Abstract
GluA2-lacking, calcium-permeable α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPARs) have unique properties, but their presence at excitatory synapses in pyramidal cells is controversial. We have tested certain predictions of the model that such receptors are present in CA1 cells and show here that the polyamine spermine, but not philanthotoxin, causes use-dependent inhibition of synaptically evoked excitatory responses in stratum radiatum, but not s. oriens, in cultured and acute hippocampal slices. Stimulation of single dendritic spines by photolytic release of caged glutamate induced an N-methyl-d-aspartate receptor-independent, use- and spermine-sensitive calcium influx only at apical spines in cultured slices. Bath application of glutamate also triggered a spermine-sensitive influx of cobalt into CA1 cell dendrites in s. radiatum. Responses of single apical, but not basal, spines to photostimulation displayed prominent paired-pulse facilitation (PPF) consistent with use-dependent relief of cytoplasmic polyamine block. Responses at apical dendrites were diminished, and PPF was increased, by spermine. Intracellular application of pep2m, which inhibits recycling of GluA2-containing AMPARs, reduced apical spine responses and increased PPF. We conclude that some calcium-permeable, polyamine-sensitive AMPARs, perhaps lacking GluA2 subunits, are present at synapses on apical dendrites of CA1 pyramidal cells, which may allow distinct forms of synaptic plasticity and computation at different sets of excitatory inputs.
Keywords: calcium, glutamate, hippocampus, polyamines, synaptic transmission
fast excitatory synaptic transmission in the central nervous system is mediated by glutamate receptors having high affinity for α-amino-3-hydroxy-5-methylisoxazole-4-propionate (AMPA). AMPA receptors (AMPARs) are encoded by four genes, GluA1–4 (Collingridge et al. 2009; Hollmann and Heinemann 1994; Seeburg 1993). In the hippocampus, mature CA1 pyramidal cells express GluA1–3 (Gerfin-Moser et al. 1995; Keinanen et al. 1990). mRNA transcripts for GluA2 are edited so that a positively charged arginine replaces glutamine at the Q/R site common to all AMPAR gene products (Burnashev et al. 1992), preventing permeation by divalent cations, such as Ca2+. Typically, AMPARs that lack edited GluA2 subunits, such as homomeric GluA1 receptors, are Ca2+-permeable and sensitive to block by polyamines, although Ca2+-permeable AMPARs containing GluA2 subunits have been described (Meucci and Miller 1998). Whether Ca2+-permeable, polyamine-sensitive AMPARs are present at excitatory synapses in the hippocampus or, alternatively, whether all synaptic AMPARs are Ca2+-impermeable has important consequences for the properties of the synapses and has been the subject of much recent debate.
The lack of positive charge in the pore of GluA2-lacking AMPARs allows positively charged polyamines to block the channel from inside or outside in a use- and voltage-dependent manner (Washburn and Dingledine 1996) whether the channel is open or closed. Closed channel blockade is relieved by cation influx (Bowie et al. 1998). Therefore, activating GluA2-lacking AMPARs twice in close succession gives rise to a postsynaptically mediated paired-pulse facilitation (PPF; Rozov et al. 1998; Rozov and Burnashev 1999) when the current triggered by the first stimulus produces use-dependent relief of polyamine block. Conversely, polyamine block of open channels increases with depolarization, giving rise to the inward rectification of GluA2-lacking AMPARs (Bowie and Mayer 1995; Donevan and Rogawski 1995; Kamboj et al. 1995; Koh et al. 1995). Because of their Ca2+ permeability and voltage-dependent signaling, GluA2-lacking AMPARs have been implicated in several forms of synaptic plasticity.
The subunit composition of synaptic AMPARs is difficult to determine directly. Immunoprecipitation suggests that 90% of hippocampal GluA1 subunits are bound to a GluA2 subunit (Wenthold et al. 1996). Indeed, extrasynaptic AMPARs in excised patches from CA1 pyramidal cell somata display linear current-voltage relationships (I–V; Jonas and Sakmann 1992), as do multisynapse evoked excitatory postsynaptic currents (EPSCs) in CA1 cells in acute rat hippocampal slices (Hestrin et al. 1990), albeit without the replacement of endogenous polyamines. These data have led to the tenet that all pyramidal cell synaptic AMPARs are Ca2+-impermeable and are either GluA1-GluA2 or GluA2-GluA3 heteromers. However, other studies have suggested that some pyramidal cell AMPARs are Ca2+-permeable (Lerma et al. 1994; Noh et al. 2005; Ogoshi and Weiss 2003; Rozov et al. 2012; Tsubokawa et al. 1995) and that individual synapses may express both Ca2+-permeable and -impermeable AMPARs (He et al. 2009; Isaac et al. 1995; Washburn et al. 1997). Lu et al. (2009) have established that homomeric GluA1 receptors can be formed in CA1 cells and trafficked to synapses. Toomim and Millington (1998) have suggested that there may be a distinct localization of Ca2+-permeable AMPARs in apical dendrites in area CA1.
Using extracellularly applied polyamines and focal photolysis of caged glutamate (Bagal et al. 2005), we find that Ca2+-permeable AMPARs, perhaps lacking GluA2 subunits, may contribute as much as half of the current in single spines of apical, but not basal, dendrites of CA1 pyramidal neurons.
MATERIALS AND METHODS
Hippocampal slice and tissue culture preparation.
Organotypic hippocampal slice cultures were prepared using the roller tube method (Gähwiler et al. 1998). In brief, 400-μm-thick hippocampal slices were prepared from 5- to 8-day-old rat pups and attached to polylysine-coated glass coverslips in a film of clotted chicken plasma (Cocalico Biologicals, Reamstown, PA) supplemented with fibrin (Tisseel VH; Baxter Healthcare, Westlake Village, CA). Cultures were then maintained on a roller drum in horse serum-containing medium in an incubator for 14–28 days to allow for synaptic maturation. Hippocampal slices were also prepared acutely from adult male Sprague-Dawley rats using standard techniques. Rats were deeply anesthetized with urethane (2% in O2) or isoflurane and decapitated. Transverse slices were cut at 400-μm thickness on a vibratome. Slices were held at room temperature in a holding chamber with humidified 95% O2-5% CO2 for >1 h before recording. These protocols were approved by the University of Maryland School of Medicine Institutional Animal Care and Use Committee.
Electrophysiology.
Hippocampal slice cultures were transferred to a recording chamber mounted on the stage of an upright microscope (Nikon) equipped with differential interference contrast (DIC) optics. The chamber was perfused with saline containing, in mM, 137 NaCl, 2.8 KCl, 2 CaCl2, 2 MgCl2, 11.6 NaHCO3, 2 HEPES, 0.4 NaH2PO4, 0.01 phenol red, and 5.6 glucose, titrated to pH 7.4 by bubbling with 95% O2-5% CO2. Whole cell voltage-clamp recordings were made at room temperature (22–24°C) with pipettes (5–10 MΩ) containing, in mM, 90 CsCH3SO3, 50 CsCl, 0.4 HEPES, 1 MgCl2, 0.2 EGTA, and 0.1 Alexa Fluor 568 and was titrated to pH 7.3 with 1 M CsOH. The Alexa dye allowed visualization of dendritic spines. Cs+ was included in the pipette solution to reduce potassium currents in the recorded cell and improve voltage-clamp control. For Ca2+ imaging experiments, EGTA in the pipette solution was replaced with Oregon Green BAPTA-1 (OGB-1; 40–80 μM) and 10–20 μM CaCl2 was added. For acute hippocampal slices, the extracellular saline contained, in mM, 120 NaCl, 3 KCl, 2 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 25 NaHCO3, and 10 glucose and was bubbled with 95% O2-5% CO2.
Membrane currents were digitized and recorded using an Axopatch 200B amplifier and pClamp software (Invitrogen, Carlsbad, CA). The holding potential was −75 mV unless otherwise noted. Series resistance was monitored continually, and data from recordings in which the resistance changed by >15% were not included. To determine accurately the paired-pulse ratio (PPR) of photolytic EPSCs (phEPSCs), single and paired responses were elicited in alternation. Because the decay of phEPSC is slower than the 10- to 20-ms interstimulus interval (ISI) used to determine the PPR in most experiments, the amplitude of the second response was measured by digitally subtracting the mean response to the single UV flash from the response to a pair of flashes, so as to display the second response in isolation. The PPR was then calculated as the ratio of the amplitude of the digitally isolated second response divided by the amplitude of the mean single response. Extracellular field potentials were recorded with low-resistance (1–3 MΩ) pipettes filled with extracellular saline.
Photolysis.
Stimulation of single dendritic spines with photolysis of caged glutamate was performed as described previously (Bagal et al. 2005). In brief, the perfusion of extracellular saline was stopped after stable whole cell recordings had been obtained, and 1 mM caged glutamate [N-(6-nitro-7-coumarylmethyl)-l-glutamate; synthesized by J. P. Y. Kao] was added to the bath along with 40 μM bicuculline and 1 μM tetrodotoxin to block fast GABAergic inhibition and action potentials, respectively. The pH of the extracellular saline remained stable under these conditions due to the presence of HEPES. Experiments were stopped if the addition of caged glutamate caused an increase in holding current >50 pA.
UV light (351 and 364 nm) was generated by a diode-pumped solid-state laser (DPSS Lasers, Santa Clara, CA) and directed into a 25-μm-diameter quartz multimode fiber (OZ Optics, Ottawa, Ontario, Canada). The proximal end of the fiber was focused at a conjugate focal plane using relay lenses. The light was then directed to the preparation through the high-power (×60, numerical aperture = 1.0) objective of the microscope via a dichroic mirror so that photolysis could be performed while simultaneously using wide-field excitation from a conventional mercury arc (HBO) lamp to excite the OGB-1 or Alexa Fluor 568 dyes. As reported previously (Bagal et al. 2005), this system produces UV illumination at depth in the culture within a spot having a diameter at half-maximal amplitude of ∼1 μm, close to the observed diffraction limit. Fluorescence emission was imaged using a charge-coupled device (CCD) camera (Orca ER II; Hamamatsu; effective pixel size = 0.012 μm2). Image acquisition was controlled by SimplePCI software (Hamamatsu).
Cobalt loading.
Permeability of AMPA receptors to divalent cations was examined using the Co2+ staining technique, as described previously (Aurousseau et al. 2012). In brief, after recovering from slicing for 1 h in normal extracellular saline, 400-μm-thick hippocampal slices were placed in mesh-bottom cups and submerged in a series of solutions titrated to pH 7.4 by bubbling with 95% O2-5% CO2 at room temperature. Slices were first equilibrated in assay buffer [57.5 mM NaCl, 5 mM KCl, 20 mM NaHCO3, 12 mM D(+)glucose, 139 mM sucrose, 0.75 mM CaCl2, 2 mM MgCl2, 30 μM cyclothiazide (CTZ), and 0.01 mM phenol red] for 20 min. The cup containing the slices was then transferred to assay buffer containing 5 mM CoCl2 and 10 mM glutamate for 20 s to activate AMPARs followed by 40 s in assay buffer containing Co2+ alone. This 20-s Glu-on-40-s Glu-off protocol was repeated 10 times over 10 min to minimize desensitization of AMPARs. The slices were then transferred to assay buffer containing 2 mM EDTA for 5 min to chelate the extracellular Co2+ and then washed in assay buffer for 5 min. Finally, the slices were treated with 0.24% (NH4)2S in assay buffer to precipitate intracellular Co2+ and washed again in assay buffer before being stored in 4% paraformaldehyde in 0.1 M PBS at 4°C overnight.
Each 400-μm slice was embedded in a small cube of 10% gelatin in 0.1 M PBS and resectioned on a vibratome into 50-μm slices, which were then subjected to 60 min of silver intensification (GE Healthcare IntenSE M Silver Enhancement kit) to enhance Co2+ visualization. After incubation, the slices were washed thoroughly in water to arrest the action of the silver reagents and then imaged with DIC microscopy using a CCD camera (Orca ER II) and SimplePCI software.
Calcium imaging.
To calculate the ΔF/F values, the baseline fluorescence emission intensity (F) was calculated from background-subtracted gray values of circular regions of interest (ROIs) surrounding the spine head and averaged over 10–25 continuously acquired image frames (∼25-ms frame-to-frame interval) corresponding to 500 ms preceding the uncaging stimulus. This baseline average was then subtracted from each gray value of the spine head before and after the uncaging stimulus to calculate the ΔF. Background subtraction was achieved by measuring gray values in ROIs adjacent to the stimulated spine head over 10–25 frames. This value was then subtracted from the gray value of the dendritic spine head for each image frame.
Integrals of the ΔF/F signal traces were calculated by computing the three-point average of the ΔF/F values and multiplying the three-point average by the frame-to-frame interval. The sum of the product was taken over 500 ms, or 10–25 values, following the uncaging stimulus. Signals were excluded from analysis if the time from the beginning of the signal (i.e., when ΔF/F values 1st became >0.05) to end of the signal (when ΔF/F values < 0.05) was not ≥350 ms and the signal-to-noise ratio was not ≥2. The signal-to-noise ratio was calculated by dividing the root mean square of the ΔF/F values over 100 ms (signal) following the uncaging stimulus by the root mean square of the ΔF/F values over 500 ms preceding the uncaging stimulus (noise).
All data are presented as means ± SE. Significance values were determined using paired and unpaired, two-tailed t-tests as appropriate, unless the requirements for performing parametric tests were not met in the data set. In these cases, nonparametric tests were used, as noted in the text. Spine volume was calculated by tracing spine heads manually, measuring their area with SimplePCI software, and converting this measurement to volume, assuming spherical dimensions. All chemicals were obtained from Sigma (St. Louis, MO), Tocris (Ballwin, MO), or Invitrogen.
RESULTS
Synaptic responses are sensitive to the polyamine spermine but not philanthotoxin.
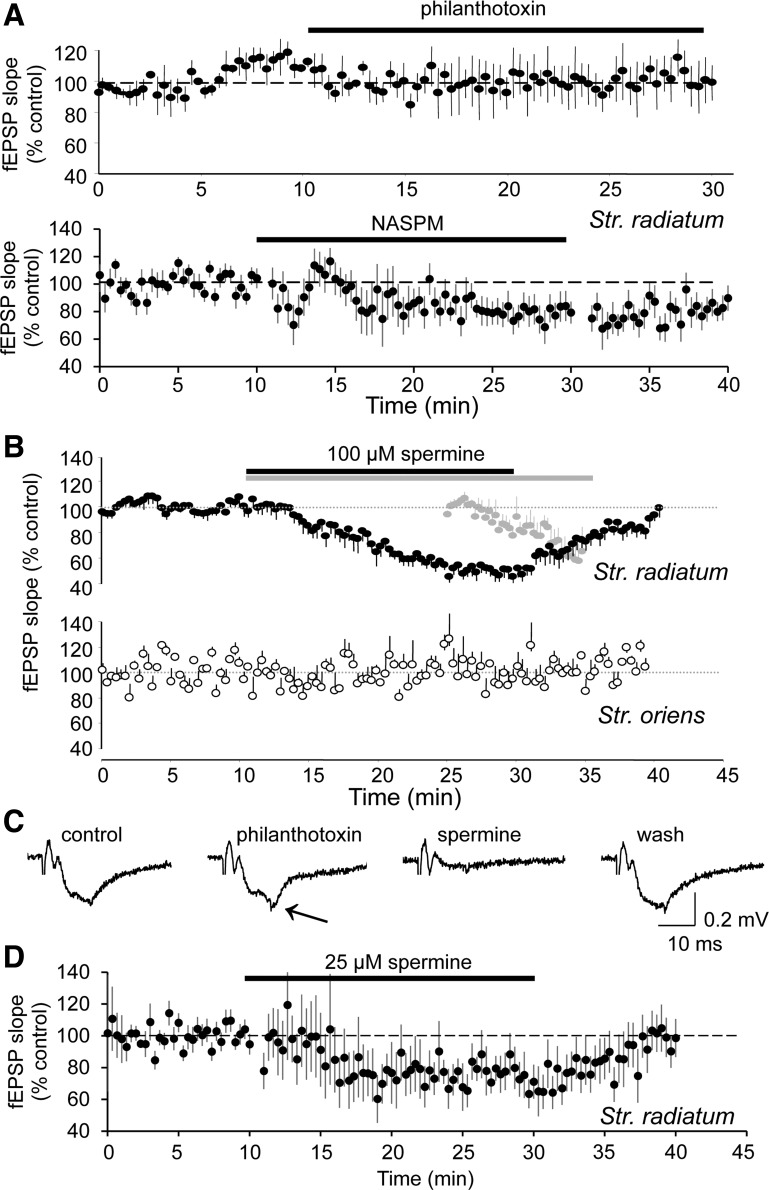
Previous studies reported that the polyamines philanthotoxin-433 (PhTx) and 1-naphthyl-acetyl-spermine (NASPM) have little or no effect on EPSCs in area CA1 (Adesnik and Nicoll 2007; Moult et al. 2010; Noh et al. 2005; Plant et al. 2006; Rozov et al. 2012). We repeated these experiments in acute hippocampal slices from adult (>250 g) rats with extracellular recording electrodes in stratum radiatum. Because N-methyl-d-aspartate receptors (NMDARs) are also sensitive to polyamines (Rock and Macdonald 1992), all experiments were performed in the presence of 40 μM amino-5-phosphonovaleric acid (AP-5). We observed that 10 μM PhTx did not affect field excitatory postsynaptic potential (fEPSP) amplitude (Fig. 1, A and C; n = 4 slices). In some experiments, PhTx promoted the appearance of population spikes (Fig. 1C), presumably because it blocked disynaptic inhibition (Toth and McBain 1998), indicating that the PhTx was pharmacologically active within the slice. Likewise, NASPM (200 μM) had a very small effect on fEPSP slope (79 ± 1% of control after 20 min, n = 16 slices; Fig. 1A), consistent with the results of Noh et al. (2005). Some types of Ca2+-permeable AMPARs are insensitive to PhTx (Osswald et al. 2007), however, and recent evidence suggests that endogenous polyamines block Ca2+-permeable AMPARs in CA1 pyramidal neurons and therefore occlude the effects of PhTx (Rozov et al. 2012). Indeed, several reports suggest that the endogenous polyamine, spermine, interacts with Ca2+-permeable AMPARs and can inhibit hippocampal excitatory synaptic transmission when applied either intra- or extracellularly (DiScenna et al. 1994; Ferchmin et al. 1995; Rozov et al. 2012). Because Ca2+-permeable AMPARs might be selectively enriched in s. radiatum (Toomim and Millington 1998), we performed these experiments using a two-pathway design in which one extracellular stimulating and recording electrode pair was placed in s. radiatum and another independent pair was placed in s. oriens so that AMPAR-mediated fEPSPs could be assessed at apical and basal synapses alternatingly in the same slices. Bath application of 100 μM spermine reduced the slope of s. radiatum fEPSPs by ∼50% without affecting s. oriens fEPSPs (Fig. 1B; n = 6 slices), whereas application of 25 μM spermine reduced fEPSP slope in s. radiatum to 76 ± 2% of control (Fig. 1D; n = 11 slices). Extracellular application of spermine did not reduce fEPSPs in the absence of stimulation, and only became effective when stimulation was restarted (Fig. 1B; n = 3 slices), as is consistent with the use-dependent polyamine block of recombinant GluA2-lacking AMPARs (Washburn and Dingledine 1996).
Fig. 1.
Selective use-dependent inhibition of evoked excitatory postsynaptic currents (EPSCs) in stratum radiatum by the polyamine spermine in hippocampal brain slices. A: extracellularly recorded field excitatory postsynaptic potentials (fEPSPs) in s. radiatum of acutely prepared hippocampal slices from adult rats were largely unaffected by the polyamines philanthotoxin (PhTx; 10 μM; n = 4 slices; top graph) or 1-naphthyl-acetyl-spermine (NASPM; 200 μM; n = 16 slices; bottom graph). B: the smaller polyamine, spermine, reduced the slope of fEPSPs recorded in s. radiatum in response to s. radiatum stimulation (closed circles) without affecting simultaneously recorded fEPSPs in s. oriens in response to s. oriens stimulation (open circles; n = 6 slices). In a separate set of slices, spermine did not affect fEPSP slope in s. radiatum during 15 min of continuous application if no stimulation was delivered but produced a progressive decrease on restarting stimulation (gray circles; n = 3 slices). C: representative recordings from an experiment in which PhTx had no effect on fEPSP slope, but subsequent application of spermine did decrease fEPSP slope reversibly. Note the slight enhancement of the response in the presence of PhTx (arrow), consistent with PhTx inhibition of GluA2-lacking α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptors (AMPARs) at excitatory synapses in inhibitory interneurons or perhaps inhibition of K+ channels. D: 25 μM spermine produces a small inhibition of fEPSP slope in s. radiatum (n = 11 slices).
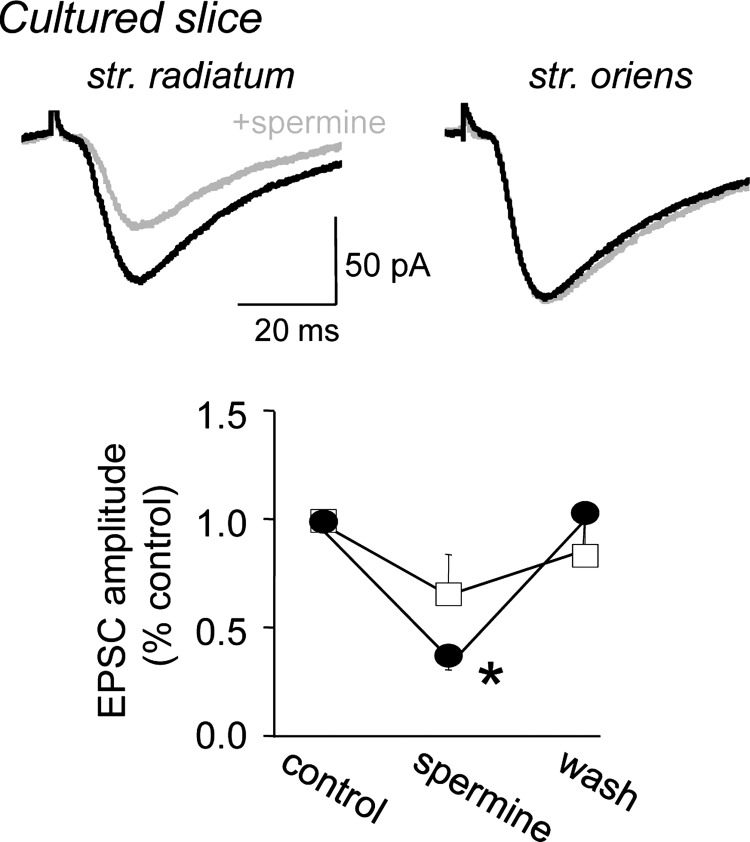
We also evoked EPSCs in rat hippocampal slice cultures with synaptic stimulation (0.05–0.1 Hz) delivered alternatingly in s. radiatum and s. oriens using two stimulation pipettes placed within 5–10 μm of the Alexa Fluor 568-filled dendrites of voltage-clamped CA1 cells. Extracellular application of spermine (25 μM) reversibly depressed the amplitude of synaptic EPSCs evoked with stimulation in s. radiatum (mean = 72 ± 8% of control, n = 6 cultures; P < 0.01, Mann-Whitney U test) but did not significantly decrease synaptic EPSCs elicited with stimulation in s. oriens (Fig. 2; n = 5 cultures).
Fig. 2.
Spermine inhibits EPSCs in hippocampal slice cultures. Whole cell recordings of EPSCs in rat hippocampal slice cultures in response to alternating stimulation in s. radiatum (left) and s. oriens (right) before (black) and after (gray) application of 25 μM spermine. Pooled data (right) indicating that spermine produced a significant decrease in the amplitude of s. radiatum EPSCs (closed circles) but not s. oriens EPSCs (squares; *P < 0.01, Mann-Whitney U test; n = 5 cultures).
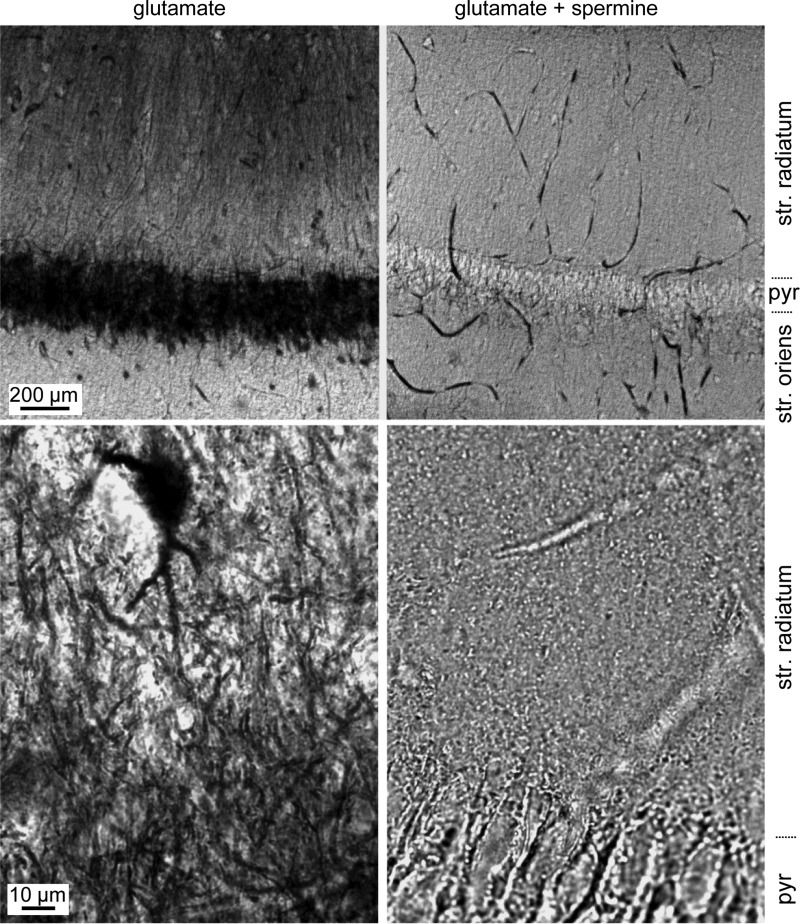
These results could be explained by a selective pre- or postsynaptic action of spermine. Specifically, apical dendritic synapses might contain some Ca2+-permeable AMPARs that are sensitive to spermine but not PhTx or NASPM. Alternatively, spermine, but not PhTx or NASPM, might inhibit some presynaptic channels and thereby reduce transmitter release probability specifically at Schaffer collateral synapses in s. radiatum. As an independent test for the presence of Ca2+-permeable AMPARs in the apical dendrites of CA1 pyramidal cells in adult rat brain, we next used the Co2+-loading approach, as described by Aurousseau et al. (2012). Acute hippocampal slices were placed in saline containing 5 mM CoCl2 and 10 mM glutamate for 20 s to activate AMPARs followed by 40 s in saline containing Co2+ alone. This 20-s-on/40-s-off cycle was repeated 10 times over 10 min. Both solutions contained CTZ (30 μM) to minimize AMPAR desensitization. Slices were then fixed, resectioned, and stained for the presence of Co2+ using silver intensification. Consistent with the electrophysiological results, strong loading of Co2+ was observed in dendrites in s. radiatum, but not s. oriens (Fig. 3; n = 6 slices), as well as in presumed interneurons located outside of s. pyramidale. Glutamate-induced Co2+ loading was negligible when performed in the presence of 25 μM spermine (Fig. 3; n = 6 slices) and was greatly reduced in the absence of CTZ. These data support the localization of Ca2+-permeable, polyamine-sensitive AMPARs (or kainate receptors) potentially in the apical dendrites of CA1 pyramidal cells.
Fig. 3.
Loading of CA1 cell dendrites with Co2+. Acute hippocampal slices were submerged in saline containing 5 mM CoCl2 and 10 mM glutamate for 20 s to activate AMPARs, followed by 40 s in saline containing Co2+ alone, repeated 10 times over 10 min. Slices were then fixed, resectioned, and stained for the presence of Co2+ using silver intensification. Low-magnification images of area CA1 (top row) and high-magnification images of s. radiatum (bottom row) of slices treated with glutamate alone (left column) or glutamate and spermine (right column). Intense labeling was seen in the somata and dendrites of slices exposed to glutamate, and this staining was absent when glutamate was applied in the presence of spermine. A heavily stained s. radiatum interneuron is visible in the high-power image at left. pyr, S. pyramidale.
Differences in AMPAR-mediated responses from apical and basal spines.
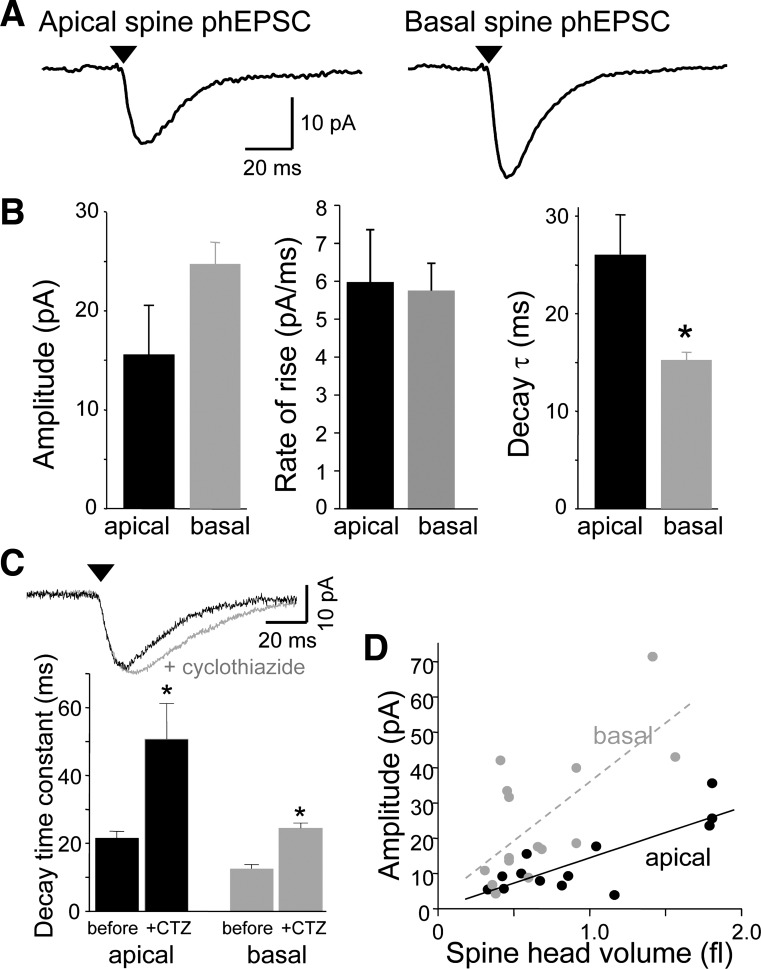
We further tested the hypothesis that some AMPARs in single dendritic spine heads are Ca2+-permeable using microphotolysis of caged glutamate in the presence of TTX with NMDARs and GABAA receptors blocked pharmacologically (Bagal et al. 2005). This method has the advantage of activating postsynaptic AMPARs without involving presynaptic factors. The UV light was focused to a nearly diffraction-limited spot (∼1-μm diameter) and likely activated both synaptic and extrasynaptic receptors confined to the targeted spine head. We focused the laser spot at dendritic spines located on apical dendrites, extending into s. radiatum, or basal dendrites, extending into s. oriens. phEPSCs recorded from either apical or basal dendritic spines were eliminated by 6,7-dinitroquinoxaline-2,3-dione (DNQX; 40 μM; data not shown), indicating that they were mediated solely by AMPARs. There were no significant differences in the mean amplitudes or rates of rise of phEPSCs elicited from spines on dendrites in apical or basal dendrites (unpaired t-test; n = 21, 27 spines; Fig. 4, A and B). The decay of AMPAR-mediated phEPSCs at both locations was well-fit with a single exponential. The mean decay time constant of phEPSCs elicited from basal spines was faster than for phEPSCs from apical spines (P < 0.01, unpaired t-test). Bath application of CTZ (10 μM) to slow AMPAR desensitization had no effect on amplitudes or rates of rise of phEPSCs elicited from either apical or basal dendritic spines but roughly doubled their decay time constants (P < 0.05, paired t-test; n = 5, 3; Fig. 4C). The decay of basal spine phEPSCs remained faster than that of apical spine phEPSCs in the presence of CTZ.
Fig. 4.
Comparison of the basic properties of photolysis-induced EPSCs (phEPSCs) at single dendritic spines on apical and basal dendrites. A: averaged phEPSCs at single apical and dendritic spines. B: mean amplitude, rate of rise, and decay time constant of phEPSCs at apical and basal spines (n = 21, 27 spines). Only the difference in decay time constant was significant (*P < 0.01, unpaired t-tests). C: cyclothiazide (CTZ) prolongs apical and basal spine responses to similar extents. Responses of a basal spine to photostimulation before and after application of CTZ are shown above. Summary data below illustrate that CTZ decreased the decay rate of phEPSCs at both apical and basal spines significantly (*P < 0.05, paired t-test; n = 5, 3). D: the amplitude of responses at individual apical (black) and basal (gray) spines (n = 13, 15 spines, respectively) is plotted as a function of spine head volume. In both cases, the relationship was linear (r = 0.64 and 0.69, respectively). In this and all subsequent figures, arrowhead indicates delivery of UV pulse.
There was a strong correlation between the volume of the dendritic spine head targeted with the laser and the amplitude of the resulting phEPSC (Fig. 4D) at both apical and basal spines, although the slope was slightly steeper for basal spines. AMPAR “silent” synapses, which express NMDARs but lack functional AMPARs, have been observed in acute and cultured hippocampal slices (Isaac et al. 1995; Liao et al. 1995; Montgomery et al. 2001). However, after photostimulation of many hundreds of dendritic spines in cultured slices >14 days in vitro, we have never observed a single instance in which a mature spine, having a clearly distinguishable head and neck region, failed to respond to photorelease of glutamate with an AMPAR-mediated phEPSC, provided the health of the cell could be established by eliciting phEPSCs from other spines.
Apical spine phEPSCs display properties of Ca2+-permeable AMPARs.
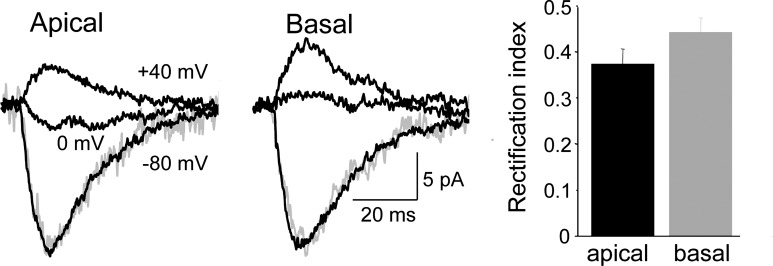
Most Ca2+-permeable, GluA2-lacking AMPARs display a polyamine-mediated, rectifying I–V, hence lack of rectification is commonly taken as evidence that these receptors are absent (Adesnik and Nicoll 2007; Plant et al. 2006; but see: Bowie 2012; Rozov et al. 2012). We therefore compared the rectification of phEPSPCs elicited from apical and basal spines using pipette solutions that contained 10 μM spermine. Only dendritic spines within 50 μm of the somatic recording electrode were sampled, and cesium was used in the pipette solution to minimize voltage- and space-clamp errors. The rectification index (RI) was measured as the phEPSC amplitude at +40 mV divided by its amplitude at −80 mV so that, assuming a reversal potential of 0 mV, a linear I–V would have an RI of 0.5. No significant difference in the RIs of phEPSCs from apical spines and basal spines was detected, although stronger rectification was observed at apical spines (apical RI = 0.37 ± 0.03, n = 15, vs. 0.44 ± 0.03, n = 8; P > 0.05, Mann-Whitney U test; Fig. 5). There was no difference in the rates of rise or decay time constants of phEPSCs at −80 and +40 mV. The observation of RIs <0.5 might suggest that apical and basal spines express some Ca2+-permeable AMPARs. Alternatively, the currents often failed to reverse at 0 mV (mean current at 0 mV = 3.9 ± 1.0 and 1.7 ± 0.3 pA for apical and basal spines, n = 7 and 3, respectively), suggesting that these RI measurements might be compromised by imperfect voltage control at the stimulated spine. We therefore tested several other predictions of the presence of Ca2+-permeable AMPARs.
Fig. 5.
Apparent rectification of phEPSCs is comparable at apical and basal spines. Responses of apical and basal spines to photostimulation with the cell voltage-clamped to −80, 0, and +40 mV. The phEPSC at +40 mV was inverted, scaled, and shown in gray superimposed on the phEPSC at −80 mV. The rise and decay rates were not voltage-dependent. Pooled data at right indicate that the rectification ratio was not significantly different in the 2 data sets (unpaired t-test; n = 15, 8 spines).
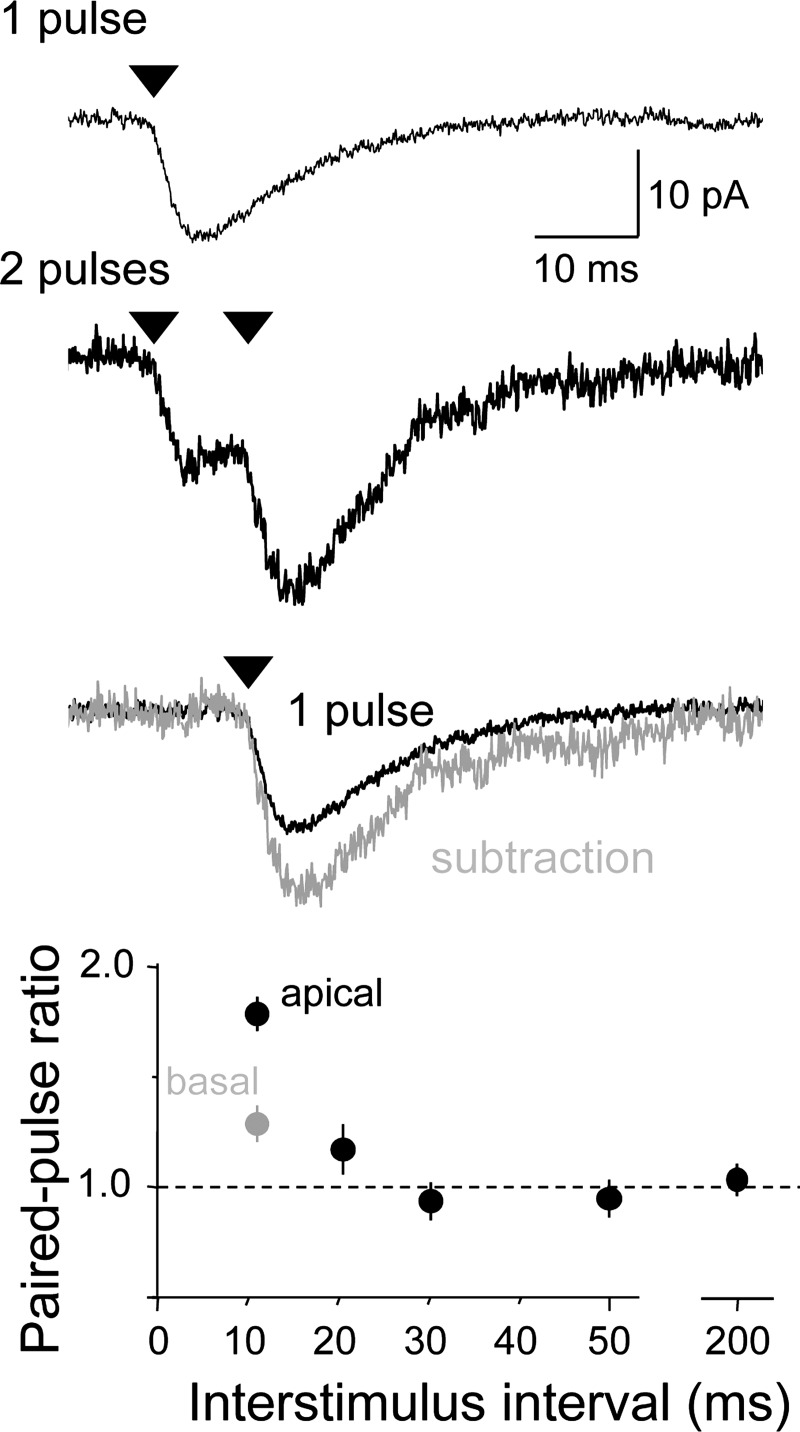
Ca2+-permeable, polyamine-sensitive AMPARs display PPF due to activity-dependent relief from intracellular polyamine block (Rozov et al. 1998; Rozov and Burnashev 1999; Shin et al. 2005). We therefore tested the effects of paired photostimuli in recordings with pipette solutions containing 10 μM spermine. Reponses were collected using a two-stimulus protocol and an ISI that varied between 10 and 200 ms (Fig. 6). The PPR was then calculated by taking the mean amplitude of the second responses and dividing them by the mean amplitude of the first responses. The amplitude of the second response was consistently greater than the amplitude of the first response at ISIs of 10–20 ms at apical dendritic spines. Basal spines also displayed PPF but significantly less than in apical spines (for ISI = 10 ms, PPR = 1.72 ± 0.06 at apical spines vs. 1.34 ± 0.05 at basal spines; n = 21, 27; P < 0.001, Mann-Whitney U test).
Fig. 6.
Apical spine phEPSCs display significant paired-pulse facilitation (PPF). Responses of a single apical dendritic spine to a single photostimulus (top) or a pair of photostimuli separated by 10 ms (middle). Paired-pulse ratios (PPRs) were calculated in all experiments by digitally subtracting the averaged response to a single stimulus from the averaged response to pairs of stimuli (bottom trace). The PPR was calculated as the amplitude of the isolated 2nd response to the amplitude of the 1st response and is plotted as a function of interstimulus interval for apical (black) and basal (gray) spines below. The PPR of apical and basal spines is significantly different at 10 ms (unpaired t-test, P < 0.001; n = 14, 20 spines, respectively).
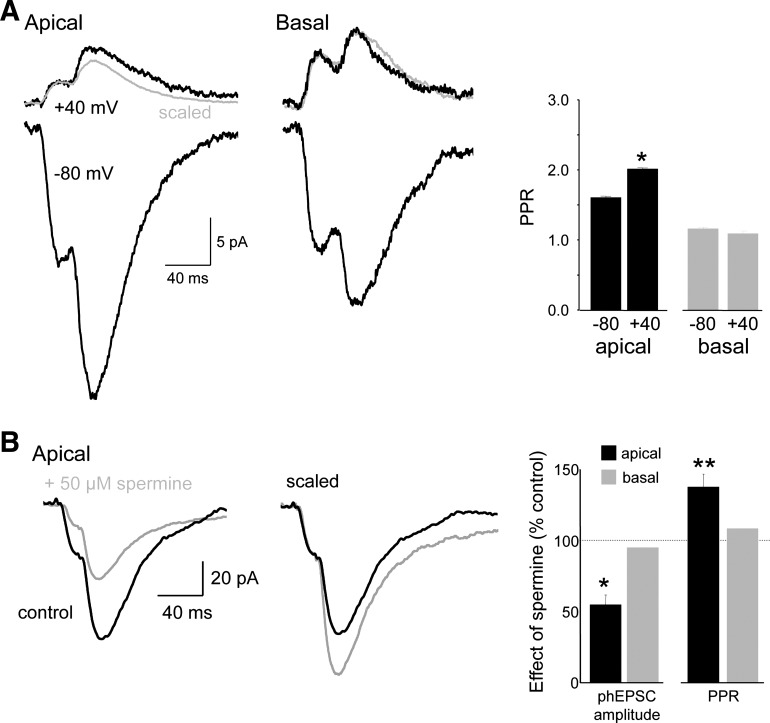
If the PPF of apical spine responses results from a relief of endogenous polyamine block of Ca2+-permeable AMPARs, then the PPR should be increased at depolarized membrane potentials because of the increased block of Ca2+-permeable AMPARs by intracellular polyamines at those potentials. We tested this prediction by depolarizing the membrane from −80 to +40 mV in cells recorded with spermine-containing intracellular solutions and found that this significantly increased the PPR, measured at an ISI of 20 ms (PPR = 1.64 ± 0.04 at −80 vs. 2.03 ± 0.09 at +40 mV; n = 5; P < 0.01; Fig. 7A). Even with imperfect voltage-clamp, the voltage dependence of the PPR at apical spines suggests that relief of polyamine block accounts for the observed PPF.
Fig. 7.
The polyamine spermine selectively inhibits apical spine responses. A: depolarization from −80 to +40 mV increased the PPR of apical (left) but not basal (right) spines, as can be seen in the inverted and scaled response at −80 mV shown in gray superimposed on the response at +40 mV. Summary graph indicates that apical spine, but not basal spine, PPRs were increased by depolarization. B: responses to pairs of photostimuli at an apical spine are shown before (black) and after (gray) bath application of 50 μM spermine (left). Spermine reduced the amplitude of the 1st response and increased the PPR, as seen most clearly after scaling the responses so that the amplitudes of the 2 1st responses are the same (right). Summary graph indicates that apical spine, but not basal spine, phEPSC amplitudes were significantly depressed by spermine and that only the PPR of apical spine phEPSCs was affected by spermine (n = 8 apical and 5 basal spines). *P < 0.05; **P < 0.01; paired t-tests.
Differences in PPR at apical and basal spines might reflect differences in the relative proportion of polyamine-sensitive AMPARs or differences in polyamine-independent processes, such as receptor mobility (Heine et al. 2008) or receptor desensitization. The PPR of phEPSCs at basal spines was not affected significantly by application of CTZ (PPR = 1.2 ± 0.1 before and 1.3 ± 0.2 after CTZ; n = 4; paired t-test). The difference in facilitation of phEPSCs at the two locations cannot therefore be attributed to differences in AMPAR desensitization. If PPF is larger at apical spines than basal spines because apical spines have more Ca2+-permeable AMPARs than basal spines, then the PPR of basal spines should be less voltage-dependent than apical spines. Indeed, the PPR, measured at an ISI of 20 ms, at basal spines was not significantly different at the two membrane potentials (PPR = 1.23 ± 0.08 at −80 mV vs. 1.18 ± 0.08 at +40 mV; n = 8; not significant, paired t-test; Fig. 7A). The smaller voltage dependence of PPF at basal spines is consistent with a smaller contribution of Ca2+-permeable AMPARs.
We next tested the effects of extracellularly applied polyamines. If apical spines contain Ca2+-permeable AMPARs, then apical dendritic spine phEPSCs should be depressed by extracellular polyamines (Washburn and Dingledine 1996). Bath application of spermine (50 μM) produced a 70% decrease in the amplitude of apical spine phEPSCs (mean amplitude = 24.1 ± 6.6 pA before vs. 6.9 ± 1.6 pA after spermine; n = 8; P < 0.05, paired t-test; Fig. 7B).
If the reduction in apical spine phEPSC amplitude by spermine is caused by block of Ca2+-permeable AMPARs, then the phEPSC PPR should increase because postsynaptic PPF results from the relief of both intra- and extracellular polyamine block (Rozov and Burnashev 1999). Indeed, the decrease in phEPSC amplitude was accompanied by a significant increase in PPR at apical spines (PPR = 1.56 ± 0.09 before vs. 2.11 ± 0.15 after spermine; n = 8; P < 0.005, paired t-test). In contrast, extracellular spermine did not alter the amplitude or PPR of phEPSCs elicited from basal spines significantly (phEPSC amplitude = 95 ± 2% of control; PPR = 108 ± 5% of control; n = 6; paired t-test; Fig. 7B).
Some apical AMPARs are resistant to block of GluA2 insertion.
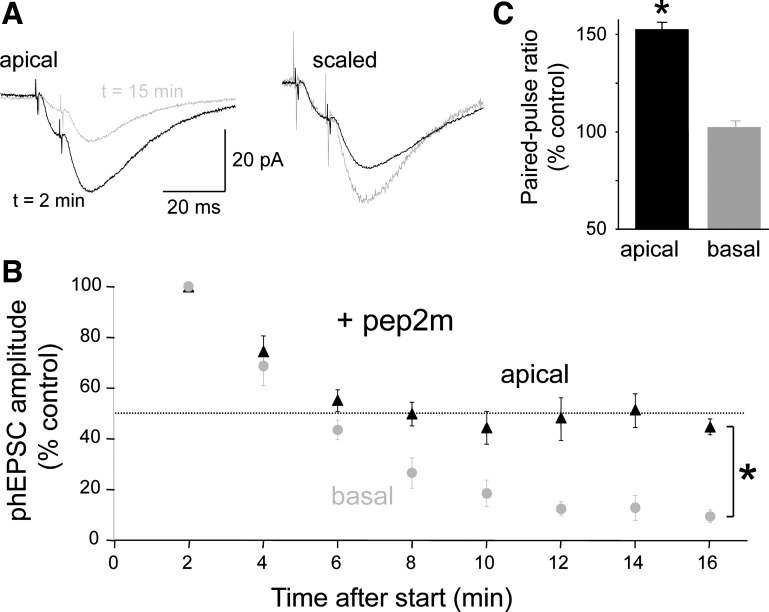
Synaptic delivery of GluA2-containing, but not GluA2-lacking, AMPARs is mediated through a cytoplasmic interaction with N-ethylmaleimide-sensitive factor (NSF; Lüthi et al. 1999; Nishimune et al. 1998). When applied intracellularly, a peptide consisting of the NSF-binding domain of GluA2, pep2m, competes with the endogenous GluA2 ligand for NSF and disrupts replenishment of GluA2-containing receptors, thereby depleting the synapses of putative GluA2-containing receptors. We observed that dialysis of pep2m (150 μM) into the cell from the patch pipette decreased AMPAR-mediated currents at both apical (Fig. 8A) and basal spines within 15 min after break-in to the whole cell recording mode. In agreement with previous studies in acute hippocampal slices (Lüthi et al. 1999; Nishimune et al. 1998), the decline in apical spine phEPSC amplitude reached a steady-state of ∼50% of the control amplitude (mean amplitude at 16 min = 45 ± 3% of starting amplitude; n = 5 cells; Fig. 8B). In striking contrast, however, pep2m almost eliminated phEPSCs elicited from basal spines within 15 min (mean amplitude at 16 min = 10 ± 2% of starting amplitude; n = 5 cells; Fig. 8B). The decrease at basal spines was significantly greater than the decrease at apical spines (P < 0.05, Mann-Whitney U test). Dialysis of the scrambled control peptide, pep4c (150 μM), from the pipette had no effect on the amplitude of phEPSCs elicited from apical spines (mean amplitude 16 min after = 99 ± 2% of starting amplitude; n = 3) or basal spines (mean amplitude 16 min after = 98 ± 3% of starting amplitude; n = 5). We conclude that a larger proportion of AMPARs at basal spines depend on GluA2-dependent recycling than at apical spines.
Fig. 8.
Inhibition of GluA2 trafficking reduces apical spine responses and abolishes basal spine responses. A: responses of an apical spine to paired photostimulation are shown at 2 and 16 min after initiation of photostimulation for a cell recorded with an electrode containing the peptide pep2m, which prevents GluA2 trafficking by competing for its binding to the protein N-ethylmaleimide-sensitive factor (NSF), in the patch pipette solution (150 μM; left). Pep2m dialysis also increased the PPR of the apical spine response, as illustrated by rescaling the traces so that the amplitudes of the 1st response are the same (right). B: summary graph showing that pep2m dialysis caused a gradual decrease in apical spine phEPSCs to a steady-state amplitude ∼50% of the control amplitude (black triangles). Basal spine phEPSCs, in contrast, were almost eliminated within ∼15 min after whole cell break-in when pipettes contained pep2m (gray circles). For each spine, 12 responses were averaged at each time point (n = 6, 5 cells). The difference at 16 min was significant (*P < 0.05, Mann Whitney U test). C: consistent with the hypothesis that the apical spine phEPSCs that persist after pep2m dialysis are mediated primarily by GluA2-lacking AMPARs, there was a significant increase in the PPR of apical spine phEPSCs (*P < 0.05, paired t-test), but not basal spine phEPSCs, after 15 min of dialysis.
There are two possible explanations for the partial reduction in apical spine phEPSC amplitude caused by pep2m. The remaining phEPSC could be mediated by GluA2-containing AMPARs that are not retrieved after block of GluA2 insertion (Lüthi et al. 1999; Nishimune et al. 1998). If this hypothesis is true, then there should be no change in PPR as the phEPSC amplitude declines. Alternatively, the persistent phEPSC could be mediated largely by AMPARs that are insensitive to pep2m because they lack GluA2 subunits. If this hypothesis is true, then the PPR of these phEPSCs should increase as removal of GluA2-containing AMPARs increases the fraction of the current mediated by GluA2-lacking AMPARs. Indeed, we observed an increase in the PPR of apical spine phEPSCs to 151 ± 23% of the control value (n = 5; P < 0.05, paired t-test) as the dialysis with pep2m caused them to become depressed (Fig. 8, A and C). At basal spines, in contrast, there was no significant change in the PPR of phEPSCs (PPR = 101 ± 7% of control, n = 5). These results are thus consistent with the hypothesis that apical spines express a considerable fraction of GluA2-lacking AMPARs (presumably GluA1 heteromers). The 50% reduction in apical spine phEPSCs resulting from pep2m dialysis suggests that perhaps as much as half of the current generated at apical spines is mediated by GluA2-lacking AMPARs.
Ca2+ influx through AMPARs at apical spines.
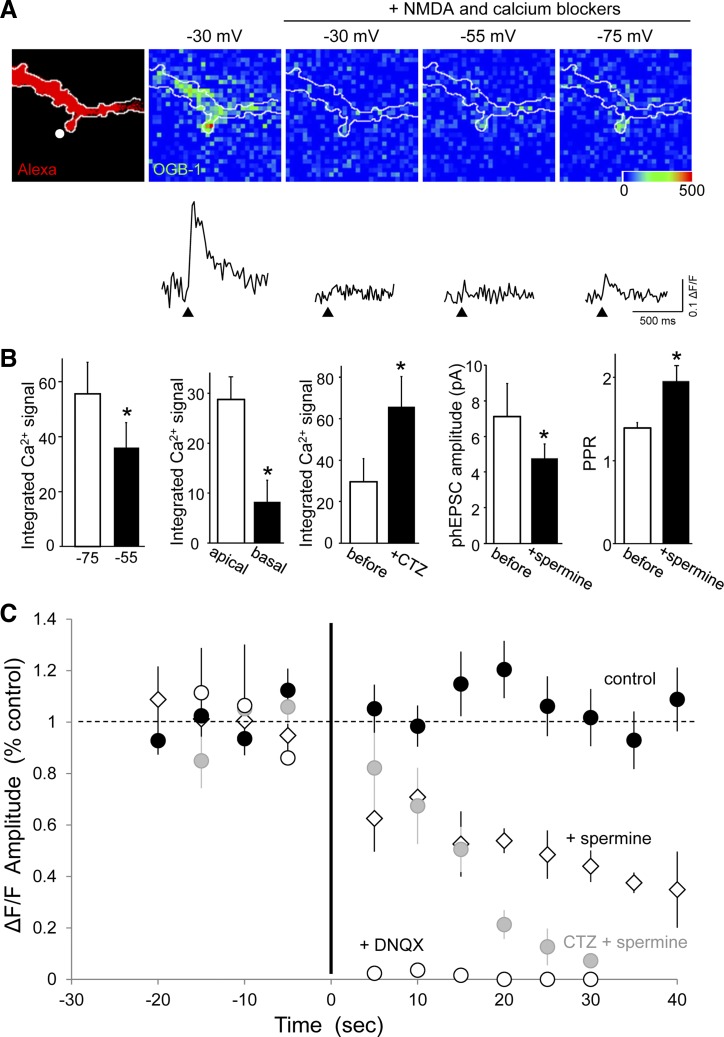
If Ca2+-permeable, polyamine-sensitive GluA2-lacking AMPARs are present at apical dendritic spines, then AMPAR activation should be accompanied by a local, polyamine-sensitive increase in the intracellular Ca2+ concentration. We anticipated that these responses would be small and difficult to detect because of the low Ca2+-to-Na+ permeability ratio of the channels, their short open time, and the small number of channels likely to be present at a single spine. Whole cell recordings from CA1 cells were made with pipette solutions containing the high-affinity Ca2+ indicator OGB-1 (40–80 μM; Fig. 9). As a positive control for the health of the cell and the sensitivity of the Ca2+ imaging conditions, phEPSCs were first elicited with the cell voltage-clamped at −30 mV to relieve Mg2+ block of NMDARs. Only spines displaying clear responses under these conditions were studied further. To isolate potential AMPAR-mediated Ca2+ influx, antagonists of NMDARs (160 μM AP-5 and 40 μM MK-801; Fig. 9A) were applied together with a cocktail of antagonists to block Ca2+-induced Ca2+ release (CICR; 20 μM ryanodine and 1 μM thapsigargin), metabotropic GluRs (100 μM LY 341495), and R-, P-/Q- and N-, T-, and L-type voltage-dependent Ca2+ channels (VDCCs; 0.3 μM SNX 482, 1 μM ω-conotoxin MVIIC, 1 μM mibefradil, and 40 μM nimodipine, respectively). Responses at −30 mV were greatly reduced under these conditions. To increase the chances of detecting AMPAR-mediated Ca2+ influx, pairs of laser pulses were delivered at an ISI of 20 ms to relieve polyamine block and maximally activate Ca2+-permeable AMPARs, as described above.
Fig. 9.
AMPAR-mediated Ca2+ influx at apical dendritic spines. A: photomicrograph of a dendritic segment of a CA1 pyramidal cell filled with Alexa Fluor 568 (Alexa; far left) and Oregon Green BAPTA-1 (OGB-1) followed by pseudocolored images of the peak change in OGB-1 fluorescence emission (ΔF/F) after photolysis of caged glutamate directed to the single prominent spine before and after the addition of a mixture of N-methyl-d-aspartate (NMDA) receptor, mGluR, voltage-dependent Ca2+ channel (VDCC), and Ca2+-induced Ca2+ release (CICR) blockers (see text). There was a large signal visible at −30 mV that was strongly reduced by the blockers. A smaller signal was apparent at more hyperpolarized voltages, and it was larger at −75 than −55 mV. The amplitude and time course of the Ca2+ responses shown in the ΔF/F traces are illustrated below the images. B: summary data indicating that Ca2+ influx, as quantified from integrated ΔF/F traces, was significantly greater 1) at apical spines at −75 than at −55 mV (n = 4; *P < 0.05, paired t-test), 2) in apical spines than in basal spines (n = 5; *P < 0.05, paired t-test), and 3) in the presence of CTZ (n = 5; *P < 0.05, paired t-test). Additional data indicate that the inhibition of Ca2+ influx by spermine was accompanied by a reduction in phEPSC amplitude (n = 4; *P < 0.01, paired t-test) and increased PPF (n = 4; *P < 0.05, paired t-test). All experiments were performed in the presence of the blocker mixture. C: summary data showing the peak amplitude of Ca2+ response in the presence and absence of CTZ. Responses were abolished by 6,7-dinitroquinoxaline-2,3-dione (DNQX; 20 μM; n = 4) and reduced in a use-dependent manner by spermine (50–100 μM; n = 5). The use-dependent decrease in Ca2+ influx was more complete in the presence of CTZ (20–100 μM; n = 5 cells, 7 spines). Responses to repeated stimulation in control artificial cerebrospinal fluid (black circles) with (n = 7) or without CTZ (n = 7, pooled) were stable in response to the same stimulation regime.
Under these conditions, significant increases (>5% lasting >100 ms) in OGB-1 emission were detected in 37 of 56 (∼66%) of the apical spines examined (Fig. 9A). The mean change in OGB-1 fluorescence was 15 ± 3% (ΔF/F; n = 10 spines), considerably less than the 60% increase in fluorescence elicited with single-spine NMDAR activation at −30 mV. In addition, the duration of the intracellular Ca2+ elevation in the presence of the blocker cocktail was significantly shorter than for NMDAR-mediated Ca2+ responses (304 ± 17 ms for AMPAR influx vs. 1,337 ± 152 ms for NMDAR influx; n = 5, 5; paired t-test, P = 0.005). Increases in OGB-1 emission (>5% lasting >100 ms) were detected in only ∼40% of basal spines tested. When responses were obtained in both apical and basal spines of the same cell, the integrals of the Ca2+ signals were significantly larger in apical spines than in basal spines (n = 5; Fig. 9B).
Although the experiments were performed under voltage-clamp in the presence of antagonists of VDCCs, we tested the hypothesis that residual AMPAR-mediated depolarization activated some population of unblocked VDCCs (Bloodgood et al. 2009; Heine et al. 2008) by comparing responses at −75 and −55 mV. If VDCCs contribute, then Ca2+ responses should be larger at −55 mV because it is closer to their activation threshold. We observed, however, that the integrals of the Ca2+ responses were significantly greater at −75 than at −55 mV (Fig. 9B; n = 4; P < 0.05, paired t-test). This is consistent with Ca2+-permeable AMPARs as the source of the Ca2+ influx because the driving force for Ca2+ influx via this pathway is greater at −75 mV.
Several other lines of evidence indicated that the responses of apical dendritic spines were mediated by Ca2+ influx directly via Ca2+-permeable AMPARs. First, Ca2+ signals at apical dendritic spines were abolished by the competitive AMPAR antagonist DNQX (20 μM; n = 4), indicating that they were totally dependent on AMPAR activation (Fig. 9C). Second, the size of the Ca2+ response integral was significantly larger when AMPAR channel open time was prolonged by CTZ (20–100 μM; Fig. 9B; n = 7 spines, 5 cells; P < 0.05, paired t-test).
Finally, if Ca2+ responses in apical spines are mediated by Ca2+-permeable AMPARs, then they should be blocked by extracellular application of spermine in a use-dependent manner. After recording baseline responses for 10 or more paired-pulse stimuli in control saline, spermine (50–100 μM) decreased the amplitude of the Ca2+ responses gradually (n = 6 spines; Fig. 9C). As expected for a use-dependent block of AMPAR-gated channels, abolition of Ca2+ responses occurred more effectively when experiments were repeated in the presence of CTZ, which prevented the desensitization of AMPARs and therefore held channels open longer (n = 5 spines). Ca2+ responses in control saline were stable with and without CTZ (n = 7 spines each, pooled).
DISCUSSION
We conclude that apical dendritic spines in CA1 pyramidal cells possess a population of Ca2+-permeable, polyamine-sensitive AMPARs in both acutely prepared and cultured hippocampal slices. Classically, AMPARs with these properties are found to lack GluA2 subunits, and we therefore suggest that GluA2 subunits are absent in a significant fraction of synaptic AMPARs on apical dendritic spines, rendering them Ca2+-permeable and sensitive to voltage- and use-dependent block by polyamines. Nevertheless, we do not know the actual subunit composition of the receptors mediating our responses and cannot therefore exclude the possibility that these responses are mediated by GluA2-containing, polyamine-sensitive, Ca2+-permeable AMPARs (Bowie 2012).
AMPAR-mediated Ca2+ influx.
The sine qua non of GluA2-lacking AMPARs is Ca2+ permeability. We observed that many apical spines exhibited a Ca2+ signal in response to uncaging stimuli in the presence of a cocktail of NMDAR, mGluR, and VDCC antagonists as well as inhibitors of CICR. Several lines of experimental evidence confirm that these Ca2+ signals were mediated by AMPARs. First, they were abolished by DNQX. Second, they were smaller at −55 than at −75 mV. This is inconsistent with alternative explanations for the Ca2+ influx, such as an AMPAR-triggered activation of VDCCs (Bloodgood et al. 2009; Heine et al. 2008). The larger signal at hyperpolarized potentials is consistent with greater Ca2+ influx through open GluA2-lacking AMPARs as the result of an increased driving force. Third, inhibiting AMPAR desensitization with CTZ increased the size of the Ca2+ signals. Finally, Ca2+ responses were inhibited by spermine in a use- and CTZ-sensitive manner. We observed that Ca2+ responses were reduced progressively after spermine was added, requiring more than eight stimuli for full block. When channel open time was increased by CTZ, the rate of inhibition by spermine was faster, requiring only six stimuli for full block. These results, taken together, support strongly the hypothesis that Ca2+-permeable AMPARs are present at spines on apical dendrites.
Ca2+ signals were evident in 66% of apical spines. This could indicate that not all spines express Ca2+-permeable AMPARs. Alternatively, the AMPAR-triggered Ca2+ signals we observed were small, at least fivefold less than the NMDAR-mediated signals at the same spines, and may have been below our detection level at some spines. Ca2+ signals mediated by AMPARs are expected to be smaller than NMDAR-mediated responses, due to the more rapid inactivation, shorter channel open times, and lower Ca2+-to-Na+ permeability ratio of AMPARs. Ca2+ signals were less commonly observed at basal spines, and when they were, the integrals of the Ca2+ signals were significantly smaller than those at apical spines. These responses may be due to incomplete block of other sources of Ca2+ entry or to the presence of some Ca2+-permeable AMPARs. Taken together with the lack of Co2+ loading in s. oriens, measurements of Ca2+ signals support our conclusion that Ca2+-permeable AMPARs are preferentially localized to apical spines.
Polyamine block of AMPARs.
We confirmed earlier observations that the polyamines PhTx and NASPM have little or no effect on Schaffer collateral fEPSPs in s. radiatum in acutely prepared hippocampal slices from adult rats (Adesnik and Nicoll 2007; Plant et al. 2006; Rozov et al. 2012). Another polyamine, spermine, did reduce Schaffer collateral fEPSPs, however, indicating that some of the receptors are polyamine-sensitive. Moreover, the spermine-induced inhibition of fEPSPs was use-dependent, as predicted for responses mediated by GluA2-lacking AMPARs. Spermine is an endogenous polyamine that affects several ion channels, but our use of NMDAR antagonists and the range of spermine concentrations we used are more consistent with the high affinity of polyamines for AMPARs (Washburn and Dingledine 1996) than with the low affinity of Ca2+ or K+ channels (Lopatin et al. 1994). Spermine also blocked glutamate-induced Co2+ loading in acute hippocampal slices, an indicator of Ca2+-permeable AMPARs.
Inhibition of fEPSPs by spermine, but not PhTx, was unexpected because PhTx is a potent antagonist of Ca2+-permeable AMPARs at many synapses (Washburn and Dingledine 1996), including both excitatory synapses on GABAergic interneurons (Toth and McBain 1998) and excitatory synapses in GluA2 knockout mice (Adesnik and Nicoll 2007; Rozov et al. 2012). However, the PhTx molecule is much larger than spermine (7.5 vs. 5 Å) and has a slower binding rate than spermine (Bowie et al. 1998). Thus PhTx may not have equal access to all Ca2+-permeable channels. The sensitivity of Schaffer collateral CA1 synapses to PhTx has only been observed when endogenous polyamines were dialyzed from CA1 pyramidal cells and not before dialysis or when spermine was contained in the patch solution (Rozov et al. 2012). There is, however, evidence for PhTx-insensitive, Ca2+-permeable AMPARs in the developing rat retina (Osswald et al. 2007). This evidence, combined with our other data, argues that PhTx insensitivity does not provide unambiguous confirmation of a lack of Ca2+-permeable AMPARs at a given synapse.
Focal photolysis of caged glutamate at single dendritic spines made possible critical tests of the hypothesis that some AMPARs on CA1 pyramidal neurons are Ca2+-permeable. Because excitatory synapses on basal dendrites in acute and cultured slices were insensitive to spermine, we hypothesized that these synapses expressed fewer Ca2+-permeable AMPARs and could thus serve as controls for tests on apical spines. Kinetic analysis revealed that basal spine phEPSCs decayed more quickly than apical phEPSCs. Deactivation and desensitization are faster for GluA2-lacking AMPARs (Geiger et al. 1995). If there is a greater number of Ca2+-permeable AMPARs at apical spines, as our other, more direct experiments indicate, then it might be expected that apical spine phEPSCs would decay faster than basal spine phEPSCs. This was not observed.
Rectification of GluA2-lacking AMPARs is attributed to voltage-dependent channel block by intracellular polyamines. Several studies of AMPAR-mediated currents in CA1 neurons failed to detect inward rectification in the absence of added intracellular polyamines (Adesnik and Nicoll 2007; Hestrin et al. 1990; Plant et al. 2006; Rozov et al. 2012), suggesting the absence of Ca2+-permeable AMPARs. However, when endogenous polyamines were dialyzed into hippocampal CA1 neurons from wild-type mouse hippocampi, inward rectification was observed, and this I–V deviated from the nonrectifying I–V observed in neurons from mutant mice lacking GluA1 or expressing GluA1 with a point mutation in the Q/R site (Rozov et al. 2012). It should be noted that AII amacrine cells in the retina express Ca2+-permeable AMPARs that are insensitive to extracellular PhTx and do not rectify (Osswald et al. 2007), demonstrating that Ca2+ permeability is not always synonymous with polyamine-sensitivity (for review, see Bowie 2012). We used single-spine photolysis to assess the rectification properties of AMPARs and observed modest rectification of phEPSCs at both apical and basal spines, like those reported for synaptic responses in some studies (Hayashi et al. 2000). We noted that the phEPSC reversal potential was more negative than 0 mV, suggesting inadequate control of spine membrane potential at depolarized voltages. These experiments were thus not conclusive. Our finding of strong voltage- and spermine-sensitive PPF indicates that Ca2+-permeable AMPARs in CA1 cell apical spines are sensitive to intra- and extracellular polyamines. To our knowledge, this profile of characteristics is uniquely associated with GluA2-lacking AMPARs, but positive identification must await molecular analysis of these synapses.
Pep2m, a peptide derived from the COOH-terminal region of GluA2 that interacts with the trafficking protein NSF, prevents the constitutive cycling of GluA2-containing, but not GluA2-lacking, AMPARs to the synapse (Lüthi et al. 1999; Nishimune et al. 1998). Pep2m abolished AMPAR-mediated phEPSCs elicited from basal spines when dialyzed into CA1 pyramidal cells but depressed phEPSC amplitudes from apical spines by only ∼50%. The decrease in phEPSC amplitude was accompanied by an increase in PPF. These data thus lend further support to the hypothesis that the Ca2+-permeable, polyamine-sensitive responses that we have recorded are mediated by GluA2-lacking AMPARs and that as much as half of the total AMPAR-mediated current may be carried by these AMPARs. If these channels are GluA1 homomers, then their number may be small because of their relatively large single-channel conductance (Rozov et al. 2012; Swanson et al. 1997).
Intracellular polyamines block closed GluA2-lacking AMPARs and are released when the channels open (Bowie and Mayer 1995; Koh et al. 1995), giving rise to PPF (Rozov et al. 1998; Rozov and Burnashev 1999). We observed that the PPRs of phEPSCs recorded from apical spines were considerably larger than those from basal spines. In addition, the PPF observed at apical spines was increased at depolarized potentials, whereas the PPF at basal spines was voltage-independent. Furthermore, extracellular spermine reduced the amplitude of phEPSCs recorded at apical spines and increased the PPF of these phEPSCs, whereas the amplitude and PPR of phEPSCs recorded from basal spines were not affected. These data further support the hypothesis that apical spines contain Ca2+-permeable, polyamine-sensitive AMPARs. Although basal synapses were polyamine-insensitive, we observed some evidence of Ca2+ permeability, the source of which is unknown. It is interesting to speculate that some basal synapses may express Ca2+-permeable, GluA2-containing AMPARs (Bowie 2012).
Comparison with previous studies.
Our conclusion that Ca2+-permeable AMPARs are present on apical dendritic spines of CA1 pyramidal neurons is the opposite of that reached in several other studies (Adesnik and Nicoll 2007; Gray et al. 2007; Plant et al. 2006). We have employed a combination of methods that has not been used to test this hypothesis previously, including single-spine microphotolysis of caged glutamate and microfluorometric Ca2+ imaging. In general, the previous studies based their conclusions on negative findings, such as a lack of rectification and a lack of PhTx sensitivity. In contrast, Rozov et al. (2012) demonstrated that when endogenous polyamines were dialyzed out from CA1 pyramidal neurons, AMPAR-mediated EPSCs increased in amplitude over time, became sensitive to PhTx, and exhibited decreased rectification. Thus endogenous polyamines appear to occlude the effect of PhTx, thereby accounting for the lack of PhTx sensitivity in some previous studies. Washout of endogenous polyamines may account for the apparently small contribution of Ca2+-permeable AMPARs to the synaptic I–V, as concluded from the nonrectifying I–V curve described in previous studies (Rozov et al. 2012). Wenthold et al. (1996) demonstrated that GluA1/2 and GluA2/3 heteromers constitute the majority of hippocampal AMPARs but also noted that 10% consisted of homomeric GluA1. CA1 pyramidal cells are capable of synthesizing GluA1 homomeric receptors and inserting them into the synaptic plasma membrane (Lu et al. 2009). Indeed, insertion of GluA2-lacking AMPARs is a major homeostatic response to reduced synaptic activity (Harms et al. 2005; Hou et al. 2008; Sutton et al. 2006; Thiagarajan et al. 2005).
He et al. (2009) have demonstrated that GluA2-lacking AMPARs are present at perisynaptic sites that were likely to be activated by glutamate microphotolysis in our experiments. Assuming that spermine inhibits EPSCs by acting solely at Ca2+-permeable AMPARs, our evidence that spermine reduces fEPSPs in s. radiatum of adult rat hippocampal slices indicates that at least some spermine-sensitive Ca2+-permeable AMPARs are accessible to synaptically released glutamate under our stimulation conditions. Further experiments, such as manipulations of intracellular polyamine concentrations, would be required to test this hypothesis more fully. Ca2+-permeable AMPARs are regulated developmentally in the neocortex, decreasing in number with maturity (Shin et al. 2005). The organotypic hippocampal slice cultures used in our photolysis experiments may mature more slowly than the intact brain; however, we observed comparable sensitivity of s. radiatum synaptic responses to spermine in both cultured and acute hippocampal slices as well as significant glutamate-induced, spermine-sensitive Co2+ loading in acute slices.
Conclusion.
The presence of Ca2+-permeable AMPARs in the dendritic spines of apical dendrites is of considerable functional importance. The amount of Ca2+ that enters the cell via this route is low, however, as demonstrated in our Ca2+ imaging results, so the existence of GluA2-lacking AMPARs is consistent with the overwhelming evidence that NMDAR-mediated Ca2+ influx is the predominant source of synaptic Ca2+ for conventional synaptic plasticity. Nevertheless, Ca2+-permeable AMPARs may take part in synaptic plasticity under other conditions, such as after chronic inactivity, and may be upregulated under pathological conditions (e.g., Liu and Zukin 2007). The presence of Ca2+-permeable AMPARs at apical but not basal spines provides evidence of the complexity and diversity of synaptic glutamate receptors.
GRANTS
This work was supported by stipends to H. A. Mattison (F31-MH-079668) and grants from the National Institutes of Health (R01-DA-014625 and R01-MH-077277 to B. E. Alger, R01-GM-056481 to J. P. Y. Kao, and R01-MH-65488 and R01-MH-086828 to S. M. Thompson).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.A.M., A.A.B., M.M., N.S.P., C.G.R., B.E.A., J.P.Y.K., and S.M.T. conception and design of research; H.A.M., A.A.B., M.M., N.S.P., and S.M.T. performed experiments; H.A.M., A.A.B., M.M., N.S.P., C.G.R., B.E.A., and S.M.T. analyzed data; H.A.M., A.A.B., M.M., N.S.P., C.G.R., B.E.A., and S.M.T. interpreted results of experiments; H.A.M., A.A.B., N.S.P., and S.M.T. prepared figures; H.A.M. and S.M.T. drafted manuscript; H.A.M., N.S.P., and S.M.T. edited and revised manuscript; H.A.M., A.A.B., M.M., N.S.P., C.G.R., B.E.A., J.P.Y.K., and S.M.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dina Popovkina, Leepeng Mok, and Yvonne Logan for their expert assistance with the preparation and maintenance of the slice cultures and Dr. Cha-Min Tang for development of the photolysis apparatus.
REFERENCES
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598–4602, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurousseau MR, Osswald IK, Bowie D. Thinking of Co2−-staining explant tissue or cultured cells? How to make it reliable and specific. Eur J Neurosci 35: 1201–1207, 2012 [DOI] [PubMed] [Google Scholar]
- Bagal AA, Kao JP, Tang CM, Thompson SM. Long-term potentiation of exogenous glutamate responses at single dendritic spines. Proc Natl Acad Sci USA 102: 4434–4439, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood BL, Giessel AJ, Sabatini BL. Biphasic synaptic Ca influx arising from compartmentalized electrical signals in dendritic spines. PLoS Biol 7: 1–10, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D. Redefining the classification of AMPA-selective ionotropic glutamate receptors. J Physiol 590: 49–61, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Lange GD, Mayer ML. Activity-dependent modulation of glutamate receptors by polyamines. J Neurosci 18: 8175–8185, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie D, Mayer ML. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron 15: 453–462, 1995 [DOI] [PubMed] [Google Scholar]
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron 8: 189–198, 1992 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology 56: 2–5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiScenna PG, Ferchmin PA, Eterovic VA, Teyler TJ. Spermine depresses NMDA, K/AMPA and GABAA-mediated synaptic transmission in the rat hippocampal slice preparation. Brain Res 647: 353–356, 1994 [DOI] [PubMed] [Google Scholar]
- Donevan SD, Rogawski MA. Intracellular polyamines mediate inward rectification of Ca(2+)-permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Proc Natl Acad Sci USA 92: 9298–9302, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchmin PA, Eterović VA, Rivera EM, Teyler TJ. Spermine increases paired-pulse facilitation in area CA1 of hippocampus in a calcium-dependent manner. Brain Res 689: 189–196, 1995 [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Thompson SM, McKinney RA, Debanne D, Robertson RT. Organotypic slice cultures of neural tissue. In: Culturing Nerve Cells (2nd ed.), edited by Banker G, Goslin K. Cambridge, MA: MIT Press, 1998, p. 461–498 [Google Scholar]
- Geiger JR, Melcher T, Koh DS, Sakmann B, Seeburg PH, Jonas P, Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron 15: 193–204, 1995 [DOI] [PubMed] [Google Scholar]
- Gerfin-Moser A, Grogg F, Rietschin L, Thompson SM, Streit P. Alterations in glutamate but not GABAA receptor subunit expression as a consequence of epileptiform activity in vitro. Neuroscience 67: 849–865, 1995 [DOI] [PubMed] [Google Scholar]
- Gray EE, Fink AE, Sarinana J, Vissel B, O'Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol 98: 2488–2492, 2007 [DOI] [PubMed] [Google Scholar]
- Harms KJ, Tovar KR, Craig AM. Synapse-specific regulation of AMPA receptor subunit composition by activity. J Neurosci 25: 6379–6388, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267, 2000 [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci USA 106: 20033–20038, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Thoumine O, Mondin M, Tessier B, Giannone G, Choquet D. Activity-independent and subunit-specific recruitment of functional AMPA receptors at neurexin/neuroligin contacts. Proc Natl Acad Sci USA 105: 20947–20952, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol 422: 203–225, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci 17: 31–108, 1994 [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci USA 105: 775–780, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron 15: 427–434, 1995 [DOI] [PubMed] [Google Scholar]
- Jonas P, Sakmann B. Glutamate receptor channels in isolated patches from CA1 and CA3 pyramidal cells of rat hippocampal slices. J Physiol 455: 143–171, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamboj SK, Swanson GT, Cull-Candy SG. Intracellular spermine confers rectification on rat calcium-permeable AMPA and kainate receptors. J Physiol 486: 297–303, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science 249: 556–560, 1990 [DOI] [PubMed] [Google Scholar]
- Koh DS, Burnashev N, Jonas P. Block of native Ca2+-permeable AMPA receptors in rat brain by intracellular polyamines generates double rectification. J Physiol 486: 305–312, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Morales M, Ibarz JM, Somohano F. Rectification properties and Ca2+ permeability of glutamate receptor channels in hippocampal cells. Eur J Neurosci 6: 1080–1088, 1994 [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375: 400–404, 1995 [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci 30: 126–134, 2007 [DOI] [PubMed] [Google Scholar]
- Lopatin AN, Makhina EN, Nichols CG. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature 372: 366–369, 1994 [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62: 254–268, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüthi A, Chittajallu R, Duprat F, Palmer MJ, Benke TA, Kidd FL, Henley JM, Isaac JT, Collingridge GL. Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluA2 interaction. Neuron 24: 389–399, 1999 [DOI] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. Dissociation between the Joro spider toxin sensitivity of recombinant alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors and their ability to increase intracellular calcium. Neuropharmacology 37: 1431–1443, 1998 [DOI] [PubMed] [Google Scholar]
- Montgomery JM, Pavlidis P, Madison DV. Pair recordings reveal all-silent synaptic connections and the postsynaptic expression of long-term potentiation. Neuron 29: 691–701, 2001 [DOI] [PubMed] [Google Scholar]
- Moult PR, Cross A, Santos SD, Carvalho AL, Lindsay Y, Connolly CN, Irving AJ, Leslie NR, Harvey J. Leptin regulates AMPA receptor trafficking via PTEN inhibition. J Neurosci 30: 4088–4101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune A, Isaac JT, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron 21: 87–97, 1998 [DOI] [PubMed] [Google Scholar]
- Noh KM, Yokota H, Mashiko T, Castillo PE, Zukin RS, Bennett MV. Blockade of calcium-permeable AMPA receptors protects hippocampal neurons against global ischemia-induced death. Proc Natl Acad Sci USA 102: 12230–12235, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogoshi F, Weiss JH. Heterogeneity of Ca2+-permeable AMPA/kainate channel expression in hippocampal pyramidal neurons: fluorescence imaging and immunocytochemical assessment. J Neurosci 23: 10521–10530, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osswald IK, Galan A, Bowie D. Light triggers expression of philanthotoxin-insensitive Ca2+-permeable AMPA receptors in the developing rat retina. J Physiol 582: 95–111, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluA2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci 9: 602–604, 2006 [DOI] [PubMed] [Google Scholar]
- Rock DM, Macdonald RL. The polyamine spermine has multiple actions on N-methyl-d-aspartate receptor single-channel currents in cultured cortical neurons. Mol Pharmacol 41: 83–88, 1992 [PubMed] [Google Scholar]
- Rozov A, Burnashev N. Polyamine-dependent facilitation of postsynaptic AMPA receptors counteracts paired-pulse depression. Nature 401: 594–598, 1999 [DOI] [PubMed] [Google Scholar]
- Rozov A, Sprengel R, Seeburg PH. GluA2-lacking AMPA receptors in hippocampal CA1 cell synapses: evidence from gene-targeted mice. Front Mol Neurosci 5: 1–10, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A, Zilberter Y, Wollmuth LP, Burnashev N. Facilitation of currents through rat Ca2+-permeable AMPA receptor channels by activity-dependent relief from polyamine block. J Physiol 511: 361–377, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci 16: 359–365, 1993 [DOI] [PubMed] [Google Scholar]
- Shin J, Shen F, Huguenard JR. Polyamines modulate AMPA receptor-dependent synaptic responses in immature layer V pyramidal neurons. J Neurophysiol 93: 2634–2643, 2005 [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell 125: 785–799, 2006 [DOI] [PubMed] [Google Scholar]
- Swanson GT, Kamboj SK, Cull-Candy SG. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J Neurosci 17: 58–69, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47: 725–737, 2005 [DOI] [PubMed] [Google Scholar]
- Toomim CS, Millington WR. Regional and laminar specificity of kainate-stimulated cobalt uptake in the rat hippocampal formation. J Comp Neurol 402: 141–154, 1998 [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci 1: 572–578, 1998 [DOI] [PubMed] [Google Scholar]
- Tsubokawa H, Oguro K, Robinson HP, Masuzawa T, Kawai N. Single glutamate channels in CA1 pyramidal neurones after transient ischaemia. Neuroreport 6: 527–530, 1995 [DOI] [PubMed] [Google Scholar]
- Washburn MS, Dingledine R. Block of α-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid (AMPA) receptors by polyamines and polyamine toxins. J Pharmacol Exp Ther 278: 669–678, 1996 [PubMed] [Google Scholar]
- Washburn MS, Numberger M, Zhang S, Dingledine R. Differential dependence on GluR2 expression of three characteristic features of AMPA receptors. J Neurosci 17: 9393–9406, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci 16: 1982–1989, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]