Abstract
Stability is an important concern during human walking and can limit mobility in clinical populations. Mediolateral stability can be efficiently controlled through appropriate foot placement, although the underlying neuromechanical strategy is unclear. We hypothesized that humans control mediolateral foot placement through swing leg muscle activity, basing this control on the mechanical state of the contralateral stance leg. Participants walked under Unperturbed and Perturbed conditions, in which foot placement was intermittently perturbed by moving the right leg medially or laterally during the swing phase (by ∼50–100 mm). We quantified mediolateral foot placement, electromyographic activity of frontal-plane hip muscles, and stance leg mechanical state. During Unperturbed walking, greater swing-phase gluteus medius (GM) activity was associated with more lateral foot placement. Increases in GM activity were most strongly predicted by increased mediolateral displacement between the center of mass (CoM) and the contralateral stance foot. The Perturbed walking results indicated a causal relationship between stance leg mechanics and swing-phase GM activity. Perturbations that reduced the mediolateral CoM displacement from the stance foot caused reductions in swing-phase GM activity and more medial foot placement. Conversely, increases in mediolateral CoM displacement caused increased swing-phase GM activity and more lateral foot placement. Under both Unperturbed and Perturbed conditions, humans controlled their mediolateral foot placement by modulating swing-phase muscle activity in response to the mechanical state of the contralateral leg. This strategy may be disrupted in clinical populations with a reduced ability to modulate muscle activity or sense their body's mechanical state.
Keywords: biomechanics, locomotion, muscle activity, stability
maintaining stability is traditionally considered a basic requirement for functional human gait (Woollacott and Tang 1997). Among many clinical populations, mobility is limited by the increased fall risk and fear of falling that accompany decreased stability (Jorgenson et al. 2002; Schmid and Rittman 2009). To address this issue, several metrics have been proposed to quantify gait stability (e.g., stride period variability, Lyapunov exponents, Floquet multipliers), each of which have been associated with fall risk (Granata and Lockhart 2008; Hausdorff et al. 2001; Lockhart and Liu 2008). However, interpretation of these metrics is difficult (Bruijn et al. 2013; Monsch et al. 2012; van Schooten et al. 2011), as causal relationships with stability have not been established. For example, increases in kinematic variability have been interpreted as a sign of both increased (Bauby and Kuo 2000) and decreased (Sawers and Hahn 2012) active control of stability. Rather than focusing on potential indicators of fall risk, the present study investigates the neuromechanical strategy that healthy, uninjured humans use to maintain mediolateral gait stability and prevent falls. A clearer understanding of this typical stabilization strategy may lead to methods better able to directly identify the cause of increased fall risk among clinical populations.
Gait stability requires appropriate mechanical interactions with the environment. While walking, humans continuously experience mechanical perturbations due to motor control errors or environmental factors. Model simulations have demonstrated that small anteroposterior perturbations can be stabilized by the body's passive dynamics, while mediolateral perturbations require active stabilization (Kuo 1999). This may explain why frontal-plane motor control appears to play a dominant role in gait stability (Allet et al. 2012). Both models and experiments have indicated that gait can be efficiently stabilized through appropriate mediolateral foot placement (Bauby and Kuo 2000; Kuo 1999; MacKinnon and Winter 1993), as controlled through frontal-plane positioning of the leg during the swing phase (Hurt et al. 2010; Krishnan et al. 2013). However, simply being able to accurately control swing leg position may not be sufficient for mediolateral stability, as humans must also identify a mechanically appropriate target location for swing leg placement. For example, during a typical right step, placing the swing foot to the left of both the stance foot and the center of mass (CoM) would likely lead to a loss of balance, no matter how accurately the foot was guided toward this position. The appropriate foot placement target may be derived from the mechanical state of the stance leg or trunk (Hurt et al. 2010; Kuo 1999). Supporting this proposal, the body's extrapolated center of mass (a metric combining CoM position and velocity) generally predicts mediolateral foot placement for a variety of walking conditions (Hof et al. 2007; Rosenblatt et al. 2012). However, the underlying neural mechanism through which stance leg mechanics contributes to the control of swing leg placement is unclear (Hof et al. 2010).
Active control of lateral stability implies a neural strategy to meet the mechanical requirements of walking. While it can be difficult to differentiate between gait characteristics caused by passive mechanics and active control, muscle activation patterns can provide insight into the underlying control strategies (Chvatal and Ting 2012). To convincingly demonstrate the existence of an active control strategy for mediolateral foot placement, we must first show that muscle activity influences foot placement location. Subsequently, we must determine which factors humans use to choose the level of this muscle activity, providing insight into how the target foot placement location is determined. Recent cat experiments have demonstrated that during walking muscle activation patterns and foot placement are influenced by the mechanical state of the contralateral stance leg (Musienko et al. 2012b). However, it is presently unclear how humans control swing-phase activity and foot placement during a given step.
The purpose of this project was to investigate the neuromechanical strategy used by healthy, uninjured humans to control their mediolateral foot placement. Our primary interest was in the strategy used during what is often termed “steady-state” walking, in which no external mechanical perturbations are applied but humans must continuously respond to the effects of motor control errors. To quantify this behavior, we correlated step-to-step metrics of gait kinematics and muscle activity during unperturbed walking. While this approach can reveal relationships between various gait metrics, it is unable to demonstrate a causal relationship between the body's mechanical state and subsequent muscle activity. Therefore, we applied mediolateral foot placement perturbations during the swing phase of walking (altering the body's mechanical state) and determined whether the subsequent response matched the correlations present during unperturbed walking. Our use of perturbations to test specific correlation-based predictions differentiates the present work from the many previous studies that have focused on the immediate effects of mechanical perturbations during human walking (e.g., Bachmann et al. 2008; Berger et al. 1984; Chvatal and Ting 2012; Field-Fote and Dietz 2007; Hof and Duysens 2013; Misiaszek et al. 2000; Shinya et al. 2009; Stanek et al. 2011; Tang et al. 1998).
We hypothesized that humans actively control mediolateral foot placement by modulating swing leg muscle activity and base the target of this control on the mechanical state of the stance leg and torso. Specifically, we expected that the swing-phase activity of hip muscles that primarily act in the frontal plane would control the mediolateral foot placement. We also expected that the mechanical state of the stance leg (displacement, velocity, and acceleration of the CoM relative to the stance foot) would predict the muscle activity in the contralateral swing leg.
MATERIALS AND METHODS
Participants
Ten healthy, right-leg-dominant adults [8 women, 2 men; age = 23 ± 1 yr, height = 1.70 ± 0.09 m, mass = 63.8 ± 12.1 kg (mean ± SD)] participated in this study. Participants provided informed consent, and the protocol was approved by the Medical University of South Carolina Institutional Review Board.
Experimental Procedure
For all trials, participants walked on a treadmill at 1.25 m/s and were instructed to keep their gaze directed forward. Participants first performed a 5-min warm-up trial in order to become accustomed to walking on the treadmill (Zeni and Higginson 2010). Participants then performed a 5-min Unperturbed walking trial and a 5-min Perturbed walking trial, in randomized order. The Unperturbed trial was used to identify associations between metrics of gait mechanics and active control (in the form of muscle activity) during typical, unperturbed walking. In contrast, the purpose of the Perturbed trial was to more directly test causality between these metrics by applying mechanical perturbations and quantifying the behavioral responses. A rest period of 5 min was provided between trials. During walking trials, participants wore a harness attached to an overhead rail for safety. This harness did not provide any body weight support but would have prevented a fall in case of a loss of balance.
In the Unperturbed walking trial, participants were instructed to walk normally. In the Perturbed walking trial, the mediolateral placement of the right foot was intermittently perturbed. Unlike perturbations applied by pushing the CoM (Hof et al. 2010; Hof and Duysens 2013) or translating the support surface under the stance foot (Chvatal and Ting 2012; Oddsson et al. 2004), the present type of perturbation mimics the mechanical effects of errors in foot placement control. Foot placement perturbations were applied by an experimenter moving the right leg by hand slightly medially (defined as a medial perturbation) or laterally (a lateral perturbation) during the swing phase prior to heel strike. Participants were instructed to ignore the perturbations as much as possible and not to resist or assist the experimenter. By applying the perturbations during the swing phase, relatively small forces were sufficient to influence foot placement location. A lightweight padded cuff was strapped around the right leg just above the ankle, so participants were unable to detect pressure applied by the experimenter's hand. Pressure-sensitive switches (Motion Lab Systems, Baton Rouge, LA) on the medial and lateral sides of the cuff were used to detect when a perturbation was applied. The same experimenter applied the perturbations to all participants after an extensive training period (8 preliminary data collection sessions) of practicing delivery of these perturbations. There were no apparent trends for the number or average size of the delivered perturbations to change over time from the first to the tenth participant (Spearman correlations: P = 0.39 for number of perturbations; P = 0.16 for lateral perturbation size; P = 0.82 for medial perturbation size).
To prevent participants from predicting the application of perturbations, the direction (medial or lateral) and timing (every 5–10 strides) of the perturbations were randomized by the experimenter applying the perturbations. Across participants, 17 ± 2 (mean ± SD) medial perturbations and 20 ± 3 lateral perturbations were applied. Medial perturbations had an amplitude of 53 ± 9 mm (mean ± SD), while lateral perturbations had an amplitude of 98 ± 14 mm, as quantified by the change in mediolateral foot placement compared with steady-state walking. As humans appear to recover from mediolateral perturbations within a single stride (Hof et al. 2010), the perturbation timing allowed participants to return to steady state between perturbations.
Data collection and processing.
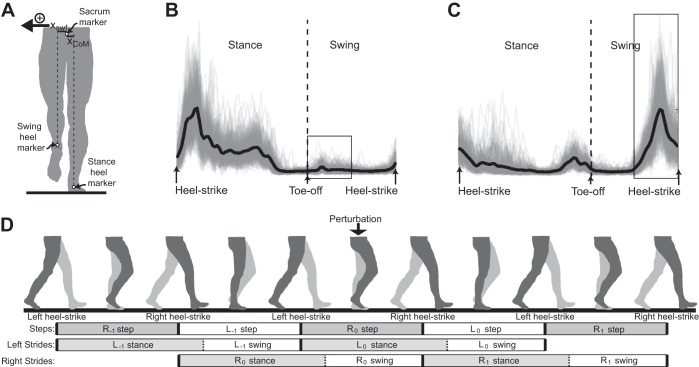
Spatiotemporal gait characteristics were quantified with active LED markers (PhaseSpace, San Leandro, CA) sampled at 120 Hz and placed on the sacrum, right heel, and left heel. The sacrum marker was used to represent the mediolateral position of the body's CoM. While this relatively simple method does not account for frontal-plane motion of the extremities, the timing of mediolateral motion of the pelvis is very similar to that of the body's entire CoM (Whittle 1997). The mediolateral displacement of the swing foot was measured with respect to the CoM, while the mediolateral displacement of the CoM was measured with respect to the stance foot (Fig. 1A). Velocities were calculated by low-pass filtering (20 Hz) and differentiating the displacement data. Accelerations were calculated by performing the same process with the velocity data. The anteroposterior velocities of the heel markers were used to identify heel-strike and toe-off events (Zeni et al. 2008), thus defining the stance and swing phases of the gait cycle. Strides were defined for each leg as the period from one heel strike to the next heel strike with the same leg, while steps were defined as the period from one heel strike to the next heel strike with the opposite leg.
Fig. 1.
Spatiotemporal gait characteristics and patterns of muscle activity were quantified during Unperturbed and Perturbed walking. A: mediolateral swing foot displacement (xswf) was measured as the mediolateral distance from the sacrum to the swing heel. Center of mass displacement (xCoM) was measured as the mediolateral distance from the stance heel to the sacrum. For all steps, the positive direction was defined as the direction toward the swing foot. B: the gluteus medius (GM) activity pattern during Unperturbed walking is illustrated for a typical participant. The GM was most strongly active during stance but also exhibited a burst of activity during the first half of swing in some strides (boxed area). Individual strides (n = 250) are shown as thin lines, and the average stride is shown as a thick dark line. C: Unperturbed adductor magnus (AM) activity is illustrated for a typical participant. The AM was consistently active in the second half of swing (boxed area) and typically also exhibited smaller bursts of activity during early and late stance. Again, the thick dark line indicates the average of the illustrated 250 strides. D: during Perturbed walking, periods of gait were classified on the basis of their timing relative to the perturbation, as illustrated in a simple schematic. Steps were defined using heel-strike events from both legs (i.e., a right step was defined as the period from left heel strike to the next right heel strike). Strides were defined using heel-strike events from a single leg (i.e., from left heel strike to the next left heel strike) and were divided into stance and swing phases depending on whether this leg was on the ground. Perturbations were applied to the right leg during a right step (R0 step), while the left leg was in stance (L0 stance) and the right leg was in swing (R0 swing). Negatively labeled strides (e.g., L−1) occur before the perturbation, while positively labeled strides (e.g., R1) occur after the perturbation.
Muscle activity was quantified with surface electromyography (EMG) electrodes (Motion Lab Systems) sampled at 1,000 Hz. We measured bilateral gluteus medius (GM) activity and bilateral adductor magnus (AM) activity with electrode placement based on previously published guidelines (Hermens et al. 1999; Lovell et al. 2012). Prior to data collection, we visually inspected the EMG data to confirm that GM was most strongly active during isolated hip abduction contractions and AM was most strongly active during isolated hip adduction contractions. The EMG data from each muscle were processed by high-pass filtering at 20 Hz, rectifying, and smoothing with a low-pass filter at 50 Hz. EMG data were then divided into strides based on heel-strike timing. The average EMG trace during a stride was calculated for the Unperturbed walking trial (Fig. 1, B and C), and all EMG data were normalized by the peak value of this average trace.
Data Analysis and Statistics: Unperturbed Walking
Data analysis was performed for both the right and left legs of each participant (n = 20 legs) for the final 250 strides of the Unperturbed walking trial.
Contributors to mediolateral foot placement.
Our first goal was to determine whether swing leg muscle activity predictably influenced mediolateral foot placement. Based on the typical patterns of muscle activity, average swing-phase GM activity was calculated during the first half of swing (Fig. 1B) and average swing-phase AM activity was calculated during the second half of swing (Fig. 1C). To account for the initial mechanical state of the swing leg, we quantified the mediolateral displacement, velocity, and acceleration of the swing foot relative to the CoM at the time of toe-off. It should be noted that the velocity values were dominated by motion of the CoM, as mediolateral velocity of the foot is low at this point in the gait cycle. Mediolateral foot placement was quantified as the mediolateral displacement of the foot relative to the CoM at the time of heel strike.
We performed a multiple linear regression to calculate the step-by-step contribution of each of the independent variables [GM swing-phase activity (GMsw), AM swing-phase activity (AMsw), initial swing foot displacement (xswf), initial swing foot velocity (vswf), initial swing foot acceleration (aswf)] to the dependent variable (mediolateral foot placement). We included 250 strides per leg for each of the 20 tested legs, for a total of 5,000 data points in the regression. To account for differences in gait characteristics across individual legs (e.g., different average foot placement locations or patterns of muscle activation), we included the leg number in our regression as a “dummy” indicator variable. Therefore, the results of the regression focused on step-to-step differences in the measured variables, not differences across individuals. To more easily interpret the regression output, each data distribution was normalized by subtracting its mean and dividing by its standard deviation (Schielzeth 2010). The standardized regression coefficient thus provides an indication of the strength of the relationship. The variance inflation factors for this regression were all <1.5 (mean = 1.19), substantially smaller than the value of 10 typically cited as an indicator of problematic multicollinearity (Belsey et al. 1980). P values of <0.05 were interpreted as significant. It should be noted that this statistical approach produces identical results as analyzing the data with generalized estimation equations with an equicorrelated structure.
Predictors of swing-phase muscle activity.
Our second goal was to determine whether swing-phase GM activity was predicted by the state of the contralateral stance leg and torso. This goal was based on our initial finding that swing-phase GM activity was the best predictor of mediolateral foot placement (see results for details). In comparison, swing-phase AM activity had only a minor effect on foot placement. For each stride, we quantified average GM activity during the first half of swing. During the same time period, we quantified the average mediolateral displacement, velocity, and acceleration of the CoM (relative to the contralateral stance foot) as well as GM activity of the contralateral stance leg.
Following the methods described above, we performed a multiple linear regression to calculate the contribution of each of the independent variables [CoM displacement (xCoM), CoM velocity (vCoM), CoM acceleration (aCoM), contralateral stance-phase GM activity (GMst)] to the dependent variable (ipsilateral swing-phase GM activity). As above, a total of 5,000 data points were included in the regression, data distributions were normalized, all variance inflation factors were <1.5 (mean = 1.21), and P values of <0.05 were interpreted as significant.
Data Analysis and Statistics: Perturbed Walking
During the Perturbed walking trials, steps and strides were classified on the basis of their timing relative to the perturbations (Fig. 1D). The perturbations were delivered during a right step (R0 step), while the right leg was in swing (R0 swing) and the left leg was in stance (L0 stance). Step timing values were used to quantify step period and foot placement location at the end of each step. Stride timing values (including the division into stance and swing) were used to define the gait periods in which we calculated average muscle activity. Steps or strides just prior to the perturbation (e.g., L−1) were considered to represent steady-state behavior during the Perturbed trial. Subsequent steps or strides were used to quantify the response to the perturbations by comparing measurements of step period, mediolateral foot placement, GM activity during the swing phase, GM activity during the stance phase, and AM activity during the swing phase. Lateral perturbations and medial perturbations were analyzed separately.
To determine whether participants altered their steady-state gait pattern in anticipation of the perturbations, we performed separate ANOVAs with gait condition (Unperturbed walking, steady state during Perturbed walking) as the independent variable and spatiotemporal gait characteristic (step period, mediolateral right foot placement, or mediolateral left foot placement) as the dependent variable. The primary purpose of performing the Perturbed walking trials was to test the causality of associations identified during the Unperturbed walking trials. Therefore, we did not use similar regression methods to analyze the Perturbed walking results. Instead, specific effects of the perturbations were examined with a series of repeated-measures one-way ANOVAs. To determine whether the perturbations influenced spatiotemporal gait characteristics, we performed separate ANOVAs with stride classification as the independent variable and right mediolateral foot placement, left mediolateral foot placement, or step period as the dependent variable. To determine whether the perturbations influenced muscle activity, we performed separate ANOVAs with perturbation type (medial, lateral, or no perturbation) as the independent variable and muscle activity (right stance-phase GM activity, right swing-phase GM activity, left stance-phase GM activity, left swing-phase GM activity, right swing-phase AM activity, or left swing-phase AM activity) as the dependent variable. For each of the ANOVAs described above, post hoc paired t-tests were performed where appropriate, with Bonferroni corrections for multiple pairwise comparisons applied to the resultant P values. Corrected P values of <0.05 were interpreted as significant.
RESULTS
During both Unperturbed and Perturbed walking, swing-phase GM activity was related to the subsequent mediolateral foot placement. This swing-phase activity was predicted by the state of the contralateral stance leg and torso, particularly by the mediolateral position of the CoM relative to the stance foot. These results provide direct evidence for a neuromechanical strategy based on the swing-phase adjustment of mediolateral foot placement used by humans to maintain gait stability.
Foot Placement Control During Unperturbed Walking
Contributors to mediolateral foot placement.
Mediolateral foot placement varied from step to step and was influenced by swing-phase muscle activity (Fig. 2A). Swing-phase GM activity was the strongest predictor of mediolateral foot placement (P < 0.0001, standardized regression coefficient β = 0.32), as increased GM activity was associated with more lateral foot placement. The effect of swing-phase AM activity was smaller (P = 0.0006, β = 0.05), with greater adductor activity present during steps in which the foot was placed more laterally.
Fig. 2.
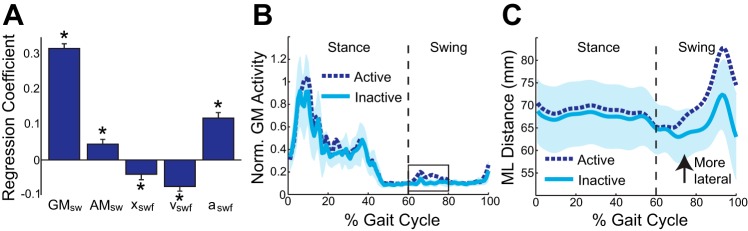
Several measures of gait kinematics and muscle activity were associated with the subsequent foot placement. A: mediolateral foot placement was significantly associated with swing-phase GM activity (GMsw), swing-phase AM activity (AMsw), and the initial mechanical state of the swing leg [xswf, swing foot velocity (vswf), swing foot acceleration (aswf)]. Error bars represent SE. *Significant effect (P < 0.05). B: active strides were defined by early swing GM activity (boxed area) exceeding 2 SDs above the minimal EMG level during a stride. Inactive strides were defined by early swing GM activity remaining within 1 SD of this minimal value. C: Active and Inactive strides were paired on the basis of the initial mechanical state of the swing leg (at ∼60% gait cycle). The mediolateral (ML) location of the swing foot is plotted with respect to the final position of the CoM. During Active strides, the swing foot landed more laterally. For B and C, the lines represent averages and the shaded area represents 2 SDs around the average Inactive stride.
Mediolateral foot placement was also influenced, albeit more weakly, by the initial mechanical state of the swing leg (Fig. 2A). Mediolateral foot placement was significantly associated with initial foot displacement (P = 0.0096, β = −0.04), velocity (P < 0.0001, β = −0.08), and acceleration (P < 0.0001, β = 0.12). Therefore, the foot tended to be placed more laterally if it began the step relatively close to the CoM, if the CoM was initially moving relatively slowly toward the contralateral stance foot, and if the foot was initially accelerating relatively slowly toward the CoM.
During steps with clear swing-phase GM activity, the leg moved more laterally during swing and landed with a more lateral foot placement. For illustrative purposes, the effects of swing-phase GM activity during strides with similar initial mechanical states of the swing leg are shown for a single participant in Fig. 2, B and C. Some strides (termed Active) exhibited a burst of GM activity during early swing, while in other strides (termed Inactive) the GM was essentially silent during this period (Fig. 2B; see figure legend for more detailed definitions). During Active strides, the swing leg trajectories diverged early in swing and remained more lateral upon foot placement (Fig. 2C).
Predictors of gluteus medius activity during swing.
Activation of the GM during swing (the strongest predictor of mediolateral foot placement) was associated with the state of the contralateral stance leg and torso (Fig. 3A). Swing-phase GM activity was most strongly associated with the mediolateral CoM displacement relative to the stance foot (P < 0.0001, β = 0.34) and more weakly associated with CoM velocity (P = 0.024, β = 0.03), CoM acceleration (P < 0.0001, β = 0.06), and contralateral stance leg GM activity (P < 0.0001, β = 0.08). Therefore, swing-phase GM activity tended to be higher during steps in which the CoM was far from the stance foot and moving and accelerating toward the stance foot slowly and the contralateral GM was strongly active.
Fig. 3.
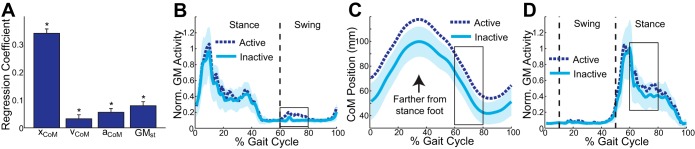
Characteristics of the stance leg and torso were associated with swing-phase GM activity in the contralateral leg. A: swing-phase GM activity was significantly associated with mediolateral CoM displacement (xCoM), velocity (vCoM), and acceleration (aCoM), as well as by the simultaneous stance-phase GM activity in the contralateral leg (GMst). Error bars represent SE. *Significant effect (P < 0.05). B: for a single participant, GM activity is illustrated for Active and Inactive strides. C: the CoM is farther from the stance foot throughout the Active strides. D: GM activity of the contralateral stance leg is higher during the Active strides than during the Inactive strides. For B–D, the lines represent averages and the shaded area represents 2 SDs around the average Inactive stride.
The swing leg GM was more strongly activated when the CoM was farther from the stance foot. As illustrated for a single participant, strides were again identified as either Active or Inactive, depending on whether substantial GM activity was present during early swing (Fig. 3B). During the Active strides, the CoM was displaced farther mediolaterally from the stance foot (Fig. 3C), indicating a more abducted stance leg. Additionally, during the Active strides the GM of the contralateral stance leg was more strongly active (Fig. 3D), paralleling the increased GM activity in the swing leg.
Foot Placement Control During Perturbed Walking
Steady-state adaptation to perturbations.
Participants adapted their steady-state gait pattern in anticipation of perturbations. During strides defined as steady state (R−1, L−1), perturbations had not been applied for at least four strides, allowing participants to return to their typical steady-state gait pattern. Steady-state foot placement with the perturbed (right) leg was significantly (P = 0.0006) more lateral during the Perturbed walking trial (86 ± 23 mm; mean ± SD) than during the Unperturbed walking trial (72 ± 24 mm). However, steady-state mediolateral foot placement with the unperturbed (left) leg was not significantly (P = 0.66) different during the Perturbed trial compared with the Unperturbed trial. The steady-state step period during the Perturbed walking trial (0.525 ± 0.031 s; mean ± SD) was slightly, but significantly (P = 0.027), shorter than the step period during the Unperturbed walking trial (0.529 ± 0.030 s).
Effects of mediolateral perturbations on gait mechanics.
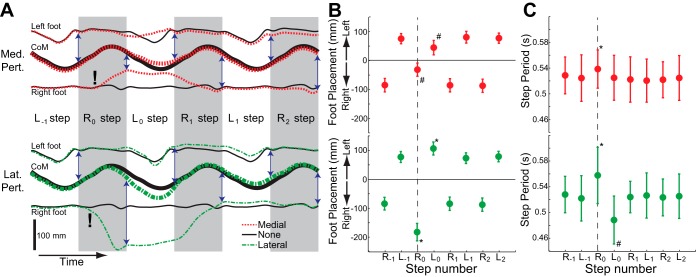
Perturbations influenced the spatiotemporal characteristics of gait, but only for two steps. The effects of these perturbations on mediolateral motion are illustrated for a single participant in Fig. 4A. Medial perturbations (Fig. 4A, top) directly caused the right foot to be placed more medially at the end of the perturbed step (R0 step), while having relatively small effects on the position of the CoM. Therefore, the CoM was much closer to the stance foot at the start of the next left step (L0 step). The left foot deviated from its normal trajectory during this step, ending the step by being placed more medially. In subsequent steps, foot placement locations appeared to return to their typical values. Lateral perturbations (Fig. 4A, bottom) caused the right foot to be placed more laterally, while again having relatively minor effects on the CoM (during R0 step). This had the effect of dramatically increasing the mediolateral distance from the CoM to the new stance foot at the start of the next left step (L0 step). Subsequently, the left foot was placed more laterally relative to the CoM at the end of this step, before the foot placement values returned to normal. Of note, participants were not strictly required to remain in the same mediolateral location on the treadmill on a step-by-step basis. For example, after the medial perturbation the illustrated participant ended up walking slightly (∼1 cm) to the right of their original position.
Fig. 4.
Perturbations influenced subsequent spatiotemporal gait characteristics. A: for a single participant, the effects of medial (top) and lateral (bottom) perturbations on the mediolateral locations of the left foot, CoM, and right foot are illustrated over 6 consecutive steps. Each trace illustrates the average response to the (∼20) perturbations delivered during a trial. Exclamation marks (!) indicate the approximate time when perturbations were applied. Right steps are shaded, and the vertical arrows indicate the measured mediolateral foot placements, defined as the mediolateral distance from the CoM to the foot at the time of heel strike. For simplicity, each step is illustrated as having the same duration. B: mediolateral foot placement was predictably influenced by both medial and lateral perturbations. The plotted values correspond to the average lengths of the vertical arrows illustrated in A across all participants. For clarity, left foot placement is plotted as positive while right foot placement is plotted as negative. C: step period was affected by medial and lateral perturbations, as the perturbed step consistently had a longer step period. For B and C, the vertical dashed line indicates the perturbed right step (R0), in which the foot is moved either medially or laterally during swing. For each participant, we calculated the average values for these gait spatiotemporal metrics preceding and following the applied perturbations. Here, the dot locations indicate mean values and the error bars represent SDs of these values across all 10 participants. *Magnitude significantly greater than steady state, #magnitude significantly less than steady state (P < 0.05; post hoc t-tests).
The effects of the perturbations illustrated in Fig. 4A were significant across participants. As expected, mediolateral foot placement was significantly influenced by both medial (P < 0.0001) and lateral (P < 0.0001) perturbations. Medial perturbations caused the perturbed right step to have a more medial foot placement than during steady-state walking. The subsequent left step was also placed more medially than normal (Fig. 4B). Similarly, lateral perturbations caused both the perturbed right step and the subsequent left step to have a more lateral foot placement than during steady-state walking (Fig. 4B). Step period was also significantly influenced by medial perturbations (P = 0.009) and lateral perturbations (P < 0.0001). Both perturbation types caused the perturbed right step to have a longer period, while lateral perturbations also caused the subsequent left step to have a shorter period (Fig. 4C).
Effects of mediolateral perturbations on muscle activity.
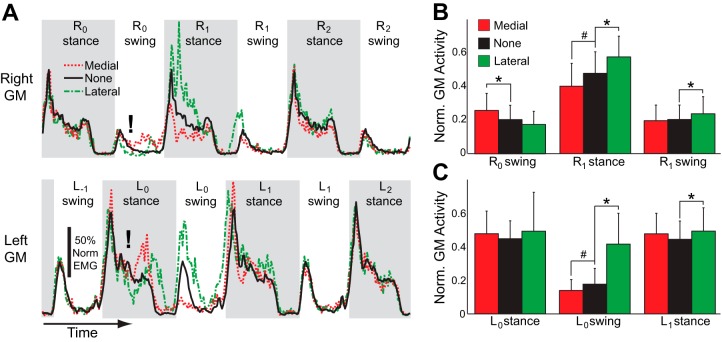
Perturbations influenced GM activity in the perturbed leg (Fig. 5A). For the perturbed (right) leg, perturbations significantly (P = 0.0006) influenced swing-phase GM activity during the perturbed step, with increased activity in response to medial perturbations (Fig. 5B, R0 swing). The effects of the perturbations were even more apparent once the foot was placed on the ground. The perturbations significantly (P < 0.0001) influenced GM activity during the subsequent stance phase on the right leg, as medial perturbations caused a decrease in GM activity and lateral perturbations caused an increase in GM activity (Fig. 5B, R1 stance). Perturbations also had a significant (P = 0.013) effect on the next step with the right foot, as lateral perturbations caused this step to exhibit increased swing-phase GM activity (Fig. 5B, R1 swing), before returning to baseline.
Fig. 5.
Perturbations also influenced bilateral GM activity. A: for a single participant, the muscle activity during 3 consecutive right strides is illustrated for both legs, corresponding to the same time period illustrated in Fig. 4. Again, each trace illustrates the average response to the applied perturbations. For each leg, stance phases are indicated by shaded areas. Perturbations (indicated by exclamation marks) were delivered to the right leg when this leg was in swing (R0 swing) and the left leg was in stance (L0 stance). B: across participants, perturbations influenced right GM activity during both the perturbed stride and the subsequent stride. Generally, lateral and medial perturbations had opposite effects. C: perturbations also influenced the left GM activity. Most notably, after perturbations of the right leg the next swing phase with the left leg demonstrated substantial changes in GM activity. B and C: *significant increase in muscle activity relative to strides without a perturbation, #significant decrease (P < 0.05; post hoc t-tests).
Of perhaps greater interest, perturbations also influenced GM activity in the unperturbed leg (Fig. 5A). For the unperturbed (left) leg, perturbations significantly (P = <0.0001) influenced swing-phase GM activity during the next left step. Medial perturbations of the right leg decreased the mediolateral displacement of the CoM relative to the new stance foot (see Fig. 4), which in turn caused a decrease in swing-phase GM activity of the contralateral left leg (Fig. 5C, L0 swing). Conversely, lateral perturbations of the right leg increased the mediolateral displacement of the CoM relative to the new stance foot, and subsequently increased swing-phase GM activity in the left leg (Fig. 5C, L0 swing). The perturbations also significantly (P = 0.023) influenced GM activity of the unperturbed leg during the subsequent stance phase. Specifically, lateral perturbations increased this contralateral stance-phase GM activity (Fig. 5C, L1 stance). In contrast to the clear results observed with bilateral GM activity, the perturbations did not have a significant effect (P > 0.10) on subsequent swing-phase AM activity for either leg.
DISCUSSION
The aim of this experiment was to identify a neuromechanical foot placement control strategy that uninjured humans use to maintain lateral stability while walking. We used a combination of unperturbed and perturbed walking trials to relate movement kinematics to neural control in the form of muscle activation. Supporting our hypothesis, we found that swing-phase GM activity predictably influenced mediolateral foot placement, and that this swing-phase activity was predicted by the mechanical state of the stance leg and torso.
The present results suggest that humans use a consistent mediolateral foot placement strategy to ensure their stability during both unperturbed and perturbed walking. During Unperturbed walking trials, we identified significant associations between gait kinematics (e.g., mediolateral location of the CoM) and active control (e.g., swing-phase GM activity) by taking advantage of natural step-to-step variability in these measures. However, such regression analyses cannot determine causality, as both CoM location and swing-phase muscle activity could conceivably be adjusted in parallel by some other independent factor (e.g., altered feedforward descending commands). The Perturbed walking trials permitted us to test the causal relationships suggested by the Unperturbed associations. The applied perturbations altered the mediolateral location of the CoM relative to the new stance foot, allowing us to quantify the subsequent effects on contralateral swing-phase GM activity and foot placement. During both Unperturbed and Perturbed walking trials, an increase in the CoM displacement relative to the stance foot was accompanied by increased contralateral swing-phase GM activity. Increased swing-phase GM activity was followed by more lateral foot placement for both types of walking trials. In contrast, swing-phase AM activity had only a relatively small effect on foot placement during Unperturbed walking and was not consistently influenced by the applied perturbations during Perturbed walking. This modulation of GM activity to control foot placement, and the relatively minor role of AM activity, are consistent with the results of recent experiments in which lateral pushes were applied to the CoM during walking (Hof and Duysens 2013). Importantly, our results indicate that a similar stabilization strategy is present even in the absence of external perturbations.
Unlike previous experiments in which the CoM or the stance leg was directly perturbed (Chvatal and Ting 2012; Hof et al. 2010; Hof and Duysens 2013; Oddsson et al. 2004), our perturbations mimicked the mechanical effects of foot placement errors that could be produced by inaccurate mediolateral control of the swing leg. These perturbations influenced the CoM mediolateral displacement relative to the stance foot at the start of the next step, essentially having the effect of introducing large errors in foot placement. While such perturbations are not as commonly investigated as slips, trips, or pushes, a recent observational video study in a long-term care setting found that internal perturbations in the form of incorrect weight shifting (including improperly placed steps) were the most frequent cause of falls (Robinovitch et al. 2013). Interestingly, in the present study the amplitude of the medial perturbations was consistently smaller than the amplitude of the lateral perturbations. We believe that this is likely due to participants resisting the medial perturbations despite instructions not to, as evidenced by increased GM activity during the perturbed swing phase. Additionally, the involved experimenter may have subconsciously reduced the amplitude of the applied medial perturbations because of the perception that these perturbations were more destabilizing.
Our results provide direct experimental evidence for a neuromechanical strategy used to actively control lateral stability during human walking. Within each stride, the swing phase allows control over the next point of contact with the environment. Such a foot placement strategy permits center of pressure adjustments an order of magnitude larger than those possible through control of the stance leg ankle (Hof et al. 2010). During the swing phase, humans modulate hip muscle activity to control the mediolateral trajectory of the swing leg, as increased abductor activity early in swing causes the leg to move more laterally. While this muscle action is logical, standard gait analysis textbooks typically describe the GM as being inactive during this phase of gait (Perry 1992; Vaughan et al. 1999; Winter 1987). The present results suggest that GM activity during early swing, while small compared with stance-phase activity, is an important contributor to the production of stable gait patterns even during unperturbed walking. The slight increase in adductor activity observed with more lateral foot placement during Unperturbed walking is likely a result of using this muscle to slow the swing leg prior to the impact at heel strike. A similar increase in both abductor and adductor activity with more lateral steps was previously observed in walking cats (Karayannidou et al. 2009).
The swing leg's initial mechanical state (displacement, velocity, and acceleration) influenced its eventual placement location, although not as strongly as GM activity. This influence may be partially attributable to passive mechanics, as pendular movement of the swing leg would be expected to depend on its initial state. The effect of the initial mechanical state could help to explain some aspects of swing leg motion during the Perturbed trials that may otherwise seem confusing. For example, after a lateral perturbation of the right leg, the small change in mediolateral CoM position caused the left foot to begin its swing phase slightly farther from the CoM (see Fig. 4A). Based on our Unperturbed associations, we would expect this to lead to more medial movement of the left leg. Indeed, after lateral perturbations we commonly observed the left leg to move more medially during midswing (see Fig. 4A). The final foot placement in a more lateral location can be attributed to the intervening increased swing-phase GM activity. The opposite effect was often seen after medial perturbations. The influence of the initial mechanical state of the swing leg could also explain why we observed increased swing-phase GM activity during stride R1 but this did not cause more lateral foot placement; the initial large displacement of the right foot relative to the CoM may have been sufficient to cancel out any effects of increased GM activity.
Simultaneous with the active swing leg control, humans must identify a mechanically appropriate foot placement target for the ongoing step. Unlike typical upper extremity reaching tasks, the ideal end point is not entirely determined by environmental constraints. Additionally, accurately moving the swing leg to the identical frontal-plane angle for each step would not allow for adjustments to variation in CoM motion. Instead, the mediolateral foot placement must account for the mechanical state of the body in order to redirect the CoM back toward the midline (Hof et al. 2010). We found that humans base their active control of mediolateral foot placement on the mechanical state of the stance leg and torso, specifically the mediolateral displacement of the CoM from the stance foot. Similar strategies have previously been proposed for efficient stabilization in model simulations (Kuo 1999) and have successfully stabilized bipedal walking robots (Hobbelen and Wisse 2009). Additionally, swing-phase activity and subsequent foot placement are predictably influenced by the state of the stance leg in walking cats (Musienko et al. 2012a, 2012b). However, direct evidence for this type of mechanics-dependent active neural control has not previously been demonstrated in unperturbed walking humans.
While humans appear to partially control their mediolateral foot placement by modulating swing-phase abductor and adductor muscle activity, the sensory information used to execute this control is unclear. Humans may control the mediolateral trajectory of the swing leg by monitoring proprioceptive feedback, as occurs during accurate upper extremity movements (Sarlegna and Sainburg 2009). For example, muscle spindle feedback from the swing leg hip musculature could be used by the central nervous system to estimate frontal-plane hip position, followed by appropriate muscle activation to minimize errors between the sensed and desired positions. Future experiments could provide insight into the neural mechanism underlying swing leg control by investigating whether foot placement is influenced by perturbing proprioceptive feedback from the swing leg, such as by applying muscle vibration to activate muscle spindles (Roll and Vedel 1982). In clinical populations, gait function may be limited by an inability to modulate hip abductor and adductor activity during swing to control foot placement. Indeed, an inability to quickly recruit the GM has previously been associated with fall risk (Brauer et al. 2000). Function could also be limited by an inability to sense the position of the swing leg, as proprioception accuracy at the hip is reduced in many stroke patients (Connell et al. 2008).
The proposed neuromechanical gait stabilization strategy requires humans to develop a perception of the mechanical state of their stance leg and torso. Our finding that the mediolateral displacement of the CoM plays a major role in the active control of foot placement provides further support for the proposed importance of CoM mechanics in motor control (Scholz and Schoner 1999). While the body may not have specific receptors to sense CoM mechanics, humans are able to generate novel perceptual representations of the body's state through multisensory integration (Green and Angelaki 2010). Future experiments could extend previous investigations of sensory integration during standing posture (Bingham et al. 2011; Goodworth and Peterka 2012) to walking. Several types of sensory feedback may contribute to the perceptual representation of CoM mechanics during gait, including proprioceptive (e.g., spindle and Golgi tendon organ) feedback from the stance hip (Popov et al. 1999), knee (Cammarata and Dhaher 2011), or ankle (Kavounoudias et al. 1999), cutaneous feedback from the sole of the stance foot (Kavounoudias et al. 2001), vestibular feedback (Bent et al. 2005), or visual feedback (O'Connor and Kuo 2009). While less well understood, the sense of effort (McCloskey et al. 1974) required for the stance leg abductors to support the pelvis may also contribute to the perception of CoM mechanics, which could explain the observed relationship between the simultaneous GM activity in the stance and swing legs. Importantly, neural circuits have the potential to differentiate and integrate sensory signals over time (Aksay et al. 2001), so our observed primary importance of CoM displacement does not necessarily implicate sensory sources that directly provide positional information (e.g., secondary muscle spindles). In clinical populations, functional gait could be limited by inaccuracy in any of the sensory modalities described above, or by a reduced ability to integrate this information.
Variation in mediolateral foot placement appears to be both a cause of and a solution to lateral instability during human walking. Our results indicate that errors in mediolateral foot placement require clear subsequent adjustments in active control, which could be interpreted as suggesting that variability in foot placement should be minimized to prevent the need for such corrections. However, the corrections themselves often take the form of adjustments to mediolateral foot placement, suggesting that step-to-step variability is necessary. For example, externally applied lateral foot placement perturbations (the error) are followed by more lateral foot placement with the opposite leg (the correction). This is not a novel concept, as both abnormally low and abnormally high step width variability have previously been associated with increased fall risk (Brach et al. 2005). Additionally, recent work has begun to categorize variability of frontal-plane gait motions as “good variance” or “bad variance” with uncontrolled manifold analyses of kinematic measures (Krishnan et al. 2013; Rosenblatt et al. 2014). The present results support the proposal that some variation in mediolateral foot placement can be a beneficial response to altered CoM motion and provide direct evidence that such “good variance” is actively controlled by the nervous system through appropriate muscle recruitment.
The anticipatory gait adaptations observed during Perturbed walking trials were indicative of a more conservative gait strategy, suggesting that participants were less confident in their foot placement. When anticipating perturbations, participants walked with shorter, faster steps, matching both previous experimental results in uninjured participants (Cappellini et al. 2010; Monsch et al. 2012) and the cautious gait pattern often observed in clinical populations (Wall et al. 1991). More interestingly, participants placed their right leg more laterally when expecting perturbations but did not adjust placement of their left leg. More lateral foot placement of the leg that participants expected to be perturbed was observed even though both medial and lateral perturbations were applied during the trial and the lateral perturbations were actually larger in amplitude. This was likely because the more lateral foot placement was safer (Hof et al. 2007) given the uncertainty in where the foot would land. Essentially, placing the foot more laterally decreases the control accuracy required to maintain stability (Kuo 1999). Similar behavior has been observed in individuals who have experienced a stroke, as the paretic leg is typically placed more laterally than the nonparetic leg (Balasubramanian et al. 2010). Our results suggest that more lateral foot placement may be due to reduced confidence in foot placement accuracy, possibly as a result of decreased proprioceptive accuracy. More lateral foot placement would likely increase the mechanical demands on the hip abductors of the paretic leg during stance, which are typically already weak after a stroke (Neckel et al. 2006). In turn, this may contribute to the reduced time stroke patients spend in single-leg stance on the paretic leg and exacerbate gait asymmetry.
In summary, we found that humans use a consistent neuromechanical strategy to maintain lateral stability during unperturbed and perturbed walking. Specifically, humans sense the mechanical state of the stance leg and torso and use this information to actively control the mediolateral placement of the swing leg by modulating muscle activity. These results are consistent with a stabilizing strategy previously proposed by model simulations, used in bipedal walking robots, and observed in walking cats. Our findings could have clinical implications for populations in which limited sensation accuracy or sensory integration could cause deficits in functional mobility.
GRANTS
This study was partially supported by the Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development Service through Grant 1IK2RX000750-01A1. This study was also partially supported by the National Institute of Child Health and Human Development through Grant 1R21 HD-064964-01A1.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s). The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
Author contributions: B.L.R., S.K.B., and J.C.D. performed experiments; B.L.R., S.K.B., and J.C.D. analyzed data; B.L.R., S.K.B., and J.C.D. interpreted results of experiments; B.L.R., S.K.B., and J.C.D. drafted manuscript; B.L.R., S.K.B., and J.C.D. edited and revised manuscript; B.L.R., S.K.B., and J.C.D. approved final version of manuscript; J.C.D. conception and design of research; J.C.D. prepared figures.
REFERENCES
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci 4: 184–193, 2001 [DOI] [PubMed] [Google Scholar]
- Allet L, Kim H, Ashton-Miller JA, Richardson JK. Which lower limb frontal plane sensory and motor functions predict gait speed and efficiency on uneven surfaces in older persons with diabetic neuropathy? PMR 4: 726–733, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann V, Muller R, van Hedel HJ, Dietz V. Vertical perturbations of human gait: organisation and adaptation of leg muscle responses. Exp Brain Res 186: 123–130, 2008 [DOI] [PubMed] [Google Scholar]
- Balasubramanian CK, Neptune RR, Kautz SA. Foot placement in a body reference frame during walking and its relationship to hemiparetic walking performance. Clin Biomech 25: 483–490, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech 33: 1433–1440, 2000 [DOI] [PubMed] [Google Scholar]
- Belsey DA, Kuh H, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New York: Wiley, 1980 [Google Scholar]
- Bent LR, McFadyen BJ, Inglis JT. Vestibular contributions during human locomotor tasks. Exerc Sport Sci Rev 33: 107–113, 2005 [DOI] [PubMed] [Google Scholar]
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol 357: 109–125, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham JT, Choi JT, Ting LH. Stability in a frontal plane model of balance requires coupled changes to postural configuration and neural feedback control. J Neurophysiol 106: 437–448, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, Berlin JE, Van Swearingen JM, Newman AB, Studenski SA. Too much or too little step width variability is associated with a fall history in older persons who walk at or near normal gait speed. J Neuroeng Rehab 2: 21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer SG, Burns YR, Galley P. A prospective study of laboratory and clinical measures of postural stability to predict community-dwelling fallers. J Gerontol A Biol Sci Med Sci 55: M469–M476, 2000 [DOI] [PubMed] [Google Scholar]
- Bruijn SM, Meijer OG, Beek PJ, van Dieen JH. Assessing the stability of human locomotion: a review of current measures. J R Soc Interface 10: 20120999, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarata ML, Dhaher YY. Proprioceptive acuity in the frontal and sagittal planes of the knee: a preliminary study. Eur J Appl Physiol 111: 1313–1320, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellini G, Ivanenko YP, Dominici N, Poppele RE, Lacquaniti F. Motor patterns during walking on a slippery walkway. J Neurophysiol 103: 746–760, 2010 [DOI] [PubMed] [Google Scholar]
- Chvatal SA, Ting LH. Voluntary and reactive recruitment of locomotor muscle synergies during perturbed walking. J Neurosci 32: 12237–12250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil 22: 758–767, 2008 [DOI] [PubMed] [Google Scholar]
- Field-Fote EC, Dietz V. Single joint perturbation during gait: preserved compensatory response pattern in spinal cord injured subjects. Clin Neurophysiol 118: 1607–1616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodworth AD, Peterka RJ. Sensorimotor integration for multisegmental frontal plane balance control in humans. J Neurophysiol 107: 12–28, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granata KP, Lockhart TE. Dynamic stability differences in fall-prone and healthy adults. J Electromyogr Kinesiol 18: 172–178, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AM, Angelaki DE. Multisensory integration: resolving sensory ambiguities to build novel representations. Curr Opin Neurobiol 20: 353–360, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Rios DA, Edelberg HK. Gait variability and fall risk in community-living older adults: a 1-year prospective study. Arch Phys Med Rehabil 82: 1050–1056, 2001 [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, Merletti R, Stegeman D, Blok J, Rau G, Disselhorst-Klug C, Hagg G. European Recommendations for Surface Electromyography. Enschede, The Netherlands: Roessingh Research and Development, 1999 [Google Scholar]
- Hobbelen DG, Wisse M. Active lateral foot placement for 3D stabilization of a limit cycle walker prototype. Int J Humanoid Robotics 6: 93–116, 2009 [Google Scholar]
- Hof AL, Duysens J. Responses of human hip abductor muscles to lateral balance perturbations during walking. Exp Brain Res 230: 301–310, 2013 [DOI] [PubMed] [Google Scholar]
- Hof AL, van Bockel RM, Schoppen T, Postema K. Control of lateral balance in walking. Experimental findings in normal subjects and above-knee amputees. Gait Posture 25: 250–258, 2007 [DOI] [PubMed] [Google Scholar]
- Hof AL, Vermerris SM, Gjaltema WA. Balance responses to lateral perturbations in human treadmill walking. J Exp Biol 213: 2655–2664, 2010 [DOI] [PubMed] [Google Scholar]
- Hurt CP, Rosenblatt N, Crenshaw JR, Grabiner MD. Variation in trunk kinematics influences variation in step width during treadmill walking by older and younger adults. Gait Posture 31: 461–464, 2010 [DOI] [PubMed] [Google Scholar]
- Jorgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke 33: 542–547, 2002 [DOI] [PubMed] [Google Scholar]
- Karayannidou A, Zelenin PV, Orlovsky GN, Sirota MG, Beloozerova IN, Deliagina TG. Maintenance of lateral stability during standing and walking in the cat. J Neurophysiol 101: 8–19, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavounoudias A, Gilhodes JC, Roll R, Roll JP. From balance regulation to body orientation: two goals for muscle proprioceptive information processing? Exp Brain Res 124: 80–88, 1999 [DOI] [PubMed] [Google Scholar]
- Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol 532: 869–878, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Rosenblatt NJ, Latash ML, Grabiner MD. The effects of age on stabilization of the mediolateral trajectory of the swing foot. Gait Posture 38: 923–928, 2013 [DOI] [PubMed] [Google Scholar]
- Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robotic Res 18: 917–930, 1999 [Google Scholar]
- Lockhart TE, Liu J. Differentiating fall-prone and healthy adults using local dynamic stability. Ergonomics 51: 1860–1872, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell GA, Blanch PD, Barnes CJ. EMG of the hip adductor muscles in six clinical examination tests. Phys Ther Sport 13: 134–140, 2012 [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Winter DA. Control of whole body balance in the frontal plane during human walking. J Biomech 26: 633–644, 1993 [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Ebeling P, Goodwin GM. Estimation of weights and tensions and apparent involvement of a “sense of effort.” Exp Neurol 42: 220–232, 1974 [DOI] [PubMed] [Google Scholar]
- Misiaszek JE, Stephens MJ, Yang JF, Pearson KG. Early corrective reactions of the leg to perturbations at the torso during walking in humans. Exp Brain Res 131: 511–523, 2000 [DOI] [PubMed] [Google Scholar]
- Monsch ED, Franz CO, Dean JC. The effects of gait strategy on metabolic rate and indicators of stability during downhill walking. J Biomech 45: 1928–1933, 2012 [DOI] [PubMed] [Google Scholar]
- Musienko P, Courtine G, Tibbs JE, Kilimnik V, Savochin A, Garfinkel A, Roy RR, Edgerton VR, Gerasimenko Y. Somatosensory control of balance during locomotion in a decerebrated cat. J Neurophysiol 107: 2072–2082, 2012a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Zelenin PV, Lyalka VF, Gerasimenko YP, Orlovsky GN, Deliagina TG. Spinal and supraspinal control of the direction of stepping during locomotion. J Neurosci 32: 17442–17453, 2012b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckel N, Pelliccio M, Nichols D, Hidler J. Quantification of functional weakness and abnormal synergy patterns in the lower limb of individuals with chronic stroke. J Neuroeng Rehabil 3: 17, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor SM, Kuo AD. Direction-dependent control of balance during walking and standing. J Neurophysiol 102: 1411–1419, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddsson LI, Wall C, McPartland MD, Krebs DE, Tucker CA. Recovery from perturbations during paced walking. Gait Posture 19: 24–34, 2004 [DOI] [PubMed] [Google Scholar]
- Perry J. Gait Analysis: Normal and Pathological Function. Thorofare, NJ: SLACK, 1992 [Google Scholar]
- Popov KE, Kozhina GV, Smetanin BN, Shlikov VY. Postural responses to combined vestibular and hip proprioceptive stimulation in man. Eur J Neurosci 11: 3307–3311, 1999 [DOI] [PubMed] [Google Scholar]
- Robinovitch SN, Feldman F, Yang Y, Schonnop R, Leung PM, Sarraf T, Sims-Gould J, Loughin M. Video capture of the circumstances of falls in elderly people residing in long-term care: an observational study. Lancet 381: 47–54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res 47: 177–190, 1982 [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Hurt CP, Grabiner MD. Sensitivity of dynamic stability to changes in step width during treadmill walking by young adults. J Appl Biomech 28: 616–621, 2012 [DOI] [PubMed] [Google Scholar]
- Rosenblatt NJ, Hurt CP, Latash ML, Grabiner MD. An apparent contradiction: increasing variability to achieve greater precision? Exp Brain Res 232: 403–413, 2014 [DOI] [PubMed] [Google Scholar]
- Sarlegna FR, Sainburg RL. The roles of vision and proprioception in the planning of reaching movements. In: Progress in Motor Control: A Multidisciplinary Perspective, edited by Sternad D. New York: Springer, 2009, p. 317–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers A, Hahn ME. Regulation of whole-body frontal plane balance varies within a step during unperturbed walking. Gait Posture 36: 322–324, 2012 [DOI] [PubMed] [Google Scholar]
- Schielzeth H. Simple means to improve the interpretability of regression coefficients. Methods Ecol Evol 1: 103–113, 2010 [Google Scholar]
- Schmid AA, Rittman M. Consequences of poststroke falls: activity limitation, increased dependence, and the development of fear of falling. Am J Occup Ther 63: 310–316, 2009 [DOI] [PubMed] [Google Scholar]
- Scholz JP, Schoner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126: 289–306, 1999 [DOI] [PubMed] [Google Scholar]
- Shinya M, Fujii S, Oda S. Corrective postural responses evoked by completely unexpected loss of ground support during human walking. Gait Posture 29: 483–487, 2009 [DOI] [PubMed] [Google Scholar]
- Stanek JM, McLoda TA, Csiszer VJ, Hansen AJ. Hip and trunk muscle activation patterns during perturbed gait. J Sport Rehabil 20: 287–295, 2011 [DOI] [PubMed] [Google Scholar]
- Tang PF, Woollacott MH, Chong RK. Control of reactive balance adjustments in perturbed human walking: roles of proximal and distal postural muscle activity. Exp Brain Res 119: 141–152, 1998 [DOI] [PubMed] [Google Scholar]
- van Schooten KS, Sloot LH, Bruijn SM, Kingma H, Meijer OG, Pijnappels M, van Dieen JH. Sensitivity of trunk variability and stability measures to balance impairments induced by galvanic vestibular stimulation during gait. Gait Posture 33: 656–660, 2011 [DOI] [PubMed] [Google Scholar]
- Vaughan CL, Davis BL, O'Connor JC. Dynamics of Human Gait (2nd ed.). Cape Town, South Africa: Kiboho, 1999 [Google Scholar]
- Wall JC, Hogan DB, Turnbull GI, Fox RA. The kinematics of idiopathic gait disorder. A comparison with healthy young and elderly females. Scand J Rehabil Med 23: 159–164, 1991 [PubMed] [Google Scholar]
- Whittle MW. Three-dimensional motion of the center of gravity of the body during walking. Hum Mov Sci 16: 347–355, 1997 [Google Scholar]
- Winter DA. The Biomechanics and Motor Control of Human Gait. Waterloo, Canada; Waterloo Biomechanics, 1987 [Google Scholar]
- Woollacott MH, Tang PF. Balance control during walking in the older adult: research and its implications. Phys Ther 77: 646–660, 1997 [DOI] [PubMed] [Google Scholar]
- Zeni JA, Higginson JS. Gait parameters and stride-to-stride variability during familiarization to walking on a split-belt treadmill. Clin Biomech 25: 383–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeni JA, Richards JG, Higginson JS. Two simple methods for determining gait events during treadmill and overground walking using kinematic data. Gait Posture 27: 710–714, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]