Abstract
Mate attraction in Aplysia involves a long-distance water-borne signal (the protein pheromone attractin), which is released during egg laying. Aplysia californica attractin attracts species that produce closely related attractins, such as Aplysia brasiliana, whose geographic distribution does not overlap that of A. californica. This finding suggests that other mollusks release attractin-related pheromones to form and maintain breeding aggregations. We describe four additional members of the attractin family: A. brasiliana, Aplysia fasciata, Aplysia depilans (which aggregates with A. fasciata aggregations), and Aplysia vaccaria (which aggregates with A. californica aggregations). On the basis of their sequence similarity with A. californica attractin, the attractin proteins fall into two groups: A. californica, A. brasiliana, and A. fasciata (91–95% identity), and A. depilans and A. vaccaria (41–43% identity). The sequence similarity within the attractin family, the conserved six cysteines, and the compact fold of the NMR solution structure of A. californica attractin suggest a common fold for this pheromone family containing two antiparallel helices. The second helix contains the IEECKTS sequence conserved in Aplysia attractins. Mutating surface-exposed charged residues within this heptapeptide sequence abolishes attractin activity, suggesting that the second helix is an essential part of the receptor-binding interface.
Pheromones are thought to play an important role in coordinating reproductive behavior in many aquatic species, but only two water-borne peptide pheromones have been chemically and behaviorally characterized in invertebrates in detail: attractin from the marine opisthobranch gastropod mollusk Aplysia californica (1–5) and nereithione from the marine polychaete worm Nereis (6, 7). A. californica is a simultaneous hermaphrodite that does not normally fertilize its own eggs. Field studies have shown that A. californica are solitary animals most of the year, but during the reproductive season, they move into breeding aggregations (“brothels”), mate, and lay eggs. The aggregations usually occur where egg cordons are laid, often deposited one on top of another. Egg cordons are attractive in T-maze assays, and attraction is due to chemical, rather than visual, cues (8).
After ovulation, eggs travel to the fertilization chamber, which is surrounded by the albumen gland (refs. 9 and 10, and Fig. 4 and Supporting Text, which are published as supporting information on the PNAS web site). This gland packages eggs into a long string-like cordon, which has a high surface-to-volume ratio. Egg cordons are considered to be a source of both water-borne and contact pheromones, which attract animals to the area and induce them to mate and lay eggs (8, 11). Of the pheromones produced by the albumen gland, only attractin has been identified.
Attractin, a 58-residue protein with three intramolecular disulfide bonds (2, 3), was isolated from A. californica, a Pacific Coast species, but was bioassayed with Aplysia brasiliana, a species found in the Gulf of Mexico, for several reasons: A. californica locomote slowly or not at all during T-maze attraction assays and tend to crawl out of T-mazes before being exposed to chemical test stimuli; A. brasiliana is genetically closely related to A. californica despite their different geographic locations; and A. brasiliana are rapid swimmers that often reach test stimuli in T-maze assays within 10–15 seconds. A. brasiliana attractin differs from A. californica attractin at only three positions (this study). A. californica attractin is attractive to A. brasiliana, reduces the latency to mating, and increases the time spent mating; the peptide also stimulates hermaphroditic mating, reduces the latency to hermaphroditic mating, and increases the duration of the activity (2, 5). These effects may result from attractin stimulating both animals to mate as males. These findings are consistent with field observations in which multiple Aplysia species are often found in the same egg-laying and mating aggregations, for example, A. californica and Aplysia vaccaria from the Pacific Coast (12, 13), which occasionally mate with each other (S. LePage, Marine Research and Educational Products, Carlsbad, CA, personal communication); and Aplysia fasciata and Aplysia depilans from the Mediterranean Sea, in which egg-laying and mating aggregations have been repeatedly observed (14), although interspecific mating has not. T-maze attraction assays have shown that attractin acts as part of a bouquet of water-borne odors: (i) egg cordons are attractive in the absence of Aplysia; and (ii) attractin is attractive only when Aplysia are part of the stimulus (5); the animal does not need to be a conspecific.
In previous work (3, 4), we used mass spectroscopy and NMR to determine the three disulfide bonds of recombinant A. californica attractin (C4-C41, C13-C33, and C20-C26). The NMR solution structure of Aplysia attractin has a compact folded structure with two antiparallel helices (4). Aplysia attractin has a different helix packing compared with the peptide pheromones from the ciliated protozoan Euplotes (15, 16). However, the second helix of attractin, which contains a highly conserved sequence motif, IEECKTS, is structurally similar to the third helix of the Euplotes raikovi pheromones (Er), which is involved in receptor recognition in the ciliated protozoan. In this study, we tested the hypothesis that the charged surface-exposed residues of this motif are essential for attraction by bioassaying mutant proteins in T-maze attraction assays.
We hypothesized that each species secretes a unique attractin. In this study, therefore, we characterized attractins from four Aplysia species and have identified the residues that are important for attraction activity. We conclude that, in contrast to the species-specific, water-borne peptide pheromonal attractants in amphibians (17–20), the attractins are an unusual family of protein pheromones in invertebrates that are not species specific.
Materials and Methods
Animals. Animals were obtained from the following locations: A. californica and A. vaccaria (Pacific Coast of California; Marine Research and Educational Products, Escondido, CA); A. fasciata and A. depilans (Mediterranean coast of Israel); A. brasiliana (Gulf of Mexico, South Padre Island, TX).
Characterization of Attractin-Related Proteins. Albumen glands were removed from A. brasiliana, A. fasciata, A. vaccaria, and A. depilans, extracted, and purified by RP-HPLC, and attractin was characterized as described for A. californica attractin (2). Attractin and related fragments were subjected to N-terminal sequence analysis. In addition, we sequenced fragments obtained upon digestion with endoproteinase Glu-C (21), cyanogen bromide, or endoproteinase Lys-C. A. vaccaria attractin residues 49–52 and A. depilans attractin residues 25, 49, and 50 were determined by 3′-RACE (see Supporting Text).
Protein Expression and Site-Directed Mutagenesis. A. californica attractin was expressed and purified as described (3, 5). By using A. californica attractin cDNA (1), two attractin mutants were generated and expressed in a baculovirus expression system. The first triple mutant contained mutations in the three charged residues in the sequence IEECKTS that are strictly conserved in all five Aplysia species: attractin E31Q, E32Q, K34Q. The second triple mutant contained mutations in three other charged residues that are either strictly conserved or contain conservative substitutions: attractin D5A, D22A, E39A (see Supporting Text).
Egg Cordon Elutions. To demonstrate that egg laying would trigger attractin secretion, egg laying was induced in A. brasiliana by injecting A. californica atrial gland extract (9), which contains egg-laying hormone-related peptides (22–24), into the hemocoel; egg laying in A. vaccaria was induced by injecting A. vaccaria atrial gland extract. One hour after injection and at 30-min intervals thereafter, egg cordons were removed, briefly rinsed in fresh artificial seawater (ASW), and transferred to fresh ASW for elution. Eluates were filtered (0.45 μm) and purified on C18 Sep-Pak Vac cartridges and rinsed with 0.1% heptafluorobutyric acid, and samples were eluted sequentially with 10% and 50% acetonitrile/0.1% heptafluorobutyric acid, and lyophilized. The 50% eluate fractions were resuspended in 0.1% heptafluorobutyric acid and fractionated by analytical Vydac C18 RP-HPLC (2).
Pheromonal Attraction of Aplysia. The T-maze and conduction of T-maze assays using A. brasiliana have been described (2, 5, 8) (see Supporting Text).
Results
Purification and Characterization of Attractin-Related Proteins and the Structural Basis for Their Attraction. The final purification of attractin from four species is shown in Fig. 5 (which is published as supporting information on the PNAS web site). Edman sequence analyses revealed a single distinct attractin-related protein in albumen gland extracts of A. brasiliana, A. fasciata, A. vaccaria, and A. depilans. Additional sequence information was obtained by sequencing endoproteinase Glu-C, endoproteinase Lys-C, and cyanogen bromide peptides and by 3′-rapid amplification of cDNA ends (Table 1, which is published as supporting information on the PNAS web site). The complete sequences of the attractin proteins are shown in Fig. 1. The six cysteines, three charged residues (Asp-5, Asp/Glu-22, and Glu-39), and the sequence Ile-30-Glu-31-Glu-32-Cys-33-Lys-34-Thr-35-Ser-36 (IEECKTS) was conserved in all five Aplysia attractins. A. brasiliana, A. fasciata, A. vaccaria, and A. depilans share 95%, 91%, 43%, and 41% sequence identity, respectively, with A. californica attractin. The attractin proteins fall into two groups according to their sequence similarity with A. californica: A. californica, A. brasiliana, and A. fasciata (91–95% identity), and A. vaccaria and A. depilans (41–43% identity). The attractins we have characterized are representatives of three subgenera that comprise 32 of the 35 known Aplysia species (Table 2, which is published as supporting information on the PNAS web site) and ≈15% of all Aplysia species (Fig. 1).
Fig. 1.
Comparison of amino acid sequences of A. californica, A. brasiliana, A. fasciata, A. vaccaria, and A. depilans attractin. Identities are shaded black. Asterisks indicate amino acids (Glu-31, Glu-32, and Lys-34) substituted in one A. californica attractin triple mutant; plus signs indicate amino acids (Asp-5, Asp-22, and Glu-39) substituted in a second A. californica attractin triple mutant.
Three of the attractin-related proteins are posttranslationally modified in the N-terminal region (Asn-8, A. brasiliana, A. fasciata; Asn-25, A. depilans) and two in the C-terminal region (Ser-49, Ser-50, Thr-51, Thr-52, A. vaccaria; Ser-49, Ser-50, A. depilans). N-glycosylation at Asn-8 of A. californica attractin is not required for attraction (2, 3, 5). Because A. californica attractin is progressively degraded from the C terminus but not from the N terminus, and only the N-terminal 41–47 aa are required for attraction activity (2), posttranslational modifications in the C-terminal region of A. vaccaria and A. depilans attractin may serve to prolong the half-life and biological activity of the proteins or to serve some other unknown function, but this was not examined.
MS Characterization of A. vaccaria and A. depilans Attractins. Mass spectra of reduced and alkylated A. vaccaria and A. depilans attractin did not have peaks corresponding to the predicted masses of the unmodified, but alkylated, attractins. Instead, significantly higher masses are observed. As shown in Fig. 6 (which is published as supporting information on the PNAS web site), A. vaccaria spectra had a high-mass peak grouping, similar to that seen in spectra of A. californica attractin (2), with a major peak at m/z 7962, followed by five lower-intensity peaks spaced 163 ± 4 Da apart, suggesting a glycosylated protein with variable numbers of hexose units. A. depilans spectra also suggest significant posttranslational modification.
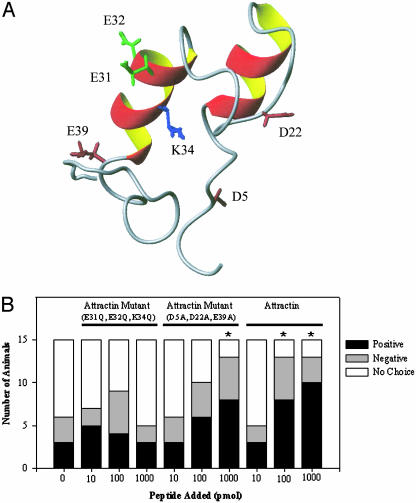
Residues Important for Attraction Activity. Conservation of Asp-5, Asp/Glu-22, Glu-31, Glu-32, Lys-34, and Glu-39 in five Aplysia attractins suggested that some of these charged residues might be important for attraction activity. To test this hypothesis, three charged amino acids within the IEECKTS region were substituted in A. californica attractin (Gln for Glu-31, Glu-32, and Lys-34; Figs. 1 and 2A). This triple mutant attractin was inactive in T-maze assays (Fig. 2B), indicating that Glu-31, Glu-32, and perhaps Lys-34 are important for attraction activity. Three other charged amino acids were substituted in a second triple mutant attractin (Ala for Asp-5, Asp-22, and Glu-39; Figs. 1 and 2 A). In contrast, this triple mutant attractin retained activity (Fig. 2B). These data suggest that at least one, and possibly all, of the charged residues in the IEECKTS region are important for attraction activity. The effects of mutating uncharged residues (Ile-30, Cys-33, Thr-35, Ser-36) in the IEECKTS region were not examined.
Fig. 2.
NMR structure of A. californica attractin and biological activity of attractin mutants in T-maze attraction assays. (A) NMR structure showing the side chains of the residues mutated in these studies. (B) The number of A. brasiliana attracted to a nonlaying conspecific (0 pmol) was not significantly increased when 10, 100, or 1,000 pmol of the attractin triple mutant with three substituted charged amino acids (Gln for Glu-31, Glu-32, and Lys-34) was placed in the adjacent ASW. In contrast, the number of A. brasiliana attracted to a nonlaying conspecific (0 pmol) was significantly increased (*, P < 0.001) when 1,000 pmol of a second attractin triple mutant with three other substituted charged amino acids (Ala for Asp-5, Asp-22, and Glu-39) was placed in the adjacent ASW. In control experiments, when 100 pmol of recombinant A. californica attractin was tested, 8 of 15 animals (53%) were attracted to attractin (*, P < 0.001); when 1,000 pmol was tested, 10 of 15 animals (67%) were attracted to attractin (*, P < 0.001). This bar graph is based on 150 single-arm experiments, 15 per stimulus. In each experiment, animals chose between a stimulus in one arm and no stimulus in the other.
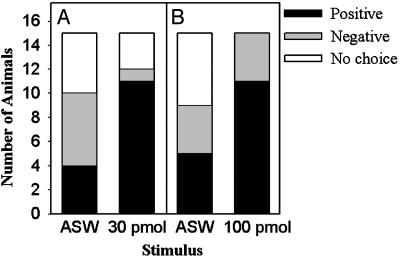
A. vaccaria Attractin Attracts A. brasiliana. In control assays [nonlaying conspecific (A. brasiliana), no added pheromone], four animals entered the arm of the T-maze containing the stimulus animal and remained, six went to the opposite arm, and five did not make a choice (Fig. 3A). These control assays established chance levels of attraction at four animals.
Fig. 3.
A. vaccaria attractin attracts A. brasiliana. (A) The number of A. brasiliana attracted to a nonlaying conspecific (artificial seawater; ASW) increased when 30 pmol A. vaccaria attractin was added to the adjacent seawater; fewer animals went to the opposite arm (negative) of the T-maze, and fewer failed to make a choice (no choice). (B) Same as A, except that 100 pmol of A. vaccaria attractin was added.
The response pattern changed when 30 pmol of A. vaccaria attractin was placed in the ASW adjacent to the stimulus animal: 11 of 15 animals (73%) entered the arm of the T-maze containing A. vaccaria attractin, one went to the opposite arm, and three did not make a choice. The response pattern differed significantly from that to a nonlaying conspecific alone [χ2(2) = 17.22; P < 0.001]. Due to the limited seasonal availability and lifespan of A. brasiliana, assays testing 30 and 100 pmol of A. vaccaria attractin were performed in different years, and a second set of control assays was performed: five animals entered the arm of the T-maze containing the stimulus animal and remained, four went to the opposite arm, and six did not make a choice (Fig. 3B); these control assays established chance levels of attraction at five animals. When 100 pmol of A. vaccaria attractin was tested, 11 of 15 animals (73%) entered the arm of the T-maze containing A. vaccaria attractin, and four went to the opposite arm. The response pattern differed significantly from that to a nonlaying conspecific alone [χ2(2) = 13.20; 0.001 < P < 0.005]. The response pattern to 10 pmol of A. vaccaria attractin was not significantly different from that to a nonlaying conspecific (n = 15; data not shown). In summary, despite the sequence dissimilarity of A. brasiliana and A. vaccaria attractin, A. vaccaria attractin was attractive to A. brasiliana.
We examined whether egg laying would trigger attractin secretion in A. brasiliana and A. vaccaria. RP-HPLC purification of A. brasiliana and A. vaccaria egg cordon eluates is shown in Fig. 7, which is published as supporting information on the PNAS web site. Peaks were pooled based on absorbance, then lyophilized, and analyzed by N-terminal sequence analysis. The partial sequences of the peaks indicated in Fig. 7 A and B corresponded to the five N-terminal residues of A. brasiliana and A. vaccaria attractin, respectively, demonstrating that they are released during egg laying in these species.
Discussion
Based on sequence comparison and an NMR structure of A. californica attractin (3, 4), we previously proposed that a conserved region of the protein is responsible for interaction with cellular receptors. The results shown here offer further evidence that the heptapeptide sequence, IEECKTS, conserved in attractins from five different Aplysia species, is indeed important for biological activity. Altering the three charged amino acids in this sequence, which forms the second helix of the 3D structure, effectively abolished the activity of attractin in T-maze assays. In contrast, mutating three conserved charged residues at other areas of the protein reduced but did not destroy attractin activity. All attractins characterized to date have six conserved cysteines and three acidic residues corresponding to Asp-5, Glu-31, and Glu-32 of A. californica. These three residues are solvent exposed in the 3D NMR solution structure (ref. 4 and Fig. 2 A). The 3D structure allowed us to distinguish residues that are conserved for structural reasons (e.g., cysteines, the core residue Ile-30) from those whose surface exposure could reflect a role in receptor recognition (Asp-5, Ser-11, Met-15, Asp/Glu-22, Glu-31, Glu-32, Glu-39) (4). The triple mutant A. californica attractin E31Q, E32Q, K34Q (Fig. 2 A) lacks activity in T-maze assays, suggesting that Glu-31, Glu-32, and Lys-34 may be involved in receptor binding and pheromonal attraction and may account for the interspecific attraction activity of attractin.
We further showed that even attractin from a genetically distant Aplysia species, A. vaccaria, is attractive to A. brasiliana. The responses to 30 and 100 pmol of A. vacarria attractin (Fig. 3 A and B) may have been maximal because they were comparable to the maximal response obtained for A. californica attractin (Fig. 2B). In these T-maze assays, it should be noted that, because attractin is delivered as a pulse and the seawater is stationary during the assay, the attractin concentration gradually changes with time, and the concentration detected by A. brasiliana depends on when they reach the stimulus (range: ≈10 seconds to 20 min). In the present study, the attractiveness of A. vacarria attractin to A. brasiliana suggests that, despite only ≈40% sequence conservation in some cases, Aplysia attractins share a common 3D structure and receptor-binding site that is probably in the conserved C-terminal helical domain. We therefore conclude that the attractins are part of a signaling/chemotaxis mechanism that is common to many marine organisms.
The signaling system may be even more fundamental. As noted in previous work (3), attractins have similar sequence and structural properties to those of the well-studied pheromones of the ciliated protozoan Euplotes. In particular, the second helix of attractin, containing the IEECKTS motif, is quite similar to the third helix of Er-11 (4). This third helix of the Euplotes pheromones is involved in receptor recognition (25, 26). Although Euplotes pheromone sequences differ significantly, sharing only six cysteines and an N-terminal aspartic acid, all have the same compact “pyramid” 3D structure of three α-helices (25–29). The pheromone Er-1 can bind to the mammalian receptor for interleukin-2 (30), raising the possibility that water-borne pheromones may be ancestors of cytokines in higher organisms.
In most organisms, sex pheromones attract potential mates (e.g., ref. 18). If mate attraction were the sole function of attractin, one might expect that the pheromone would attract only conspecifics. However, our data demonstrate that attractin is a relatively promiscuous signal. This finding suggests that the 3D structure of attractin (4) has been conserved during evolution. Other additional functions, for example the reduced latency to mating and the stimulation of hermaphroditic mating (5), may prevent alteration of the attractin structure. There may be other behavioral functions attributable to attractin.
The benefits derived from the aggregation of multiple Aplysia species may exceed those derived solely by accessing potential mates. One benefit may be defense from predators. Consistent with this hypothesis, other communal animals that are thought to herd for defense may form aggregates composed of multiple species (31–34). Secretion of a pheromone that attracts individuals of a different species may still be useful in attracting a potential mate, if the individual that is attracted subsequently lays eggs and releases an attractin signal that attracts a conspecific. The concentration of attractin released from the egg cordons of multiple individuals should be higher than that from a cordon laid by a single individual. Because attractin is partially degraded within 30 min of eluting from egg cordons (2), a higher concentration of active attractin may be sustained over longer distances, thereby increasing the possibility that additional individuals will be recruited to breeding aggregations. Some of the new recruits may be appropriate partners for mating.
This work adds further support to the hypothesis that the attraction mechanism is similar to a signaling mechanism conserved in unicellular organisms. Attractins may also be regulators of other functions, as indicated by their ability to reduce the latency to mating and stimulate hermaphroditic mating. Thus, attractins are proving a multifaceted system to study signaling in marine invertebrates.
Supplementary Material
Acknowledgments
We thank Drs. M. J. Greenberg and J. Koene for constructive comments on an earlier version of the manuscript, Dr. P. Luporini for insights into Euplotes pheromones, and the University of Texas Medical Branch Protein Chemistry Lab and Recombinant DNA Sequencing Core. This work was supported by National Science Foundation Grants IBN-9985778 (to S.D.P.), DBI-9714937 (to W.B.), and IBN-0314377 (to G.T.N.); by National Institutes of Health Grants NS31609 (to J.V.S.) and R01-NS5546 and GM-08224 (to M.W.M.); by U.S.–Israel Binational Science Foundation Grant 2000-344 (to A.J.S.); and by John Sealy Memorial Endowment Fund for Biomedical Research Grant 2579-02R (to G.T.N.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ASW, artificial seawater; Er, Euplotes raikovi pheromone.
Data deposition: The attractin sequences have been deposited in the Protein Information Resource database, http://pir.georgetown.edu [accession nos. A59061 (A. californica), B59060 (A. brasiliana), A59447 (A. fasciata), A59446 (A. depilans), and A59424 (A. vaccaria)].
References
- 1.Fan, X., Wu, B., Nagle, G. T. & Painter S. D. (1997) Mol. Brain Res. 48, 167-170. [DOI] [PubMed] [Google Scholar]
- 2.Painter, S. D., Clough, B., Garden, R. W., Sweedler, J. V. & Nagle, G. T. (1998) Biol. Bull. 194, 120-131. [DOI] [PubMed] [Google Scholar]
- 3.Schein, C. H., Nagle, G. T., Page, J. S., Sweedler, J. V., Xu, Y., Painter, S. D. & Braun, W. (2001) Biophys. J. 81, 463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garimella, R., Xu, Y., Schein, C. H., Rajarathnam, K., Nagle, G. T., Painter, S. D. & Braun, W. (2003) Biochemistry 42, 9970-9979. [DOI] [PubMed] [Google Scholar]
- 5.Painter, S. D., Clough, B., Black, S. & Nagle, G. T. (2003) Biol. Bull. 205, 16-25. [DOI] [PubMed] [Google Scholar]
- 6.Zeeck, E., Müller, C. T., Beckmann, M., Hardege, J. D., Papke, U., Sinnwell, V., Schroeder, F. C. & Francke, W. (1998) Chemoecology 8, 33-38. [Google Scholar]
- 7.Ram, J. L., Müller, C. T., Beckmann, M. & Hardege, J. D. (1999) FASEB J. 13, 945-952. [DOI] [PubMed] [Google Scholar]
- 8.Painter, S. D., Chong, M. G., Wong, M. A., Gray, A., Cormier, J. G. & Nagle, G. T. (1991) Biol. Bull. 181, 81-94. [DOI] [PubMed] [Google Scholar]
- 9.Coggeshall, R. E. (1972) Tissue Cell 4, 105-127. [DOI] [PubMed] [Google Scholar]
- 10.Painter, S. D., Kalman, V. K., Nagle, G. T., Zuckerman, R. A. & Blankenship, J. E. (1985) J. Morphol. 186, 167-194. [DOI] [PubMed] [Google Scholar]
- 11.Begnoche, V. L., Moore, S. K., Blum, N., van Gils, C. & Mayeri, E. (1996) J. Neurophysiol. 75, 2161-2166. [DOI] [PubMed] [Google Scholar]
- 12.Kupfermann, I. & Carew, T. (1974) Behav. Biol. 12, 317-337. [DOI] [PubMed] [Google Scholar]
- 13.Pennings, S. C. (1991) J. Exp. Mar. Biol. Ecol. 149, 249-266. [Google Scholar]
- 14.Achituv, Y. & Susswein, A. J. (1985) J. Exp. Mar. Biol. Ecol. 85, 113-122. [Google Scholar]
- 15.Luginbuhl, P., Ottiger, M., Mronga, S. & Wüthrich, K. (1994) Protein Sci. 3, 1537-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zahn, R., Damberger, F., Ortenzi, C., Luporini, P. & Wüthrich, K. (2001) J. Mol. Biol. 313, 923-931. [DOI] [PubMed] [Google Scholar]
- 17.Kikuyama, S., Toyoda, F., Ohmiya, Y., Matsuda, K., Tanaka, S. & Hayashi, H. (1995) Science 267, 1643-1645. [DOI] [PubMed] [Google Scholar]
- 18.Kikuyama, S., Yamamoto, K., Iwata, T. & Toyoda, F. (2002) Comp. Biochem. Physiol 132, 69-74. [DOI] [PubMed] [Google Scholar]
- 19.Wabnitz, P. A., Bowie, J. H., Tyler, M. J., Wallace, J. C. & Smith, B. P. (1999) Nature 401, 444-445. [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto, K., Kawai, Y., Hayashi, T., Ohe, Y., Hayashi, H., Toyoda, F., Kawahara, G., Iwata, T. & Kikuyama, S. (2000) FEBS Lett. 472, 267-270. [DOI] [PubMed] [Google Scholar]
- 21.Coligan, J. E., Dunn, B. M., Ploegh, H. L., Speicher, D. W. & Wingfield, P. T. (1997) Current Protocols in Protein Science (Wiley, New York).
- 22.Nagle, G. T., Painter, S. D., Blankenship, J. E., Dixon, J. D. & Kurosky, A. (1986) J. Biol. Chem. 261, 7853-7859. [PubMed] [Google Scholar]
- 23.Nagle, G. T., Painter, S. D., Blankenship, J. E. & Kurosky, A. (1988) J. Biol. Chem. 263, 9223-9237. [PubMed] [Google Scholar]
- 24.Rothman, B. S., Hawke, D. H., Brown, R. O., Lee, T. D., Dehghan, A. A., Shively, J. E. & Mayeri, E. (1986) J. Biol. Chem. 261, 1616-1623. [PubMed] [Google Scholar]
- 25.Weiss, M. S., Anderson, D. H., Raffioni, S., Bradshaw, R. A., Ortenzi, C., Luporini, P. & Eisenberg, D. (1995) Proc. Natl. Acad. Sci. USA 92, 10172-10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luporini, P., Miceli, C., Ortenzi, C. & Vallesi, A. (1996) in Signaling Mechanisms in Protozoa and Invertebrates, eds. Csaba, G. & Muller, W. E. G. (Springer, Heidelberg), pp. 80-104.
- 27.Mronga, S., Luginbühl, P., Brown, L. R., Ortenzi, C., Luporini, P., Bradshaw, R. A. & Wüthrich, K. (1994) Protein Sci. 3, 1527-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luginbühl, P., Wu, J., Zerbe, O., Ortenzi, C., Luporini, P. & Wüthrich, K. (1996) Protein Sci. 5, 1512-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miceli, C., La Terza, A., Bradshaw, R. A. & Luporini, P. (1991) Eur. J. Biochem. 202, 759-764. [DOI] [PubMed] [Google Scholar]
- 30.Vallesi, A. Giuli, G., Ghiara, P., Scapigliati, G. & Luporini, P. (1998) Exp. Cell. Res. 241, 253-259. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton, W. D. (1971) J. Theor. Biol. 31, 295-311. [DOI] [PubMed] [Google Scholar]
- 32.Bshary, R. & Noe, R. (1997) Anim. Behav. 54, 1461-1474. [DOI] [PubMed] [Google Scholar]
- 33.Noe, R. & Bshary, R. (1997) Proc. R. Soc. London B 264, 253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson, R. M., Aspbury, A. S. & McDaniel, L. L. (2002) Proc. R. Soc. London B 269, 2503-2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.