Abstract
To determine whether the spacer region between the -35 and -10 elements plays any sequence-specific role, we randomized the GC-rich sequence (-20CCGGCTCG-13) within the spacer region of the cAMP-dependent lac promoter and selected an activator-independent mutant, which showed extraordinarily high intrinsic activity. The hyperactive promoter is obtained by incorporation of a specific 10-bp-long AT-rich DNA sequence within the spacer, referred to as the -15 sequence, which must be juxtaposed to the upstream end of the -10 sequence for the hyperactivity. The transcription enhancement functions only in the presence of a -35 element. The spacer sequence enhanced both RNA polymerase binding and open complex formation. Isolated in the lac promoter, it also enhanced transcription when placed at two other unrelated promoters. Sequence analysis shows a low GC content and an abundance of stereochemically flexible TG:CA and TA:TA dimeric steps in the -18/-9 region and a strong correlation between the presence of flexible dimeric steps in this region and the intrinsic strength of the promoter.
A promoter is a stretch of DNA sequence that encodes information for RNA polymerase binding and initiation of transcription. Genetic and statistical analysis of promoters in Escherichia coli defined two kinds of Eσ70 RNA polymerase holoenzyme-dependent promoters (1). Both kinds require a 6-bp -10 sequence (consensus 5′-TATAAT-3′) located ≈7 bp 5′ to the transcription start site. Functionally, the -10 element participates in RNA polymerase binding by interacting with the region 2.3–2.4 of σ70 (2–10) and is part of an ≈15-nt putative single-stranded region in the open complex (4, 8, 11–15). The first kind of promoters (-35 promoters) is more common and characterized by the presence of the -10 element as well as a 6-bp (consensus sequence 5′-TTGACA-3′) in the -35 position (16). The -35 element also helps RNA polymerase binding through interaction with region 4.2 of σ70 (2, 5, 17). It is believed that the spacer region between -35 and -10 (optimal length 17 bp) does not have any specific sequence requirement and simply facilitates the spatial alignment of the -10 and -35 elements in binding to the 2.4 and 4.2 regions of the σ factor (7, 17, 18). The second kind, called “extended -10” promoters, contains an extra 2-bp 5′-TG-3′ sequence located 1 bp 5′ to the -10 element, (consensus sequence -15TGNTATAAT-7) (19). The DNA sequence immediately upstream of an extended -10 element may enhance the activity of the extended -10 promoter (20, 21). The TG element also binds to RNA polymerase by contacting the 3.0 (formerly 2.5) segment of σ70 (22). The -35 promoters may be improved by the presence of the extended -10 sequence and vice versa.
Many naturally occurring promoters are more or less inefficient because of the presence of non-consensus sequence elements or suboptimal spacer lengths and are resurrected by extra regulatory factors (23). The extra factor may be a DNA sequence, e.g., an UP element (24–26). The UP element, located immediately 5′ to the -35 element, has a recognizable pattern of AT-rich sequences. It enhances RNA polymerase binding by complexing with α subunits and stimulates the intrinsic transcription observed without an UP element (27, 28). In most cases, however, transcription of weak promoters is enhanced by regulatory proteins that act by binding to cognate and specific DNA sequences and stimulating one or more steps of transcription initiation.
To investigate whether the spacer segment between the -10 and the -35 elements in the -35 promoters can have a sequence-specific role, we randomized the spacer segment of the intrinsically weak E. coli lac promoter, and screened for mutants with high levels of transcription in vivo in the absence of cAMP receptor protein (CRP), which normally activates the promoter by binding to the -60 region (29). We arbitrarily divided the 18-bp spacer (-30 to -13) of the lac promoter into two segments: -30 to -21 and -20 to -13, and randomized the latter 8-bp region adjacent to the 15-bp segment, which is distorted in the open complex and thus may be more interesting for a systematic analysis. A mutant of the lac promoter, resulting in a specific 10-bp sequence around the -15 position, was hyperactive in the absence of CRP. We showed that this sequence enhances both RNA polymerase binding and open complex formation. We also analyzed the putative structural role of the sequence in transcription initiation.
Experimental Procedures
Strains, Plasmids, and Protein. E. coli 12 strains were as follows: MG1655 (wild type); DH5α (supE44ΔlacU169(ϕ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1); G947 (DH5α::rec+, F′, Δcrp). Plasmids were as follows: pUC19 and pUC19-DP (see below). Proteins were as follows: wild-type RNA polymerase, purified to >95% pure from E. coli MG1655 (30). We used mutant RNA polymerase containing IT439, RC441, and EN458 changes in σ, reconstituted by mixing core with the mutant σ expressed from a σ clone (a gift of S. Busby, University of Birmingham, Birmingham, U.K.), and we used CRP, purified to 98% homogeneity from the N6165S carrying the crp gene in a plasmid (31).
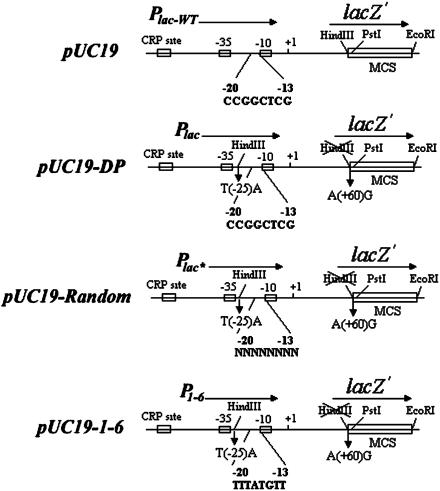
Selection of cAMP-Independent Mutant lac Promoters by Mutations in the Spacer Region. Randomized mutations at the lac spacer region were generated in pUC19 plasmid (Fig. 1). The HindIII site, present as part of the multiple cloning site in lacZα in pUC19, was deleted by the changing the codon TTG (Leu) to CTG (Leu) in Plac-wt. An HindIII site was then introduced into the spacer region in Plac-wt by replacing the -25T by an A (-26AAGCTT-21). The altered promoter in the mutant plasmid (pUC19-DP) was named Plac as opposed to Plac-wt (Fig. 1). Next, a primer with a random sequence from -20 to -13 (-31ACTTTAAGCTTNNNNNNNNTATGTTGT GTGGAATTGTG+7) was used to generate randomized mutations at the GC-rich region (-20CCGGCTGG-13) by PCR. The PCR-amplified fragments were ligated between the HindIII and PstI sites of pUC19-DP. The recombinant plasmids were used to transform the strain G947 (crp-) to look for Lac+ cells.
Fig. 1.
Schematic illustration of randomized mutagenesis of 8-bp DNA sequence in the spacer of the lac promoter in the plasmid pUC19-DP. A primer with random sequence in the -20 and -13 segment of the lac promoter was used for PCR amplification as shown in pUC19-Random. In pUC19-1-6, the 8 GC-rich sequence (CCGGCTCG) was substituted by the AT-rich sequence (TTTATGTT).
Site-Directed Mutagenesis of P1–6. The derivatives of P1–6 were created by site-directed mutagenesis. The mutations were confirmed by DNA sequencing.
In Vitro Transcription. All promoters were tested for function by runoff transcription of PCR-amplified fragments as templates. The PCR-amplified DNA fragments were 390 bp, which contained 314 bp upstream of the transcription start site (+1) of Plac and 76 bp downstream of +1. Reactions were carried out at 37°C (32, 33). The transcripts were quantified with a Molecular Dynamics Storm gel and blot imaging system using imagequant; the data for each species were corrected for background.
Electrophoresis Mobility Assays (EMA). EMA were carried out as described (34). RNA polymerase and 32P-labeled 390-bp PCR-amplified DNA fragments (also used in transcription) were incubated at 37°C for 30 min or indicated in 1× transcription buffer with 5% sucrose and 0.1 mg/ml BSA. Heparin was added to 50 μg/ml, and reaction mixtures were loaded in a 4.0% polyacrylamide gel for electrophoresis.
KMnO4 Footprinting. The experiments followed a modification of a protocol described before (33, 35). DNA (10 nM, 390-bp PCR-amplified fragments) and RNA polymerase (200 nM) were incubated for 30 min at different temperatures ranging from 5–37°C before KMnO4 (12.5 mM) was added. The reactions were quenched by 2-mercaptoethanol. The primers were extended by using a PCR cycle to amplify signals. The products were electrophoresed in an 8% polyacrylamide-7M urea sequencing gel.
Results
Isolation of P1–6. The DNA sequence of the -20 to -13 region of Plac in plasmid pUC19-DP was randomized as in Experimental Procedures. G947 (Lac-Crp-) cells transformed with mutagenized plasmid DNA preparations were plated on LB-Ampicillin plates containing 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-gal) and isopropyl β-d-thiogalactoside (IPTG), and the plates were incubated at 37°C for 1–2 days. The transformed colonies were screened for blue (Lac+) color. Eighty-two candidates were picked from ≈4,000 transformants. Ten of them with high intensities of blue color were assayed for β-galactosidase, and their promoter region was sequenced (data not shown). From among those with high β-galactosidase activities, four independent clones had the same sequence (TTTATGTT) in the “randomized” region of the promoter. One was used for further study in vitro. The mutant promoter was named P1–6.
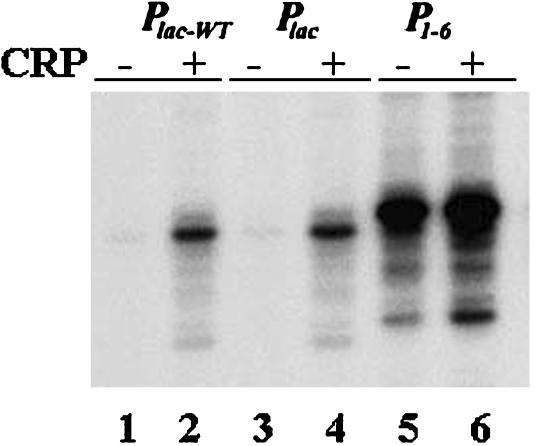
In Vitro Transcription. We quantified 76- to 77-nt long run-off RNA made in vitro. The results of transcription with Plac-wt, Plac, and P1–6 in the absence and presence of CRP are shown in Fig. 2. It is clear that the change in 1 bp in Plac-wt spacer to create an HindIII site in making Plac did not change the transcription activity. Henceforth, Plac was used as a control in evaluating P1–6. The P1–6 promoter showed >40-fold greater RNA synthesis compared with Plac in the absence of CRP. Whereas CRP (50 nM) stimulated Plac transcription 10-fold, it showed 2-fold further stimulation of P1–6. Incidentally, unlike Plac, in which transcription initiated mostly at the +1 position, the major transcription start in P1–6 shifted to the position -1. The mechanism of shifting of the transcription start site is currently being studied.
Fig. 2.
In vitro transcription of Plac-wt, Plac, and P1–6 promoters. Lanes 1, 3, and 5 were in the absence of CRP, and lanes 2, 4, and 6 were in the presence of CRP.
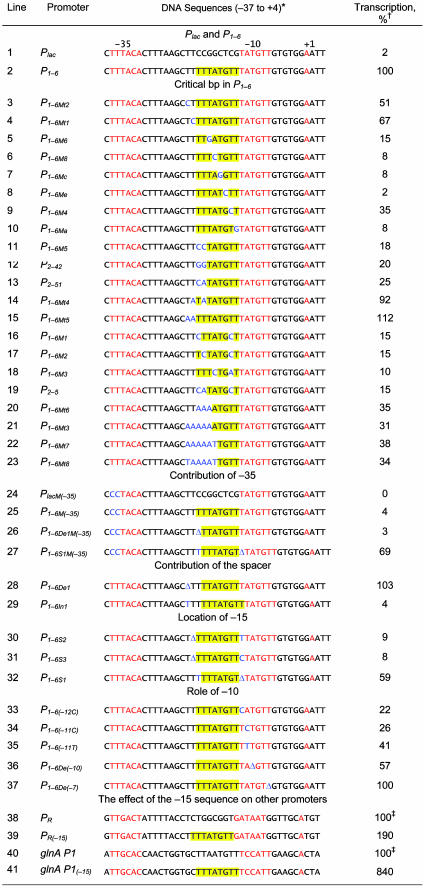
Critical Base Pairs in P1–6. To identify the critical base pairs in the -20TTATGTT-13 sequence of P1–6 that contribute to the high promoter activity, site-directed mutagenesis was used to make systematic single or multiple base pair changes in the spacer segment. Because of the presence of two additional T residues (-22TT-21) in the parental Plac, which is now 5′ to the -20TTTATGTT-13 sequence of P1–6, we scanned the entire segment from -22 to -13 (TTTTTATGTT). The DNA sequence alterations and the results of in vitro transcription are shown in Table 1. Transcription results of representative sample templates are shown in Fig. 3. It was apparent that the nature of the base pairs at 5 of the 10 positions is more critical for the high activity of the promoter than the others. Substitution of -18T, -17A, -16T, -15G, or -13T substantially reduced the promoter enhancement whereas substitution of -22T, -21T, -20T, -19T, or -14T showed less drastic effects. In summary, these and additional results (Table 1, lines 3–23, and data not shown) showed that the 10-bp segment (from position -22 to -13) in P1–6 with a consensus sequence WWTWTATGYT is responsible for the strong intrinsic transcription of P1–6. The TATGTT part of the sequence seems to be a duplication of the native -10 element of Plac. However, it is not acting as a new -10 element because it was not sensitive to KMnO4 treatment (shown below).
Table 1. Relative amounts of transcription from Plac, P1-6 and P1-6 derivatives.
*The yellow outlines show the sequences of the -15 sequence in P1-6 or parts of it. The -10 and -35 elements are in red. The transcription start point (+1) of the parental Plac is also in red. The mutations introduced by site-directed mutagenesis are in blue. The sequence of the -47/-38 region in Plac and P1-6 is GGCACCCCAGG.
†The number of transcripts was normalized to the percentage of that of P1-6, which was taken as 100%. The data were averaged from three individual experiments. Errors were <10%.
‡The number of transcripts was normalized to the percentage of those of the wild-type promoters, which were taken as 100%. The data were averaged from three individual experiments. Errors were <10%.
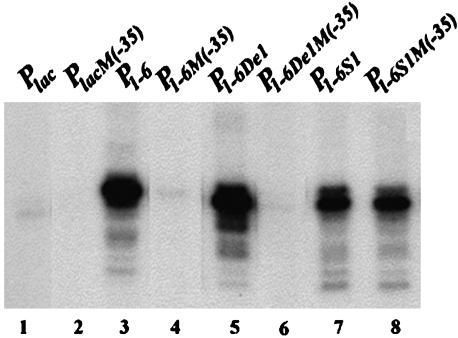
Fig. 3.
In vitro transcription of lac promoters with defective -35 elements. Lanes 1, 3, 5, and 7 were promoters with an active -35 element; lanes 2, 4, 6 and 8 were promoters with a mutant -35 element.
Role of the -35 Element and the Spacer Length in P1–6 Activity. Although the sequence of the -35 element in Plac is not perfect (TTTACA vs. consensus TTGACA) and is located 18 bp (instead of the usual 17 bp) away from the -10 element, it is an essential element of the Plac promoter; change of TT to CC at the 5′ end made the promoter totally defective (Table 1, line 24 and Fig. 3, lane 2). To determine the contribution of the -35 element toward the P1–6 activity, the same TT to CC changes were made in the latter promoter. The changes completely eliminated the enhanced activity of P1–6, establishing, like in the parent, the essentiality of the -35 sequence in P1–6 function (Table 1, line 25 and Fig. 3, lane 4). It also showed that the -15 sequence does not substitute the role of the -35 element in promoter function. Additionally, when the length of the spacer between -35 and -10 elements was changed from 18 to 17 bp by deleting a base pair between the -35 and -15 elements, the P1–6 activity was slightly enhanced (Table 1, line 28). However, increasing the length to 19 bp decreased the P1–6 transcription level to 4% (Table 1, line 29). The DNA template with the 17-bp spacer, when combined with a defective -35 element, also completely lost its high transcription (Table 1, line 26 and Fig. 3, lane 6).
Location of the AT-Rich -15 Sequence. In P1–6, the (-22TTTTTATGTT-13) sequence is located immediately upstream of the -10 element (-12TATGTT-7). When the -15 sequence was moved 1 bp further upstream by inserting T or C between the -13 and -12 positions and deleting a T in the -22 to -18 region, transcription enhancement disappeared (Table 1, lines 30 and 31). Interestingly, when T at position -13 was deleted, thus making the -10 element and the -15 sequence overlap by 1 bp (both 3′ end of–15 and 5′ end of -10 being T), the high level of P1–6 transcription was reduced to only 40% (Table 1, line 32); the 18-bp spacer between -10 and -35 elements was maintained by adding an extra T in the -20 to -17 region. However, when the -35 sequence of the latter template was inactivated by the TT to CC changes described above, transcription did not decrease; if anything, it increased slightly (Table 1, line 27, and Fig. 3, lane 8). Incidentally, the removal of the -13T created a 5′TG3′ sequence 1 bp upstream of the -10 element, a signature of the extended -10 promoters, which are already known to be capable of functioning quite efficiently without a -35 sequence (19).
Generality of the -15 Sequence Activity. The authenticity of the -15 sequence in promoter enhancement was further supported by the fact that, when the TTTATGTT sequence was engineered into two other promoters, the strong bacteriophage λ PR promoter, and the moderately active E. coli glnA P1 promoter, the intrinsic transcription was enhanced by ≈2- and 8-fold, respectively (Table 1, lines 38–41). Note that PR is one of the strongest promoters in E. coli.
Role of the -10 Element. The -12T, -11A, -10T, and -7T bp are critical in the functioning of the -10 element in all promoters (1). Substitution of any one of them brings about substantial decrease in promoter activities (16). In the case of P1–6, a change of -12T to C, -11A to C or T, or a deletion of -10T reduced the corresponding promoter activity, although not as much as expected (Table 1, lines 33–36). A deletion of the -7T affected the promoter only marginally (Table 1, line 37).
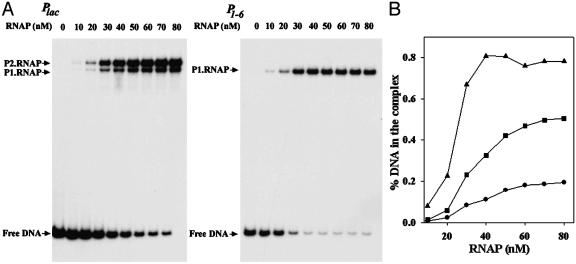
RNA Polymerase Binding. We compared the affinity of RNA polymerase binding to Plac and P1–6 by electrophoresis mobility assays. Different concentrations of RNA polymerase were mixed with 32P-labeled promoters at 37°C to allow open complex formation, and the number of open complexes were estimated after their separation from free DNA by gel electrophoresis. The results showed concentration-dependent binding of RNA polymerase in both cases (Fig. 4). The Plac DNA also contains another overlapping promoter P2lac (36). In this template, as expected, two promoter–RNA polymerase complexes were observed; the P2lac complex was of the slower mobility, and the Plac had the faster mobility (Fig. 4A). In case of P1–6, only one complex was seen. Note that P2lac was transcriptionally inactive in P1–6. The base substitutions in the -15 region of the latter inactivated the former. Quantification showed that the affinity of RNA polymerase to the three promoters, as determined by the this method, differs significantly: the relative affinities of Plac, P2lac, and P1–6 were 1, 2.7, and 10, respectively (Fig. 4B and Table 2).
Fig. 4.
Electrophoresis mobility assays of RNA polymerase binding. (A) Varying amounts of RNA polymerase binding to 32P-labeled Plac or P1–6 DNA. (B) Percentage of DNA in the complex at different RNA polymerase concentrations. •, Plac; ▪, P2lac; ▴, P1–6. Binding reactions contained 10 nM DNA and 0–80 nM RNA polymerase.
Table 2. RNA polymerase binding and open complex formation at different promoters.
| Plac | P2lac | P1-6 | |
|---|---|---|---|
| Relative affinity | 1 | 2.7 | 10 |
| kobs | 0.10 ± 0.05 | 0.14 ± 0.06 | 0.41 ± 0.06 |
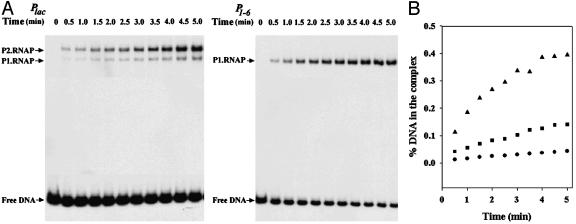
Kinetics of Open Complex Formation. We determined the relative rates of open complex formation at the promoters by quantifying the number of open complexes formed in the binding reactions described above at different times by electrophoretic separation of the complexes as shown in Fig. 5A. Using the single exponential equation F = A × [1 - exp(-kobs × t)] + C to fit the percentages of the DNA in each complex, we determined the kobs for the different products (Fig. 5B). The rate of open complex formation (kobs) was much higher in case of P1–6 compared with both Plac and P2lac (Fig. 5B and Table 2).
Fig. 5.
Kinetics of open complex formation. (A) RNA polymerase binding to 32P-labeled Plac or P1–6 DNA vs. time. (B) Percentage of DNA in the complex. •, Plac; ▪, P2lac; ▴, P1–6. Binding reactions containing 10 nM DNA and 30 nM RNA polymerase were carried out at 37°C. The reactions were terminated by addition of 50 μg/ml heparin at different time intervals.
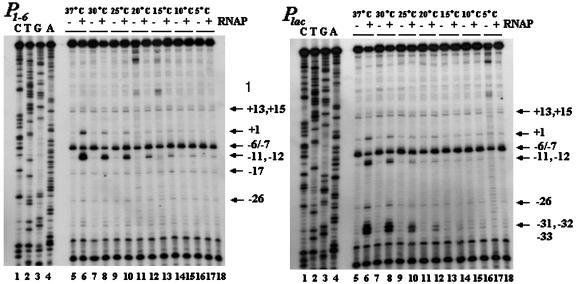
KMnO4 Sensitivity of Open Complex. Open complexes formed at the promoters were also probed by KMnO4-mediated cleavage of distorted pyrimidines, as described by Sasse-Dwight and Gralla for Plac-wt (35), at temperatures from 5–37°C. Excess RNA polymerase (200 nM) was used to overcome any binding lag. The results are shown in Fig. 6. Because of the closeness of the bands in the sequencing gels, our identification of the KMnO4-sensitive sites in some cases may be off by a nucleotide. KMnO4 sensitivity was observed at discrete positions at all temperatures even in the absence of RNA polymerase, suggesting the existence of sequence-dependent DNA “soft spots” (Fig. 6). The sensitivity of these sites to KMnO4 was weak except at position -6/-7. The intensity of the KMnO4-sensitive spots in naked DNA depended on the nature of PCR enzyme used to detect the cleavage products; use of the Taq enzyme made the cleavages intense, but that of the Klenow enzyme did not (data not shown). In the presence of RNA polymerase, strong pyrimidine distortions were visible in Plac at several of the sites (-33, -32, -31, -26, -12, -11, -10, and +1) that were weakly sensitive in the absence of RNA polymerase. KMnO4 sensitivity observed at positions -12, -11, and +1 were because of RNA polymerase binding to Plac whereas that at sites -33, -32, and -31 were because of RNA polymerase binding to P2lac. Note that the -33, -32, and -31 correspond to -12, -11, and -10 positions of P2lac.
Fig. 6.
Detection of open complex formation by KMnO4 at varying temperatures. The reaction mixtures were incubated at 5–37°C for 30 min before modification by KMnO4. Lanes 1–4, the sequences of the P1–6 or Plac promoter; lanes 6, 8, 10, 12, 14, 16, and 18, KMnO4 treatments in the absence of RNA polymerase; and lanes 5, 7, 9, 11, 13, 15, and 17, KMnO4 treatments in the presence RNA polymerase.
The pattern of KMnO4 sensitivity in Plac reported here was somewhat different from the one observed by Sasse-Dwight and Gralla (35), very likely because the current experiments were done with linear DNA whereas those by the other authors were with supercoiled DNA. RNA polymerase forms open complex at P2lac strongly but does not initiate transcription whereas at Plac it forms open complex less but initiates efficiently (36). Our results of RNA polymerase binding and open complex formation at these two promoters and the KMnO4 results that RNA polymerase make the +1 site of Plac KMnO4 sensitive whereas it did not do so at +1 of P2lac confirm that. Nevertheless, open complex formation, as judged by KMnO4 sensitivity, at both Plac and P2lac was detectable only at temperatures 20°C and above. The KMnO4-sensitive patterns, both in the absence and presence of RNA polymerase at P1–6, were the same as in Plac except the following: (i) open complexes were observed, as judged by pyrimidine distortions, at 20°C and above in Plac whereas the complexes were formed even at 5°C in P1–6; (ii) no obvious open complex formation at P2lac site was observed in P1–6; (iii) an intrinsic KMnO4-sensitive site was observed at position -17 of P1–6; and (iv) the intensity of the KMnO4 sensitivities at positions -11 and -12 was very high in P1–6 compared with that in Plac. When using a lower concentration of RNA polymerase (30 nM, same as that used in vitro transcription assays) at 37°C, the intensities were significantly different (data not shown). The intensity at P1–6 was ≈10-fold higher than that at Plac.
Sequence Analysis of the Spacer Region. To elucidate putative relationships between relative promoter strength and structural properties of the spacer region, we analyzed GC content, helical twist, and overall DNA flexibility and bendability of the 18-bp-long spacer. Our analysis showed the following.
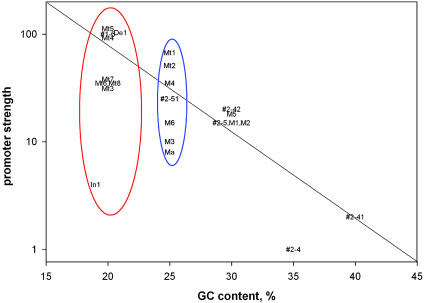
GC content. The promoters with GC-rich spacers were weaker than the promoters with AT-rich spacers (Fig. 7). Note that there are no points in the upper-right corner of the graph. How does the GC content of the spacer influence the promoter strength? It is believed that immediately downstream of the spacer, only a 15-bp segment (-12 to +3), and not the spacer sequence itself, becomes “single stranded” during open complex formation among the -35 element-containing promoters (37). However, it is possible that RNA polymerase deforms the spacer region in the open complex in a way that is insensitive to KMnO4 treatment. If the spacer regions were distorted in some way, then a higher GC content would create an energy barrier to such a distortion, decreasing promoter strength. Alternatively, the high GC content may be, directly or indirectly, inhibitory to distortion of a neighboring AT-rich region. The influence of a GC-rich sequence on the melting of a contiguous AT-rich region has been reported (38, 39). The fact that the strength of the promoter varied significantly among the group of promoters with the same GC content (25%) indicated that the exact DNA sequence in the spacer is also influential in determining promoter strength determination (Fig. 7).
Fig. 7.
Relative promoter strength as a function of GC content of the spacer. Promoters with low GC content (20%) are within a red oval and those with intermediate GC content are within a blue oval. Only promoters with an 18-bp spacer having the TGNT motif and intact -10 and -35 elements were selected from Table 1 for spacer sequence analysis. The promoters 2-4 (TTGGTCTGAT) and P2-41 (TTCCGGTGAT) are not included in Table 1.
Twist angle. There was a moderate negative correlation between promoter strength and the overall helical twist of the spacer DNA; the equilibrium helical twisting of the spacer in strong promoters was lower than that in weak promoters (data not shown). The observation that the helical twist of the spacer sequence influenced the promoter strength may reflect the previously proposed spatial alignment for interaction of the -35 and -10 promoter elements to the corresponding 4.2 and 2.4 DNA binding regions of σ70 (40). The alignment is critical for the DNA–protein interaction and strand separation.
Flexible dimeric steps. The -15 sequence contained two pyrimidinepurine steps, TA:TA and TG:CA. These dimeric steps are known to be soft spots in causing DNA distortion, such as, under- and over-twisting, kinking, base pair opening, etc. (41–44). Interestingly, the KMnO4 sensitivity observed in the absence of RNA polymerase occurs in the TG:CA and TA:TA steps. Note that the flexible TA:AT and TG:CA steps are frequently located in the promoter positions (45). The occurrence of these dimeric steps in the -15 sequence, in addition to those in the -10 element, may actually be one of the major determinants in making the P1–6 promoter hyperactive by facilitating the isomerization step. Additionally, the dimeric steps in the -15 region may also be responsible for making the -10 region somewhat less vulnerable to substitutions in P1–6.
DNA structure and bendability. In principle, the promoter strength may be related to deformation energy required for fitting the DNA surface around the RNA polymerase surface (23). In this regard, note that in the two strongest promoters analyzed here, P1–6 and P1–6Mt5 (Table 1, lines 2 and 18), the middle segment of the spacer region contains the sequences (TTTTT or AATTT) that are the most easily bendable in an intrinsically narrow minor groove (43, 44). By contrast, the Plac promoter that contains the -20CCGG-17 sequence strongly resists such a bending and has a wide minor groove (43, 44). Thus, if the DNA in the initiation complex were to bend into the minor groove in this vicinity, then the sequences in the promoters P1–6 and P1–6Mt5 would be much more advantageous compared with that in Plac.
Discussion
Overall, the -10 proximal part of the spacer region in native promoters in E. coli is GC-rich (46). It was suggested that stretches of Gs or Cs in the center of the spacer region inhibit open complex formation (40). The significance of the inhibitory effect of the GC-richness may be related to the idea that many promoters are naturally defective and need to be resurrected by regulators (23). We report here that a specific 10-bp AT-rich sequence, when present in the spacer and juxtaposed to the 5′ end of the -10 element of the very weak lac promoter, enhanced transcription >40-fold. In contrast, the same lac promoter is stimulated only 10-fold by its cognate activator CRP under identical conditions. The demonstration that a specific sequence at -15 can enhance the promoter strength in vitro showed that the increased transcription is also an intrinsic property of the promoter rather than resurrection of the promoter by a putative activator protein binding to the -15 sequence in vivo. The -15 sequence does not put the P1–6 promoter into a new class, among the σ70-dependent E. coli promoters; the -15-containing promoter is entirely dependent upon the presence of the -35 element and upon a strict distance between -35 and -10 elements. Because the -35 group of promoters can function without the -15 sequence, the latter, like an UP element, is an enhancer rather than an intrinsic part of the promoter. P1–6 is still subject to regulation by an activator. For comparison, the extended -10 promoters, unlike P1–6, do not require a -35 element, and thus the spacer length is irrelevant in the latter case (19). In fact, by sliding the TG sequence by 1 bp close to the -10 element, we were able to convert P1–6 into an extended -10 type, which showed no dependency on the -35 element (Fig. 3, lane 8). The juxtaposition of the -15 sequence in the spacer to the -10 element is vital; shifting the -15 sequence even by 1 bp upstream made the sequence element inactive (Table 1, lines 30–31).
Although the -35 proximal segment (from -30 to -21) of the spacer was not systematically analyzed and may influence the property of the -15 region in an unknown way; nevertheless, we established a strong linkage between the stereochemical property of the entire spacer sequence and the intrinsic strength of the promoter.
Revelation of the -15 sequence provides an example of a promoter in which a specific sequence in the spacer region can contribute in a major way in promoter function. The -15 sequence resulted in a stronger open complex formation. It increased affinity for RNA polymerase 10-fold and the rate of open complex formation 4-fold, predicting a 40-fold synergistic effect on transcription, which was precisely observed. The enhancing effect of -15 sequence on open complex formation especially at lower temperatures is more interesting and needs further investigation. Most E. coli promoters, including the wild-type lac, are inactive at temperatures below 20°C, very likely due to the thermal energy requirement for DNA duplex deformation (12, 47) although, once isomerized, they remain open at lower temperatures (48). It indicates that P1–6 has a reduced energy barrier for isomerization during formation of the open complex.
The enhancement of RNA polymerase binding and open complex formation is likely the consequence of contacts between RNA polymerase and the -15 sequence. From the 6.5-Å resolution structure of Taq RNA polymerase–DNA complex (49), two types of contacts are feasible: (i) the σ 3.0 region is within interacting distance of the major groove of the -17/-12 segment, and (ii) residues in the β′ Zn2+ binding domain are close for minor groove contacts in the -22/-18 region. The Arg-35 residue in the β′ Zn2+ binding domain seems to be favorably located (49) to penetrate into the narrow minor grooves of the TTTTT and AATTT sequences in the -15 region discussed above. It will be important to investigate the major and minor groove contacts, which are on the same face of DNA.
Abbreviation: CRP, cAMP receptor protein.
References
- 1.Lisser, S. & Margalit, H. (1993) Nucleic Acids Res. 21, 1507-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegele, D. A., Hu, J. C., Walter, W. A. & Gross, C. A. (1989) J. Mol. Biol. 206, 591-603. [DOI] [PubMed] [Google Scholar]
- 3.Waldburger, C., Gardella, T., Wong, R. & Susskind, M. M. (1990) J. Mol. Biol. 215, 267-276. [DOI] [PubMed] [Google Scholar]
- 4.Roberts, C. W. & Roberts, J. W. (1996) Cell 86, 495-501. [DOI] [PubMed] [Google Scholar]
- 5.Gardella, T., Moyle, H. & Susskind, M. M. (1989) J. Mol. Biol. 206, 579-590. [DOI] [PubMed] [Google Scholar]
- 6.Zuber, P., Healy, J., Carter, H. L., 3rd, Cutting, S., Moran, C. P., Jr., & Losick, R. (1989) J. Mol. Biol. 206, 605-614. [DOI] [PubMed] [Google Scholar]
- 7.deHaseth, P. L. & Helmann, J. D. (1995) Mol. Microbiol. 16, 817-824. [DOI] [PubMed] [Google Scholar]
- 8.Juang, Y. L. & Helmann, J. D. (1994) J. Mol. Biol. 235, 1470-1488. [DOI] [PubMed] [Google Scholar]
- 9.Panaghie, G., Aiyar, S. E., Bobb, K. L., Hayward, R. S. & de Haseth, P. L. (2000) J. Mol. Biol. 299, 1217-1230. [DOI] [PubMed] [Google Scholar]
- 10.Helmann, J. D. & deHaseth, P. L. (1999) Biochemistry 38, 5959-5967. [DOI] [PubMed] [Google Scholar]
- 11.Siebenlist, U., Simpson, R. B. & Gilbert, W. (1980) Cell 20, 269-281. [DOI] [PubMed] [Google Scholar]
- 12.Kirkegaard, K., Buc, H., Spassky, A. & Wang, J. C. (1983) Proc. Natl. Acad. Sci. USA. 80, 2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh, W. C., Ross, W. & Record, M. T., Jr. (1993) Science 259, 358-361. [DOI] [PubMed] [Google Scholar]
- 14.Zaychikov, E., Denissova, L., Meier, T., Gotte, M. & Heumann, H. (1997) J. Biol. Chem. 272, 2259-2267. [DOI] [PubMed] [Google Scholar]
- 15.Helmann, J. D. & Chamberlin, M. J. (1988) Annu. Rev. Biochem. 57, 839-872. [DOI] [PubMed] [Google Scholar]
- 16.McClure, W. R. (1985) Annu. Rev. Biochem. 54, 171-204. [DOI] [PubMed] [Google Scholar]
- 17.Young, B. A., Gruber, T. M. & Gross, C. A. (2002) Cell 109, 417-420. [DOI] [PubMed] [Google Scholar]
- 18.Voskuil, M. I. & Chambliss, G. H. (1998) Nucleic Acids Res. 26, 3584-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bown, J., Barne, K., Minchin, S. & Busby, S. (1997) Nucleic Acids Mol. Biol. 11, 41-52. [Google Scholar]
- 20.Burr, T., Mitchell, J., Kolb, A., Minchin, S. & Busby, S. (2000) Nucleic Acids Mol. Biol. 28, 1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell, J. E., Zheng, D., Busby, S. J. & Minchin, S. D. (2003) Nucleic Acids Mol. Biol. 31, 4689-4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barne, K. A., Bown, J. A., Busby, S. J. & Minchin, S. D. (1997) EMBO J. 16, 4034-4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adhya, S., Gottesman, M., Garges, S. & Oppenheim, A. (1993) Gene 132, 1-6. [DOI] [PubMed] [Google Scholar]
- 24.Ross, W., Aiyar, S. E., Salomon, J. & Gourse, R. L. (1998) J. Bacteriol. 180, 5375-5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrem, S. T., Gaal, T., Ross, W. & Gourse, R. L. (1998) Proc. Natl. Acad. Sci. USA 95, 9761-9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flatow, U., Rajendrakumar, G. V. & Garges, S. (1996) J. Bacteriol. 178, 2436-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ross, W., Gosink, K. K., Salomon, J., Igarashi, K., Zou, C., Ishihama, A., Severinov, K. & Gourse, R. L. (1993) Science 262, 1407-1413. [DOI] [PubMed] [Google Scholar]
- 28.Helmann, J. D. (1995) Nucleic Acids Res. 23, 2351-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolb, A., Busby, S., Buc, H., Garges, S. & Adhya, S. (1993) Annu. Rev. Biochem. 62, 749-795. [DOI] [PubMed] [Google Scholar]
- 30.Sukhodolets, M. V. & Jin, D. J. (1998) J. Biol. Chem. 273, 7018-7023. [DOI] [PubMed] [Google Scholar]
- 31.Ryu, S., Kim, J., Adhya, S. & Garges, S. (1993) Proc. Natl. Acad. Sci. USA 90, 75-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Geanacopoulos, M. & Adhya, S. (1997) J. Bacteriol. 179, 228-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu, M., Gupte, G., Roy, S., Bandwar, R. P., Patel, S. S. & Garges, S. (2003) J. Biol. Chem. 278, 39755-39761. [DOI] [PubMed] [Google Scholar]
- 34.Ryu, S., Fujita, N., Ishihama, A. & Adhya, S. (1998) Gene 223, 235-245. [DOI] [PubMed] [Google Scholar]
- 35.Sasse-Dwight, S. & Gralla, J. D. (1989) J. Biol. Chem. 264, 8074-8081. [PubMed] [Google Scholar]
- 36.Peterson, M. L. & Reznikoff, W. S. (1985) J. Mol. Biol. 185, 535-543. [DOI] [PubMed] [Google Scholar]
- 37.deHaseth, P. L., Zupancic, M. L. & Record, M. T. J. (1998) J. Bacteriol. 180, 3019-3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burd, J. F., Wartell, R. M., Dodgson, J. B. & Wells, R. D. (1975) J. Biol. Chem. 250, 5109-5113. [PubMed] [Google Scholar]
- 39.Early, T. A., Kearns, D. R., Burd, J. F., Larson, J. E. & Wells, R. D. (1977) Biochemistry 16, 541-551. [DOI] [PubMed] [Google Scholar]
- 40.Warne, S. E. & deHaseth, P. L. (1993) Biochemistry 32, 6134-6140. [DOI] [PubMed] [Google Scholar]
- 41.Bhattacharyya, D. & Bansal, M. (1990) J. Biomol. Struct. Dyn. 8, 539-572. [DOI] [PubMed] [Google Scholar]
- 42.Zhurkin, V. B., Ulyanov, N. B., Gorin, A. A. & Jernigan, R. L. (1991) Proc. Natl. Acad. Sci. USA 88, 7046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Olson, W. K., Gorin, A. A., Lu, X.-J., Hock, L. M. & Zhurkin, V. B. (1998) Proc. Natl. Acad. Sci. USA. 95, 11163-11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dickerson, R. E. (1998) Nucleic Acids Res. 15, 1906-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ozoline, O. L., Deev, A. A. & Trifonov, E. N. (1999) J. Biomol. Struct. Dyn. 16, 825-831. [DOI] [PubMed] [Google Scholar]
- 46.Walter, G., Zillig, W., Palm, P. & Fuchs, E. (1967) Eur. J. Biochem. 3, 194-201. [DOI] [PubMed] [Google Scholar]
- 47.Harley, C. B. & Reynolds R. P. (1987) Nucleic Acids Res. 15, 2343-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamali-Moghaddam, M. & Geiduschek, E. P. (2003) J. Biol. Chem. 278, 29701-29709. [DOI] [PubMed] [Google Scholar]
- 49.Murakami, K. S., Masuda, S., Campbell, E. A., Muzzin, O. & Darst, S. A. (2002) Science 296, 1285-1290. [DOI] [PubMed] [Google Scholar]