Abstract
INTRODUCTION
Metastases to the parathyroid gland are very uncommon. Although renal cell carcinoma metastasis to the head and neck region is well recognised, with a predilection for unpredictable metastasis to unusual sites such as the thyroid gland, nose, paranasal sinuses, and cranial bones, there are no reports of parathyroid gland involvement.
PRESENTATION OF CASE
We describe an unusual case of renal cell carcinoma metastasis to a parathyroid gland in a 69-year-old male who had been treated 8 years previously for a pT3b N0 M1 clear cell carcinoma of the right kidney with a right nephrectomy, and interferon immunotherapy for 18 months. The patient had originally presented to the plastic surgeons with a rapidly enlarging 3 cm superficial lesion on the ventral aspect of the left forearm, which was excised with histology revealing metastatic renal (clear) cell carcinoma.
DISCUSSION
Renal cell carcinoma has a reputation for unpredictable patterns of metastasis, and our case highlights this, with the first description in the literature of parathyroid gland metastasis. Despite the poor prognosis associated with metastatic renal cell carcinoma, our patient is still alive 10 years following original presentation, despite having metastasis to two different extra-renal sites and a shortened course of initial adjuvant systemic therapy.
CONCLUSION
In parathyroid gland metastasis, metastectomy can offer excellent local long term local control.
Keywords: Parathyroid gland, Renal cell carcinoma, Metastasis, Head and neck neoplasms
1. Introduction
Renal cell carcinoma (RCC) is the most common malignant neoplasm affecting the kidney, accounting for 3% of adult cancers.1 Approximately 30% of patients present with metastatic disease,2 often as the initial manifestation of RCC.
The most common sites of distant RCC metastasis are the lungs (60%), bone (40%), and liver (40%),3 but RCC is also renowned for unpredictable patterns of secondary spread to involve almost every other body site. Late recurrences are another feature, with lesions appearing 10 years or more following surgical treatment.4 Metastatic RCC (mRCC) has a poor prognosis with a median survival of less than 12 months.3 RCC metastasis to the head and neck region is well recognised, occurring in approximately 15% of cases.5,6 Of interest, there are no reported cases of metastasis to the parathyroid gland. We describe a case of RCC metastasis to the parathyroid gland with a review of the relevant literature.
2. Presentation of case
A 69-year-old male patient was referred to the head and neck surgery department following serial computed tomography (CT) scans of the chest that revealed an enlarging right upper mediastinal mass.
He was seen by the plastic surgeons 8 years previously with a rapidly enlarging 3 cm superficial lesion on the ventral aspect of the left forearm which revealed metastatic renal (clear) cell carcinoma on excision biopsy. He was subsequently treated for a pT3b N0 M1 clear cell carcinoma of the right kidney with a right nephrectomy, and interferon immunotherapy for 18 months. He was disease free for 7 years post treatment. A surveillance CT scan then detected a 1.1 cm enhancing upper mediastinum mass, suggestive of a ‘lymph node’ in the right cervical para-oesophageal region (Fig. 1). A repeat CT scan of the chest and abdomen 12 months later showed that the right para-oesophageal mass had increased in size to 1.4 cm, with no other lesions evident. The patient was otherwise asymptomatic, and apart from hypertension, had no other significant past medical history. Clinical examination of the head and neck region was unremarkable.
Fig. 1.
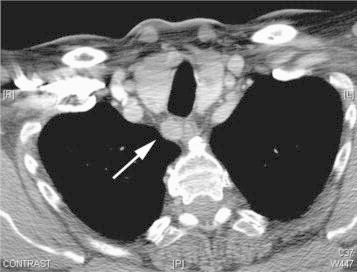
Computed tomography (CT) scan of the chest showing a 1.4 cm right upper mediastinum mass in the cervical para-oesophageal region (white arrow).
Given the suspected diagnosis of mRCC the patient was counselled for excision surgery via a transcervical approach. Intraoperatively, the right thyroid lobe was mobilised medially, and the metastatic deposit was found to lie deep to the inferior thyroid artery in the tracheo-oesophageal groove. The right recurrent laryngeal nerve was in an abnormal position, having been displaced by tumour. The tumour was excised entirely. The patient had an uneventful recovery, and was discharged on the 2nd post-operative day.
Histopathological assessment of the lesion, revealed a 2 cm × 1.5 cm × 0.5 cm firm grey soft tissue nodule. On microscopic examination, it comprised a nodule of highly vascular carcinoma comprising small lobules, cysts and clusters of cells with clear to eosinophilic cytoplasm, mildly pleomorphic nuclei and small prominent nucleoli with insignificant mitotic activity (Fig. 2). Intratumour haemorrhage and cysts were present. In this central zone the tumour cells co-expressed cytokeratin (AE1/3) and vimentin, and were positive for CD10. Around the periphery there was an almost complete rim of compressed parathyroid gland consisting of normal parathyroid chief and oxyphilic cells. In some areas these formed recognisable parathyroid gland with fat, but in other areas the thin rim of partly clear cells was highlighted by positive immunoreaction for parathyroid hormone (PTH) and chromogranin (Fig. 3), which were negative in the central zone. There was no vimentin co-expression or CD10 in the parathyroid component. The excision margin ran along the edge of both the parathyroid gland and in places of the carcinoma, with no evidence of transected tumour. Overall, the histological analysis was consistent with mRCC to the parathyroid gland.
Fig. 2.
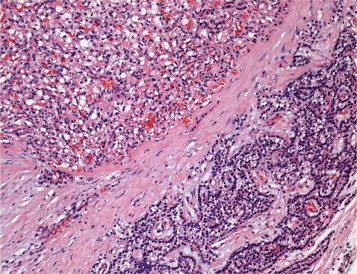
Metastatic renal cell carcinoma (upper left) surrounded by parathyroid chief and oxyphilic cells (lower right). The neoplastic cells are polygonal with clear to eosinophilic cytoplasm, mildly pleomorphic nuclei and small prominent nucleoli (haematoxylin and eosin).
Fig. 3.
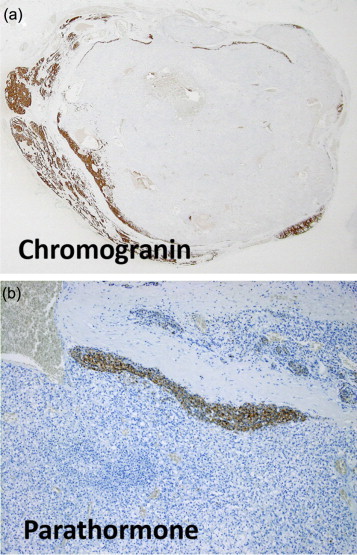
(a and b) Immunohistochemistry showing that only the rim of normal parathyroid gland surrounding the metastasis was positive for chromogranin (low power of whole lesion) and parathormone (PTH, high power).
The patient remains alive at 19 months following excision. His post-operative PTH and corrected calcium was within normal limits.
3. Discussion
RCC is the third most common infraclavicular tumour to metastasise to the head and neck region after breast and lung cancer.7–10 The therapeutic modality for mRCC depends on the head and neck subsite involved, but most efforts are directed at palliation to control symptoms, with metastectomy most often advocated.10–15 Metastectomy in cases of RCC metastases can be curative or at least offer excellent long term local control. This case demonstrates that in selected cases of RCC metastases to the head and neck region, metastectomy can be advocated to achieve excellent local control and potential cure.
Radiotherapy has also been purported to be an effective palliative treatment for RCC with excellent local symptom control.11 Other treatments include cytotoxic chemotherapy,3 immunotherapy,2,3 and more recently molecular targeted therapy,16 all with limited response rates.
To the best of our knowledge, mRCC to the parathyroid gland is extremely rare, if reported. In our patient, the previous history of mRCC made us consider RCC metastasis. However, given the discrete anatomical location, separate from the thyroid gland, our initial diagnosis was of a metastatic lymph node deposit. Intra-operative findings of a vascular lesion closely related to the inferior thyroid artery and recurrent laryngeal nerve did raise suspicion of a metastatic inferior parathyroid tumour, which was confirmed on subsequent histological analysis.
Metastases to the parathyroid glands are extremely rare with very few cases in the literature, even in the context of widespread carcinomatosis.17 A study investigating sites of metastasis in 1000 autopsies of cancer patients in 1950 did not observe a single case of parathyroid metastasis, despite noting metastases in unusual locations.18 Further work from a prospective post-mortem study of 160 patients with a range of malignant neoplasms, demonstrated metastatic involvement of at least one parathyroid gland in 19.9% of cases.19 However, the same investigators in a retrospective study of 750 necropsies identified secondary malignant involvement of the parathyroid in only 5.3% of cases. The most common primary sites in decreasing order of frequency were breast, blood (leukaemia), skin (malignant melanoma), lung, soft tissue (spindle cell sarcomas), and lymphomas.19 Interestingly, in both series, secondary neoplastic involvement of the parathyroid was part of widespread metastasis, however, it should be noted that the methods employed to identify the primary tumour site appear ambiguous, and their results need to be interpreted with caution. A further post-mortem investigation of metastasis to endocrine glands detected only 3 cases out of 1900 patients with involvement of the parathyroid gland,20 and like the previous studies mentioned, none of the primary sites of origin included the kidney.
Despite the rich vascular supply of the parathyroid glands, it would appear that there is no predilection for mRCC. There was no clinical evidence of altered calcium balance in our case preoperatively and post-operative calcium and PTH levels were normal. With the involvement of one parathyroid gland, there is nothing in the literature to guide management of the remaining parathyroids with regards to close monitoring for further metastatic deposits versus prophylactic parathyroidectomy to prevent the development of further metastatic deposits. Given the bizarre presentation of mRCC in our case, the patient is being followed up closely, with a view to localised treatment of any further metastatic deposits as and when they arise, depending on his symptoms and performance status.
4. Conclusion
RCC has a reputation for unpredictable patterns of metastasis, and our case highlights this, with the first description in the literature of RCC metastasis to the parathyroid gland. Our patient presented initially with a cutaneous RCC metastasis that upon further investigation resulted in detection of the right kidney primary. Despite the poor prognosis associated with mRCC, our patient is still alive 10 years following original presentation, despite having metastasis to two different extra-renal sites and a shortened course of initial adjuvant systemic therapy. In mRCC to the parathyroid gland, metastectomy can offer excellent long term local control.
Conflict of interest
None declared.
Funding
None declared.
Ethical approval
Written informed consent was obtained from the patient for publication of this case report and case series and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Author contributions
All authors (Enyinnaya Ofo, Rishi Mandavia, Jean-Pierre Jeannon, Edward Odell and Ricard Simo) contributed to the case data collection, analysis and writing of this paper.
References
- 1.Jemal A., Siegel R., Ward E., Murray T., Xu J., Smigal C. Cancer statistics. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Lam J.S., Leppert J.T., Belldegrun A.S., Figlin R.A. Novel approaches in the therapy of metastatic renal cell carcinoma. World J Urol. 2005;23:202–212. doi: 10.1007/s00345-004-0466-0. [DOI] [PubMed] [Google Scholar]
- 3.Gupta K., Miller J.D., Li J.Z., Russell M.W., Charbonneau C. Epidemiologic and socioeconomic burden of metastatic renal cell carcinoma (mRCC): a literature review. Cancer Treat Rev. 2008;34:193–205. doi: 10.1016/j.ctrv.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Sountoulides P., Metaxa L., Cindolo L. Atypical presentations and rare metastatic sites of renal cell carcinoma: a review of case reports. J Med Case Rep. 2011;5:429. doi: 10.1186/1752-1947-5-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pritchyk K.M., Schiff B.A., Newkirk K.A., Krowiak E., Deeb Z.E. Metastatic renal cell carcinoma to the head and neck. Laryngoscope. 2002;112:1598–1602. doi: 10.1097/00005537-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto R., Helmus C. Hypernephroma metastatic to the head and neck. Laryngoscope. 1973;83:898–905. doi: 10.1288/00005537-197306000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Boles R., Cerny J. Head and neck metastases from renal carcinomas. Mich Med. 1971;70:616–618. [PubMed] [Google Scholar]
- 8.Nakhjavani M.K., Gharib H., Goellner J.R., van Heerden J.A. Metastasis to the thyroid gland. A report of 43 cases. Cancer. 1997;79:574–578. doi: 10.1002/(sici)1097-0142(19970201)79:3<574::aid-cncr21>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Di Stasi V., D’Antonio A., Caleo A., Valvano L. Metastatic renal cell carcinoma to the thyroid gland 24 years after the primary tumour. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-007569. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bugalho M.J., Mendonca E., Costa P., Santos J., Silva E., Cantarino A. A multinodular goiter as the initial presentation of a renal cell carcinoma harbouring a novel VHL mutation. BMC Endocr Disord. 2006;6:6. doi: 10.1186/1472-6823-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simo R., Sykes A.J., Hargreaves S.P., Axon P., Birzgalis A., Slevin N. Metastatic renal cell carcinoma to the nose and paranasal sinuses. Head Neck. 2000;22:722–727. doi: 10.1002/1097-0347(200010)22:7<722::aid-hed13>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 12.Lee H.M., Kang H.J., Lee S.H. Metastatic renal cell carcinoma presenting as epistaxis. Eur Arch Otorhinolaryngol. 2005;26:69–71. doi: 10.1007/s00405-003-0671-2. [DOI] [PubMed] [Google Scholar]
- 13.Azam F., Abubakerr M., Gollins S. Tongue metastasis as an initial presentation of renal cell carcinoma: a case report and literature review. J Med Case Rep. 2008;2:249. doi: 10.1186/1752-1947-2-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mrena R., Leivo I., Passador-Santos F., Hagstrom J., Makitie A.A. Histopathological findings in parotid gland metastases from renal cell carcinoma. Eur Arch Otorhinolaryngol. 2008;265:1005–1009. doi: 10.1007/s00405-008-0679-8. [DOI] [PubMed] [Google Scholar]
- 15.Sabatini P., Ducic Y. Bilateral lacrimal gland masses: unusual case of metastatic renal cell carcinoma. J Otolaryngol Head Neck Surg. 2009;38:E1–E2. [PubMed] [Google Scholar]
- 16.Motzer R.J., Hutson T.E., Tomczak P., Michaelson M.D., Bukowski R.M., Rixe O. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 17.Gattuso P., Khan N.A., Jablokow V.R., Kathuria S. Neoplasms metastatic to parathyroid glands. South Med J. 1988;81:1467. doi: 10.1097/00007611-198811000-00039. [DOI] [PubMed] [Google Scholar]
- 18.Abrams H.L., Spiro R., Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Horwitz C.A., Myers W.P., Foote F.W., Jr. Secondary malignant tumors of the parathyroid glands. Report of two cases with associated hypoparathyroidism. Am J Med. 1972;52:797–808. doi: 10.1016/0002-9343(72)90086-1. [DOI] [PubMed] [Google Scholar]
- 20.Rivadeneyra J., Lira-Puerto V., Aguilar-Parada E. Metastasis to the endocrine glands (review of 1900 autopsies) Ginecol Obstet Mex. 1972;31:139–150. [PubMed] [Google Scholar]


