Abstract
We utilized a novel ratiometric nanoquantum dot fluorescence resonance energy transfer (NQD-FRET) optical sensor to quantitatively measure oxygen dynamics from single cell microdomains during hypoxic episodes as well as during 4-aminopyridine (4-AP)-induced spontaneous seizure-like events in rat hippocampal slices. Coupling oxygen sensing with electrical recordings, we found the greatest reduction in the O2 concentration ([O2]) in the densely packed cell body stratum (st.) pyramidale layer of the CA1 and differential layer-specific O2 dynamics between the st. pyramidale and st. oriens layers. These hypoxic decrements occurred up to several seconds before seizure onset could be electrically measured extracellularly. Without 4-AP, we quantified a narrow range of [O2], similar to the endogenous hypoxia found before epileptiform activity, which permits a quiescent network to enter into a seizure-like state. We demonstrated layer-specific patterns of O2 utilization accompanying layer-specific neuronal interplay in seizure. None of the oxygen overshoot artifacts seen with polarographic measurement techniques were observed. We therefore conclude that endogenously generated hypoxia may be more than just a consequence of increased cellular excitability but an influential and critical factor for orchestrating network dynamics associated with epileptiform activity.
Keywords: hippocampus, hypoxia, epilepsy, FRET, sensors, preseizure state, seizure termination
the mammalian brain is dependent on an adequate and continuous supply of oxygen (Hochachka and Guppy 1987). Its necessity stems from a central role to produce adenosine triphosphate (ATP), the primary source of cellular energy required to maintain structural and functional integrity. With minimal O2 storage ability, a tightly regulated balance between supply and demand is necessary to maintain proper brain functioning, where too little or too much can cause serious consequences. This low tolerance to changes in oxygen concentration ([O2]) renders the central nervous system exceedingly vulnerable to its O2 supply, and when the balance between metabolism and delivery become compromised, so does physiological activity (Schiff and Somjen 1987).
Although all brain cells perish during prolonged hypoxia, the initial cellular response will inevitably cause membrane depolarization before function is arrested and cell death ensues (Czéh et al. 1993; Somjen 2004). This initial hypoxic depolarization becomes increasingly important during pathological brain states such as epilepsy where excessive neuronal firing produces brief hypoxic states within the interstitium (Folbergrova et al. 1981; Bahar et al. 2006). Previous reports suggest hypoxia as a source for cellular hyperexcitability (Schiff and Somjen 1985a) and as the leading cause for certain epileptic attacks (Rossen et al. 1943; Madison and Niedermeyer 1970; Volpe 2001), yet pinpointing ictal onset to changes in [O2] remains difficult to test in vivo as confounding responses to system level hypoxia, such as hypocapnia and an increase in blood flow complicate interpretation. To study purely hypoxic responses, we utilized a hippocampal slice in which seizures could be studied as a consequence of pharmacologic exposure or changes in oxygen concentration within the perfusate.
To accurately evaluate O2 during epilepsy one must be able to measure direct [O2] with high spatiotemporal resolution. We seek to overcome some of the spatial, temporal, and sensitivity limitations from previous methodologies that either indirectly monitor hemodynamic (Ogawa et al. 1990; Vanzetta and Grinvald 1999; Wolf et al. 2007) and metabolic changes (LaManna et al. 1987; Lakowicz et al. 1992) or more direct [O2] quantification using polarography (Clark 1956; Silver 1973), binding of nitroimidazole based components (Koch 2002), and electron paramagnetic resonance (Swartz and Clarkson 1998). To improve spatial and temporal resolution, more recent advances in direct O2 sensing have applied optical methods utilizing phosphorescence quenching dyes that linearly and reversibly change luminescence depending on [O2] (Kautsky and Hirsch 1931; Vanderkooi et al. 1987; Papkovsky and O'Riordan 2005; Sakadzic et al. 2010; Ingram et al. 2013). In the following study we utilized a variety of phosphorescence quenching oxygen sensors to measure extracellular [O2] dynamics with several hundred-millisecond temporal resolution within excitatory and inhibitory microdomains during spontaneous seizure-like events (SLEs). In a companion paper (Wei et al. 2014), we explore the computational modeling required to better understand these complex dynamics.
METHODS
Animals.
Experiments were performed on male Sprague-Dawley rats (P21-P35) under a Pennsylvania State University Institutional Animal Care and Use Committee-approved protocol.
Electrophysiology.
Animals were anesthetized using diethyl-ether and decapitated, brains removed, the hippocampus isolated, and transverse 350- to 400-μm sections cut in cold dissection buffer (in mM: 2.6 KCl, 1.23 NaH2PO4, 24 NaHCO3, 0.1 CaCl2, 2 MgCl2, 205 sucrose, and 20 glucose, 3°C) using a vibratome.
Slices were then incubated for a minimum of 1 h in artificial cerebral spinal fluid (aCSF in mM: 130 NaCl, 2 MgSO4, 3.5 KCl, 2 CaCl2, 10 glucose, 2.5 NaH2PO4, 24 NaHCO3 at pH 7.3, 30°C) oxygenated with 95% O2-5% CO2. Recordings were performed using (aCSF in mM: 130 NaCl, 0.6 MgSO4, 3.5 KCl, 1 CaCl2, 10 glucose, 2.5 NaH2PO4, and 24 NaHCO3 at pH 7.3, 32–34°C) oxygenated with 95% O2-5% CO2 at a chamber perfusion rate of 1 ml/min.
Our experiments were carried out in submersion configuration to have accurate control over the oxygen concentration that the tissue is exposed to, in contrast to an interface configuration where separate gas and liquid phases are used and the oxygen extraction from the different phases is dissimilar.
Extracellular recordings were performed using pulled borosilicate glass micropipettes (1–3 MΩ, 0.9% NaCl) and current-clamp recordings using micropipettes (4–7 MΩ) containing the following (in mM): 116 K gluconate, 6 KCl, 0.5 EGTA, 20 HEPES, 10 phosphocreatine, 0.3 NaGTP, 2 NaCl, 4 MgATP, and 0.3% Neurobiotin at pH 7.25, 295 mOsm. For intracellular experiments, the membrane potential of CA1 st. pyramidale pyramidal cells or st. oriens interneurons was held at −75 to −80 mV and negative and positive square wave current pulses were injected in increments of 50–100 pA for 500 ms to determine the membrane properties of the cells to further confirm the identity of each cell type. Electrical recordings were performed using an Axoclamp 92 2B MCC700 (Molecular Devices) amplifier filtered (5-kHz whole cell, 1-kHz extracellular) and digitized at 10 kHz (Digidata and Pclamp7; Molecular Devices).
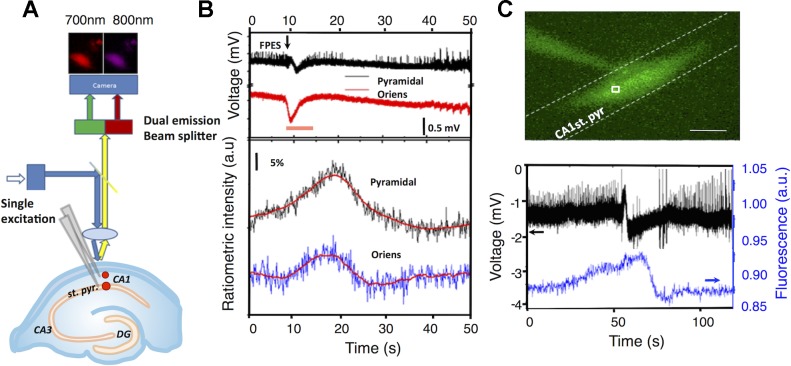
Spontaneous seizures were evoked by bath application of the potassium channel blocker 4-AP (200 μM) and raising the bath temperature to 34°C (Perreault and Avoli 1991). Alignment of seizure onset times for analysis was determined by the consistently identifiable electrical fast positive extracellular shift (FPES; 1–5 mV; Žiburkus et al. 2006, 2013). Seizure termination was determined by the cessation of spike activity and a return to baseline membrane potential. Experiments were conducted on a Zeiss Axioskop2 FSPlus (Hg bulb illumination using a Archoplan 10×/0.30w objective) microscope equipped with a dual emission beam splitter (Optical Insights, Santa Fe, NM) and Sensicam EM camera (Cooke Instruments) driven with IPLab software (BioVision Technologies) at an acquisition rate of 5 frames/second.
Probes encapsulated by biologically localized embedding.
Spherical oxygen-sensitive probes encapsulated by biologically localized embedding (PEBBLEs; 30–60 nm) were constructed using the oxygen-sensing dye Pd-meso-tetra-(4-carboxyphenyl) porphyrin (Oxyphor G2; emission 800 nm) and Hilyte Fluor 680 (emission 680 nm) reference dye entrapped within a polyacrylamide matrix (Koo et al. 2010).
In a stable ground state with no excitation, an oxygen-sensing dye has all of its molecular orbitals occupied with electrons. During absorption, one electron moves into a higher molecular orbital known as singlet state that can then relax into a lower energy triplet state (Lichtman and Conchello 2005). As both states relax back to ground state they release excess energy via light emission. As an electron returns to ground state a photon is emitted during the fast nanosecond singlet fluorescence state as well as during a longer lived microsecond phosphorescence triplet state (Bergman 1968). Oxygen sensing occurs by a process of energy transfer, where the excited triplet state of the oxygen-sensing dye chromophore is linearly and reversibly quenched by an electron orbital overlap from the triplet ground state of oxygen.
PEBBLEs were dissolved in phosphate-buffered saline (PBS) at a concentration of 10 mg/ml, and picoliter concentrations were injected into the CA1 st. pyramidale and st. oriens using a syringe pump (UMC4; World Precision Instruments, Sarasota, FL). For ratiometric imaging, a single excitation frequency (450 ± 30 nm) was used to simultaneously excite both dyes and the emission beam was passed through a dual view emission beam splitter and recorded at 700 ± 30 nm (Hilyte Fluor 680) and 800 ± 40 nm (Oxyphor G2).
PEBBLEs containing platinum 120 (II) octaethylporphine ketone (PtOEPK; emission 720 nm) and Bodipy (emission 620 nm) were excited at a wavelength of 577 nm.
Ru(phen)3-encapsulated liposomes.
Unilamminar liposomes were prepared using a 4:2:1 mixture of dipalmitoylphosphadidylcholine (DPPC):distearoylphosphatidyl-ethanolamine-N-poly 2000 (DSPE-PEG):cholesterol (Avanti Polar Lipids, Alabaster, AL) (Marik et al. 2007) dissolved in a 2:1 mixture of choloroform:methanol that was evaporated under nitrogen or rotoevaporation to form a thin lipid film. The film was then stored under vacuum overnight to remove any organic residue and frozen under argon at −20°C until use. Daily, films consisting of 10 mg DPPC, 5 mg DSPE-PEG, and 2.4 mg cholesterol were hydrated with 1 ml of 1× PBS containing 10 mM of the oxygen-sensing dye Ru(phen)3 (Sigma-Aldrich) (Carraway et al. 1991; McNamara et al. 1999). The lipid mixture was then sonicated to clarity in a cylindrical sonicator (Laboratory Supplies, Hicksville, NY). To remove unencaspulated Ru(phen)3, liposomes were then centrifuged through Sephadex G-50 as described by Fry et al. (1978). To prepare the Sephadex column, 10 g of Sephadex G-50 (Sigma-Aldrich) were mixed with 0.9% NaCl and allowed to swell overnight. A glass wool plug was placed at the bottom of an empty 1-ml syringe, and 1 ml of the Sephadex G-50 solution was added to the syringe. The syringe was then centrifuged at 2,000 rpm for 3 min to remove all excess 0.9% NaCl and form a dry solid Sephadex column. Additional Sephadex solution was then added to the column and centrifuged until the column filled 1 ml of the syringe. Then, 200 μl of the liposome and dye mixture were added to the column and centrifuged at 2,000 rpm for 3 min so that the excess free dye was contained within the Sephadex column and the flow through contained encapsulated liposomes. The encapsulated liposomes were then passed through the column two additional times to ensure excess free dye was removed. Finally, the dye encapsulated liposomes were passed through a mini-extruder (Avanti Polar Lipids, Alabaster, Alabama) three times each in 100-nm and 50-nm pore size filters.
Fluorescence resonance energy transfer-excited ratiometric oxygen sensors.
Complete technical details on the fabrication of a ratiometric nanoquantum dot (NQD) fluorescence resonance energy transfer (FRET)-based biological oxygen sensor can be found in Ingram et al. (2013). Briefly, a biologically inert and oxygen permeable optode matrix was prepared by dissolving 22 mg of polyvinyl chloride (PVC) and 44 mg of bis 2-ethylhexyl sebacate in 1.5 ml tetrahydrofuran (THF). Then, 10 ng of the phosphorescence quenching dye PtOEPK and 3.3 ng ZnS-coated CdSe NQDs (6 nm; Ocean NanoTeach) were added to the polymer mixture, thoroughly mixed, and evaporated to 1 ml.
To fabricate optode-coated microelectrodes, 150 pulled borosilicate microelectrodes (1–3 MΩ) were filled with 0.9% NaCl, mounted onto a micromanipulator, and submerged 10 times within the optode matrix to deposit a thin (<50 nm) homogeneous coating on the distal portion of the electrode (Barker et al. 1998). The electrode was then thoroughly rinsed with deionized water and dried under a stream of N2 to ensure complete removal of THF. The optode-coated microelectrode was then mounted onto a micromanipulator and placed at a depth of ∼100 μm into extracellular matrices of the CA1 st. pyramidale and st. oriens. A single 450-nm excitation was used to excite the NQDs. NQD emission occurs at 590 nm, the same wavelength that PtOEPK absorbs. Due to this spectral overlap and the close acceptor donor interactions from the PVC matrix, FRET transfer from NQDs can be then used to excite PtOEPK. For ratiometric recordings, the emission beam was then passed through a dual view emission beam splitter and the luminescence at 590 nm (NQDs) and 750 nm (PtOEPK) was recorded.
All statistical results are reported as means ± SD.
RESULTS
Oxygen sensing during epileptiform activity utilizing PEBBLEs and liposome nanosensors.
We begin with a reporting of results that did not work adequately. Despite considerable interest and activity in the creation of nanoparticle-sensing technology in biology, we found that for this particular set of experiments that such particles appeared infeasible for a number of reasons which we shall detail. We report small numbers of anecdotal results and make a strong case for the use of the electro-optode technology that enabled us to make reproducible layer-specific oxygen and electrical measurements.
Simultaneous optical and electrical activity were recorded from oxygen-sensitive PEBBLEs injected in the st. pyramidale and st. oriens during recurrent SLEs during exposure to 4-AP (n = 2; ≥5 s ictal-like network events with substantial extracellular potential shifts; Fig. 1B). Changes in [O2] were monitored by recording changes in the luminescence intensity of Oxyphor G2, which linearly and reversibly quenches depending on [O2] (Dunphy et al. 2002). To account for light, concentration, or equipment variability, the luminescence ratio between the oxygen insensitive Hilyte 680 over Oxyphor G2 luminescence was used to monitor absolute changes in [O2]. An increase in Oxyphor G2 luminescence signaling decrease in [O2] was observed before the fast positive excitatory spike (FPES), an electrical signature used to align seizure start times (Žiburkus et al. 2006, 2013). After ictal onset, the greatest intensity increase was recorded within the densely packed cell body layer of the st. pyramidale. In both layers, the luminescence would once again reach baseline values after ictal termination. To confirm the PEBBLE response, Ru(phen)3-encapsulated liposomes were injected and localized with in the CA1 st. pyramidale (Fig. 1C, top). During a SLE, Ru(phen)3 luminescence again increased before the FPES and returned to baseline after ictal termination (Fig. 1C, bottom; n = 3).
Fig. 1.
Probes encapsulated by biologically localized embedding (PEBBLE) and liposome nanosensing during spontaneous seizure events. A: PEBBLE nanosensors were injected into the CA1 stratum (st.) pyramidale and st. oriens. Absorption at 450 ± 30 nm was used to excite the reference (HiLyte 680) and oxygen sensitive dye (Oxyphor G2) and the emission beam was split into two wavelengths (700 ± 30 and 800 ± 40 nm) through a dual emission beam splitter and captured on a single CCD camera. Simultaneous electrical activity was recorded using local field potential electrodes in the st. oriens and st. pyramidale. B: ratiometric intensity (bottom) and local field potential recordings (top) during a 4-aminopyridine (4-AP)-induced spontaneous seizure-like event (orange bar) in the CA1 st. oriens and st. pyramidale (n = 2). C: picoinjection of liposomes within CA1 st. pyramidale. Optical recordings were monitored over a 25 × 25 μm region of interest. Scale bar = 100 μm. Simultaneous recording of neuronal activity and Ru(phen)3 intensity within the CA1 pyramidal layer during 4-AP-induced spontaneous seizure event, demonstrating a decrease in oxygen in advance of the electrical correlate of seizure-like event (n = 3). FPES, fast positive extracellular shift; DG, dentate gyrus; a.u., arbitrary units.
Both PEBBLE and liposome techniques were cumbersome in this setting. We avoided utilizing techniques such as a gene gun with PEBBLEs (Koo et al. 2004), since we wished to specifically localize our sensor within the extracellular space about neurons. The pressure injection technique employed above produced inactive tissue within the core of the PEBBLE bolus, and our recordings were therefore made at the edge of a bolus that spanned the layers of interest. In contrast, although liposomes could diffuse within the extracellular space, they suffered from both mechanical stability, as well as rapid photobleaching. Furthermore, we cannot exclude the possibility that despite extracellular tissue injection that liposomes or PEBBLEs did not get taken up intracellularly. If there was fractional cellular uptake of sensors, then our local mean-field measurements from regions of interest would represent a lower bound on the extracellular oxygen pressure. We were unable to find a reliable means to insert nanoscaled sensors into these slices. Accordingly, these experiments were not further pursued.
To address these technical issues, we incorporated a novel NQD-FRET-sensing technology that we recently developed (Ingram et al. 2013).
Quantitative CA1 layer-specific oxygen requirements.
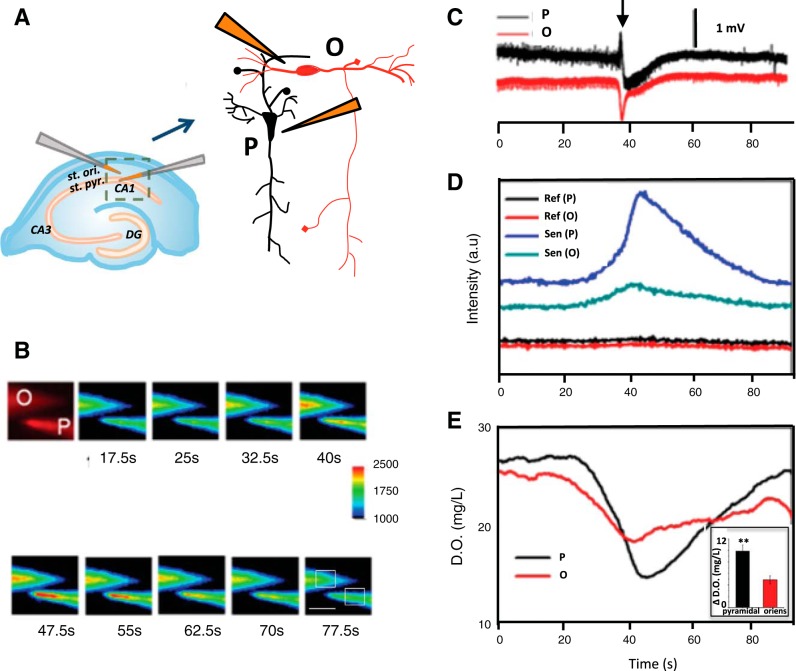
To more directly sample CA1 layer-specific [O2] during neuronal activity, optode-coated microelectrodes were positioned within the st. pyramidale and st. oriens and simultaneous optical and electrical traces were recorded by monitoring the luminescence from a 25 × 25 μm region of interest from the optode coated microelectrode tip (Fig. 2, A and B). Before seizure onset, basal [O2] was calculated to be 23.8 ± 2.7 mg/l at a depth of 100 μm within the st. pyramidale and 25.5 ± 3.7 mg/l in the st. oriens in the presence of 200 μM 4-AP. Because 4-AP increases electrical activity within the tissue, it will decrease the concentration of oxygen measured. During an individual SLE (n = 16 slices, 26 SLEs), further luminescence changes were found in the oxygen-sensitive dye while the NQDs luminescence showed no change (Fig. 2C). Maximum changes in [O2] were 6.5 ± 3.9 mg/l [O2] within the st. pyramidale and were significantly less (Student's t-test, P < 0.05) in the st. oriens, corresponding to 4.1 ± 2.6 mg/l [O2]. An increase in O2 consumption was found before FPES onset by 6.7 ± 6.8 s within the st. pyramidale and 6.8 ± 7.0 s within the st. oriens. Hypoxic durations (30 ± 10s) substantially exceeded the duration of the SLE (10.4 ± 7.2 s) in both layers before returning to baseline.
Fig. 2.
Dual-layer recording with optode coated microelectrodes. A: diagram of the experimental configurations for dual-layer recording within the st. oriens (O) and st. pyramidale (P). B: luminescence changes from oxygen-sensitive dye emission (750 ± 30 nm) recorded during a seizure event. Scale bar = 100 μm. C: extracellular electrical recording in the CA1 st. pyramidale and st. oriens during a single seizure event. FPES onset occurred at arrowhead. D: raw luminescence intensities recorded from platinum 120 (II) octaethylporphine ketone (PtOEPK; Sen, sensing) and nanoquantum dots (NQDs; Ref, reference) from st. oriens (O) and st. pyramidale (P). E: calibrated dissolved O2 (D.O.) concentration during a seizure-like event (SLE). Inset: demonstrates maximum average O2 concentration ([O2]) change during 26 SLEs in each layer (Student's t-test, **P < 0.05).
Hypoxia and neuronal activity.
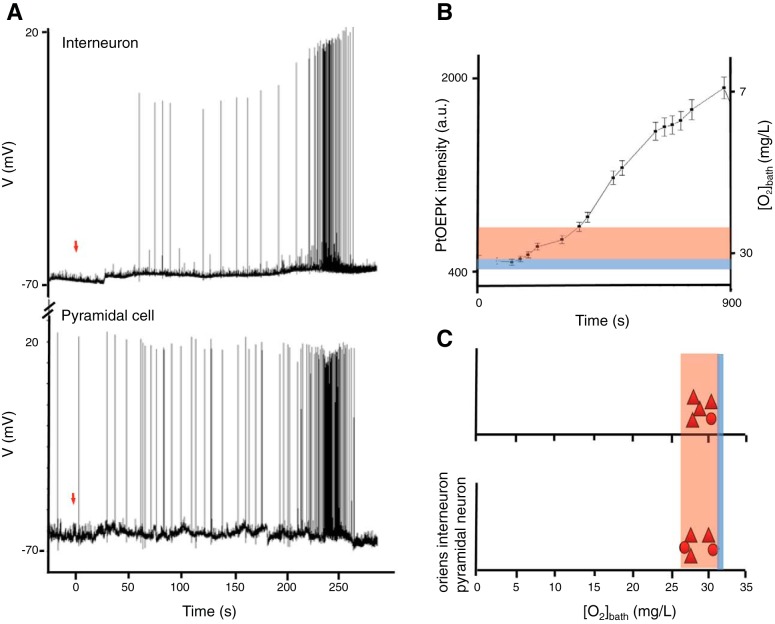
To correlate the neuronal response to hypoxia, we quantified how much of a hypoxic decrease was required to impose changes in neuronal activity. In the absence of a slice, the recording chamber was first saturated by perfusing aCSF aerated with 95% O2-5% CO2 at a perfusion rate of 1 ml/min for 30 min. An optode-coated microelectrode was then submerged in the recording chamber. After saturation, the perfusate was switched to one aerated with 95% N2-5% CO2 (t = 0 s). As N2 infused perfusate slowly entered the experimental setup, the ratiometric optode intensity was monitored for a duration of 5 s every 30–45 s (Fig. 3B). Within 3 min, oxygen-sensitive dye luminescence began to increase indicating O2 was decreased in the recording chamber. Saturation with N2-infused perfusate was reached in ∼12 min. This same experiment was replicated while patch clamping individual pyramidal cells (n = 5) or oriens interneurons (n = 5). After 2 min of recording baseline cellular activity the external bath solution was switched from 95% O2-5% CO2 to 95% N2-5% CO2 and the electrical activity was recorded. Experiments were conducted on both cell types in the absence of 4-AP or cells bathed in 200 μM 4-AP for more than 20 min with no previous SLEs.
Fig. 3.
Seizures induced by hypoxia. A: simultaneous patch-clamp recording from 2 CA1 neurons: an oriens interneuron (top) and a pyramidal neuron (bottom) during hypoxia. SLEs occurred when the bath [O2] was lowered to 31 mg/l. Red arrow (t = 0) indicates the time when 95% O2-5% CO2 was switched to 95% N2-5% CO2. B: quantitative monitoring of [O2] within the bath recording chamber when switching between artificial cerebral spinal fluid (aCSF) bubbled with 95% O2-5% CO2 and 95% N2-5% CO2. In contrast to A, these data reflect the full course of hypoxia, through the region where seizures are observed and throughout the region where seizures are no longer supported when oxygen pressures are too low. Next to the normoxic chamber levels of [O2] (blue bar) is the band of hypoxia (orange band) that supported all SLEs during patch clamp recordings of individual cells. C: [O2] bath in which SLEs occurred during patch clamp recordings of CA1 pyramidal (n = 4 without 4-AP and n = 1 with 4-AP) or oriens interneurons (n = 3 without 4-AP and n = 2 with 4-AP). Baseline values of 32 mg/l are denoted by the blue bar.
In fully saturated buffer, chamber [O2] was 32–33 mg/l. After we switched to the N2-infused perfusate, the initial cellular response demonstrated epileptiform-like activity (Fig. 3A) in pyramidal cells (n = 4 without 4-AP and n = 1 with 4-AP; Fig. 3C) and oriens interneurons (n = 3 without 4-AP and n = 2 with 4-AP; Fig. 3C). Increases in epileptiform like activity occurred when the chamber [O2] was 31–27 mg (Fig. 3, B and C, in red). No electrical signs of spreading depression were observed.
DISCUSSION
Using a series of phosphorescent quenching O2 sensors, we were able to probe extracellular [O2] before, during, and following termination of spontaneous SLEs. The optical response in all sensors revealed that basal [O2] levels in the hippocampus remain stable until several seconds before an electrically identifiable marker for seizure onset. This preseizure decrease in [O2] suggests an increased metabolic demand of the tissue that precedes measures of extracellular electrical seizure activity. Although the O2 requirements in the brain have been extensively studied, they have primarily focused on metabolism during increased states of firing (Attwell and Laughlin 2001; Attwell and Iadecola 2002; Lennie 2003). Here we demonstrate the first experimental evidence that a local hypoxic decrease can occur seconds before changes reflected in the macroscopic local field potential activity.
If widespread increased neuronal firing is not responsible for increased metabolism, then what causes a decrease in [O2] before FPES onset? Such a result may in part be the summation of active oxygen-consuming transport mechanisms that are attempting to restore ionic gradients before SLE onset. In addition, the sparse firing of interneurons with widespread connectivity to other neurons could account for an increased metabolic demand out of proportion to local field potential changes. In similar in vitro SLEs, a subset of oriens interneurons increase firing before FPES onset (Žiburkus et al. 2006, 2013). These inhibitory interneurons project GABAergic terminals to the apical dendrites of the CA1 pyramidal neurons, which during increased firing cause Cl− to flood pyramidal cells. Increased release of GABA by a variety of other inhibitory interneurons also causes Cl loading (Dzhala and Staley 2003), and may reflect an inhibitory veto on excessive excitatory activity (Jiruska et al. 2013). This influx of Cl− must be removed by coupled transport (i.e., Cl− coupled with K+, HCO3−, and Na+), aided by K+ transport through the Na+-K+-ATPase pump. Additionally, excessive glutamatergic innervations from the CA3 also synapse on CA1 pyramidal neurons (Li et al. 1994), which are highly active during 4-AP seizure initiation (Penă and Tapia 1999). If metabolic ion restoration fails to keep up with ion flux, or in vivo if local blood flow failed to keep up with metabolic demand, [O2] could play a previously unrecognized role in spontaneous seizure initiation.
This preseizure hypoxic event also raises the question of what defines the point of ictal onset or from a network perspective what changes render seizure onset unavoidable? Seizure initiation may be best described as an irreversible network state from which an ictal event will ensue, but this point is typically determined electrically from the initial preceding EEG deflection (or in slices by the FPES). We propose that electrical markers of seizure onset may be only one aspect of determining when a seizure is about to begin. If seizures are triggered by relatively sparse firing, then macroscopic O2 measurements may be an alternative to multiple microscopic single cell measurements to monitor onset (Cyberblit-Sabba and Schiller 2010). The possibility of a potential preseizure state has been described elsewhere (Adelson et al. 1999; Mäkiranta et al. 2005; Hawco et al. 2007; Hoshi and Tamura 1992; Litt et al. 2001; Elger and Lehnertz 1998). Although the biological basis underlying why a metabolic change would occur before ictal onset remains incompletely characterized, our data supports that a metabolic preictal marker may have a role in influencing network stability.
Our second main finding (Fig. 3) is that brief externally induced hypoxia, at a range of bath [O2] similar to that measured 100 μm deep within tissue before FPES onset, was sufficient to induce epileptiform activity. We observed a narrow band of mild hypoxia that reliably supported SLEs. This was observed regardless of the presence of 200 μM 4-AP in the perfusate.
Our third main finding is that the greatest reduction in [O2] was observed in the densely packed cell body layer of the CA1 st. pyramidale. This implies that the increased metabolic demand within this region metabolizes O2 faster than O2 diffusion from the bath is able to compensate. Likely contributing is the packing density of CA1 pyramidal neurons (Boss et al. 1987; McBain et al. 1990). The cells within the st. pyramidale of CA1 are much more tightly packed than the sparsely populated st. oriens. Within the st. pyramidale, compared with CA3, CA1 has a 50% higher density of neurons with 50% lower interstitial volume, so even if an identical change in ion flux occurred for each cell, the greater change in total ionic redistribution and subsequent O2 metabolism would be associated with the CA1 region. Focal O2 consumption may also be more prominent in this region due to the high Ca2+ conductances on the apical dendrites of CA1 pyramidal cells (Schwartzkroin and Wyler 1980), along with a high density of glutamate receptors (McDonald and Johnston 1990). N-methyl-D-aspartate receptor density is also substantially higher in CA1 than the surrounding areas (Cotman and Iversen 1987). Glutamate transport itself is a highly energetic process (Attwell and Laughlin 2001) and its excessive release during seizure may further underlie CA1 specific hypoxia. These CA1-specific responses may also be intensified by the effects of preseizure hypoxia, as depolarization, increases in extracellular [K+], and cell swelling during low O2 states are consistently higher in the CA1 than CA3 (Kawasaki et al. 1990; Pérez-Pinzón et al. 1995).
Our fourth main finding is the differential layer-specific O2 dynamics between the st. pyramidale and st. oriens. Our initial motivation to measure [O2] within these two layers was to begin to explain why oriens interneuron (oriens lacunosum-moleculare) firing failed during the excitatory inhibitory interplay seen during in vitro SLEs (Žiburkus et al. 2006, 2013). We hypothesized that the increased interneuron firing before seizure onset could deplete the interstitial [O2] in the st. oriens, induce cell-specific hypoxic spreading depression, and place the interneuron in a temporary state of inactivation or depolarization block. Although selective vulnerability to hypoxia has been reported between neuronal cell-types (Aitken and Schiff 1986; Krnjevíc et al. 1991; Zhu and Krnjevic 1994), the several micrometer resolution of our sensors cannot attribute interstitial O2 changes from a single identified cell. Nevertheless, our thin film optode data are consistent with interstitial hypoxia occurring within in the st. oriens before the SLE, which may contribute to increased neuronal firing and further drive the oriens lacunosum-moleculare into depolarization block. Such a phenomenon may help explain the failure of the inhibitory veto seen at the onset of experimental (Trevelyan et al. 2006) and human (Schevon et al. 2012) seizures. Unexpectedly, we found that as the seizure intensifies the st. oriens has a greater O2 availability than the st. pyramidale. A more available supply of [O2] in the st. oriens could help restore ionic gradients disrupted during depolarization block allowing the membrane potentials to be restored and permit return of inhibitory control (Jiruska et al. 2013). The greater hypoxic effect within the st. pyramidale also suggests that pyramidal cells in the st. pyramidale may deplete their O2 supply as the inhibitory layer restores activity and further contribute to seizure termination.
After seizure termination the observed hypoxic deficit would slowly return to baseline if not disrupted by a subsequent seizure event. We attribute this prolonged hypoxic deficit to two processes: the prolonged time scale to restore ionic gradients and the time scale of O2 diffusion through tissue. First, restoring ionic gradients following intense activity can considerably outlast (Chub et al. 2006) the period of intense firing and rapid gradient collapse. Second, based on the rate of diffusion of O2 in living tissue (2 × 10−5 cm2/s or 20 μm2/s) (Hlatky and Aplen 1985), it would take a time scale on the order of 18 s (350 μm/20 μm/s) for O2 reperfusion through 350 μm of tissue. This diffusion gradient is in strong agreement with our experimental data, where it took ∼20 s to return [O2] back to basal values after the maximum hypoxic deficit. In comparison, recordings from optode coated electrodes placed 100 μm within the tissue detected shorter hypoxic durations than 2D surface optode position beneath such slices (see Ingram et al. 2013), consistent with the longer diffusion distances required to reach the 2D surface optodes underneath the slice.
The reperfusion response from our NQD-FRET sensors is quite different than those previously observed using clark-style polarographic electrodes. Polarographic electrodes actively consume O2, and their measurements are diffusion limited. Such substantial consumption contributes to spurious oxygen depth profiles within tissue. With oxygen-sensitive dye, consumption is reduced to that of single molecule binding, which, although potentially measurable, is both much less than with polarographic techniques and reverses upon oxygen unbinding. In addition, a lack of specificity can create O2 overshoots during reoxygenation when using polargraphic electrodes. This has been confirmed in both brain slices (Schiff and Somjen 1985b) as well as during 4-AP induced epileptic events in vivo (Bahar et al. 2006). This posthypoxic overshoot has been attributed to other reactive O2 species, especially in vitro where the vascular system is absent, as well as in vivo to increased blood flow carrying oxygenated hemoglobin (Pérez-Pinzón et al. 1997). The phosphorescent NQD-FRET sensors we tested in this report never generated spurious posthypoxic overshoots.
In the companion paper (Wei et al. 2014), we use computational modeling to further interpret and explain these experimental results.
GRANTS
This work was supported by National Institutes of Health Grants R01-MH-50006 and K02-MH-01493 (to S. J. Schiff) and F32-NS-051072 (to J. R. Cressman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.I., C.Z., J.R.C., and S.J.S. conception and design of research; J.I., C.Z., J.R.C., and A.H. performed experiments; J.I. and C.Z. analyzed data; J.I., C.Z., A.H., Y.-E.K., J.Z., R.K., J.X., and S.J.S. interpreted results of experiments; J.I., C.Z., and J.R.C. prepared figures; J.I. and C.Z. drafted manuscript; J.I., C.Z., Y.W., and S.J.S. edited and revised manuscript; J.I., C.Z., and S.J.S. approved final version of manuscript.
ACKNOWLEDGMENTS
Present address of J. Ingram: Dept. of Biology, Pennsylvania College of Technology, Williamsport, PA (e-mail: jmi2@pct.edu).
Present address of Y.-E. Koo: Dept. of Chemistry, Hanyang University, Seoul, Korea (e-mail: yeleekoo@gmail.com).
REFERENCES
- Adelson P, Nemoto E, Scheuer M, Painter M, Morgan J, Yonas H. Noninvasive continuous monitoring of cerebral oxygenation periictally using near infrared spectroscopy: a preliminary report. Epilepsia 40: 1484–1489, 1999 [DOI] [PubMed] [Google Scholar]
- Aitken P, Schiff SJ. Selective neuronal vulnerability to hypoxia in vitro. Neurosci Lett 67: 92–96, 1986 [DOI] [PubMed] [Google Scholar]
- Attwell D, Iadecola C. The neural basis of functional brain imaging signals. Trends Neurosci 25: 621–625, 2002 [DOI] [PubMed] [Google Scholar]
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 21: 1133–1145, 2001 [DOI] [PubMed] [Google Scholar]
- Bahar S, Suh M, Zhao M, Schwartz TH. Intrinsic optical signal imaging of neocortical seizures: the “epileptic dip”. Neuroreport 17: 499–503, 2006 [DOI] [PubMed] [Google Scholar]
- Barker S, Thorsrud B, Kopelman R. Nitrite- and chloride-selective fluorescent nano-optodes and in vitro application to rat conceptuses. Anal Chem 70: 100–104, 1998 [DOI] [PubMed] [Google Scholar]
- Bergman I. Rapid-response atmospheric oxygen monitor based on fluorescence quenching. Nature 218: 396, 1968 [Google Scholar]
- Boss B, Turlejski K, Stanfield B, Cowan W. On the numbers of neurons in fields CA1 and CA3 of the hippocampus of Sprague-Dawley and Wistar rats. Brain Res 406: 280–287, 1987 [DOI] [PubMed] [Google Scholar]
- Carraway E, Demas J, DeGraff B, Bacon J. Photophysics and photochemistry of oxygen sensors based on luminescent transition metal complexes. Anal Chem 63: 337–342, 1991 [Google Scholar]
- Chub N, Mentis G, O'Donovanm M. Chloride-sensitive MEQ fluorescence in chick embryo motoneurons following manipulations of chloride and during spontaneous network activity. J Neurophysiol 95: 323–330, 2006 [DOI] [PubMed] [Google Scholar]
- Clark L. Monitor and control of blood and tissue oxygen tension. Trans Am Soc Artif Internal Organs 2: 41–48, 1956 [Google Scholar]
- Cotman C, Iversen L. Excitatory amino acids in the brain focus on NMDA receptors. Trends Neurosci 10: 263–265, 1987 [Google Scholar]
- Cyberblit-Sabba A, Schiller Y. Network dynamics during development of pharmacologically induced epileptic seizures in rats in vivo. J Neurosci 30: 5078–5090, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh G, Aitken PG, Somjen GG. Membrane currents in CA1 pyramidal cells during spreading depression (SD) and SD-like hypoxic depolarization. Brain Res 632: 195–208, 1993 [DOI] [PubMed] [Google Scholar]
- Dunphy I, Vinogradov S, Wilson D. Oxyphor R2 and G2: phosphors for measuring oxygen by oxygen dependent quenching of phosphorescence. Anal Biochem 310: 191–198, 2002 [DOI] [PubMed] [Google Scholar]
- Dzhala V, Staley K. Excitatory actions of endogenously released GABA contribute to initiation of ctal epileptiform activity in the developing hippocampus. J Neurosci 23: 1840–1846, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elger C, Lehnertz K. Seizure prediction by non-linear time series analysis of brain electrical activity. Eur J Neurosci 10: 786–789, 1998 [DOI] [PubMed] [Google Scholar]
- Folbergrova J, lngvar M, Siesjo B. Metabolic changes in cerebral cortex, hippocampus and cerebellum during sustained bicuculline-induced seizures. J Neurochem 37: 1228–1238, 1981 [DOI] [PubMed] [Google Scholar]
- Fry D, White J, Goldman I. Rapid separation of low molecular weight solute from liposomes without dilution. Anal Biochem 90: 809–815, 1978 [DOI] [PubMed] [Google Scholar]
- Hawco C, Bagshaw A, Lu Y, Dubeau F, Gotman J. BOLD changes occur before epileptic spikes seen on scalp EEG. Neuroimage 35: 1450–1458, 2007 [DOI] [PubMed] [Google Scholar]
- Hlatky L, Alpen E. Two-dimensional diffusion limited system for cell growth. Cell Tissue Kinet 18: 597–611, 1985 [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Guppy M. Metabolic Arrest and the Control of Biological Time. Cambridge, MA: Harvard Univ Press, 1987 [Google Scholar]
- Hoshi Y, Tamura M. Cerebral oxygenation state in chemically induced seizures in the rat; study by near infrared spectrophotometry. Adv Exp Med Biol 316: 137–142, 1992 [DOI] [PubMed] [Google Scholar]
- Ingram JM, Zhang C, Xu J, Schiff SJ. FRET excited ratiometric oxygen sensing in living tissue. J Neurosci Methods 214: 45–51, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiruska P, de Curtis M, Jefferys JG, Schevon CA, Schiff SJ, Schindler K. Synchronization and desynchronization in epilepsy: controversies and hypotheses. J Physiol 591: 787–797, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kautsky H, Hirsch A. 384 Interactions of excited dye molecules and oxygen. Ber Dtsch Chem Ges 64: 2677–2686, 1931 [Google Scholar]
- Kawasaki K, Traynelis S, Dingledine R. Different responses of CA1 and CA3 regions to hypoxia in rat hippocampal slice. J Neurophysiol 63: 385–394, 1990 [DOI] [PubMed] [Google Scholar]
- Koch C. Measurement of absolute oxygen levels in cells and tissues using oxygen sensors and 2-nitroimidazole EF5. Methods Enzymol 352: 3–31, 2002 [DOI] [PubMed] [Google Scholar]
- Koo YL, Cao Y, Kopelman R, Koo SM, Brasuel M, Philbert MA. Real-time measurements of dissolved oxygen inside live cells by organically modified silicate fluorescent nanosensors. Anal Chem 76: 2498–2505, 2004 [DOI] [PubMed] [Google Scholar]
- Koo YL, Ulbrich E, Kim G, Hah H, Strollo C, Fan W, Gurjar R, Koo S, Kopelman R. Near infrared luminescent oxygen nanosensors with nanoparticle matrix tailored sensitivity. Anal Chem 82: 8446–8455, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevíc K, Xu Y, Zhang L. Anoxic block of GABAergic IPSPs. Neurochem Res 16: 279–284, 1991 [DOI] [PubMed] [Google Scholar]
- Lakowicz J, Szmacinski H, Nowaczyk K, Johnson M. Fluorescence lifetime imaging of free and protein-bound NADH. Proc Natl Acad Sci USA 89: 1271–1275, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna J, Sick T, Pikarsky S, Rosenthal M. Detection of an oxidizable fraction of cytochrome oxidase in intact rat brain. Am J Physiol Cell Physiol 253: C477–C483, 1987 [DOI] [PubMed] [Google Scholar]
- Lennie P. The cost of cortical computation. Curr Biol 13: 493–497, 2003 [DOI] [PubMed] [Google Scholar]
- Li X, Somogyi P, Ylinen A, Buzsaki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol 339: 181–208, 1994 [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Conchello JA. Fluorescence microscopy. Nat Methods 2: 910–919, 2005 [DOI] [PubMed] [Google Scholar]
- Litt B, Esteller R, Echauz J, D'Alessandro M, Shor R, Henry T, Pennell P, Epstein C, Bakay R, Dichter M, Vachtsevanos G. Epileptic seizures may begin hours in advance of clinical onset: a report of five patients. Neuron 30: 51–64, 2001 [DOI] [PubMed] [Google Scholar]
- Madison D, Niedermeyer E. Epileptic seizures resulting from acute cerebral anoxia. J Neurol Neurosurg Psychiatry 33: 381–386, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkiranta M, Ruohonen J, Suominen K, Niinimäki J, Sonkajärvi E, Kiviniemi V, Seppänen T, Alahuhta S, Jäntti V, Tervonen O. BOLD signal increase preceeds EEG spike activitya dynamic penicillin induced focal epilepsy in deep anesthesia. Neuroimage 27: 715–724, 2005 [DOI] [PubMed] [Google Scholar]
- Marik J, Tartis MS, Zhang H, Fung JY, Kheirolomoom A, Sutcliffe JL, Ferrara KW. Long-circulating liposomes radiolabeled with [18F]fluorodipalmitin ([18F]FDP). Nucl Med Biol 34: 165–171, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain CJ, Traynelis SF, Dingledine R. Regional variation of extracellular space in the hippocampus. Science 249: 674–7, 1990 [DOI] [PubMed] [Google Scholar]
- McDonald J, Johnston M. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev 15: 41–70, 1990 [DOI] [PubMed] [Google Scholar]
- McNamara K, Rosenzweig N, Rosenzweig Z. Liposome-based optochemical nanosensors. Microchim Acta 131: 57–64, 1999 [Google Scholar]
- Ogawa S, Lee T, Kay A, Tank D. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 87: 9868–9872, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkovsky D, O'Riordan T. Emerging applications of phosphorescent metalloporphyrins. J Fluoresc 15: 569–584, 2005 [DOI] [PubMed] [Google Scholar]
- Pérez-Pinzón M, Mumford P, Rosenthal M, Sick T. Antioxidants, mitochondrial hyperoxidation and electrical recovery after anoxia in hippocampal slices. Brain Res 754: 163–170, 1997 [DOI] [PubMed] [Google Scholar]
- Pérez-Pinzón M, Tao L, Nicholson C. Extracellular potassium, volume fraction and tortuosity in rat hippocampal CA1, CA3 and cortical slices during ischemia. J Neurophysiol 74: 565–573, 1995 [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol 65: 771–785, 1991 [DOI] [PubMed] [Google Scholar]
- Penă F, Tapia R. Relationships among seizures, extracellular amino acid changes, and neurodegeneration induced by 4-aminopyridine in rat hippocampus: a microdialysis and electroencephalographic study. J Neurochem 72: 2006–2014, 1999 [DOI] [PubMed] [Google Scholar]
- Rossen R, Kabat H, Anderson J. Acute arrest of cerebral circulation in man. Arch Neurol Psychiatry 50: 510–528, 1943 [Google Scholar]
- Sakadzic S, Roussakis E, Yaseen M, Mandeville E, Srinivasan V, Arai K, Ruvinskaya S, Devor A, Lo E, Vinogradov S, Boas D. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods 7: 755–759, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevon C, Weiss S, McKhann G, Goodman R, Yuste R, Emerson R, Trevelyan A. Evidence of an inhibitory restraint of seizure activity in humans. Nat Commu 3: 1060, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff SJ, Somjen GG. Hyperexcitability following moderate hypoxia in hippocampal tissue slices. Brain Res 337: 337–340, 1985a [DOI] [PubMed] [Google Scholar]
- Schiff SJ, Somjen GG. Overshoot of oxygen pressure in post-hypoxic brain tissue: a re-evaluation. Brain Res 344: 150–153, 1985b [DOI] [PubMed] [Google Scholar]
- Schiff SJ, Somjen GG. The effect of graded hypoxia on the hippocampal slice: an in vitro model of the ischemic penumbra. Stroke 18: 30–37, 1987 [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P, Wyler A. Mechanisms underlying epileptiform burst discharge. Ann Neurol 7: 96–107, 1980 [DOI] [PubMed] [Google Scholar]
- Silver A. The oxygen micro-electrode. Adv Exp Med Biol 37A: 7–15, 1973 [DOI] [PubMed] [Google Scholar]
- Somjen GG. Ions in the Brain. New York: Oxford Univ Press, 2004 [Google Scholar]
- Swartz H, Clarkson R. The measurement of oxygen in vivo using EPR techniques. Phys Med Biol 43: 1957–1975, 1998 [DOI] [PubMed] [Google Scholar]
- Trevelyan A, Sussillo D, Watson B, Yuste R. Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J Neurosci 26: 12447–12455, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J, Maniara G, Green T, Wilson D. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem 262: 5476–5482, 1987 [PubMed] [Google Scholar]
- Vanzetta I, Grinvald A. Increased cortical oxidative metabolism due to sensory stimulation: implications for functional brain imaging. Science 286: 1555–1558, 1999 [DOI] [PubMed] [Google Scholar]
- Volpe J. Neurology of the Newborn. Philadelphia, PA: W B Saunders, 2001 [Google Scholar]
- Wei Y, Ullah G, Ingram J, Schiff SJ. Oxygen and seizure dynamics: II. Computational modeling. J Neurophysiol. First published March 26, 2014; 10.1152/jn.00541.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Ferrari M, Quaresima V. Progress of near-infrared spectroscopy and topography for brain and muscle clinical applications. J Biomed Opt 12: 062104, 2007 [DOI] [PubMed] [Google Scholar]
- Zhu P, Krnjevic K. Anoxia selectively depresses excitatory synaptic transmission in hippocampal slices. Neurosci Lett 166: 27–30, 1994 [DOI] [PubMed] [Google Scholar]
- Žiburkus J, Cressman JR, Barreto E, Schiff SJ. Interneuron and pyramidal cell interplay during in vitro seizure-like events. J Neurophysiol 95: 3948–3954, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Žiburkus J, Cressman JR, Schiff SJ. Seizures as imbalanced up states: excitatory and inhibitory conductances during seizure-like events. J Neurophysiol 109: 1296–306, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]