Abstract
The capacity for humans to learn a new walking pattern has been explored with a split-belt treadmill during single sessions of adaptation, but the split-belt treadmill can also be used to study longer-term motor learning. Although the literature provides some information about motor learning after stroke, existing studies have primarily involved the upper extremity and the results are mixed. The purpose of this study was to characterize learning of a novel locomotor task in stroke survivors. We hypothesized that the presence of neurological dysfunction from stroke would result in slower learning of a locomotor task and decreased retention of what was learned and that these deficits would be related to level of sensorimotor impairment. Sixteen participants with stroke and sixteen neurologically intact participants walked on a split-belt treadmill for 15 min on 5 consecutive days and during a retention test. Step length and limb phase were measured to capture learning of the spatial and temporal aspects of walking. Learning the spatial pattern of split-belt treadmill walking was slowed after stroke compared with neurologically intact subjects, whereas there were no differences between these two groups in learning the temporal pattern. During the retention test, poststroke participants demonstrated equal retention of the split-belt treadmill walking pattern compared with those who were neurologically intact. The results suggest that although stroke survivors are slower to learn a new spatial pattern of gait, if given sufficient time they are able to do so to the same extent as those who are neurologically intact.
Keywords: stroke, locomotion, learning, adaptation
motor learning is traditionally defined as a persistent change in a movement that occurs over long-term practice and experience and that results in stable performance (Schmidt 1988; Schmidt and Wrisberg 2000). The process of learning begins with exposure to the task, followed by consolidation, which is a set of processes that involve changes in the central nervous system leading to a long-term memory that is resistant to disruption or interference by other motor activity (Krakauer and Shadmehr 2006; Stickgold and Walker 2007). These processes ultimately lead to the ability to recall the newly learned motor skill in the appropriate context or environment.
The capacity for humans to learn a new walking pattern has been explored with a split-belt treadmill (Malone et al. 2011) and a rotating treadmill (Earhart et al. 2001). During walking on a rotating treadmill, subjects adapt their walking pattern while walking in place on the perimeter of a rotating disk. After ∼30 min of exposure, when the subjects are blindfolded and asked to walk under normal conditions they demonstrate an involuntary and significant curvature of their walking trajectory. The split-belt treadmill is a treadmill in which there are two independently controlled belts, one under each foot, that travel at two different speeds. When subjects adapt their walking pattern to these conditions for 10–15 min, significant asymmetries in step length, double-support times, and limb phase angles are present when normal walking conditions are resumed (Reisman et al. 2005, 2007, 2009). These responses, of changing interlimb dynamics over a series of steps during one practice period on the rotating or split-belt treadmill, are a form of motor adaptation; participants are adjusting an already well-learned motor skill over a period of trial-and-error practice, as they are exposed to a novel and perturbing environment (Martin et al. 1996b). This adaptation has been extensively studied (Earhart et al. 2001; Malone and Bastian 2010; Reisman et al. 2005, 2007, 2009, 2010; Torres-Oviedo and Bastian 2010; Vasudevan and Bastian 2010) and is thought to be a necessary component of longer-term motor learning (Bastian 2008).
The split-belt treadmill adaptation paradigm can be used to study longer-term motor learning, as has been done with upper extremity adaptation paradigms (Caithness et al. 2004; Krakauer et al. 2005; Shadmehr and Brashers-Krug 1997). To use the split-belt treadmill to study longer-term motor learning, participants walk on the split-belt treadmill over a series of days. As has been recently shown in neurologically intact subjects (Malone et al. 2011), if participants learn something about how to walk more symmetrically in this novel environment, then with each day of practice less asymmetry is observed at the start of split-belt walking and a more rapid readaptation is observed with each day of practice (Malone et al. 2011). Thus through evaluation of the rate and magnitude of learning one can determine whether subjects successfully learned a new walking pattern appropriate to the conditions of the split-belt treadmill.
While improvements in gait in poststroke locomotor studies support the idea that locomotor learning occurs after stroke, the literature provides limited information about the process of relearning to walk (locomotor learning) after stroke. Studies of poststroke motor learning have primarily involved the upper extremity, and in many studies subjects utilize the nonparetic arm to complete the task (Boyd et al. 2007; Boyd and Winstein 2001, 2003, 2004, 2006; Cirstea and Levin 2007; Winstein et al. 1999). Given the substantial difference between the control of locomotion and the control of upper extremity movement, it is unclear whether these findings apply to learning of a walking task. Moreover, the control of motor learning has been suggested to involve multiple areas of the brain that are commonly affected by stroke (Boyd and Linsdell 2009; Hadipour-Niktarash et al. 2007; Hotermans et al. 2008; Muellbacher et al. 2002; Perez et al. 2008; Vidoni et al. 2010; Vidoni and Boyd 2007).
Therefore, the aim of this study was to characterize learning of a novel locomotor task after stroke. We hypothesized that the participants with stroke would require additional days of practice to achieve the same level of performance on the locomotor task. Additionally, we hypothesized that the participants with stroke would recall less of the newly learned locomotor pattern after a 2-day break from training. We further hypothesized that this slowed learning would be related to level of sensorimotor impairment.
MATERIALS AND METHODS
Participants
Participants with chronic stroke and age- and sex-matched neurologically intact participants were recruited from Delaware and surrounding states with the assistance of local physicians, physical therapists, and advertising. All participants provided written informed consent, and the protocol was approved by the University of Delaware Human Subjects Review Board. To be included, participants with stroke must have had a single stroke at least 6 mo prior to study participation and be able to walk independently with or without bracing at a treadmill speed ≥0.4 m/s with no more than light touch on the handrail. The neurologically intact participants must have been free from any neurological dysfunction and any musculoskeletal problem that had the potential to impact ambulation and have an age ±5 yr as and be the same sex as their stroke participant counterpart. Exclusion criteria for both participant groups included uncontrolled blood pressure or diabetes, cardiovascular or arthritic dysfunction exacerbated by exercise, and active cancer.
Participants with stroke underwent clinical testing that included the lower extremity portion of the Fugl-Meyer assessment (Fugl-Meyer et al. 1975) and the timed 6-m walk test. Sensation of the hemiparetic great toe was tested with graded monofilaments ranging from 6.65 g to 2.83 g. Each monofilament was presented five times, and the number of times the participant correctly reported perceiving the monofilament was recorded. A participant's sensation level was recorded as the smallest monofilament that was correctly identified at least four out of five times. The clinical examination was completed on a day that was separate from the rest of the experimental protocol.
Instrumentation and Procedures
This was a multiday study in which participants walked on the split-belt treadmill for 15 min at a speed ratio of 2:1 for 5 consecutive days. Before the first split-belt session, baseline data were collected with participants walking with the belts traveling at the same speeds. Otherwise, each day consisted of 15 min of split-belt treadmill walking at the same speed and ratio day to day. One final 15-min split-belt treadmill exposure was completed after 2 days without exposure. This final exposure was done to evaluate retention, and the 2-day time frame was chosen in accordance with other similarly structured learning studies (Boyd and Winstein 2006; Meehan et al. 2011; Vidoni and Boyd 2009). The structures of the data collections are shown in Fig. 1.
Fig. 1.
Experimental protocol.
All participants walked on a split-belt treadmill instrumented with two independent 6 degree of freedom force platforms (AMTI, Watertown, MA) from which ground reaction force data were continuously collected at 2,000 Hz. Kinematic data were continuously collected with an eight-camera Vicon Motion Capture System (Vicon MX, Los Angeles, CA) at 100 Hz. Retroreflective markers (14-mm diameter) were placed on rigid shells over the pelvis, bilateral thigh, shank, and foot segments. Single markers were placed on the medial and lateral iliac crests, greater trochanters, knee joint line, and malleoli. During walking, all participants rested fingertips on an instrumented handrail that provided real-time quantitative data for vertical force exerted by the participant during all walking trials. To keep handrail use consistent from day to day, the researcher monitored these vertical forces and provided verbal cues to participants if a change in force above those recorded at baseline was observed.
All participants wore a safety harness around their chest for fall prevention; it did not provide body weight support. Participants' blood pressure, heart rate, and rate of perceived exertion (RPE) (Borg 1970) were monitored, and participants with stroke were permitted to have standing or sitting rests during testing if necessary but did not dismount from the treadmill.
For each participant, the speeds used during the split-belt walking portion were set in a ratio of 2:1. The speeds were determined in the following manner. For the participants with stroke, the fastest treadmill speed was determined by slowly increasing the treadmill speed until 1) the participant reported that he/she could not tolerate any further increase or 2) the researcher determined that it was unsafe to increase the speed any further. This speed was then used to set the fast belt speed used during split-belt treadmill walking, and 50% of this speed was used as the slow belt speed. For our participants with stroke, the split-belt configuration (which leg was assigned to the fast belt) was chosen in order to induce exaggeration of baseline asymmetries. All participants who were neurologically intact walked at the same speeds as their stroke participant counterparts, the rest break structure was maintained, and leg assignment was randomized.
Data Analysis
All kinematic and kinetic data were exported from Vicon-Nexus software and further processed with Visual 3D (C-Motion, Germantown, MD). The gait events of foot strike and liftoff were determined for each limb individually with an automatic algorithm in Visual 3D. Foot strike was identified when the vertical ground reaction force exceeded 20 N for at least eight frames, and liftoff was identified when the vertical ground reaction force dropped below 20 N for at least eight frames. After the algorithm was run, all gait events were visually checked for accuracy.
Dependent variables.
Previous studies have found that spatial and temporal variables respond differently to split-belt walking and that their response is dissociable and is retained differently with repeated exposure to split-belt walking (Malone et al. 2011, 2012; Malone and Bastian 2010). Therefore both spatial (step length) and temporal (limb phasing) variables were evaluated in this study. Step length and limb phasing were calculated for each leg continuously during each phase of the protocol. Step length was calculated as the sagittal distance between the right and left heel markers at foot strike. Step length was labeled paretic or nonparetic (or slow or fast, respectively, for control subjects) based on the leading leg. Step asymmetry was calculated as the difference between the paretic step length and the optimal step length, as follows:
where optimal step length = (paretic step length + nonparetic step length)/2. To calculate limb phase, continuous limb angle was calculated for each limb as the angle between a vector connecting the greater trochanter and the lateral malleoli and the lab vertical. Peak limb flexion and extension were then located for each stride, and lag time for a given limb was calculated as the difference in time between contralateral peak flexion and ipsilateral peak extension. This value was normalized by ipsilateral stride time to get limb phase. Stride-by-stride limb phase symmetry was calculated by dividing the limb phase value for the leg on the slow belt by that on the fast belt.
To evaluate learning in our participants, we calculated three measures of learning for both step length and limb phasing: daily magnitude of early asymmetry, daily rate of adaptation (Malone et al. 2011), and stride-by-stride variability (Albouy et al. 2012; Muller and Sternad 2004).
DAILY MAGNITUDE OF EARLY ASYMMETRY.
One way to evaluate learning of this novel walking pattern over days was by evaluating how the magnitude of initial asymmetry changes from day to day. If participants learned something about how to walk on the split-belt treadmill on day 1 of practice, then on day 2 of practice we would expect to see less step length or limb phase asymmetry when the participant begins split-belt walking (Malone et al. 2011). That is, if they had learned something about this novel walking pattern, they should have been less perturbed by the split belts (less asymmetric, signifying a decrease in “error”) on day 2. To calculate the daily magnitude of early asymmetry, the first 30 strides of symmetry data during split-belt walking were averaged and the average of the entire baseline was subtracted from this value for each day.
RATE OF ADAPTATION.
Another way to evaluate learning was by examining the amount of practice required to resolve the initial asymmetry observed on each day. The time required to resolve the early asymmetry and achieve a stable pattern in step length or limb phase symmetry on a given day was called the rate of adaptation (Huang et al. 2011). If a participant had learned something about how to walk on the split-belt treadmill on the first day of practice, then he would have been able to resolve the asymmetry (adapt his walking pattern and achieve symmetry) more quickly on day 2.
Daily rate of adaptation was calculated for each variable with a customized MATLAB (MathWorks, Natick, MA) program that found the time required to achieve a plateau in symmetry of step length or limb phase each day (Fig. 2). The rate was determined to be 0 if there was no statistical difference between the first and last 30 strides of symmetry data for that day (P > 0.05). In all other cases, the rate was calculated as demonstrated in Fig. 2. Stride-by-stride symmetry data was evaluated by creating bins of 5 strides and comparing their values to that of the final 30 strides. Starting with the first bin on a given day, the program moved sequentially through the data until an agreement was found between the current bin's values and the final 30 strides, according to the following criterion: 4 of 5 strides in the bin fell within ±1 standard deviation of the average of the final 30 strides. When this criterion was met for four consecutive bins, this was determined to be the plateau in the adaptation curve. The number of strides required to achieve this agreement was recorded as the rate of adaptation for that day and was reported as a percentage of total practice strides for that day.
Fig. 2.
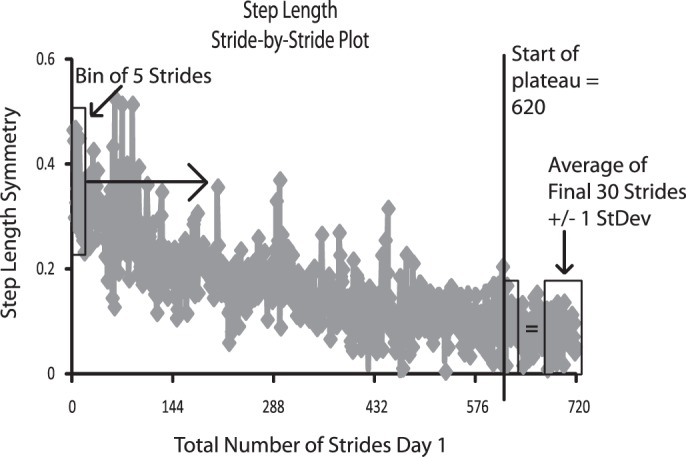
Illustration of rate calculation. First bin of 5 strides is represented by a rectangle at far left. Progressing from left to right in chronological order, the contents of each consecutive bin of 5 strides were compared to the average of the final 30 strides. When 4 of 5 strides in the current bin fell within ±1 SD of the average of the final 30 strides, a plateau was determined to have been achieved. The number of the first stride in this bin was identified as the number of strides of practice required to achieve plateau, which we called the rate of adaptation.
STRIDE-BY-STRIDE VARIABILITY.
Because motor learning is associated with a reduction in the variability of task performance (Albouy et al. 2012; Muller and Sternad 2004), we also examined the change in step length and limb phasing asymmetry for each day. To evaluate how step-to-step variability changed over days, variability was calculated as the standard deviation of step length or limb phase symmetry for each participant on each day.
Clinical measures.
To evaluate factors that may influence learning, clinical measures of lower extremity Fugl-Meyer and sensation scores were correlated with the amount of learning that occurred on day 1. The amount of learning that occurred on day 1 was measured by the change in magnitude of early asymmetry on day 2 compared with day 1 and the change in rate on day 2 compared with day 1.
Statistical Analysis
Normality of the data distribution was confirmed with the Kolmogorov-Smirnov test for normality. All statistical testing was completed with SPSS v19. Statistical tests were completed for all dependent variables (daily magnitude of early asymmetry, rate of adaptation, and stride-by-stride variability for step length symmetry and limb phase symmetry) unless otherwise indicated.
Before testing our hypothesis that participants with stroke would require additional days of practice to achieve the same level of performance on the locomotor task, we needed to evaluate whether the two groups responded similarly to the split-belt treadmill on the first day (e.g., were perturbed a similar amount initially and adapted completely by the end of the first session). To determine whether the two groups were perturbed similarly by the split-belt treadmill on the first day, we compared day 1 magnitude of early asymmetry for both step length and limb phase between the stroke and neurologically intact groups, using an independent-samples t-test. To assist with interpretation of nonsignificant differences in these comparisons, effect sizes were calculated and evaluated for each comparison. A small effect size indicated that the nonsignificant difference was less likely due to a type II error than a true similarity between the groups.
Similarly, to confirm that both groups completely adapted their walking pattern on day 1, we compared the average step length or limb phase asymmetry of the last 30 strides of split-belt walking on day 1 to the average baseline asymmetry. No differences between the end of split-belt walking on day 1 and baseline asymmetry would indicate that the subjects fully adapted back to their baseline walking pattern by the end of the 15-min session. Finally, to determine whether both groups responded similarly to the split-belt treadmill on the final day of practice, day 5 magnitude of early asymmetry and rate of adaptation for both step length and limb phase were compared between the stroke and neurologically intact groups with an independent-samples t-test. No differences between the stroke and neurologically intact groups on day 5 would indicate that the two groups learned the same amount about split-belt treadmill walking by the fifth day of practice.
To test our hypothesis that participants with stroke would require additional days of practice to achieve the same level of performance on the locomotor task, we completed a paired-samples t-test for each of our dependent measures comparing each day to day 5. We reasoned that when the comparison between the value for a given day and day 5 was not significantly different and remained not different over the ensuing days, this would indicate that the full amount of learning that would occur over the 5 days had been completed. If a greater number of days was required to achieve this complete learning in the stroke group, our hypothesis would be supported. A Bonferroni correction was used to control for repeated comparisons, resulting in statistical significance being accepted when P < 0.0125.
To test our hypothesis that the participants with stroke would recall less of the newly learned locomotor pattern after a 2-day break from training, retention of learning for each group was evaluated with a paired-samples t-test between values for day 5 and the day of the retention test.
A general linear regression was used to assess the association between learning on day 1 and lower extremity Fugl-Meyer score and sensory score for both step length and limb phase in the participants with stroke only.
RESULTS
Sixteen participants with stroke (62.75 ± 8.24 yr) and sixteen participants who were neurologically intact (64.56 ± 7.93 yr) participated in this study. Table 1 contains participant demographics and speeds for the participants with stroke.
Table 1.
Participant demographics and speeds
| Participant Number | Age, yr | Sex | Months Since Stroke | Hemiparetic Side | Speeds, m/s | Fugl-Meyer Score | Lesion Location |
|---|---|---|---|---|---|---|---|
| 61 | 60 | M | 57 | L | 0.6/0.3 | 12 | R lenticular nucleus, internal capsule, frontal and temporal lobes |
| 128 | 66 | F | 43 | R | 0.6/0.3 | 20 | L thalamus |
| 138 | 59 | M | 31 | L | 0.6/0.3 | 24 | R temporal lobe |
| 177 | 67 | M | 8 | L | 0.8/0.4 | 26 | R cerebral intraparenchymal |
| 186 | 57 | F | 37 | L | 0.4/0.2 | 20 | R pontine basis |
| 187 | 57 | F | 17 | L | 0.8/0.4 | 26 | R hemi pons |
| 192 | 55 | F | 24 | L | 0.6/0.3 | 27 | R posterolateral parietal cortex |
| 194 | 77 | F | 21 | R | 0.8/0.4 | 27 | L frontal lobe |
| 196 | 69 | F | 13 | R | 1.0/0.5 | 29 | L frontoparietal region |
| 200 | 60 | M | 12 | R | 1.0/0.5 | 25 | L cerebral hemisphere-frontoparietal region |
| 202 | 60 | F | 38 | L | 1.0/0.5 | 33 | R basal ganglia |
| 241 | 69 | F | 151 | L | 0.6/0.3 | 14 | R cerebral white matter infarct with cortical involvement |
| 257 | 77 | F | 94 | L | 0.4/0.2 | 24 | R basal ganglia |
| 265 | 49 | M | 6 | R | 1.2/0.6 | 20 | L pons |
| 291 | 67 | M | 19 | L | 0.4/0.2 | 12 | R parietal, temporal, frontal, and lateral occipital lobes |
| 294 | 55 | F | 12 | R | 1.0/0.5 | 21 | L medial upper pons and lower midbrain |
Daily Magnitude of Early Asymmetry
Figure 3 represents magnitude of early asymmetry for step length and limb phase across days for each group. Magnitude of early asymmetry is calculated as the amount of asymmetry over the first 30 strides each day minus the average baseline asymmetry, so a larger number indicates greater asymmetry relative to baseline.
Fig. 3.
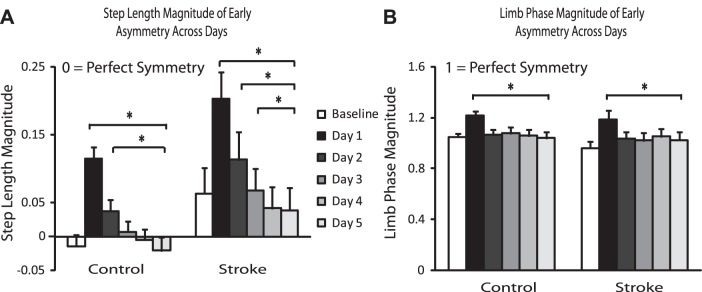
Step length and limb phase magnitude across days. A: step length magnitude of early asymmetry across days. B: limb phase magnitude of early asymmetry across days. Error bars represent SE. *Statistical significance (P < 0.0125).
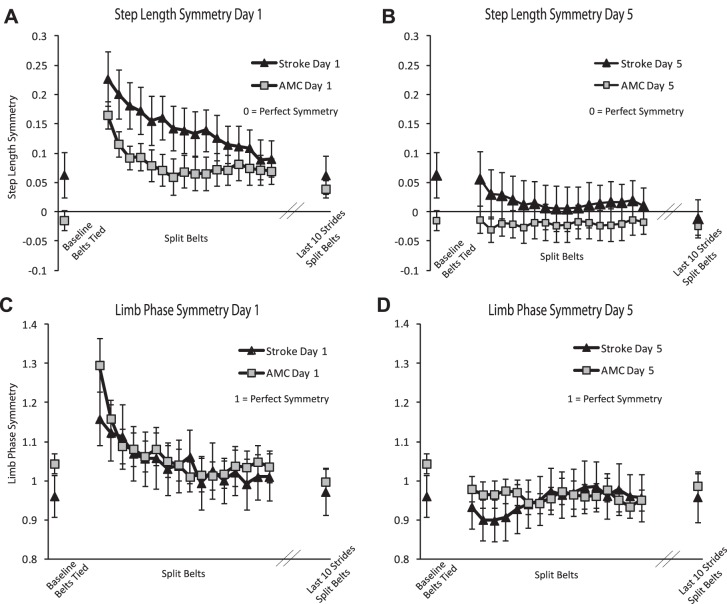
There were no differences in magnitude of early asymmetry between the groups on day 1 or day 5 [Fig. 4; step: P = 0.658, effect size (ES) = 0.143; P = 0.471, ES = 0.252; phase: P = 0.299, ES = 0.379; P = 0.224, ES = 0.429, respectively], demonstrating that participants with stroke and participants who were neurologically intact were equally perturbed by the split-belt treadmill on day 1 and day 5 for both step length and limb phase. There were also no differences in step length or limb phase asymmetry between the end of day 1 and baseline for either the stroke or control group (step: P = 0.296, ES = 0.108; P = 0.057, ES = 0.54; phase: P = 0.757, ES = 0.046; P = 0.283, ES = 0.202, respectively). This demonstrates that each group/variable completely adapted and returned to its baseline (a)symmetry by the end of day 1.
Fig. 4.
Step length and limb phase symmetry. A and B: step length symmetry on the first day of practice (A) and the fifth day of practice (B) walking on split belts for the stroke and control (AMC) groups. C and D: limb phase symmetry on the first day of practice (C) and the fifth day of practice (D) of walking on the split belts for the stroke and control groups. Baseline is the average of walking with belts tied. Each data point in the split-belt period is the average of 10 consecutive strides (1–160), and the final point represents the average over the last 10 strides of split-belt walking.
As expected, the largest magnitude of early asymmetry for step length was observed on day 1, followed by a reduction in this early perturbation on subsequent days (Fig. 3A), demonstrating that participants learn something about how to walk on the split-belt treadmill with repeated exposure (Malone et al. 2011). For both groups early step length asymmetry differed between day 1 and day 5 (Fig. 3A; P < 0.001, both control and stroke) and between day 2 and day 5 (Fig. 3A; P = 0.001, P < 0.001 for control and stroke, respectively). For the group with stroke there was also a difference between day 3 and day 5 (Fig. 3A; P = 0.003).
Similarly for limb phase, magnitude of early asymmetry is largest on day 1, followed by a reduction with repeated exposure. For both groups early limb phase asymmetry differed between day 1 and day 5 only (Fig. 3B; P = 0.005, P = 0.010 for control and stroke, respectively).
Rate of Adaptation
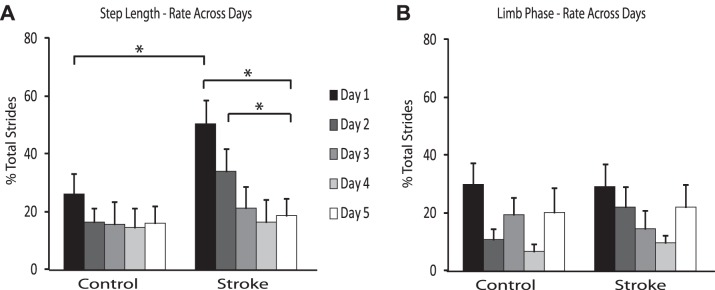
Rate of adaptation is represented as the percentage of total daily strides to reach plateau for the day, so a larger number represents slower adaptation. There was a difference in the rate of adaptation of step length asymmetry on day 1 between the groups, with a slower rate of adaptation in the group with stroke (Fig. 4A; P < 0.01). By day 5, however, this difference had resolved (Fig. 4B), indicating that with repeated exposure the stroke survivors learned to adapt step length asymmetry as quickly as the neurologically intact participants.
As expected, the adaptation rate for step length was slowest on day 1, followed by a reduction in adaptation rate on subsequent days (Malone et al. 2011). However, the rate of step length adaptation on any day compared with day 5 was not significantly different in the neurologically intact subjects (Fig. 5A). In the group of participants with stroke, the rate of adaptation of step length asymmetry was different between day 1 and day 5 and between day 2 and day 5 (Fig. 5A; P < 0.001 and P = 0.010, respectively).
Fig. 5.
Step length and limb phase rate across days. A: step length rate of adaptation across days. B: limb phase rate of adaptation across days. Error bars represent SE. *Statistical significance (P < 0.0125).
In contrast to step length rate of adaptation, there was no difference in the rate of adaptation of limb phase on day 1 or day 5 between the group with stroke and the control group (Fig. 4, C and D). Also, unlike the step length rate of adaptation findings, there were no differences in the rates between any day and day 5 in either group (Fig. 5B).
Retention
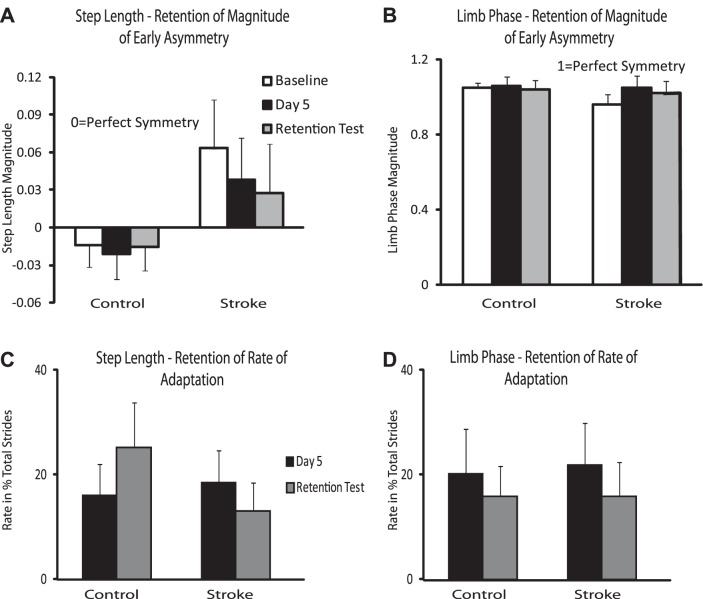
Figure 6 represents retention of magnitude of early asymmetry for step length and limb phase and retention of rate of adaptation. Perfect retention would be represented by equality in magnitude or rate between day 5 and the day of retention, while a higher value on the day of retention compared with day 5 would represent a relative loss of learning over the 2-day break.
Fig. 6.
Retention of magnitude of early asymmetry and rate of adaptation. A: step length retention of magnitude of early asymmetry. B: limb phase retention of magnitude of early asymmetry. C: step length retention of adaptation rate. D: limb phase retention of adaptation rate. Baseline magnitude is represented by open bars, day 5 magnitude/rate is represented by black bars, and the retention day test is represented by gray bars. Error bars represent SE.
Both of our participant groups demonstrate no difference in retention for magnitude of early asymmetry and rate of adaptation for step length and limb phase. In other words, participants with stroke and those who are neurologically intact retain the newly learned locomotor task even after 2 days without practice.
Figure 7 demonstrates the step length and limb phase variability across days. For step length, there was a reduction in variability between day 1 and day 5 for both groups (P = 0.001 for control, P < 0.001 for stroke). In addition, the stroke group also showed a reduction in variability between days 2 and 5 (P = 0.004). For limb phase, there was a reduction in variability between day 1 and day 5 only for both groups (P < 0.001 for control, P = 0.003 for stroke).
Fig. 7.
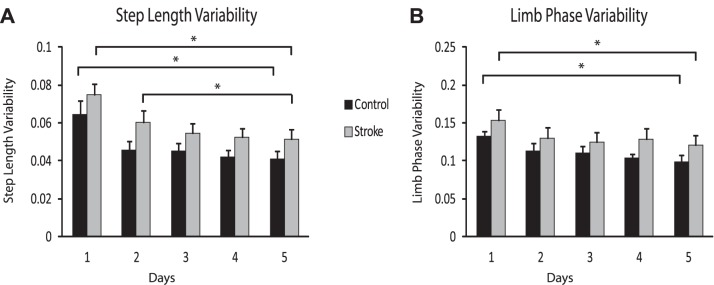
Variability of step length and limb phase across days. A: step length variability across days. B: limb phase variability across days. Error bars represent SE. *Statistical significance (P < 0.0125).
Factors Influencing Learning: Level of Impairment
We hypothesized that level of impairment, as measured by the lower extremity Fugl-Meyer and/or sensory score, would result in a reduced rate and magnitude of learning in the participants with stroke. The general linear regression revealed no significant relationships between learning as measured by magnitude or rate of either variable and the level of sensorimotor impairment.
DISCUSSION
The results of this study demonstrate that learning a novel walking pattern appears to proceed differently in persons with chronic stroke compared with their neurologically intact counterparts. Specifically, our measures of learning suggest that the learning process associated with modifying a spatial parameter of gait is slowed after stroke. However, if given sufficient practice time, persons with chronic stroke are able to demonstrate learning that is similar to that of those who are neurologically intact. Furthermore, survivors of stroke retain the same amount as those who are neurologically intact. This has implications for rehabilitation, as it informs clinicians of the expectations for ambulatory practice and performance after stroke.
The results of this study are particularly important and novel because previous studies of poststroke motor learning have primarily involved the upper extremity, and the results of those studies have been mixed. Some studies showed intact motor learning after stroke (Winstein et al. 1999), and others found specific learning deficits (Boyd et al. 2007; Boyd and Winstein 2001, 2003, 2006). Given the differences in the neural substrates that underlie control of the unilateral upper extremity, compared with the interlimb control of the lower extremity, conclusions cannot be drawn about the learning of a locomotor task after stroke based on the previously published upper extremity motor learning literature.
For both step length and limb phase, there was no difference between groups in the magnitude of early asymmetry on day 1 or on day 5, demonstrating that both groups were initially perturbed the same amount and learned to resolve this early asymmetry to the same degree by the last day of practice. What differed, however, was the number of days required by each group to achieve the learning observed by day 5. For the spatial variable of step length, it took our participants with stroke an additional day of practice to achieve the amount of learning observed by day 5. Similarly, when learning was measured by examining the change in rate of adaptation from day to day, those with stroke required an additional day of practice to achieve the amount of learning observed by day 5.
This slowed learning for step length may be at least partially due to the slower rate of step length adaptation on day 1 in the stroke survivors, despite equivalent perturbations. The adaptation curve for an individual day (Fig. 4) typically has a period in which the participant is adjusting his step length on a stride-by-stride basis, followed by a period of step symmetry that is closest to baseline. Since the rate of adaptation captures the point at which participants reach this plateau in symmetry, a slower rate indicates a larger amount of time spent in stride-by-stride adjustment compared with practice of a plateaued symmetry state. Greater practice of a relatively stable pattern has been shown to lead to better performance at the next exposure during a prism adaptation task in monkeys (Yin and Kitazawa 2001) and during a visuomotor adaptation task in humans (Krakauer et al. 2005). In a recent visuomotor adaptation study it was shown that after a person undergoes error-driven adaptation, error-free repetition of the plateaued pattern engages an additional learning system that reinforces the newly adapted pattern. They showed that it is specifically these reinforcement-based learning mechanisms that are responsible for retention of the adapted pattern from one training session to the next (Huang et al. 2011). Thus it is possible that because our stroke participants were slower to adapt on day 1 and therefore had less reinforcement practice, they retained less the next day and thus required additional practice time to achieve maximum learning.
The differences we observe between learning in poststroke participants and those who are neurologically intact may be related to the presence of cerebral damage. Although multiple studies have demonstrated that the cerebellum is the essential brain component for the trial-and-error process of movement adjustment during adaptation (Baizer and Glickstein 1974; Lang and Bastian 1999; Martin et al. 1996a; Morton and Bastian 2004, 2006; Tseng et al. 2007; Yanagihara and Udo 1994), the control of motor learning has been shown to involve multiple areas of the cerebral cortex (Hadipour-Niktarash et al. 2007; Hotermans et al. 2008; Muellbacher et al. 2002; Vidoni et al. 2010; Vidoni and Boyd 2007). The primary motor cortex (M1) is of particular importance, as studies have shown that transcranial magnetic stimulation (TMS) disruption of this area interferes with consolidation and retention of a newly learned motor task (Hadipour-Niktarash et al. 2007; Muellbacher et al. 2002). In addition, the supplemental motor area, the premotor cortex, and the somatosensory cortex have also been proposed to be involved in learning (Boyd and Linsdell 2009; Perez et al. 2008; Vidoni et al. 2010). Because motor learning appears to involve contributions from multiple areas within the cerebrum, it is probable that the damage from the stroke impacted one or more aspects of the motor learning pathway. Furthermore, damage from the stroke may have also impacted sensory pathways that are responsible for providing key error signals during learning. This lack of appropriate error signal delivery may provide further explanation for the differences seen between our participants after stroke and those who are neurologically intact (Vidoni and Boyd 2009).
It is also possible that learning to modify step length asymmetry was slowed in the group of poststroke participants because persons after stroke are less able to adjust their walking pattern in response to environmental demands. Evidence for this was found in a study that looked at carryover of an adapted walking pattern to overground walking (Reisman et al. 2009). In this study, participants with stroke were observed to have carried over ∼60% of their newly learned walking pattern from the split-belt treadmill to a new context (overground), compared with 30% in those who were neurologically intact, suggesting that the stroke survivors were less able to use environmental cues to appropriately modify their walking pattern.
Differences in learning between the groups were observed for the spatial variable of step length but not for the temporal variable of limb phase. Considering the biomechanics of walking, it is possible that the differences we see here may simply be because a deviation in limb phasing too far from optimal would be incompatible with maintaining upright. Thus our results may simply be a reflection of the fact that the biomechanics of walking allow for greater deviation from optimal for step length than for limb phasing.
However, the fact that we see differences in learning for the spatial versus temporal variable is not entirely unexpected. A number of recent studies have shown that in neurologically intact subjects spatial and temporal variables adapt at different rates and respond differently to conscious control and manipulations in practice structure during split-belt treadmill walking (Malone et al. 2011, 2012; Malone and Bastian 2010). Neurologically intact subjects walking on the split-belt treadmill adapt limb phase at a rate twice that of step length (Malone and Bastian 2010). Moreover, temporal parameter adaptation has been shown to be much more difficult to manipulate through conscious efforts compared with spatial parameter adaptation (Malone et al. 2011, 2012; Malone and Bastian 2010). In a recent study, after various types of exposure to split-belt walking on day 1 readaptation of both step length and limb phasing was examined on day 2, to determine the effect of different practice structures on learning in neurologically intact subjects. It was found that different practice schedules influenced learning related to the spatial, but not temporal, variables (Malone et al. 2011). Together these results have led to the suggestion that the temporal and spatial aspects of gait may have separate neural control, and that the temporal aspects of gait may be less influenced by cerebral control (Malone et al. 2011). Thus the cerebral damage resulting from stroke may be more likely to affect the learning of spatial, rather than temporal, aspects of gait.
In light of the results of previous studies that showed that adaptation was slowed when afferent signals were disrupted (Guedon et al. 1998; Pipereit et al. 2006), we hypothesized that there would be a relationship between learning and level of sensorimotor impairment associated with stroke. However, the results did not support this hypothesis. A previous study of split-belt treadmill adaptation in those with chronic stroke found no relationship between the level of sensorimotor impairment and the magnitude of aftereffects in persons with chronic stroke (Reisman et al. 2007). Our results extend these findings and demonstrate that learning of a novel locomotor task over days is also not related to the level of sensorimotor impairment after stroke. This has positive implications for rehabilitation, because over multiple days of learning participants with stroke are able to achieve the same amount of learning as those who are neurologically intact, and they retain just as much as those who are healthy, regardless of their level of sensorimotor impairment.
Over the years many studies have demonstrated that motor learning is associated with a reduction in the variability of task performance (Albouy et al. 2012; Muller and Sternad 2004). These results were extended by a recent study examining an upper extremity pointing task where motor learning was defined as an improvement in the speed-accuracy trade-off (Shmuelof et al. 2012). In that study, it was determined that motor learning was primarily characterized by a slow reduction in trajectory variability. The results of the present study are consistent with these findings. Across groups, the stepwise reduction in both magnitude of early asymmetry and rate of adaptation as the days of practice progressed is mirrored by a stepwise reduction in step-to-step variability. However, just as was observed for magnitude of asymmetry and rate of adaptation of step length, the reduction of step length variability over days was slower in the group of stroke subjects, supporting our conclusion that motor learning for step length was slower in those after stroke compared with their neurologically intact counterparts.
Conclusions
The goal of this study was to characterize learning of a novel walking task in participants with stroke compared with those who are neurologically intact. To our knowledge, this is the first study to address learning of this type, over multiple days, and in this population. The results suggest that although stroke survivors are slower to learn a new spatial pattern of gait, if given sufficient practice time they are able to do so to the same extent as those who are neurologically intact. Furthermore, they retain the newly learned pattern just as well as those who are neurologically intact, and this is not related to their level of sensorimotor impairment.
GRANTS
This work was supported by National Institutes of Health (NIH) Shared Instrumentation Grant S10 RR-022396 and NIH Grants K01 HD-050582 and P20 GM-103446-13.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.M.T. and D.S.R. conception and design of research; C.M.T. performed experiments; C.M.T., E.H., and D.S.R. analyzed data; C.M.T., E.H., and D.S.R. interpreted results of experiments; C.M.T. prepared figures; C.M.T. and D.S.R. drafted manuscript; C.M.T. and D.S.R. edited and revised manuscript; C.M.T., E.H., and D.S.R. approved final version of manuscript.
REFERENCES
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Desseilles M, Boly M, Dang-Vu T, Balteau E, Degueldre C, Phillips C, Luxen A, Maquet P. Neural correlates of performance variability during motor sequence acquisition. Neuroimage 60: 324–331, 2012. [DOI] [PubMed] [Google Scholar]
- Baizer JS, Glickstein M. Proceedings: Role of cerebellum in prism adaptation. J Physiol 236: 34P–35P, 1974. [PubMed] [Google Scholar]
- Bastian AJ. Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21: 628–633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med 2: 92–98, 1970. [PubMed] [Google Scholar]
- Boyd L, Winstein C. Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J Neurol Phys Ther 30: 46–57, 2006. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Linsdell MA. Excitatory repetitive transcranial magnetic stimulation to left dorsal premotor cortex enhances motor consolidation of new skills. BMC Neurosci 10: 72, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Quaney BM, Pohl PS, Winstein CJ. Learning implicitly: effects of task and severity after stroke. Neurorehabil Neural Repair 21: 444–454, 2007. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neurosci Lett 298: 65–69, 2001. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther 83: 976–989, 2003. [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ. Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem 11: 388–396, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR. Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24: 8662–8671, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Improvement of arm movement patterns and endpoint control depends on type of feedback during practice in stroke survivors. Neurorehabil Neural Repair 21: 398–411, 2007. [DOI] [PubMed] [Google Scholar]
- Earhart G, Jones G, Horak F, Block E, Weber K, Fletcher W. Forward versus backward walking: transfer of podokinetic adaptation. J Neurophysiol 86: 1666–1670, 2001. [DOI] [PubMed] [Google Scholar]
- Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975. [PubMed] [Google Scholar]
- Guedon O, Gauthier G, Cole J, Vercher JL, Blouin J. Adaptation in visuomanual tracking depends on intact proprioception. J Mot Behav 30: 234–248, 1998. [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci 27: 13413–13419, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotermans C, Peigneux P, de Noordhout AM, Moonen G, Maquet P. Repetitive transcranial magnetic stimulation over the primary motor cortex disrupts early boost but not delayed gains in performance in motor sequence learning. Eur J Neurosci 28: 1216–1221, 2008. [DOI] [PubMed] [Google Scholar]
- Huang VS, Haith A, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron 70: 787–801, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Ghez C, Ghilardi MF. Adaptation to visuomotor transformations: consolidation, interference, and forgetting. J Neurosci 25: 473–478, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci 29: 58–64, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang CE, Bastian AJ. Cerebellar subjects show impaired adaptation of anticipatory EMG during catching. J Neurophysiol 82: 2108–2119, 1999. [DOI] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ. Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103: 1954–1962, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Bastian AJ, Torres-Oviedo G. How does the motor system correct for errors in time and space during locomotor adaptation? J Neurophysiol 108: 672–683, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone LA, Vasudevan EV, Bastian AJ. Motor adaptation training for faster relearning. J Neurosci 31: 15136–15143, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain 119: 1183–1198, 1996a. [DOI] [PubMed] [Google Scholar]
- Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119: 1199–1211, 1996b. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Randhawa B, Wessel B, Boyd LA. Implicit sequence-specific motor learning after subcortical stroke is associated with increased prefrontal brain activations: an fMRI study. Hum Brain Mapp 32: 290–303, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Prism adaptation during walking generalizes to reaching and requires the cerebellum. J Neurophysiol 92: 2497–2509, 2004. [DOI] [PubMed] [Google Scholar]
- Morton SM, Bastian AJ. Cerebellar contributions to locomotor adaptations during splitbelt treadmill walking. J Neurosci 26: 9107–9116, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature 415: 640–644, 2002. [DOI] [PubMed] [Google Scholar]
- Muller H, Sternad D. Decomposition of variability in the execution of goal-oriented tasks: three components of skill improvement. J Exp Psychol Hum Percept Perform 30: 212–233, 2004. [DOI] [PubMed] [Google Scholar]
- Perez MA, Tanaka S, Wise SP, Willingham DT, Cohen LG. Time-specific contribution of the supplementary motor area to intermanual transfer of procedural knowledge. J Neurosci 28: 9664–9669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipereit K, Bock O, Vercher JL. The contribution of proprioceptive feedback to sensorimotor adaptation. Exp Brain Res 174: 45–52, 2006. [DOI] [PubMed] [Google Scholar]
- Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005. [DOI] [PubMed] [Google Scholar]
- Reisman DS, McLean H, Bastian AJ. Split-belt treadmill training poststroke: a case study. J Neurol Phys Ther 34: 202–207, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Locomotor adaptation on a split-belt treadmill can improve walking symmetry post-stroke. Brain 130: 1861–1872, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair 23: 735–744, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics, 1988. [Google Scholar]
- Schmidt R, Wrisberg C. Motor Learning and Performance. Champaign, IL: Human Kinetics, 2000. [Google Scholar]
- Shadmehr R, Brashers-Krug T. Functional stages in the formation of human long-term motor memory. J Neurosci 17: 409–419, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 108: 578–594, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8: 331–343, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Oviedo G, Bastian AJ. Seeing is believing: effects of visual contextual cues on learning and transfer of locomotor adaptation. J Neurosci 30: 17015–17022, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YW, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ. Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98: 54–62, 2007. [DOI] [PubMed] [Google Scholar]
- Vasudevan EV, Bastian AJ. Split-belt treadmill adaptation shows different functional networks for fast and slow human walking. J Neurophysiol 103: 183–191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Acerra NE, Dao E, Meehan SK, Boyd LA. Role of the primary somatosensory cortex in motor learning: an rTMS study. Neurobiol Learn Mem 93: 532–539, 2010. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA. Achieving enlightenment: what do we know about the implicit learning system and its interaction with explicit knowledge? J Neurol Phys Ther 31: 145–154, 2007. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA. Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct 5: 36, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstein CJ, Merians AS, Sullivan KJ. Motor learning after unilateral brain damage. Neuropsychologia 37: 975–987, 1999. [DOI] [PubMed] [Google Scholar]
- Yanagihara D, Udo M. Climbing fiber responses in cerebellar vermal Purkinje cells during perturbed locomotion in decerebrate cats. Neurosci Res 19: 245–248, 1994. [DOI] [PubMed] [Google Scholar]
- Yin PB, Kitazawa S. Long-lasting aftereffects of prism adaptation in the monkey. Exp Brain Res 141: 250–253, 2001. [DOI] [PubMed] [Google Scholar]