Abstract
The vasoactive mediator, endothelin-1, elicits a novel form of hyperalgesia, stimulation-dependent hyperalgesia. Acting on its cognate receptor on the vascular endothelial cell, endothelin-1 produces a state in which mechanical stimulation now elicits release of pronociceptive mediators from endothelium that, in turn, acts at receptors on sensory neurons. The only evidence that octoxynol-9, a surface-active agent that attenuates both endothelial cell function and stimulus-dependent hyperalgesia, does not affect nociceptors is indirect (i.e., octoxynol-9 treatment did not affect behavioral nociceptive threshold or hyperalgesia induced by agents that act directly on nociceptors). To help address the question of whether the attenuation of stimulation-dependent hyperalgesia by octoxynol-9 treatment is due to alteration of nociceptor function, we used in vivo single-fiber electrophysiological recordings. Consistent with our previous behavioral observations, we observed no significant effect of octoxynol-9 on mechanical threshold in nociceptors, their response to sustained suprathreshold mechanical stimulation, conduction velocity, and change in mechanical threshold in response to the direct-acting hyperalgesic agent, PGE2. Although octoxynol-9 did not produce a biologically meaningful change in parameters of nociceptor function, we cannot exclude the possibility of a type II error. However, our data provide preliminary evidence of no effect of octoxynol-9 on nociceptors and are consistent with the suggestion that the primary action of octoxynol-9 in our studies is due to its action on the endothelium.
Keywords: endothelium, stimulus-dependent hyperalgesia, skeletal muscle, electrophysiology
effective treatment of a wide spectrum of vascular pain syndromes is a major unmet medical need. The development of new treatments for these pain syndromes is hampered by our limited understanding of the mechanisms underlying the role of blood vessels in acute and chronic pain. Although an active role of endothelial cells in peripheral pain mechanisms was already suggested several years ago (Burnstock 1999), technical challenges had prevented direct tests of this hypothesis. Recently, we adapted a method from the cardiovascular and renal vascular literatures, intravenous administration of octoxynol-9, an ethoxylated alkylphenol surfactant that impairs the endothelial cell lining of the luminal side of blood vessels (Connor and Feniuk 1989; Jamal et al. 1992; Sun et al. 1997), to attenuate endothelial cell function (McLeod and Piper 1992; Randall et al. 1991; Sun et al. 1997). We have used this method to demonstrate that the endothelial cell plays a critical role in multiple models of vascular pain syndromes (Joseph et al. 2013). We hypothesized that octoxynol-9 does not have a direct effect on nociceptor function based on two observations: octoxynol-9 did not affect either mechanical nociceptive withdrawal reflex threshold (Joseph et al. 2013) or hyperalgesia induced by pronociceptive mediators [PGE2, interleukin-6, or tumor necrosis factor-α] that act directly on the nociceptor (Joseph et al. 2013). We sought to determine whether octoxynol-9 affects nociceptor function using in vivo electrophysiological experiments to evaluate the effect of octoxynol-9 on responses of nociceptors to mechanical stimuli and to PGE2, which sensitizes nociceptors by acting at their cognate receptors on nociceptors (Kumazawa et al. 1996).
MATERIALS AND METHODS
Animals.
Experiments were performed on adult male Sprague-Dawley rats (200–250 g; Charles River Laboratories, Hollister, CA). Animals were housed three per cage, under a 12:12-h light-dark cycle, in a temperature- and humidity-controlled environment. Food and water were available ad libitum. All experimental protocols were approved by the University of California San Francisco Committee on Animal Research and conformed to National Institutes of Health Guide for the Care and Use of Laboratory Animals. A concerted effort was made to minimize the number of animals used and their suffering.
In vivo single-fiber electrophysiology.
The in vivo single-fiber electrophysiology technique for studying muscle afferents has been described by us previously (Chen et al. 2010). In brief, 4–5 h after receiving octoxynol-9 or its vehicle, rats were anesthetized with sodium pentobarbital (initially 50 mg/kg ip with additional doses given to maintain areflexia throughout the experiment), their trachea cannulated, and an electrocardiogram obtained to monitor heart rate. Animals were positioned prone, and an incision was made on the dorsal skin of the left hindleg between the midthigh and calf. The biceps femoris muscle was partially removed to expose the underlying sciatic nerve and gastrocnemius muscle, which were kept immersed under warm mineral oil. The sciatic nerve was dissected free of surrounding tissue and cut proximal to the stimulation electrode to prevent reflex stimulation of muscles in the hindlimb. Fine fascicles of axons were then dissected from the distal stump of the nerve using finely honed forceps and a filament placed on a silver-silver chloride recording electrode with a reference electrode positioned nearby. The activity of the individual units in a filament were amplified, filtered (NeuroLog Systems, Welwyn Garden City, United Kingdom), monitored, and recorded on a computer with a Micro1401 interface [Cambridge Electronic Design (CED), Cambridge, United Kingdom] for subsequent analysis. Single units were first detected by systematic stimulation of their mechanical fields in the belly of the gastrocnemius muscle with a small, blunt-tipped, glass bar and bipolar stimulating electrodes used to locate more precisely the receptive field and make an initial identification of nociceptors based on amplitude and duration of the action potential. All recorded muscle afferents had conduction velocities in the range of type III (2.5–30 m/s) or type IV (<2.5 m/s) muscle sensory fibers (Berberich et al. 1988). Mechanical threshold, determined with calibrated von Frey hairs (VFH; Ainsworth, London, United Kingdom), was defined as the lowest force that elicited at least two spikes within 1 s in ≥50% of trials. The threshold stimulus was applied five to seven times with an interstimulus interval of ≥30 s. Threshold was approached by testing with ascending intensity of the VFHs and then verified with alternate testing of the threshold VFH with the last ineffective intensity VFH. Sustained (60-s) suprathreshold (10-g) mechanical stimulation was delivered by a blunt-tipped, force-measuring transducer (Entran, Fairfield, NJ) applied with a micromanipulator. Action potentials elicited by stimulation of a receptive field were identified using the “collision” and “latency shift” methods in which mechanically and electrically evoked action potentials are collided and the latency of the electrically evoked action potential is delayed or occluded by an interaction with the mechanically evoked action potential, respectively (Hallin and Torebjörk 1974; Iggo 1958). Injections of test agents or vehicle into the receptive field were delivered in a total volume of 1.25–2.5 μl with a 30-gauge hypodermic needle (Hamilton, Reno, NV) ∼1 mm away from the center of the receptive field. In previous studies, it has been shown that even repeated introduction of the needle adjacent to the mechanical receptive field, for vehicle injections, does not change mechanical VFH threshold (Martin et al. 1987). In addition to determining changes in VFH threshold, we also quantitated the response (i.e., number of action potentials elicited) by 10-g VFH stimulation. One fiber recording was obtained from each animal.
Drugs.
The drugs used in this study were octoxynol-9 and PGE2 (Sigma-Aldrich, St. Louis, MO). Octoxynol-9 (0.5%, 1 ml/kg) was dissolved in saline and administered intravenously; PGE2 was dissolved in 0.9% saline and 100 ng in a volume of 20 μl injected at the site of the mechanical receptive field of the fiber being studied. Doses were based on data obtained in our previous studies (Chen et al. 2010; Joseph and Levine 2012).
Octoxynol-9 administration.
In the cardiovascular and renal vascular literature, a role of endothelial cells in vascular function has been evaluated, in vivo and in situ, using brief exposure to octoxynol-9. As shown by functional tests and light and electron microscopy, the intravenous or intraarterial administration of octoxynol-9 disrupts and functionally impairs the endothelial lining of blood vessels (McLeod and Piper 1992; Randall et al. 1991; Sun et al. 1997). The present study employed the same protocol of octoxynol-9 administration shown previously to attenuate endothelin-1-induced, stimulus-dependent hyperalgesia (Joseph et al. 2013): rats received a tail-vein injection of 0.5% octoxynol-9 at a dose of 1 ml/kg body wt. Injection of the saline vehicle for octoxynol-9 served as control.
We previously confirmed that this octoxynol-9 administration protocol causes loss of two endothelial cell-dependent processes: vasodilation and extravasation of plasma proteins. Specifically, following octoxynol-9 administration, we observed a loss of muscarinic cholinergic agonist-induced vasodilatation, a key diagnostic of endothelial cell function (Furchgott and Zawadzki 1980; Komori and Suzuki 1987; Medhora et al. 2001), and we also observed loss of plasma protein extravasation induced by platelet-activating factor (Green et al. 1993).
Statistics.
Group data are expressed as means ± SE of n distinct observations in control or octoxynol-9-treated rats. Statistical comparisons were made by a 1-tailed Student's t-test (for 1 or 2 independent populations) or by 1-way ANOVA for comparing multiple treatments using Prism statistical software (GraphPad, San Diego, CA). P < 0.05 was considered statistically significant.
RESULTS
Effect of octoxynol-9 on mechanical threshold.
Four to five hours after octoxynol-9 or vehicle control administration, mechanical threshold was determined for each sensory neuron; we have previously shown that endothelial function (cholinergic-induced increased blood flow) is still attenuated 24 h after octoxynol-9 administration (Joseph et al. 2013), indicating that octoxynol-9 exerts its effects within the period in which we evaluated nociceptor function. Compared with that of nociceptors in vehicle-treated control rats, octoxynol-9 did not significantly decrease mechanical threshold (0.90 ± 0.082 g, n = 44 fibers vs. 0.90 ± 0.09 g, n = 31 fibers; Student's t-test, P = 0.95; Fig. 1).
Fig. 1.
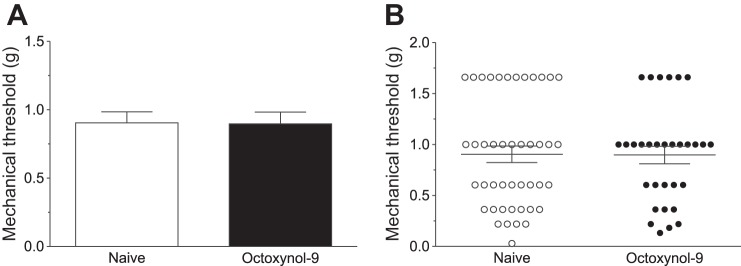
Mechanical threshold of nociceptors innervating the gastrocnemius muscle. Octoxynol-9 administration did not affect the baseline mechanical threshold of muscle nociceptors (A: mean; B: individual fibers).
Effect of octoxynol-9 on response to sustained suprathreshold stimulation.
We also tested the hypothesis that octoxynol-9 does not decrease the response of each fiber to a sustained (1-min) suprathreshold (10-g) mechanical stimulus applied to the receptive field. This measure can be useful in detecting overall enhancement of nociceptor activity. As with mechanical threshold, compared with that of nociceptors in vehicle-treated control rats, octoxynol-9 did not significantly decrease the total number of action potentials or time course of the response to a sustained suprathreshold stimulus [0–10 s: 111.1 ± 38.14 vs. 98.59 ± 20.86 spikes, Student's t-test, P = 0.77; 10–60 s: 150.3 ± 142.5 vs. 70.76 ± 26.51 spikes, Student's t-test, P = 0.55; 0–60 s: 261.4 ± 178.4 vs. 169.4 ± 41.73 spikes, Student's t-test, P = 0.59; n = 14 fibers (control) and n = 17 fibers (octoxynol-9); Fig. 2].
Fig. 2.
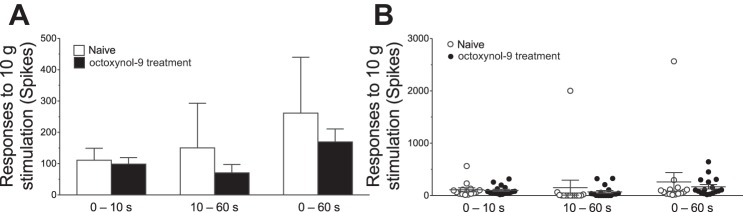
The response of nociceptors to sustained (60-s) suprathreshold (10-g) von Frey hair mechanical stimuli in muscle nociceptors. Mean values (A) and individual fibers (B) are shown from naïve control and octoxynol-9-treated rats. The responses of the nociceptors from the octoxynol-9-treated rats were not significantly different from those of control rats for any of the analysis periods (0–10, 10–60, or 0–60 s).
Effect of octoxynol-9 on conduction velocity.
Conduction velocity was measured in sensory neurons treated with octoxynol-9 or vehicle. Octoxynol-9 did not significantly affect conduction velocity compared with that of nociceptors in vehicle-treated control rats (2.23 ± 0.19, n = 44 fibers vs. 2.05 ± 0.24, n = 32 fibers; Student's t-test, P = 0.56; Fig. 3).
Fig. 3.
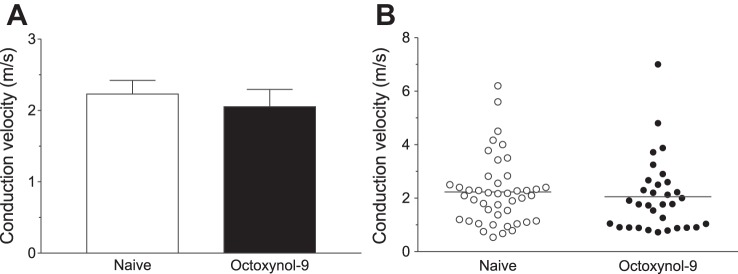
Conduction velocity. A: mean conduction velocity from octoxynol-9-treated rats were not significantly different from naïve rats. B: scattergram of conduction velocities of muscle nociceptors in naïve control and octoxynol-9-treated rats.
Effect of octoxynol-9 on PGE2-induced sensitization.
There was a significant decrease in mechanical threshold produced by PGE2 administration (F2,58 = 11.85, P < 0.0001, 2-way repeated-measures ANOVA), but there was no significant effect of octoxynol-9 treatment (F1,29 = 2.196, P = 0.15; Fig. 4).
Fig. 4.
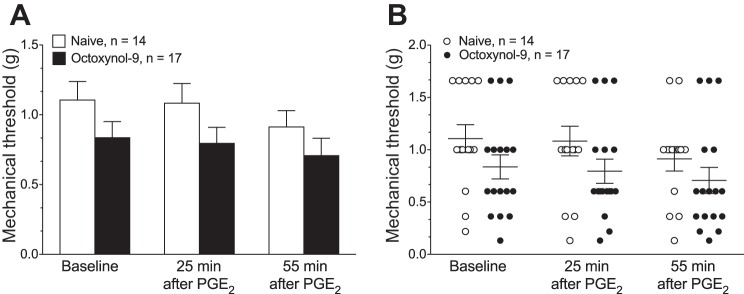
Mechanical threshold. A: mean baseline mechanical threshold and the mechanical nociceptive threshold 25 and 55 min after PGE2 administration was not significantly different in octoxynol-9- and vehicle-treated rats. B: scattergram of mechanical nociceptive threshold of muscle nociceptors in naïve control and octoxynol-9-treated rats.
Of note, to have adequate power to be able to detect what is considered to be a small effect size [i.e., d = 0.20 (Cohen 1988)] for a 2-group t-test, with 80% power and a 2-tailed alpha of 0.05, 788 fibers, 394 per group, would be required. This is not possible in single-fiber electrophysiological experiments, since at most 2–5 fibers per week are recorded. Although the current study does not have sufficient power to exclude the possibility of a type II error, an estimate of the effect size can be made with the assumption that a biologically relevant effect is a change of 20% or greater in the number of actions potentials. Using the calculation for d:
Analysis of the initial burst of action potentials (0–10 s), the relevant time period for behavioral nociceptive response to mechanical stimulation, shows that the difference between the two groups is d = 0.11, which is well below what is considered to be a small effect size (d = 0.20). Thus it is unlikely that we have a type II error and are missing some biologically relevant effect of octoxynol-9 on nociceptor function.
DISCUSSION
To address the role of the vascular endothelial cell in peripheral pain mechanisms in behavioral studies, we previously used an approach that has been employed extensively in the cardiovascular and renal vascular literature to study endothelial cell-dependent mechanisms, the functional impairment of the vascular endothelium by brief intravenous exposure to octoxynol-9 (Connor and Feniuk 1989; Jamal et al. 1992). In our previous study (Joseph and Levine 2012), octoxynol-9 treatment markedly attenuated stimulus-dependent hyperalgesia, an endothelial cell-dependent mechanical stimulus-induced hyperalgesia that can be produced by administration of two vasoactive mediators, endothelin-1 and epinephrine (Joseph et al. 2013; Joseph and Levine 2012).
We previously hypothesized that low-dose intravenous octoxynol-9 does not have a direct effect on nociceptor function based on two behavioral observations, namely that octoxynol-9 did not affect either the mechanical stimulus threshold of the nociceptive withdrawal reflex (Joseph et al. 2013) or the hyperalgesia induced by pronociceptive mediators that act directly on the nociceptor (i.e., PGE2, interleukin-6, or tumor necrosis factor-α; Joseph et al. 2013). In the present study, we sought to determine whether octoxynol-9 affected nociceptor function. Although we did not observe biologically meaningful (i.e., <20%) changes in basal measures of nociceptor function (i.e., mechanical threshold or number of action potentials generated in response to sustained suprathreshold stimulation) or sensitization of nociceptors in octoxynol-9-treated rats (i.e., decrease in mechanical threshold), our study is insufficiently powered to prove the null hypothesis. Therefore, our data provide preliminary evidence of no effect of octoxynol-9 on nociceptors and are consistent with the suggestion that the primary action of octoxynol-9 in our studies is due to its action on the endothelium. Given that intravenous octoxynol-9 affects endothelial cell function without affecting the function of the next most superficial cell in the blood vessel, the vascular smooth muscle (Joseph et al. 2013), we conclude that the effects of octoxynol-9 on peripheral pain are due to its ability to disrupt endothelial cell function. Thus intravenous administration of low-dose octoxynol-9 provides a technique to evaluate the role of the endothelial cell in peripheral vascular pain syndromes, allowing the dissociation between the effect of pronociceptive mediators on the endothelial cell from their effect on primary afferent nociceptors.
GRANTS
This work was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR-063312.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.C. and J.D.L. conception and design of research; X.C. performed experiments; X.C. and P.G.G. analyzed data; P.G.G. and J.D.L. interpreted results of experiments; P.G.G. prepared figures; J.D.L. drafted manuscript; P.G.G. and J.D.L. edited and revised manuscript; X.C., P.G.G., and J.D.L. approved final version of manuscript.
REFERENCES
- Berberich P, Hoheisel U, Mense S. Effects of a carrageenan-induced myositis on the discharge properties of group III and IV muscle receptors in the cat. J Neurophysiol 59: 1395–1409, 1988 [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat 194: 335–342, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Green PG, Levine JD. Neuropathic pain-like alterations in muscle nociceptor function associated with vibration-induced muscle pain. Pain 151: 460–466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences (2nd ed.). Hillsdale, NJ: Lawrence Erlbaum Associates, 1988 [Google Scholar]
- Connor HE, Feniuk W. Influence of the endothelium on contractile effects of 5-hydroxytryptamine and selective 5-HT agonists in canine basilar artery. Br J Pharmacol 96: 170–178, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288: 373–376, 1980 [DOI] [PubMed] [Google Scholar]
- Green PG, Luo J, Heller PH, Levine JD. Further substantiation of a significant role for the sympathetic nervous system in inflammation. Neuroscience 55: 1037–1043, 1993 [DOI] [PubMed] [Google Scholar]
- Hallin RG, Torebjörk HE. Methods to differentiate electrically induced afferent and sympathetic C unit responses in human cutaneous nerves. Acta Physiol Scand 92: 318–331, 1974 [DOI] [PubMed] [Google Scholar]
- Iggo A. The electrophysiological identification of single nerve fibers, with particular reference to the slowest-conducting vagal afferent fibers in the cat. J Physiol 142: 110–146, 1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, Bendeck M, Langille BL. Structural changes and recovery of function after arterial injury. Arterioscler Thromb 12: 307–317, 1992 [DOI] [PubMed] [Google Scholar]
- Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci 33: 2849–2859, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph EK, Levine JD. Role of endothelial cells in antihyperalgesia induced by a triptan and beta-blocker. Neuroscience 232: 83–89, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori K, Suzuki H. Electrical responses of smooth muscle cells during cholinergic vasodilation in the rabbit saphenous artery. Circ Res 61: 586–593, 1987 [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K, Koda H, Fukusako H. EP receptor subtypes implicated in the PGE2-induced sensitization of polymodal receptors in response to bradykinin and heat. J Neurophysiol 75: 2361–2368, 1996 [DOI] [PubMed] [Google Scholar]
- Martin HA, Basbaum AI, Kwiat GC, Goetzl EJ, Levine JD. Leukotriene and prostaglandin sensitization of cutaneous high-threshold C- and A-delta mechanonociceptors in the hairy skin of rat hindlimbs. Neuroscience 22: 651–659, 1987 [DOI] [PubMed] [Google Scholar]
- McLeod JD, Piper PJ. Effect of removing the endothelium on the vascular responses induced by leukotrienes C4 and D4 in guinea-pig isolated heart. Eur J Pharmacol 212: 67–72, 1992 [DOI] [PubMed] [Google Scholar]
- Medhora M, Narayanan J, Harder D. Dual regulation of the cerebral microvasculature by epoxyeicosatrienoic acids. Trends Cardiovasc Med 11: 38–42, 2001 [DOI] [PubMed] [Google Scholar]
- Randall MD, Thomas GR, Hiley CR. Effect of destruction of the vascular endothelium upon pressure/flow relations and endothelium-dependent vasodilatation in resistance beds of spontaneously hypertensive rats. Clin Sci (Lond) 80: 463–469, 1991 [DOI] [PubMed] [Google Scholar]
- Sun ZW, Wang XD, Deng XM, Wallen R, Gefors L, Hallberg E, Andersson R. The influence of circulatory and gut luminal challenges on bidirectional intestinal barrier permeability in rats. Scand J Gastroenterol 32: 995–1004, 1997 [DOI] [PubMed] [Google Scholar]


