Abstract
Central pattern generators (CPGs) produce motor patterns that ultimately drive motor outputs. We studied how functional motor performance is achieved, specifically, whether the variation seen in motor patterns is reflected in motor performance and whether fictive motor patterns differ from those in vivo. We used the leech heartbeat system in which a bilaterally symmetrical CPG coordinates segmental heart motor neurons and two segmented heart tubes into two mutually exclusive coordination modes: rear-to-front peristaltic on one side and nearly synchronous on the other, with regular side-to-side switches. We assessed individual variability of the motor pattern and the beat pattern in vivo. To quantify the beat pattern we imaged intact adults. To quantify the phase relations between motor neurons and heart constrictions we recorded extracellularly from two heart motor neurons and movement from the corresponding heart segments in minimally dissected leeches. Variation in the motor pattern was reflected in motor performance only in the peristaltic mode, where larger intersegmental phase differences in the motor neurons resulted in larger phase differences between heart constrictions. Fictive motor patterns differed from those in vivo only in the synchronous mode, where intersegmental phase differences in vivo had a larger front-to-rear bias and were more constrained. Additionally, load-influenced constriction timing might explain the amplification of the phase differences between heart segments in the peristaltic mode and the higher variability in motor output due to body shape assumed in this soft-bodied animal. The motor pattern determines the beat pattern, peristaltic or synchronous, but heart mechanics influence the phase relations achieved.
Keywords: motor pattern in vivo, motor performance, intersegmental coordination, variability, leech
rhythmic behaviors are an important feature in many animals and are often controlled by central pattern generators (CPGs). CPGs are capable of producing motor patterns in the absence of sensory feedback (fictive motor pattern). How a CPG orchestrates an ensemble of motor neurons into a functional motor pattern has been the focus of many studies in different systems, combining modeling with electrophysiological recordings in reduced preparations (reviewed in Calabrese et al. 2011; Callaway and Marder 2012). Much less is known about the step from the motor pattern to the motor output (i.e., behavior). Rhythmic behaviors may look similar across individuals and are therefore often termed stereotyped (Marder and Calabrese 1996). However, pioneered by work in isolated nervous systems, first in the stomatogastric nervous system (STNS) in decapod crustaceans (Golowasch et al. 2002) and recently in the leech heartbeat system (Goaillard 2011; Norris et al. 2011; Roffmann et al. 2012), it has become clear that there is a two- to fivefold variability in the intrinsic membrane and synaptic properties of the individual neurons of CPGs, yet we walk and breathe, crabs chew, and leech hearts keep on beating. To gain insight into how functional and robust motor performance (or behavior) is achieved despite this variability across animals, two questions need to be addressed: First, is the variation seen in motor patterns reflected in the motor performance? Second, is the motor pattern produced in vitro (i.e., the fictive motor pattern) different from that produced in vivo? To answer these questions, largely intact (in vivo) preparations are needed in which sensory feedback and modulatory control remain intact and in which simultaneous recordings from motor neurons, muscles, and the structures they operate (i.e., limbs, wings, teeth, hearts) are possible. Currently, this approach is limited to a few systems, especially small invertebrate systems with more accessible and fewer neurons than in vertebrate preparations.
To answer the above two questions, the behavior needs to be quantitatively described and the motor pattern and the behavior need to be monitored simultaneously, requiring recording from at least subsets of motor neurons and muscles executing the behavior. The latter approach has recently been used successfully to show that two distinct chewing patterns are driven by two distinct motor programs by a single CPG in crabs (Diehl et al. 2013). Studying the interface between motor pattern and motor output may reveal mechanisms that constrain variability to ensure functional output.
In the leech, the heartbeat CPG programs two functionally different and mutually exclusive patterns on the two body sides (recent review by Calabrese 2010), which makes this system a good candidate for studying how two different beat patterns are set up by two different motor patterns in the same motor neurons. Two segmented heart tubes, one on each side, propel blood through the closed circulatory system. Each heart segment is entrained by an ipsilateral heart excitatory (HE) motor neuron in the corresponding segmental ganglion (Maranto and Calabrese 1984b). The heart motor neurons are controlled by a well-characterized CPG (Norris et al. 2006, 2007). Neurons and muscles are accessible for recordings. The beat pattern of the individual heart segments, the fictive motor pattern, and the temporal pattern of the premotor heart interneurons' bursting activity display a left-right asymmetry: on one side, intersegmental coordination is rear-to-front peristaltic; on the other side, it is coordinated nearly synchronously. The entire system is bilaterally symmetrical but is coordinated in, and switches between, a left-peristaltic/right-synchronous and right-peristaltic/left-synchronous pattern in vivo and in vitro (20–40 beats per switch cycle) (Calabrese 1977; Krahl and Zerbst-Boroffka 1983; Norris et al. 2006, 2007; Thompson and Stent 1976; Wenning et al. 2004b). As a consequence, the two heart tubes alternate between propelling blood at high systolic pressure from the rear to the front in the peristaltic mode and delivering less blood at lower systolic pressure into the segmental circulation in the synchronous mode (Hildebrandt 1988; Krahl and Zerbst-Boroffka 1983; Wenning et al. 2004a; Wenning and Meyer 2007). Hence, at any given time, the leech heartbeat CPG programs two functionally different patterns on the two body sides.
As shown in isolated nerve cords (in vitro), the bursts of the premotor interneurons have a large rear-to-front intersegmental phase difference on the peristaltic side and a small front-to-rear phase difference on the synchronous side (Norris et al. 2011; Wright and Calabrese 2011). These premotor temporal patterns set the limits of the phase difference that the motor neurons can achieve, and the pattern of segment-specific synaptic weights determines what is realized within these limits (Wright and Calabrese 2011). Across individuals, despite high individual variability of synaptic weights and premotor temporal patterns, all motor patterns were functional and either peristaltic or synchronous.
To address the two questions raised above, we examined the individual variation in the motor pattern in vivo to compare it with that in vitro and we examined whether individual variation in the motor pattern is reflected in the beat pattern. To describe the behavior in vivo, we imaged intact adult animals and quantified all intersegmental phase differences between heart segments in midbody segments 7–14. Here the bilateral pairs of corresponding segmental motor neurons are controlled by the same four bilateral pairs of premotor heart interneurons residing in ganglia 3, 4, 6, and 7 (Calabrese 1979). To assess how closely the motor output follows the motor pattern, we determined the phase relation between an individual motor neuron and the constriction of the heart segment it innervates in minimally dissected leeches (in vivo), with any potential sensory feedback and neuromodulatory control still intact. Specifically, we recorded simultaneously the bursting activity of the two ipsilateral heart motor neurons and the movement or emptying of the two ipsilateral heart segments they control and compared our results with those obtained in vitro (Norris et al. 2011). We found that variation in the motor pattern is reflected in motor performance—but only in the peristaltic mode where the intersegmental phase differences of the beat pattern and the motor pattern are positively correlated. The beat patterns in both coordination modes are similar in vivo and in vitro, but the motor patterns are not. In vivo, in the synchronous mode the motor pattern has a distinct front-to-rear progression and little variability compared with that in vitro, suggesting that the motor pattern is constrained in the intact animal.
METHODS
Animals and solutions.
Adult leeches (Hirudo sp.) (Siddall et al. 2007) were obtained from commercial suppliers (Leeches USA, Westbury, NY, or Niagara Medical Leeches, www.leeches.biz/contact) and kept in artificial pond water at 16°C. Prior to all experiments, leeches were cold-anesthetized by immersion in crushed ice for ∼10 min. For dissections and during the electrophysiological experiments, leeches were kept in leech saline (in mM): 115 NaCl, 4 KCl, 1.8 CaCl2, 10 glucose, and 10 HEPES buffer, adjusted to pH 7.4 with NaOH. All electrophysiological experiments were done at room temperature (21–22°C). In what follows, ganglion and segment numbers refer to midbody segments. Body side is indicated by R and L, i.e., HE(R, 8) is the heart motor neuron in segment 8 on the right side and heart(L, 8) is the heart tube section in segment 8 on the left side.
All leeches screened for video imaging were consecutively numbered (1–68), and these numbers (followed by the experimental date) were used to label the 13 individuals used in this study (e.g., leech 13 = L13_6/9/11, leech 26 = L26_6/29/11). The minimally dissected leeches used for electrophysiological and movement recordings were identified by the experimental date.
Video imaging and analysis of filling and emptying of hearts in vivo.
The leeches imaged here were from two batches purchased in the summer of 2011, and 20% of them were transparent enough to be suitable. Leeches were removed from the ice, transferred to a Sylgard-lined clear plastic dish (www.dowcorning.com), and covered with artificial pond water. They were pinned through their anterior and posterior suckers, ventral side up, in a stretched position. For viewing of the hearts, leeches were flattened with a glass plate clamped down to the dish with screws, leaving a thin layer of water around the leech. The dish was transferred to a preparation stand on a vibration isolation table. The leech was illuminated from below with fiber optics, and the light not going through the leech was blocked to make midbody segments 7–14 visible (Fig. 1A). Leeches were prescreened the day before filming to ensure that the skin pigmentation was light enough to visualize the hearts. Any host blood in the crop, which would also interfere with imaging, was removed by lavage through the mouth (for a detailed description see Wenning et al. 1980; Zerbst-Boroffka 1973). The animal was freed and placed in artificial pond water overnight and imaged the next or a later day.
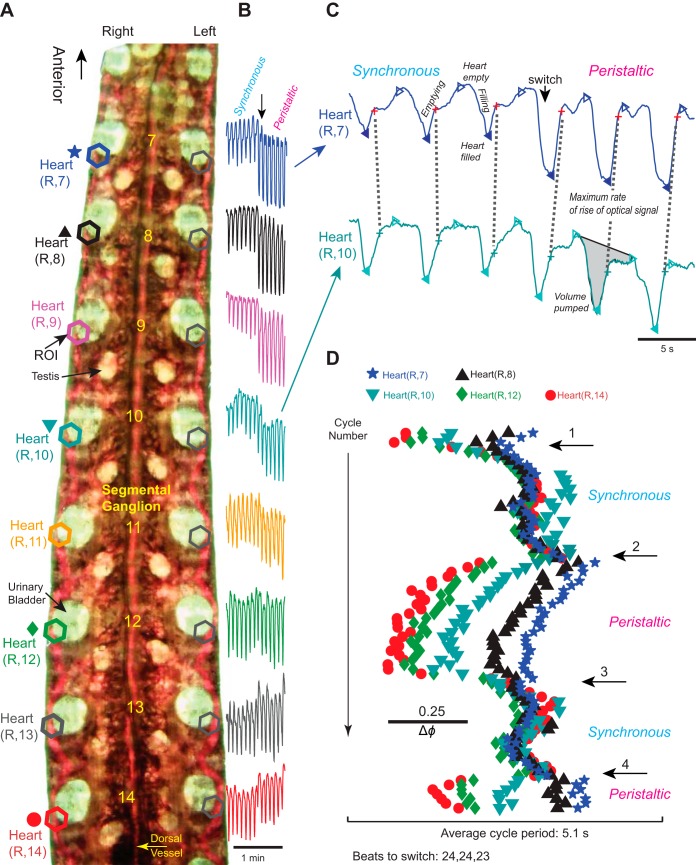
Fig. 1.
Imaging the hearts to analyze the beat pattern of adult leeches. A: midbody portion of a flattened leech, ventral side up and transilluminated (i.e., from below). Regions of interest (ROIs; hexagons) were placed over similar sections of the hearts in segments 7–14 on both sides, yielding 8 pairs of digitized optical signals. Segment numbers are placed along the chain of ganglia. Colors and symbols are shown next to the appropriate heart segment and are also used in B–D. Segments 6 and anterior and segments 15 and posterior are blocked from view. B: digitized optical signals for 14 beat cycles of 8 heart segments on the right body side [heart(R, 7) to heart(R, 14)] across a switch from the synchronous to the peristaltic mode. Note that optical signals in the hearts of segments 10 and anterior increase in size upon the switch from the synchronous to the peristaltic mode; excerpt from a longer record. C: digitized optical signals for 6 beat cycles of heart(R, 7) (top, blue) and heart(R, 10) (bottom, turquoise) across a switch from the synchronous to the peristaltic mode. Excerpt from the record shown in B. The maximum rate of rise (MRR) of the optical signal corresponds to the maximum rate of emptying of the heart segment and is indicated by a cross [red, heart(R, 7); aqua, heart(R, 12)]. Filled triangles indicate maximum fullness, and open triangles indicate maximum emptying of the hearts. Note that heart(R, 7) constricts just before heart(R, 10) during the synchronous mode but considerably later during the peristaltic mode. The volume pumped in a given heartbeat cycle corresponds to the area under the curve between 2 maxima of the optical signal (see shaded area in heart segment 10). D, top to bottom: the actogram shows the temporal relations of the MRR of emptying for the hearts on the right body side in segments 14, 12, 10, 8, and 7 across 4 switches (numbered arrows). The scale bar assists in determining the intersegmental phase differences [average cycle period of the reference segment, heart(R, 14) = 5.1 s = 1 phase unit]. Note that despite beat-to-beat variability of the phase between heart segment emptyings the coordination modes are clearly recognizable. Data from heart segments 13, 11, and 9 were omitted for clarity. Image, signals, and actogram are from the same animal.
Video clips of 10 min were taken for each animal, capturing segments 7–14 simultaneously. We used a Canon Vixia HF200 High Definition Camera (PF30 progressive, 30 f/s, MXP 24 Mbps; http://www.usa.canon.com) (Wenning et al. 2011). The temperature of the water pool around the leeches was ∼25°C by the end of imaging. The whole procedure took ∼40 min. Image processing has been described previously (Wenning et al. 2011). In brief, movies were converted into an AVI format readable by MATLAB (http://www.mathworks.com) with the Emicson MST Converter (http://www.emicsoft.com). Rhythmic filling and emptying of the heart with red blood caused oscillatory changes in the intensity of the transmitted light, and these optical signals were used in our analysis of the heartbeat. We used our own MATLAB code to determine these light intensity changes in user-defined regions of interest (ROIs), which were drawn around the heart segments in each video clip. The average intensity value of each ROI in each frame was recorded and plotted versus the frame number (i.e., the time) in the sequence (Fig. 1, B and C). Absolute values of the digitized signals depended on the size of the analysis windows and on the signal-to-noise ratio of the optical signal obtained in a given heart segment. While comparable for the same heart segment within one experiment, the optical signals were not comparable between different preparations or even between different heart segments of the same preparation.
Previously, we developed a MATLAB code to analyze digitized optical signals (described in detail in Wenning et al. 2004a). The upslope of the optical signal corresponds to emptying and the downslope to filling of the heart (Fig. 1C). We used the maximum rate of rise (MRR) of the optical signal as the phase reference for each beat (see Definitions, color code, statistics). The analysis program calculated the cycle period and the intersegmental phase difference between a given heart segment and our chosen reference segment, the heart segment 14 in the peristaltic heart tube (14p). The cycle period of segment i was defined as the interval (Ti) between the MRR of the optical signals in two consecutive heartbeat cycles of the optical signal obtained from segment i. The phase (ϕ14p-i) of a given heart segment i was determined on a cycle-by-cycle basis and was defined as the difference between the time of the MRR of the optical signal of segment i (ti) and the time of MRR of the optical signal of the reference segment (t14p) in the same cycle divided by the reference cycle period (T14p) expressed as ϕ14p-i = [(ti − t14p)/T14p]. For this study, we used 13 adult leeches. In each animal, four (except for 1 animal for which we had only 3) consecutive switch cycles were analyzed. The number of beats per switch cycle varies little in a given preparation but varies across animals (Wenning et al. 2004a). We used all suitable heartbeats in a switch cycle (up to 31); the accepted minimum was eight consecutive heartbeats per switch cycle (right peristaltic/left synchronous or left peristaltic/right synchronous).
To compare the volume entering and leaving a given heart segment per cycle between the two coordination modes, we measured the area under the curve between two maxima of the optical signal [Fig. 1C; see Wenning and Meyer (2007) for details] using Clampfit (Molecular Devices, http://www.moleculardevices.com) for 7 consecutive heartbeat cycles per coordination mode in each of the 13 adult leeches imaged.
Electrophysiological recording techniques and data acquisition.
Electrodes were pulled on a Flaming/Brown micropipette puller (P-97, Sutter Instruments, http://www.sutter.com) from borosilicate glass (1-mm OD, 0.75-mm ID; A-M Systems, http://www.a-msystems.com). For extracellular recordings, suction electrodes were filled with normal saline and placed in a suction electrode holder (E series, Warner Instruments, http://www.warneronline.com). To ensure a tight fit between cell and electrode, electrode tips were drawn to approximately the diameter of the motor neuron's soma (30 μm). The electrode tip was brought in contact with the cell body, and light suction was applied until the entire cell body was inside the electrode. Extracellular signals were monitored with a differential AC amplifier (model 1700, A-M Systems) at a gain of 1,000 with the low- and high-frequency cutoffs set at 100 and 1,000 Hz, respectively. Noise was reduced with a 60-Hz notch filter. A second amplifier (model 410, Brownlee Precision, http://www.brownleeprecision.com) amplified the signal appropriately for digitization.
Data were digitized (>5-kHz sampling rate), either with a digitizing board (Digi-Data 1200 Series Interface, Molecular Devices) and acquired with pCLAMP software (Molecular Devices) on a personal computer or digitized and acquired with PowerLab (http://www.adinstruments.com/products/data-acquisition).
Recording bursting activity of heart motor neurons and movement in corresponding heart segments in vivo.
Leeches were pinned, ventral side up, in a stretched position in a Sylgard-lined dish and covered with leech saline. To keep the leech cold during the dissection, ice cubes made of saline were added as needed. The goal of the following dissection protocol was to expose the hearts in segments 8 and 12 as well as to obtain access to the ipsilateral heart motor neurons in the same segment. Figure 2 shows the preparation for one segment.
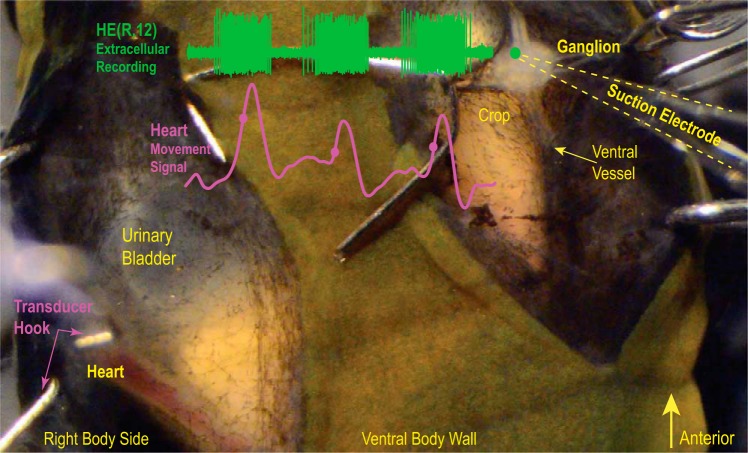
Fig. 2.
Minimally dissected leech. Ventral view of segment 12. Small hooks on both sides held the preparation in place. The exposed ganglion is seen on the right, with the green dot showing the approximate position of the HE heart motor neuron in the ganglion. The bursting activity of the heart (HE) motor neuron was recorded extracellularly (green trace), and heart movements were recorded with a movement transducer (pink trace). Filled circles indicate the MRR of the movement signal. The hook of the movement transducer cradles the heart. Note that the heart is filled with blood.
An incision was made along the ventral midline extending over one segment (5 annuli) with broken pieces of razor blades. The incision was held open by small hooks, two on each side, made of minuten pins. Muscles and connective tissue were removed until the ventral vessel, which encompasses the segmental ganglion, became visible. The hooks on the right side were removed. A second incision was made along the right lateral black stripe, this time starting at the annulus anterior to the central annulus and continuing posterior into the first annulus of the adjacent segment, held open by a pair of hooks inserted into the right body wall. Thus the two incisions in that segment were separated by a thin strip of body wall. Muscle and connective tissue were removed until the lateral heart tube became visible. Care was taken to leave enough connective tissue to prevent the heart from bulging, which disrupts heart constrictions (Wenning et al. 2004a). A longitudinal cut through the ventral vessel exposed the segmental ganglion. Two small hooks, one on each side, were inserted into the vessel lining near the segmental nerve roots to stretch and stabilize the ganglion. Next, the ventral glia sheath was removed. The second segment was prepared the same way. The preparation dish was transferred to the recording setup.
We used two methods to assess constriction-relaxation and emptying-filling of the hearts: movement recordings and imaging, respectively. For movement recordings, a small hook (made from a minuten pin) was placed underneath the heart so that it cradled the heart tube (Fig. 2). The hook was attached with thread to a displacement (semi-isotonic) transducer (model 1040, UFI, www.ufiservingscience.com). The hook and the transducer were lifted just enough to overcome the slack of the thread. A second hook and transducer were used for the other heart segment. When the movement signals were satisfactory, extracellular suction electrodes were placed over the ipsilateral heart motor neuron in segment 8 [the HE(8) heart motor neuron] and the HE(12) motor neuron to record spike activity. We were able to record both hearts and both motor neurons in eight preparations. In seven of these preparations we recorded both coordination modes, and in one we recorded only the synchronous mode. Movement recordings lasted an hour, whereas extracellular recordings lasted 25 min. The analysis is described below.
For imaging, we captured videos from the hearts in both exposed segments with a HD camera (NIKON D5100, www.nikonusa.com) mounted on the dissection scope (WILD M5, Heerbrugg). Optical signals were processed as described above. We obtained good recordings from the motor neurons and clear optical signals from the hearts in both coordination modes in two preparations.
Analysis of heart motor neuron bursting activity and heart constriction-relaxation.
We used a custom-made MATLAB code (detailed in Norris et al. 2007) to characterize the HE motor neuron activity. Spikes were detected based on threshold. For period, duty cycle, and intraburst spike frequency, spikes were grouped into bursts with an interburst interval of ≥1 s. To eliminate the effects of occasional stray spikes, groups of fewer than five spikes were not considered as bursts. We then calculated burst period (T), phase (ϕ), duty cycle (D), and intraburst spike frequencies. The burst period (T) was defined as the interval in seconds from middle spike to middle spike of consecutive bursts. The HE heart motor neuron of segment 8 served as the phase reference (ϕ = 0). The phase of the HE heart motor neuron of segment 12 was determined on a cycle-by-cycle basis and was defined as the difference between the time of its middle spike (ti) and the time of the middle spike of the reference segment (t8) in the same cycle divided by the reference cycle period (T8) expressed as ϕ8-i = [(ti − t8)/T8]. Phases calculated for individual experiments were then averaged across preparations to obtain the mean phase.
To calculate the duty cycle and for the purpose of phase box plots, we determined the mean phase of the first and last spike of the heart motor neuron bursts, as described for the middle spike. The mean duty cycle (D) is the difference between the mean first and the mean last spike phase and is displayed as a box in the phase diagrams subsequently described. The vertical line that bisects each phase box near its midpoint indicates the middle spike phase for each HE heart motor neuron. The beginning and end of each box indicate the average phase of the first and last spikes, respectively, in a series of bursts relative to the middle spike phase of the absolute phase reference cell. Error bars indicate the SD around the mean first, middle, and last spike phase in a burst. Finally, each interspike interval in a burst was converted to a frequency (reciprocal). These frequencies were binned at 0.02 phase intervals (using the middle spike of that burst as a reference) and subsequently averaged across bursts to obtain a spike frequency profile for each HE heart motor neuron in each coordination mode.
The same section of the recording used for the analysis of the heart motor neurons was used to analyze the movement signals. The upslope of the movement signal corresponds to constriction and the downslope to relaxation of the heart (Fig. 2). To extract the low-frequency movement signals, the signals were low-pass filtered (Gaussian; ∼3 Hz). We used the same MATLAB code and method described above for the optical signals to identify the point in time corresponding to the MRR of the movement signal, our reference point (see Definitions, color code, statistics). To construct the heart duty cycles, we used three additional time points: the first time point corresponds to a noticeable upslope in movement leading to the peak, the second time point is the peak, and the last time point corresponds to a noticeable downslope in the movement signal from the peak. We determined the phase difference of all four time points (beginning of the upslope, MRR of the movement signal, its peak, end of the downslope) cycle by cycle, with respect to the middle spike of the corresponding burst of the phase reference, the HE(8) heart motor neuron (see above). The mean duty cycle of the heart's constriction-relaxation is the phase difference between the beginning of the upslope and the end of the downslope of the movement signal and is displayed as a box in the phase plots. The thicker vertical line that bisects each phase box near its midpoint indicates the phase of the peak of the movement signal. The thin vertical line between the beginning of the upslope and the peak indicates the phase of the MRR of the movement signal. Error bars indicate SD.
We analyzed all suitable movement signals in a given switch cycle (11–27). The phase plots show the mean duty cycles of the constriction as well as those of the HE heart motor neuron bursts. The phase reference was the HE(8) heart motor neuron.
Aligning optical and movement signals in vivo.
In two experiments, we recorded movement signals in the hearts of segments 8 and 12 while simultaneously imaging one heart with our HD camera (see above) mounted on a stereomicroscope (WILD M5). To synchronize the movement recordings with the image data, we recorded a few brief DC pulses along with the transducer signals and played them into the audio channel of the HD camera. Analysis was as described above.
Data presentation.
Actograms (e.g., Fig. 1D) illustrate the phase relationships between events over time and were based on raster presentations similar to those used to display circadian activity patterns (Peterson and Calabrese 1982; Pittendrigh 1974). In our actograms, each symbol represents either the time of the middle spike of a burst in a heart motor neuron or, in a given heart segment, the MRR of either the movement or the optical signals. The reference period of the actogram is the mean cycle period (x-axis) of the phase reference for the stretch of record chosen. This record was then broken into a series of segments of this length that were arranged sequentially, one below the other (y-axis). Cycle periods equal to the reference period result in symbols that fall along a vertical line, while for shorter periods points drift to the left and for longer periods points drift to the right.
In the box-and-whisker plots (e.g., Fig. 3A), the median is represented by a thick black vertical line and the first and third quartiles are the left and the right edge of the box, respectively. The horizontal lines represent the range between the lowest (left) and highest (right) data point within 1.5 times the interquartile range (IQR). Data points outside 1.5IQR are marked as outliers (crosses).
Fig. 3.
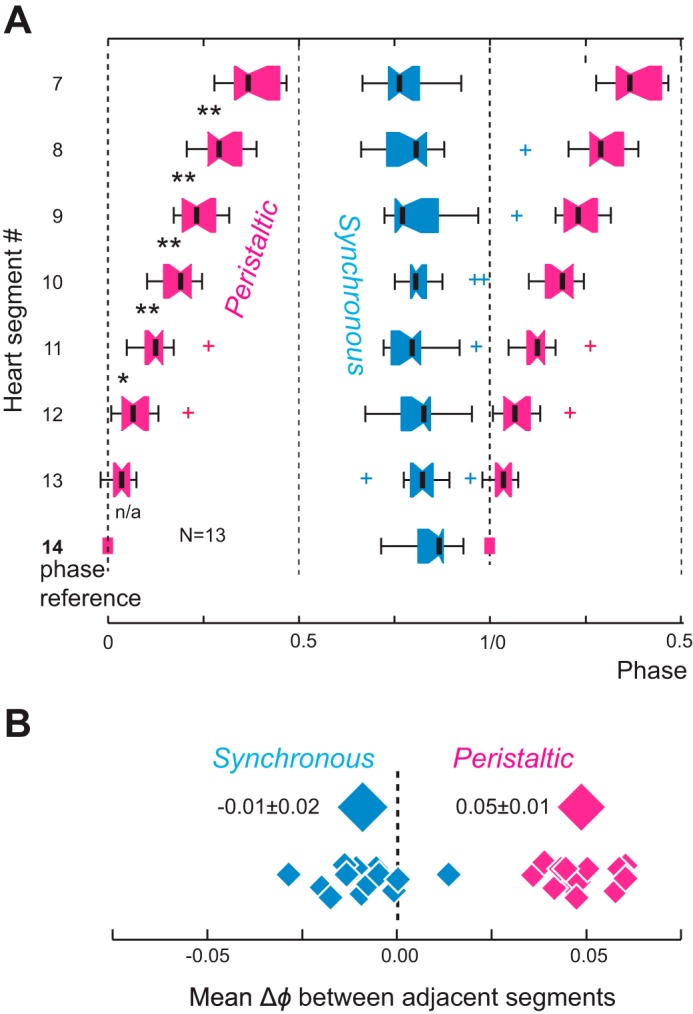
Phase relations for the MRR of emptyings between the hearts of midbody segments 7 to 14 in intact adult leeches (video imaging). A: box-and-whisker plot (see methods for details). The phase marker is heart(14) in the peristaltic mode (0 phase). Pink, peristaltic mode; blue, synchronous mode. Note that the slopes of phase progressions differ in the 2 coordination modes. There is a distinct, almost linear rear-to-front phase progression of emptyings in the peristaltic mode and, on average, no phase progression of emptying in the synchronous mode. Note that variability across animals is higher in the synchronous mode than in the peristaltic mode. n = 13 animals; 3–4 switch cycles per animal. Asterisks denote significance levels between adjacent segments (*P ≤ 0.05, **P ≤ 0.01); n/a, not applicable. B: mean phase differences (Δϕ) between adjacent segments for all 13 experiments in the synchronous and the peristaltic coordination mode. The diamonds above each distribution represent the average phase difference (±SD) between adjacent segments across all preparations. Note that across animals, the average phase differences of emptyings between adjacent segments do not overlap in the 2 coordination modes.
Definitions, color code, statistics.
Specific points in time were identified and used to establish the phase relations between the HE heart motor neuron burst and the hearts' action. For the HE bursts we used the middle spike (Cymbalyuk et al. 2002; Norris et al. 2007). When imaging the hearts we used the MRR of the optical signal, which corresponds to emptying and heart constriction (Fig. 1C). For recordings with a movement transducer we used the MRR of the movement signal, which also corresponds to emptying and constriction (Fig. 2). We refer to these points as “HE motor neuron burst,” “MRR of emptying,” and “MRR of movement.”
We used our standard color code to indicate coordination modes (pink, peristaltic; light blue, synchronous). Black was used for the heart motor neuron and heart of segment 8 and green for the HE motor neuron and heart of segment 12.
Average data are reported as means (SD). We used the following tests for statistical significance: Student's t-test, Levene's test, one-way repeated-measures ANOVA coupled to a Holm-Sidak post hoc multiple-comparisons test, and a Wilcoxon signed-rank test. Tests are specified when used in the text. The significance level was set at P ≤ 0.05.
RESULTS
Filling and emptying of hearts in adult leeches in vivo.
To study the variability of the beat pattern in vivo, we quantified the pattern of filling and emptying of the hearts of midbody segments 7 to 14 in intact adult animals. We imaged the hearts on both sides over three to four switch cycles (i.e., the time 1 side spends in 1 coordination mode) between the two modes (∼10 min; Fig. 1). We analyzed the heart section just posterior to the urinary bladder to monitor blood entering and leaving that heart segment (hexagons in Fig. 1A).
Our results corroborated earlier results from imaging juvenile leeches, where we showed that blood volume in anterior heart segments is larger in the peristaltic mode than in the synchronous mode, as indicated by an increase in amplitude of the optical signals (Wenning and Meyer 2007). Figure 1B shows this increase of the optical signals clearly in heart segments 7–9 across the switch from the synchronous to the peristaltic mode. The hearts of segments 15 to 12 constrict with a very small phase progression (Fig. 3A; Wenning et al. 2011), sending a column of blood forward that is augmented in every segment by the afferent vessels. We quantified this difference of blood volume between coordination modes in heart segments 8 and 12 (the focus of the second part of this study) in the 13 animals imaged. The volume pumped by heart segment 8 in the synchronous mode is significantly less than that of the same heart segment in the peristaltic mode (average 62 ± 23%; P ≤ 0.001, paired t-test), while the volume pumped by heart segment 12 does not differ in the two modes (98 ± 35%; P ≤ 0.86, paired t-test).
Figure 1C shows the digitized optical signals for heart segments 10 and 7 on the right body side [referred to as heart(R, 10) and heart(R, 7), respectively] across a switch in coordination modes. During the rise of the optical signal blood is leaving the heart section imaged, and during the fall of the optical signal blood is entering the heart section imaged (Wenning et al. 2004a). We used the MRR of the optical signal as the phase reference for the heart's emptying (see methods). The recording of Fig. 1C starts in the synchronous mode with heart(R, 7) emptying just before heart(R, 10) (mean Δϕ = −0.06 ± 0.06). After three beats the pattern switches to the peristaltic mode with heart(R, 10) emptying before heart(R, 7) (mean Δϕ = +0.24 ± 0.02). The actogram of Fig. 1D shows—beat for beat—the MRR of emptying in five heart segments (of the 16 segments imaged as indicated on Fig. 1A) of the right body side across four switches. The first two beats are in the peristaltic mode with heart(R, 14) emptying first, followed by the hearts in segments 12, 10, 8, and 7 in a rear-to-front progression. Then the beat pattern switched into the synchronous mode, and heart segments 14, 12, 10, 8, and 7 now emptied almost simultaneously. There was beat-to-beat variability [e.g., in heart(R, 10) in the synchronous modes between switches 1 and 2], yet emptying between segments 7 and 14 either progressed clearly rear to front (peristaltic mode; mean Δϕ for the 4 switch cycles = +0.42 ± 0.01) or occurred almost simultaneously (synchronous mode; mean Δϕ = −0.04 ± 0.02) (Fig. 1C).
Figure 3A shows averaged data for the 13 animals used in this study. In the peristaltic mode there was a significant segment effect on phase among seven segments (segment 14 was excluded because it is the reference segment and has a value of 0) (1-way repeated-measures ANOVA; F6,72 = 143.628, P ≤ 0.001). Moreover, with the exception of the phase difference between segments 13 and 12, the phase of each segment differed significantly from the phase of all other segments (P ≤ 0.03; Holm-Sidak multiple-comparisons test). When in the synchronous mode, phase differences did not differ between adjacent segments. Taken together, hearts emptied rear to front when in the peristaltic mode with an average phase difference of 0.05 ± 0.01 per segment. Hearts did not show monotonic phase progression when in the synchronous mode, and the variability of the phase differences between adjacent segments was higher (−0.01 ± 0.02 per segment) (Fig. 3B).
Across animals, variability in the range of the intersegmental phase relations of the emptying for all heart segments was quite large, as illustrated in Fig. 4 for all 13 animals imaged in this study. There were a few exceptions to the rear-to-front progression in the peristaltic mode. For example, in preparation L28_6/14/11, heart(12) and heart(13) emptied just before heart(14). Aberrations from the usual pattern could occur just on one body side, as in preparation L37_6/17/11, where heart(R, 10) emptied later than all other segments in the synchronous mode, or on both sides, as in preparation L68_7/19/11, where heart(8) and heart(9) emptied much later than all the other segments in the synchronous mode (L68 is the outlier in the synchronous mode in these segments; see Fig. 3A), suggesting that it is the CPG and/or the heart motor neurons setting up this unusual output pattern. The mean phase differences between heart segments 8 and 12 varied (Fig. 4) yet were significantly different from each other in the two modes (peristaltic mode: +0.20 ± 0.07, synchronous mode: −0.02 ± 0.12; Student's t-test P ≤ 0.001).
Fig. 4.
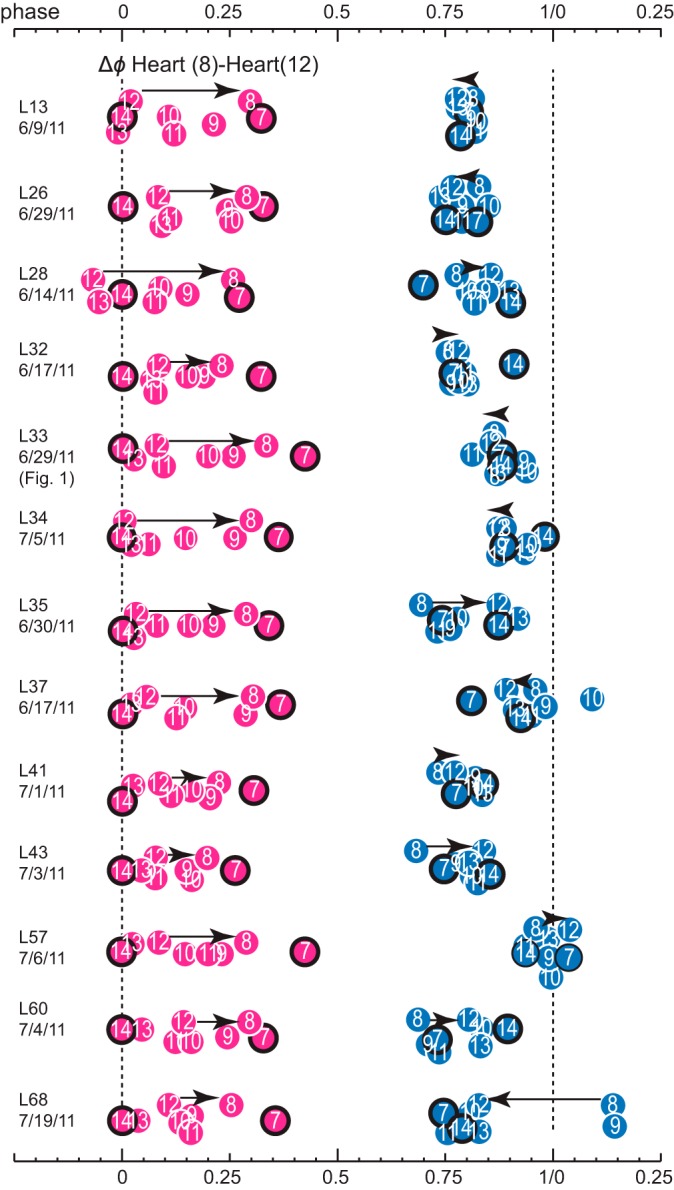
Variability of intersegmental phases of the MRR of emptyings in 13 different animals in both coordination modes (pink, peristaltic; light blue, synchronous). Animals were numbered in the order they were imaged from a batch of 68 leeches (L13, L26…, followed by the experimental date; see methods for details). Numbers in circles refer to the heart segment; anterior and posterior-most segments (7 and 14) are outlined in black. Phase reference (0 phase) is segment 14 in the peristaltic heart tube. Each circle represents the mean phase of the MRR of emptyings of all beats in a single switch cycle per animal. Black arrows connect heart segments 8 and 12 and illustrate the direction of the progression of emptyings. Note the clear rear-to-front progression in the peristaltic mode and the very variable phase differences and direction of progression of emptyings in the synchronous mode. For example, in preparation L68 (leech 68) emptyings in heart(8) and heart(9) occur later than in all other segments (L68 is the outlier on Fig. 3 for heart segments 8 and 9, synchronous mode). L33 is the preparation shown in Fig. 1.
Across the 13 animals, the two hearts switched reciprocally during the 40 switch cycles that we examined bilaterally (data not shown), confirming previous findings from blood pressure recordings of minimally dissected leeches and from imaging intact juvenile and adult leeches that the motor output in vivo, i.e., the beat pattern, is bilaterally symmetrical with reciprocal switches in coordination mode (Krahl and Zerbst-Boroffka 1983; Wenning et al. 2004a, 2011). To determine whether the phase progressions of emptying in the two hearts were equivalent in each coordination mode in a given animal, we examined the phase difference of the MRR of emptying between heart(8) and heart(12) on the two sides and in both modes (Fig. 5). Across all individuals, there was a strong correlation with a slope near unity in the phase difference between heart(12) and heart(8), suggesting that the hearts of these segments on the two body sides produced equivalent phase progressions during both coordination modes (Fig. 5; slope: 0.96, R2 = 0.78).
Fig. 5.
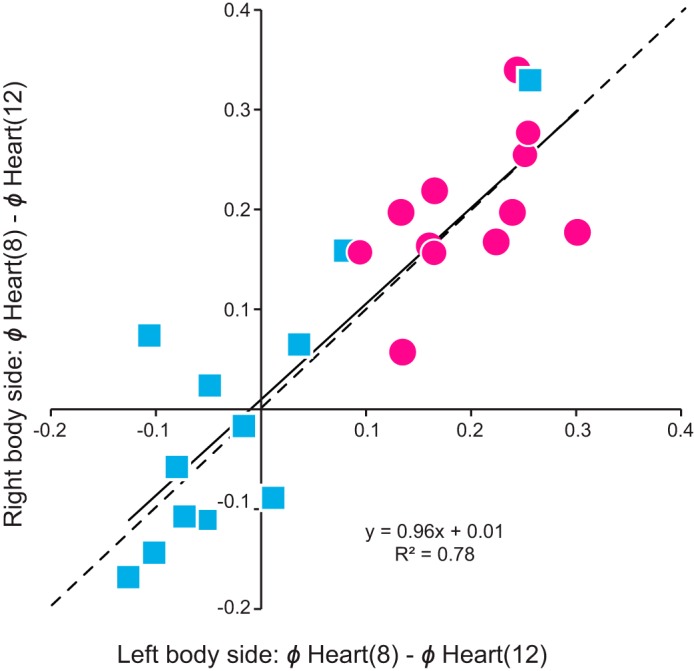
The beat pattern of the hearts in imaged adult leeches is bilaterally symmetrical. The mean phase differences of the MRR of emptyings between heart segments 12 and 8 on the right body side are plotted over those on the left body side for the peristaltic (pink circles) and synchronous (blue squares) coordination modes. Note that there is no preference for body side: data fall on both sides near the (dashed) identity line (i.e., perfect symmetry), and the slope of the (solid) fitted line is almost 1.
Aligning optical and movement signals in vivo.
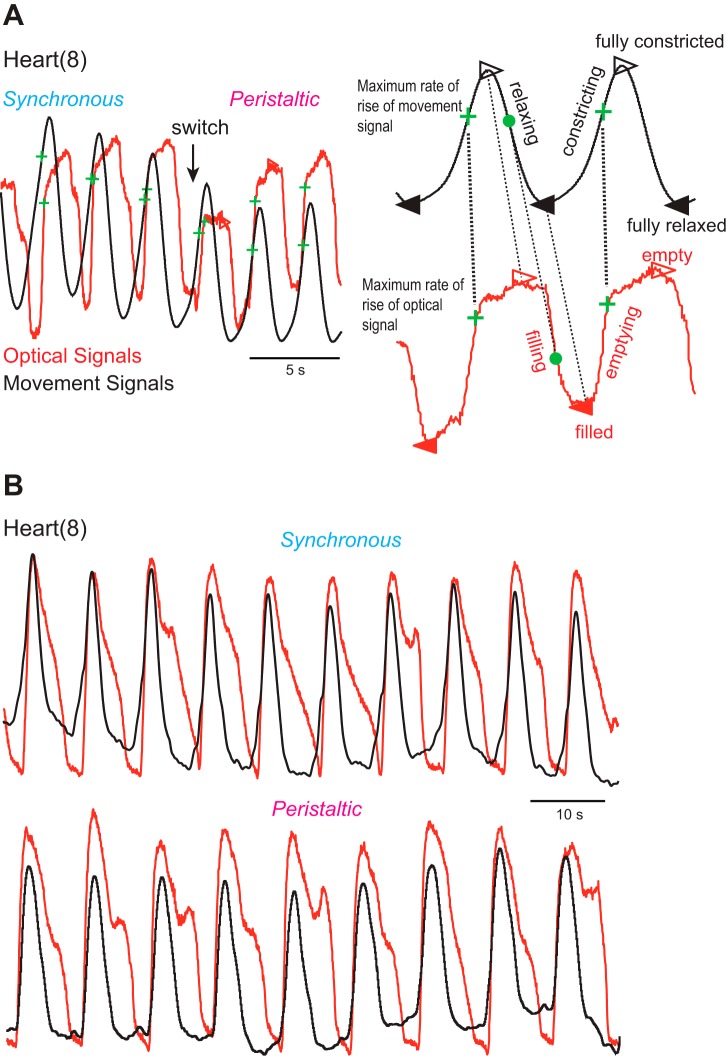
To compare the motor pattern and the motor output (heart constriction) in vivo (see Aligning motor pattern with motor output in vivo), we employed both imaging and movement recordings. Optical signals indicate filling and emptying of blood in the heart segment analyzed, while movement recordings reflect constriction and relaxation of the heart (see methods). To determine how these two signals aligned, we recorded heart movements and optical signals simultaneously. We exposed two ipsilateral heart segments (8 and 12) to assess the coordination mode. As shown in Fig. 6, the optical and movement signals aligned nearly perfectly during the upstroke (i.e., blood leaving and constriction of the heart segment). Conspicuously, filling (optical) was delayed with respect to relaxation (heart movement), and the hearts stayed devoid of blood for some period of time (Fig. 6). We noticed this prolonged lack of blood in the hearts after emptying in previous imaging studies (see Fig. 3 in Wenning et al. 2004a; Wenning and Meyer 2007). Delayed filling might simply be an artifact due to flattening of the animals for imaging, or it might result from the heart muscles still constricting or from lack of filling after the heart muscles relaxed. The minimally dissected leeches used here were not flattened, and their hearts relaxed as seen from the movement recordings, yet hearts did not fill immediately and remained devoid of blood (Fig. 6). These results show that delayed filling is not an artifact but rather that heart relaxation is not sufficient for filling, and further suggest that blood flow in the hearts is discontinuous.
Fig. 6.
Alignment of optical signals (red traces) and movement signals (black traces) in minimally dissected animals. Optical and movement signals were vertically matched in size to facilitate comparison. A: recordings are across a switch from the synchronous to the peristaltic mode in heart(8). The 2 expanded beat cycles on right show the 4 time points that the analysis code identified for the movement and the optical signals, respectively: filled triangles correspond to the trough (i.e., a relaxed and filled heart), open triangles to the peak (i.e., a constricted and empty heart), green crosses to the MRR (i.e., constricting and emptying), and filled circles to the maximum rate of decay (i.e., relaxing and filling). Note that constricting (upstroke of the movement signal) and emptying (upstroke of the optical signal) align well and that the MRR of the movement signal corresponds to the MRR of the optical signal. B: same experimental design, different animal, heart(8). Note that optical and movement traces align perfectly during constricting and emptying in both coordination modes but that filling is delayed and the heart remains empty for some time while relaxing.
The near-perfect alignment of imaging and movement recordings during emptying and constriction made it possible to use recordings interchangeably but required a common phase reference. We identified and compared four corresponding points in time for both signals (Fig. 6A). 1) The MRR of either signal corresponds to heart constriction and the hearts' emptying of blood. 2) The maximum rate of decay corresponds to heart relaxation (movement recordings) and the hearts filling with blood (optical signal). 3) The trough corresponds to a relaxed and maximally filled heart. 4) The peak corresponds to a maximally constricted and empty heart. The MRR of the optical and the movement signal aligns well for both signals and was chosen as the phase reference (Fig. 6A). Hence, optically recorded emptying is a faithful proxy for the constriction recorded with the movement transducer but not the relaxation, and these two methods for assessing heartbeat were both used in the analysis below.
Aligning motor pattern with motor output in vivo.
In the second part of this study, we determined whether the variability in the motor pattern is reflected in the beat pattern, focusing on heart segments 8 and 12. This association between heart motor neuron activity and the electrical activity of the ipsilateral heart segment had been studied previously in small heart tube sections (1 or 2 segments) left innervated in one segment by the nerve cord (Calabrese and Maranto 1984; Maranto and Calabrese 1984b). Using an isometric force transducer, those authors found that each heart motor neuron burst led to a tension signal in the heart and that, when recording intracellularly from the heart muscle cells, each spike in a heart motor neuron elicited an excitatory junction potential in the heart muscle cells. We used an in vivo preparation (minimally dissected leeches; Fig. 2) to quantify the phase relations between the heart motor neurons of segments 8 and 12 and the constriction in the corresponding heart segments. Exposing the ganglia required opening the ventral vessel, but since blood pressure is low in this vessel (Hildebrandt 1988) bleeding stopped before the recording started. As shown in Fig. 2, the heart was filled with blood. In eight preparations we recorded heart movement in both segments and the bursting activity extracellularly of both heart motor neurons, in seven of these in both coordination modes and in one only in the synchronous mode. In two experiments, we imaged emptying and filling both heart segments (8 and 12) and recorded extracellularly from both heart motor neurons in both coordination modes. The results presented here were combined from these 10 preparations, although only emptying in the imaged preparations is relevant to the constriction cycle of the heart and thus heart relaxation and duty cycle could not be assessed in these two preparations. To determine phase relations, we used the MRR of constriction (see above) as the phase reference for the hearts and the middle spike of the heart motor neuron burst as the phase reference for the motor neuron bursts (Cymbalyuk et al. 2002; Norris et al. 2007). We chose the middle spike of the HE(8) motor neuron as the absolute phase reference (assigned 0 phase).
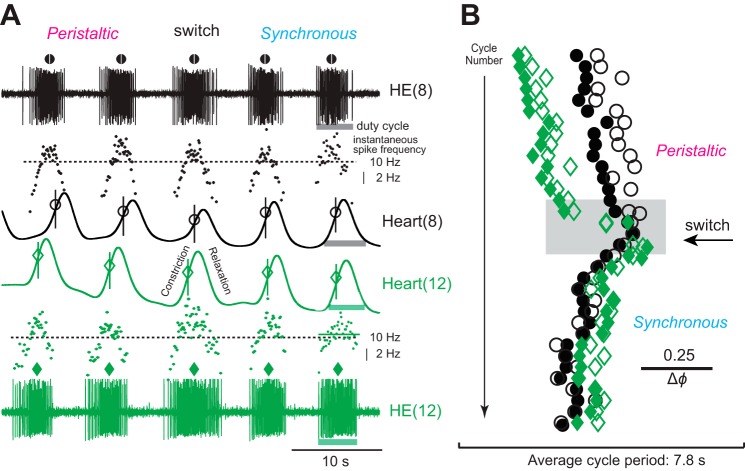
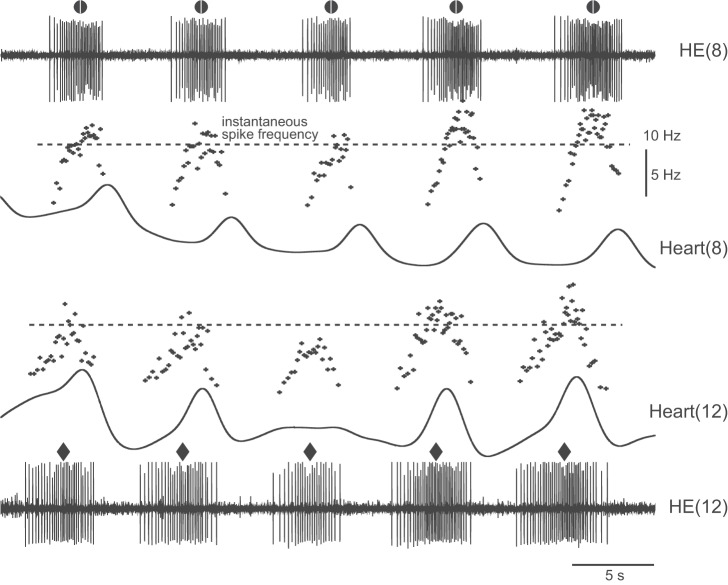
Figure 7A shows the activity of the HE(8) and HE(12) motor neurons, their intraburst frequencies, and the constrictions in the corresponding hearts across a switch in coordination mode. The MRR of constriction occurred around the middle spike of the motor neuron burst, which was usually around the highest intraburst spike frequency. In this preparation, heart(8) relaxed after the HE(8) motor neuron stopped firing, while heart(12) relaxed while the HE(12) motor neuron was still firing (Fig. 7A). The actogram of Fig. 7B shows the beat-to-beat variability in the intersegmental phase differences between the motor neurons, between the hearts, and between a heart motor neuron and its corresponding heart, yet the patterns were distinctly rear-to-front peristaltic or synchronous. The switch between coordination modes occurred concurrently in the heart motor neurons and the hearts (Fig. 7).
Fig. 7.
Extracellular recordings from 2 ipsilateral heart motor neurons and movement recordings of the corresponding heart segments in vivo (minimally dissected leeches). A: 5 bursts of the heart motor neurons in segments 8 [HE(8), black] and 12 [HE(12), green] across a switch from the peristaltic to the synchronous mode. Symbols above each motor neuron burst denote its middle spike [HE(8), circle; HE(12), diamond]. Instantaneous spike frequency is shown for each burst [HE(8), black dots; HE(12), green dots]. Dotted horizontal lines correspond to 10 Hz. Movement signals in heart(8) (black) are shown below the appropriate motor neuron burst and those of heart(12) (green) above. The MRR of movement is indicated for each beat [heart(8), circle; heart(12), diamond; vertical lines added for visual clarity]. Light-colored bars indicate the duty cycles for the motor neurons and for the heart segments (segment 8, gray; segment 12, green). In the peristaltic mode, the HE(12) heart motor neuron fires before the HE(8) heart motor neuron and heart(12) constricts before heart(8), while both motor neurons and both hearts are active almost simultaneously in the synchronous mode. Note that the MRR of movement coincides with the peak spike frequency of the corresponding heart motor neuron. Note also that hearts may begin to relax even while the motor neuron is still firing (e.g., in segment 12). B: actogram shows the phase relations of the middle spike of the motor neurons and the MRR of movement over many beat cycles across a switch from the peristaltic to the synchronous coordination mode (colors and symbols as in A). Note the cycle-to-cycle variability in the phase relation between the middle spike of a heart motor neuron and the MRR of movement in the corresponding heart segment. Note also that the intersegmental phase differences between the 2 motor neurons and those between the hearts of segments 8 and 12 vary. The gray box outlines the section of the recording shown in A.
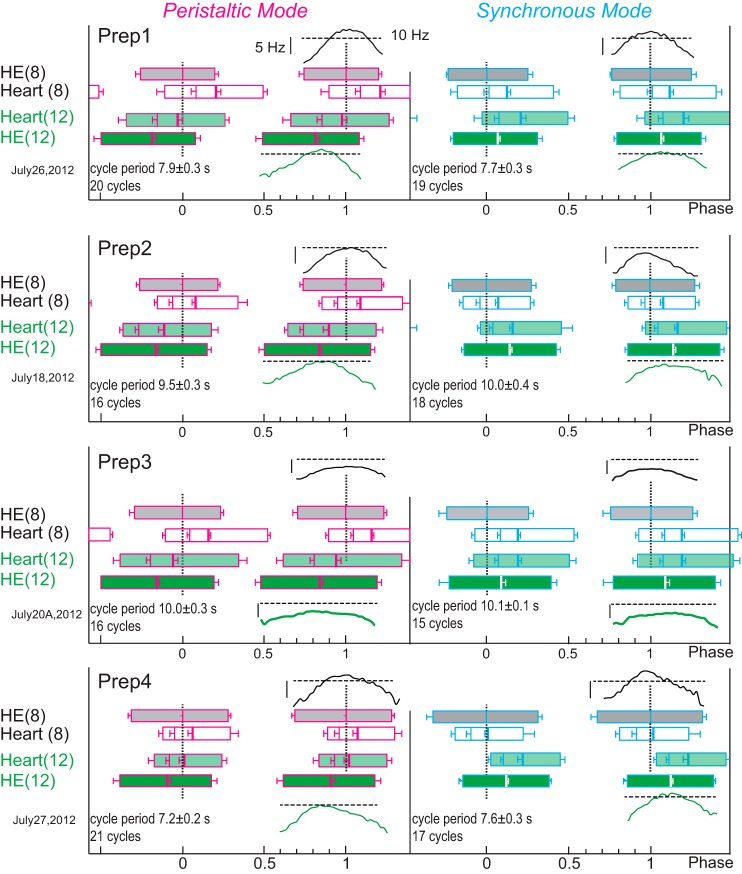
The phase plots of Fig. 8 show the average duty cycles (i.e., first to last spike for the motor neurons; start and end of the heart constrictions; see Fig. 7A), the middle spike for the motor neurons, the MRR of the constriction, and its peak of the hearts for four preparations in both coordination modes. All motor patterns and all constriction patterns were either rear-to-front peristaltic or almost synchronous. Across preparations, the MRR of constrictions aligned roughly with the middle spike, where the instantaneous intraburst spike frequency was high (Fig. 9A). The first spike of a motor neuron burst was much worse in predicting the timing of the MRR of constriction than the middle spike and resulted in wider scatter (analysis not shown). Instead, the middle spike was used as a measure of motor neuron activity because it aligned closely with constriction. There were no differences in the intraburst spike frequencies (mean or maximum) between coordination modes or segment number. Across all preparations (both heart motor neurons, both modes), the MRR of constriction occurred at a firing rate of 9.2 ± 2.1 Hz (range 6.0–15.3 Hz) in the corresponding motor neuron, which was between the mean intraburst spike frequency (6.9 Hz, range 4.9–9.1 Hz) and the maximum intraburst spike frequency (10.0 ± 2.0 Hz, range 6.7–15.3 Hz) (Fig. 8). In 14 of the 16 heart segments examined (2 per experiment) relaxation began while the heart motor neuron was still firing (e.g., preparations 2 and 3 of Fig. 8) except for two preparations where the relaxation of heart(8) began at the end of the motor neuron's burst (e.g., in the experiment shown in Fig. 7 and in preparation 1 of Fig. 8).
Fig. 8.
Phase plots from 4 different in vivo preparations (Prep1–Prep4; minimally dissected) to show the duty cycles for the heart motor neurons [HE(8), gray; HE(12), dark green] and for the corresponding heart segments [heart(8), white; heart(12), light green] in both modes (peristaltic, pink outlines; synchronous, blue outlines). Thick vertical lines in the HE and heart boxes correspond to the middle spike of the heart motor neurons and to the peak constriction in the heart segments, respectively; left edges are the first spike (motor neurons) and the start of constriction (heart segments); right edges are the last spike (motor neurons) and end of the relaxation (heart segments); the MRR of constriction is shown as the thin line between the start of the constriction and its peak (all with SD). Phase marker is the HE(8) motor neuron (0 phase). Intraburst instantaneous spike frequencies (binned in 0.02 phase intervals) are centered around the middle spike for the corresponding motor neuron and are placed above the HE(8) motor neuron and below the HE(12) motor neuron. The horizontal dashed line corresponds to 10 Hz and the vertical bar to 5 Hz. The cycle period (±SD) and the number of cycles analyzed are given for each plot. Prep1 is that shown in Fig. 7. Note that all patterns are clearly rear-to-front peristaltic or synchronous (i.e., nearly simultaneous). Note also that the MRR of constriction varies with respect to the middle spike of its motor neuron, as does the phase difference realized by motor neurons and hearts.
Fig. 9.
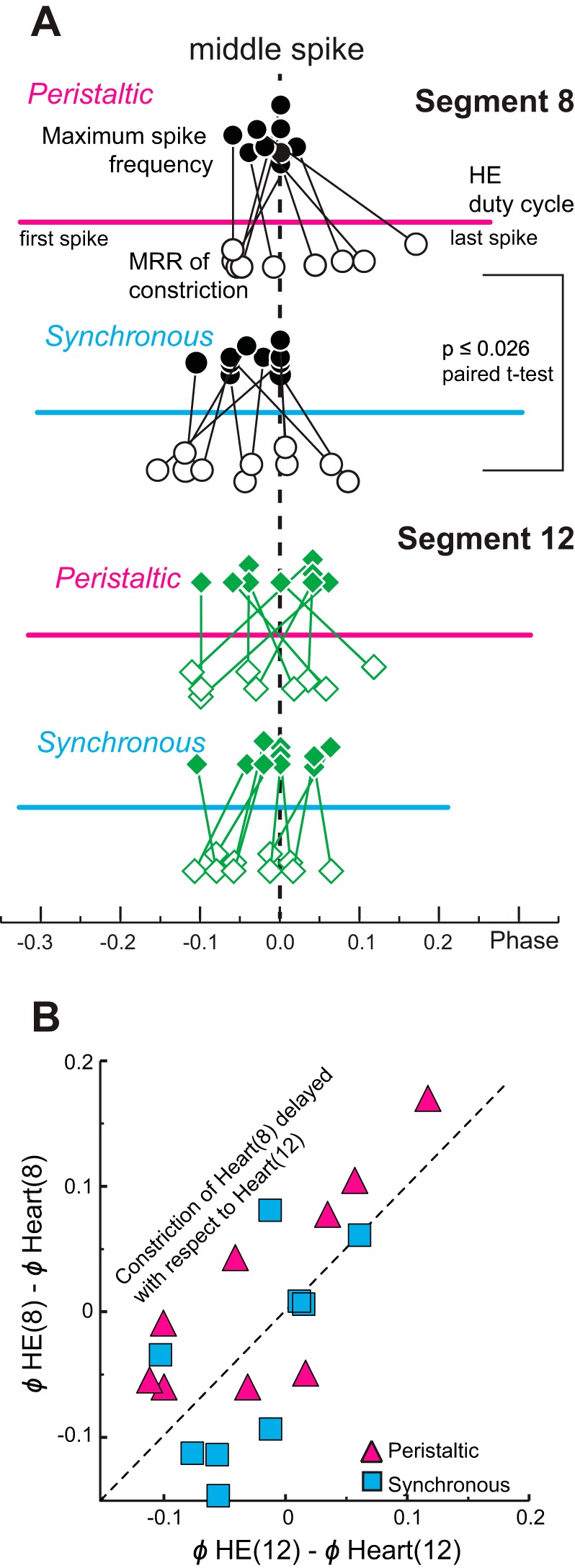
Phase relations between the heart motor neurons' firing rates and the MRR of constrictions in vivo (minimally dissected animals). A: phase relation of the maximum intraburst spike frequency and the MRR of constriction for heart segments 8 (top; circles) and 12 (bottom; diamonds) with respect to the middle spike of the corresponding heart motor neuron (dashed vertical line). The phases of the maximum intraburst spike frequency and the MRR of constriction for each individual experiment are connected by lines. Thick horizontal lines represent the average duty cycle (from first to last spike) across experiments of each heart motor neuron in each mode. The maximum intraburst spike frequency (filled symbols above the duty cycle) and the MRR of constriction (open symbols below the duty cycle) cluster around the middle spike of the corresponding heart motor neuron burst for both coordination modes and both segments. Note that the MRR of constriction of heart(8) occurs significantly later with respect to the middle spike in the peristaltic than in the synchronous mode (paired t-test). B: the phase differences between the middle spike of the HE(8) motor neuron and the MRR of constriction of heart(8) are plotted over the phase differences between the HE(12) motor neuron and the MRR of constriction of heart(12) for the peristaltic (pink triangles) and the synchronous (blue squares) coordination modes for the same experiments. The unity line represents equal phase differences in both segments. Note that in the peristaltic mode constriction of heart(8) is delayed with respect to the middle spike of the HE(8) heart motor neuron in 7 of the 9 preparations.
In reduced preparations (Calabrese and Maranto 1984) and in this study, robust bursts in a heart motor neuron reliably caused a constriction in the ipsilateral heart segment. The question arises whether the opposite is true—whether weaker bursts with fewer spikes and hence lower intraburst spike frequencies will result in a missed beat in the corresponding heart segment. Considering that we recorded on average 138 heartbeat cycles per experiment (range: 36–229), skipping a beat was a rare event: a single beat was missed in one heart segment in three preparations of the eight in which movements were recorded; in one preparation a beat was missed in both heart segments simultaneously. One example is shown in Fig. 10, where one burst of the HE(12) motor neuron had a low intraburst frequency (albeit a similar duty cycle) and the heart did not constrict. Conspicuously, the next two HE(12) motor neuron bursts after the missed beat had higher intraburst spike frequencies, and the amplitude of the movement signals increased concomitantly (Fig. 10). Hence, robust motor neuron bursting caused hearts to constrict.
Fig. 10.
Example experiment showing a skipped heartbeat: extracellular recordings from 2 ipsilateral HE motor neurons and movement recordings of the heart segments they control (peristaltic coordination mode) in vivo (minimally dissected animals). Note that the 3rd burst of the HE(12) motor neuron has fewer spikes and a lower intraburst spike frequency compared with the others and that the corresponding heart segment does not constrict in this cycle.
Finally, we asked how closely the hearts followed the activity of the motor neurons in vivo and whether the variability of their respective phase differences was comparable. Figure 11 correlates the mean phase difference between the two heart motor neurons with the mean phase difference between the MRR of constriction in the two hearts for both coordination modes. In the peristaltic mode, variances in the phase differences between the hearts and between the heart motor neurons were not significantly different (hearts: 0.0028; HE motor neurons: 0.0013; P = 0.31, Levene's test). Moreover, they were positively correlated (R2 = 0.60, P ≤ 0.015; Fig. 11A), indicating that larger motor phase progressions result in larger beat phase progressions. In contrast, the phase differences between the two heart segments during the synchronous mode had significantly higher variances than those of the heart motor neurons (hearts: 0.0031; HE motor neurons: 0.0008; P = 0.047, Levene's test) and were not correlated (Fig. 11B).
Fig. 11.
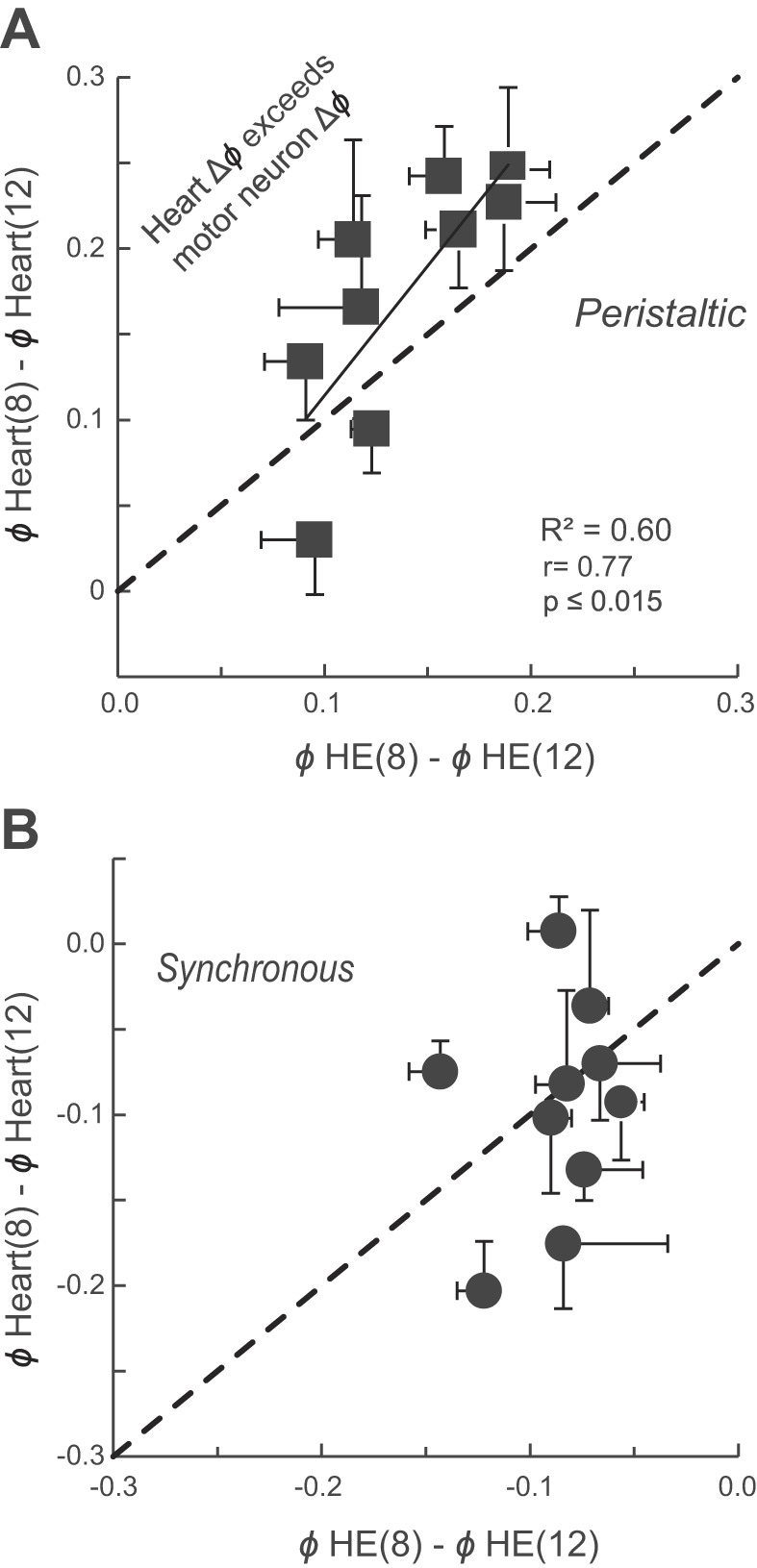
Intersegmental phase differences between the HE(8) and HE(12) motor neurons and between constriction of the corresponding hearts positively correlate in the peristaltic mode in vivo (solid line: regression line; P ≤ 0.015) (A) but do not correlate in the synchronous coordination mode (B). The (dashed) identity line represents equal phase differences between the 2 motor neurons and the 2 hearts. Note that, across preparations, in the synchronous mode the phase differences between the constrictions of the 2 hearts vary more than those between the bursts of the motor neurons. Note also that in 7 of the 9 preparations in the peristaltic mode the phase differences between the 2 heart segments exceed those of the heart motor neurons. Each symbol represents the mean ± SD of the phase differences for several beat cycles (see methods).
Interestingly, in the peristaltic mode, the phase difference between the two heart segments exceeded that between the two corresponding motor neurons in seven of the nine preparations (Fig. 11A), which means that in these seven preparations heart(8) would have to constrict later with respect to its motor neuron burst than heart(12) with respect to its motor neuron burst. The MRR of constriction was indeed delayed in heart(8) in the same seven of the nine preparations with respect to heart(12) (Fig. 9B). As stated previously, in more anterior segments blood volume is higher in the peristaltic mode because of increased filling from posterior segments (see above; Wenning and Meyer 2007), and we interpret this difference as an increase in load. Thus, while similar in the synchronous mode, the loads in heart(8) and heart(12) are different in the peristaltic mode. We attribute the phase amplification in the peristaltic mode to a load-based constriction delay, in other words, to heart tube mechanics. Moreover, with respect to its heart motor neuron burst, constriction in heart(8) occurred significantly later when in the peristaltic mode than when in the synchronous mode (Fig. 9A; Student's paired t-test; P ≤ 0.026) but was similar in heart(12) (Fig. 9A; Student's paired t-test; P ≤ 0.63).
DISCUSSION
The same CPG may program distinctly different motor outputs (Marder and Calabrese 1996). For example, at any given time, the leech heartbeat CPG programs motor neurons on one body side to execute one of two mutually exclusive coordination modes, propelling blood either rear to front with a peristaltic wave of constriction or into the periphery with almost synchronous constrictions of the individual heart segments with reciprocal switches between sides (see Calabrese 2010 for recent review). Similarly, the CPG for feeding behavior in Aplysia programs the motor neurons to execute two temporally incompatible motor patterns, one for ingesting and the other for rejecting food (Morton and Chiel 1993a, 1993b). Intrinsic membrane and synaptic properties of the individual neurons of the CPGs that set up these motor patterns may vary severalfold across animals (Calabrese et al. 2011; Callaway and Marder 2012; Roffmann et al. 2012). We asked to what extent a motor pattern and the movement it controls vary in vivo, whether the motor pattern produced in vitro (i.e., the fictive motor pattern) is different from that produced in vivo, and whether the variation seen in motor patterns has measurable consequences for the movement. To answer these questions we used two in vivo preparations (Figs. 1 and 2). In intact animals (imaging) we quantified the intersegmental phases of heart constrictions in both hearts of seven midbody segments (Figs. 3–5). In minimally dissected animals we simultaneously recorded the motor pattern and the motor performance in two segments and quantified, for the first time, their phase relations (Figs. 7–11) and aligned heart movements with filling and emptying of the hearts (Fig. 6).
Variability and constraint.
The motor patterns as well as the beat patterns in vivo showed either rear-to-front phase progression for the peristaltic mode or nearly simultaneous phase progression for the synchronous mode, and the animal-to-animal variability did not obscure the clear distinction between the two patterns produced by the same CPG and the same motor neurons (Fig. 12).
Fig. 12.
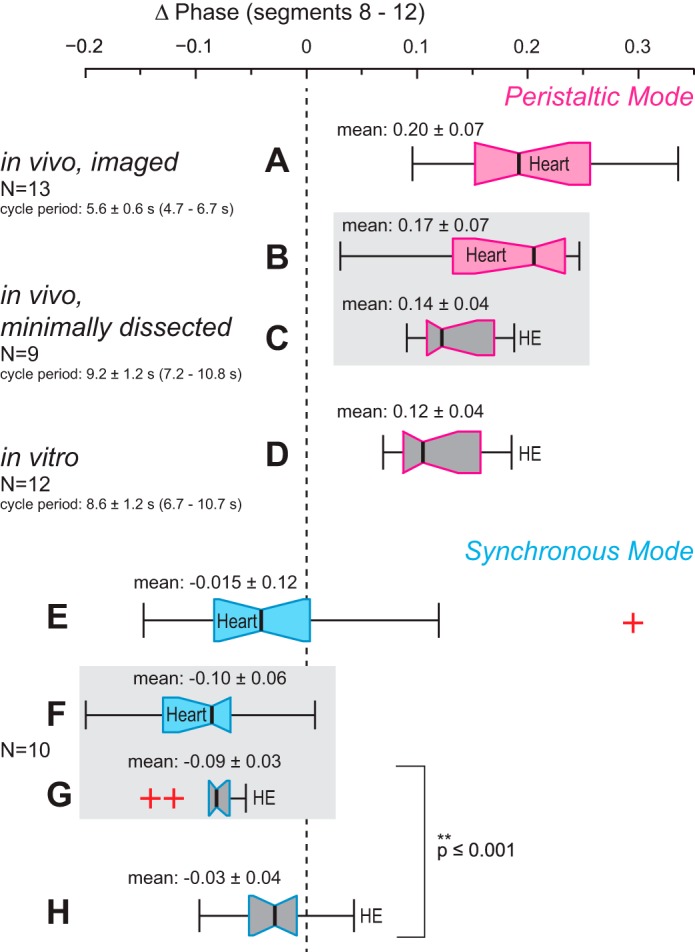
Comparison of the data of this study (in vivo) and of recent work in isolated nerve cords (in vitro; Norris et al. 2011). Box-and-whisker plots represent the intersegmental phase differences between bursts in the HE heart motor neurons (phase marker: middle spike) and heart constrictions (phase marker: MRR of constriction) of segments 8 and 12 for the peristaltic mode (top, pink outlines, A–D) and for the synchronous mode (bottom, blue outlines, E–H). Outliers are marked as red crosses. Phase differences between the heart motor neurons of segments 8 and 12 obtained in vitro do not differ from those recorded in vivo in the peristaltic mode (compare C and D) but are different in the synchronous mode (compare G and H). Phase differences between the hearts of segments 8 and 12 do not differ in the 2 in vivo preparations (compare A and B and E and F, respectively). Note that in imaged leeches the synchronous phase progression of heart constrictions matched closely the motor neuron phase progression observed in vitro in both variability and median phase difference (compare E and H). For further details see text.
Since we recorded motor output and motor performance simultaneously in vivo, we were able to examine how closely the motor performance followed the motor pattern; in other words, we asked whether the variability in the motor pattern is reflected in the motor performance. The answer depended on the coordination mode. While the phase differences between the two heart motor neurons' bursts were positively correlated with the phase differences between the MRR of constrictions in the peristaltic mode (Fig. 11A), they were not correlated in the synchronous mode (Fig. 11B).
Since we had previously recorded the fictive motor patterns in vitro (Norris et al. 2011), we were able to compare these data with our in vivo data from this study (Fig. 12) to examine whether the motor patterns produced in vivo and in vitro are different. Again, the answer depended on the coordination mode. In the peristaltic mode both the variability and the average phase difference between the two heart motor neurons were similar in vivo and in vitro (Fig. 12, compare C and D). In the synchronous mode, however, the average phase difference between the two motor neurons was significantly larger in vivo with a more pronounced front-to-rear progression in the motor pattern (P ≤ 0.001; unpaired t-test), whereas the range of intersegmental phases of the motor pattern was much larger in vitro (Fig. 12, compare G and H). These results suggest that in one coordination mode variability is constrained in vivo so that in the motor pattern there is a slight front-to-rear phase progression with a corresponding albeit more variable phase progression in the hearts commensurate with the synchronous mode. This conclusion must be tempered by the fact that in the totally intact leeches imaged in this study the synchronous phase progression of the heart constrictions matched closely the motor phase progression observed in vitro in both variability and median and mean phase difference (Fig. 12, compare E and H).
In a recent elegant study, Diehl et al. (2013) addressed the same two questions. Using the chewing rhythm generated by the STNS in the Jonah crab Cancer borealis, tension and electrophysiological recordings were combined to show that specific burst structures in a single motor neuron recorded in a semi-intact preparation yielded two distinct motor patterns and two distinct teeth movement patterns when imaged in vivo (Diehl et al. 2013). Thus the motor patterns produced in vitro matched motor output in vivo, showing that different motor patterns resulted in different associated behaviors. Sensory feedback and modulatory input are expected to be quite different in these two scenarios, and, indeed, the duty cycles of the teeth retracting and protruding were doubled and halved, respectively, and the cycle period was shorter in vivo (Diehl et al. 2013). In another example, the response of the CPG for chewing to temperature changes in the same animal was examined in the isolated STNS. C. borealis, at home in deeper waters as well as in tidal zones, has to cope with substantial temperature variations, yet the CPG showed remarkable phase constancy over a large temperature range before the rhythms broke down (“crashed”) in vitro (Tang et al. 2010). In a follow-up study, network activity was recorded extracellularly in vivo at different temperatures. Phase constancy across cycle period changes was largely maintained, but the temperature range was smaller and the rhythms crashed at lower temperatures than in vitro. On the other hand, the period was less sensitive to temperature changes in vivo than in vitro (Goeritz et al. 2013).
In the leech, the heartbeat CPG and the motor pattern are phase constant in vitro (Norris et al. 2011; Wenning et al. 2004b), as are the beat patterns in vivo [Wenning et al. 2004a; this study (data not shown)]. The duty cycles of the heart motor neurons were also similar in vitro and in vivo (data not shown). Cycle periods in vitro and in minimally dissected leeches were not significantly different (P ≤ 0.34, t-test) (Fig. 12). Cycle periods in imaged leeches, however, were significantly shorter (P ≤ 0.01, t-test), most likely because of slightly higher experimental temperatures (see methods and Arbas 1984 on temperature dependence of the heartbeat CPG).
Motor performance in vivo.
We sought to determine the requirements for setting up the two motor output patterns by considering blood flow through the system and the architecture of the hearts. Leech hearts are structured to propel blood forward along the body axis as seen in the peristaltic coordination mode. The main sphincter, a thickening in the muscular wall at the rear of each heart segment, obstructs the lumen when the heart constricts (Krahl and Zerbst-Boroffka 1983; Maranto and Calabrese 1984a; Wenning et al. 2004a; Wenning and Meyer 2007) and interferes with rearward flow. Thus to create the unidirectional, forward flow characteristic of the peristaltic mode, larger phase differences between adjacent segments are required to ensure that anterior heart segments are relaxed. To restrict longitudinal flow and thus enhance blood flow into the periphery as is characteristic of the synchronous mode (Hildebrandt 1988; Wenning and Meyer 2007), smaller differences are required. Indeed, the average phase differences in the peristaltic mode between adjacent segments were five times larger than those in the synchronous mode (0.05 ± 0.01 and −0.01 ± 0.02, respectively) (Fig. 3B).
Using two different methods to capture heart constrictions yielded an interesting result. We discovered that filling of a heart segment is delayed with respect to relaxation (Fig. 6), suggesting that heart segments fill actively (from the contractile side vessels and from the rear when in the peristaltic mode) rather than through negative pressure while relaxing.
Setting up beat pattern in vivo.
Because we recorded the motor pattern and the motor output simultaneously, we were able to determine to what extent the motor pattern determines the beat pattern and to what extent a motor neuron determines the constriction of the heart segment it controls. The heart motor neurons reliably entrained the constrictions of the heart segments to produce either one of the two distinct heart beat patterns (Figs. 7 and 8). In the peristaltic mode, the phase difference achieved by the heart is correlated with that achieved in the motor pattern (Fig. 11A), suggesting that the motor performance follows the motor pattern. The phase differences between the two hearts of segments 8 and 12, however, exceeded that of the motor neurons in these same segments in seven of the nine preparations in the peristaltic mode (Fig. 11A). We hypothesize that this disparity between the motor neuron phase difference and the heart constriction phase difference is due to heart tube mechanics. Peristaltic hearts fill from posterior heart segments (starting around segment 15); therefore there is increased filling and hence a larger load in more anterior segments (Wenning and Meyer 2007; Fig. 1B). This larger load might delay the heart tube's constriction, as is indeed the case in segment 8 (Fig. 9A), leading to the amplified phase difference between the two heart segments (Fig. 9B and Fig. 11A). In a soft-bodied animal like the leech, load varies according to body shape. Load-influenced constriction timing might also cause the larger variability of the phase differences seen between heart segments compared with those between the motor neurons (Fig. 12, compare B with C and F with G). Load-influenced constriction timing may be part of the cascade that transforms neuronal activity into the actual muscle contraction. A theoretical framework for understanding this neuromuscular transform was worked out using the feeding behavior in Aplysia (Brezina et al. 2000). A detailed analysis on the neuromuscular transform in lobster hearts in semi-intact preparations showed its predictive power for the seemingly paradoxical effects of a neuromodulator (Williams et al. 2013). In the leech, it will be interesting to characterize and then compare the neuromuscular transform in loaded and unloaded heart segments.
The intersegmental phase delays characteristic of the two distinct motor patterns are set up and maintained without sensory feedback in the leech heartbeat system (Calabrese 1977, 1979). Changes in heart rate are induced by inputs from the periphery and through interactions among central circuits without peripheral feedback (Arbas and Calabrese 1990; Davis 1986). In contrast, animals with a centralized heart adjust motor neuron activity, constriction amplitude, and cycle period often by sensory feedback as, for example, in the neurogenic heart of crustaceans (Garcia-Crescioni et al. 2010; Mahadevan et al. 2004). In the leech, sensory feedback as a mechanism to control constriction timing between heart segments 8 and 12 as studied here is hard to envision. The same four premotor interneurons of the CPG on each side control all the corresponding heart motor neurons, each of which in turn controls the corresponding heart segment. Therefore, sensory feedback cannot influence the relative timing of the premotor inputs to the different motor neurons. In contrast, the oscillatory networks of the leech swim CPG are segmentally distributed and individually process input from segmental stretch receptors to set up the intersegmental coordination of the motor neurons (Friesen and Cang 2001).
In leech heartbeat, the motor pattern determines the beat pattern, peristaltic or synchronous, and the phase differences between motor neurons determine the phase differences between heart segments. Additionally, as seen in the peristaltic mode, load-based constriction timing may amplify the phase difference realized between the heart segments.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-085006 to R. L. Calabrese.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.W., B.J.N., and R.L.C. conception and design of research; A.W., B.J.N., and A.D.-M. performed experiments; A.W., A.D.-M., and R.L.C. analyzed data; A.W., B.J.N., and R.L.C. interpreted results of experiments; A.W. prepared figures; A.W. drafted manuscript; A.W. and R.L.C. edited and revised manuscript; A.W., B.J.N., A.D.-M., and R.L.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our colleagues Drs. D. G. Lamb for helping with MATLAB coding, D. R. Stokes for designing a device for imaging adult leeches, D. Kueh for advising on statistics, and C. Günay for reading an earlier version of this manuscript. Eugenia Botezat assisted with the imaging and the subsequent analysis.
REFERENCES
- Arbas EA. Rate modification in the heartbeat central pattern generator of the medicinal leech. J Comp Physiol A 155: 783–794, 1984 [Google Scholar]
- Arbas EA, Calabrese RL. Leydig neuron activity modulates heartbeat in the medicinal leech. J Comp Physiol A 167: 665–671, 1990 [DOI] [PubMed] [Google Scholar]
- Brezina V, Orekhova IV, Weiss KR. The neuromuscular transform: the dynamic, nonlinear link between motor neuron firing patterns and muscle contraction in rhythmic behaviors. J Neurophysiol 83: 207–231, 2000 [DOI] [PubMed] [Google Scholar]
- Calabrese RL. The neural control of alternate heartbeat coordination states in the leech, Hirudo medicinalis. J Comp Physiol A 122: 11–143, 1977 [Google Scholar]
- Calabrese RL. The roles of endogenous membrane properties and synaptic interaction in generating the heartbeat rhythm of the leech, Hirudo medicinalis. J Exp Biol 82: 163–176, 1979 [DOI] [PubMed] [Google Scholar]
- Calabrese RL. The heartbeat neural control system of the leech. In: Handbook of Microcircuits, edited by Shepherd GM, Grillner S. Oxford, UK: Oxford Univ. Press, 2010, p. 450–456 [Google Scholar]
- Calabrese RL, Maranto AR. Neural control of the hearts in the leech, Hirudo medicinalis. III. Regulation of myogenicity and muscle tension by heart accessory neurons. J Comp Physiol A 154: 393–406, 1984 [Google Scholar]
- Calabrese RL, Norris BJ, Wenning A, Wright TM. Coping with variability in small neuronal networks. Integr Comp Biol 51: 845–855, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway EM, Marder E. Common features of diverse circuits. Curr Opin Neurobiol 22: 565–567, 2012 [DOI] [PubMed] [Google Scholar]
- Cymbalyuk GS, Gaudry Q, Masino MA, Calabrese RL. Bursting in leech heart interneurons: cell-autonomous and network-based mechanisms. J Neurosci 22: 10580–10592, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Influence of oxygen on the heartbeat rhythm of the leech. J Exp Biol 123: 401–408, 1986 [DOI] [PubMed] [Google Scholar]
- Diehl F, White RS, Stein W, Nusbaum MP. Motor circuit-specific burst patterns drive different muscle and behavior patterns. J Neurosci 33: 12013–12029, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WO, Cang J. Sensory and central mechanisms control intersegmental coordination. Curr Opin Neurobiol 11: 678–683, 2001 [DOI] [PubMed] [Google Scholar]
- Garcia-Crescioni K, Fort TJ, Stern E, Brezina V, Miller MW. Feedback from peripheral musculature to central pattern generator in the neurogenic heart of the crab Callinectes sapidus: role of mechanosensitive dendrites. J Neurophysiol 103: 83–96, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goaillard JM. Neural networks: more about flexibility than synaptic strength. Curr Biol 21: R276–R278, 2011 [DOI] [PubMed] [Google Scholar]
- Goeritz ML, Soofi W, Stein WA, Prinz AA, Marder E. Pyloric network responses to temperature changes in vivo (Abstract). Soc Neurosci Abstr 2013: 372.–09., 2013 [Google Scholar]
- Golowasch J, Goldman MS, Abbott LF, Marder E. Failure of averaging in the construction of a conductance-based neuron model. J Neurophysiol 87: 1129–1131, 2002 [DOI] [PubMed] [Google Scholar]
- Hildebrandt JP. Circulation in the leech, Hirudo medicinalis L. J Exp Biol 134: 235–246, 1988 [Google Scholar]
- Krahl B, Zerbst-Boroffka I. Blood pressure in the leech Hirudo medicinalis. J Exp Biol 107: 163–168, 1983 [Google Scholar]
- Mahadevan A, Lappe J, Rhyne RT, Cruz-Bermudez ND, Marder E, Goy MF. Nitric oxide inhibits the rate and strength of cardiac contractions in the lobster Homarus americanus by acting on the cardiac ganglion. J Neurosci 24: 2813–2824, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maranto AR, Calabrese RL. Neural control of the hearts in the leech, Hirudo medicinalis. I. Anatomy, electrical coupling, and innervation of the hearts. J Comp Physiol A 154: 367–380, 1984a [Google Scholar]
- Maranto AR, Calabrese RL. Neural control of the hearts in the leech, Hirudo medicinalis. II. Myogenic activity and its control by heart motor neurons. J Comp Physiol A 154: 381–391, 1984b [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. Physiol Rev 76: 687–717, 1996 [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol A 172: 17–32, 1993a [DOI] [PubMed] [Google Scholar]
- Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol A 173: 519–536, 1993b [DOI] [PubMed] [Google Scholar]
- Norris BJ, Weaver AL, Morris LG, Wenning A, Garcìa PA, Calabrese RL. A central pattern generator producing alternative outputs: temporal pattern of premotor activity. J Neurophysiol 96: 309–326, 2006 [DOI] [PubMed] [Google Scholar]
- Norris BJ, Weaver AL, Wenning A, Garcìa PS, Calabrese RL. A central pattern generator producing alternative outputs: phase relations of leech heart motor neurons with respect to premotor synaptic input. J Neurophysiol 98: 2983–2991, 2007 [DOI] [PubMed] [Google Scholar]
- Norris BJ, Wenning A, Wright TM, Calabrese RL. Constancy and variability in the output of a central pattern generator. J Neurosci 31: 4663–4674, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson EL, Calabrese RL. Dynamic analysis of a rhythmic neural circuit in the leech Hirudo medicinalis. J Neurophysiol 47: 256–271, 1982 [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. (Editor). Circadian Oscillations in Cells and the Circadian Organization of Multicellular Systems. Cambridge, MA: MIT Press, 1974, p. 437–458 [Google Scholar]
- Roffman RC, Norris BJ, Calabrese RL. Animal-to-animal variability of connection strength in the leech heartbeat central pattern generator. J Neurophysiol 107: 1681–1693, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddall ME, Trontelj P, Utevsky SY, Nkamany M, Macdonald KS. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc Biol Sci 274: 1481–1487, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang LS, Goeritz ML, Caplan JS, Taylor AL, Fisek M, Marder E. Precise temperature compensation of phase in a rhythmic motor pattern. PLoS Biol 8: e1000469, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson WJ, Stent GS. Neuronal control of heartbeat in the medicinal leech. II. Intersegmental coordination of heart motor neuron activity by heart interneurons. J Comp Physiol 111: 281–307, 1976 [Google Scholar]
- Wenning A, Cymbalyuk GS, Calabrese RL. Heartbeat control in leeches. I. Constriction pattern and neural modulation of blood pressure in intact animals. J Neurophysiol 91: 382–396, 2004a [DOI] [PubMed] [Google Scholar]
- Wenning A, Hill AA, Calabrese RL. Heartbeat control in leeches. II. Fictive motor pattern. J Neurophysiol 91: 397–409, 2004b [DOI] [PubMed] [Google Scholar]
- Wenning A, Meyer EP. Hemodynamics in the leech: blood flow in two hearts switching between two constriction patterns. J Exp Biol 210: 2627–2636, 2007 [DOI] [PubMed] [Google Scholar]
- Wenning A, Norris BJ, Doloc-Mihu A, Calabrese RL. Bringing up the rear: new premotor interneurons add regional complexity to a segmentally distributed motor pattern. J Neurophysiol 106: 2201–2215, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenning A, Zerbst-Boroffka I, Bazin B. Water and salt excretion in Hirudo medicinalis. J Comp Physiol B 139: 97–102, 1980 [Google Scholar]
- Williams AH, Calkins A, O'Leary T, Symonds R, Marder E, Dickinson PS. The neuromuscular transform of the lobster cardiac system explains the opposing effects of a neuromodulator on muscle output. J Neurosci 33: 16565–16575, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TM, Jr, Calabrese RL. Patterns of presynaptic activity and synaptic strength interact to produce motor output. J Neurosci 31: 17555–17571, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbst-Boroffka I. Osmo- und Volumenregulation bei Hirudo medicinalis nach Nahrungsaufnahme. J Comp Physiol 84: 185–204, 1973 [Google Scholar]