring (P) rats during anticipation and operant self-administration of ethanol. Alcohol Clin Exp Res 26: 318–325, 2002 [PubMed] [Google Scholar]
- Miyazaki I, Asanuma M, Diaz-Corrales FJ, Miyoshi K, Ogawa N. Direct evidence for expression of dopamine receptors in astrocytes from basal ganglia. Brain Res 1029: 120–123, 2004 [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci 17: 8147–8155, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell P, Greene J, Pabello N, Lewis BL, Grace AA. Modulation of cell firing in the nucleus accumbens. Ann NY Acad Sci 877: 157–175, 1999 [DOI] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology 38: 1617–1624, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein EH, Berkowitz BA. SCH 23390 and SK&F 83566 are antagonists at vascular dopamine and serotonin receptors. Eur J Pharmacol 108: 205–208, 1985 [DOI] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem 121: 252–262, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, Alijaniaram M, Fan T, Varghese G, Fletcher PJ, Seeman P, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J Biol Chem 285: 36625–36634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O'Dowd BF, George SR. The dopamine D1-D2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front Neuroanat 5: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature 422: 614–618, 2003 [DOI] [PubMed] [Google Scholar]
- Rajput PS, Kharmate G, Somvanshi RK, Kumar U. Colocalization of dopamine receptor subtypes with dopamine and cAMP-regulated phosphoprotein (DARPP-32) in rat brain. Neurosci Res 65: 53–63, 2009 [DOI] [PubMed] [Google Scholar]
- Rankin ML, Hazelwood LA, Free RB, Namkung Y, Rex EB, Roof RA, Sibley DR. Molecular pharmacology of the dopamine receptors. In: Dopamine Handbook, edited by Iversen LL, Dunnett SB, Iversen SD, Bjorklund A. New York: Oxford Univ. Press, 2010 [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O'Dowd BF, George SR. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc Natl Acad Sci USA 104: 654–659, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. A temporal threshold for induction of persistent alcohol preference: behavioral evidence in a rat model of intermittent intoxication. J Stud Alcohol 64: 445–449, 2003 [DOI] [PubMed] [Google Scholar]
- Robinson DL, Howard EC, McConnell S, Gonzales RA, Wightman RM. Disparity between tonic and phasic ethanol-induced dopamine increases in the nucleus accumbens of rats. Alcohol Clin Exp Res 33: 1187–1196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room R, Babor T, Rehm J. Alcohol and public health. Lancet 365: 519–530, 2005 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Lledo PM, Vincent JD. Dopamine D1 receptor modulates the voltage-gated sodium current in rat striatal neurones through a protein kinase A. J Physiol 483: 95–107, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH. Synergistically interacting dopamine D1 and NMDA receptors mediate nonvesicular transporter-dependent GABA release from rat striatal medium spiny neurons. J Neurosci 20: 3496–3503, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci 25: 10016–10024, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci 15: 264–270, 1994 [DOI] [PubMed] [Google Scholar]
- Segovia G, Mora F. Involvement of NMDA and AMPA/kainate receptors in the effects of endogenous glutamate on extracellular concentrations of dopamine and GABA in the nucleus accumbens of the awake rat. Brain Res Bull 54: 153–157, 2001 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35: 27–47, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Wightman RM. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci 29: 1735–1742, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I, Savtchenko L, Semyanov A. Tonic excitation or inhibition is set by GABAA conductance in hippocampal interneurons. Nat Commun 2: 376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci 30: 8229–8233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron 46: 661–669, 2005 [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Eberwine J, Wilson CJ, Cao Y, Stefani A, Kitai ST. Dopamine receptor subtypes colocalize in rat striatonigral neurons. Proc Natl Acad Sci USA 89: 10178–10182, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44: 269–296, 2004 [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Diab ME, Friedman R, Henze LM, Lominac KD, Bowers MS. Accumbens neurochemical adaptations produced by binge-like alcohol consumption. Psychopharmacology (Berl) 190: 415–431, 2007 [DOI] [PubMed] [Google Scholar]
- Tajuddin NF, Druse MJ. Effects of chronic alcohol consumption and aging on dopamine D2 receptors in Fischer 344 rats. Alcohol Clin Exp Res 20: 144–151, 1996 [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Neely LC, Lavin A, Seamans JK. Mechanisms underlying differential D1 versus D2 dopamine receptor regulation of inhibition in prefrontal cortex. J Neurosci 24: 10652–10659, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undieh AS. Pharmacology of signaling induced by dopamine D1-like receptor activation. Pharmacol Ther 128: 37–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Herve D, Fisone G, Girault JA. Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci 32: 538–547, 2009 [DOI] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther 267: 250–258, 1993 [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci 16: 3474–3485, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Alex FJ, Comerford CE, Morrow AL. PKCγ is required for ethanol-induced increases in GABAA receptor α4 subunit expression in cultured cerebral cortical neurons. J Neurochem 116: 554–563, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–494, 2004 [DOI] [PubMed] [Google Scholar]
- Yan Z, Song WJ, Surmeier J. D2 dopamine receptors reduce N-type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane-delimited, protein-kinase-C-insensitive pathway. J Neurophysiol 77: 1003–1015, 1997 [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol 22: 107–115, 2000 [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Yayama K, Sorimachi Y, Tani J, Ogata M, Nishimura A, Yoshida T, Ueda S, Komura S. Possibility of 5-HT3 receptor involvement in alcohol dependence: a microdialysis study of nucleus accumbens dopamine and serotonin release in rats with chronic alcohol consumption. Alcohol Clin Exp Res 20: 311A–319A, 1996 [PubMed] [Google Scholar]
- Zanassi P, Paolillo M, Montecucco A, Avvedimento EV, Schinelli S. Pharmacological and molecular evidence for dopamine D1 receptor expression by striatal astrocytes in culture. J Neurosci Res 58: 544–552, 1999 [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia 2: 5–13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]