Abstract
Background
Fermented milk is considered one of the best sources for efficient consumption of probiotic strains by hosts to promote good health. The purpose of this study was to investigate the effects of orally administering LGG-fermented milk (LGG milk) on intestinal inflammation and injury and to study the mechanisms of LGG milk's action.
Methods
LGG milk and non-LGG-fermented milk (non-LGG milk) were administered through gavage to mice before and during dextran sodium sulfate (DSS)-induced intestinal injury and colitis. Inflammatory/injury score and colon length were assessed. Intestinal epithelial cells were treated with the soluble fraction of LGG milk to detect its effects on the epidermal growth factor receptor (EGFR) and its down stream target, Akt activation, cytokine-induced apoptosis, and hydrogen peroxide (H2O2)-induced disruption of tight junctions.
Results
LGG milk treatment significantly reduced DSS-induced colonic inflammation and injury, and colon shortening in mice, compared to that in non-LGG milk-treated and untreated mice. The soluble fraction of LGG milk, but not non-LGG milk, stimulated activation of EGFR and Akt in a concentration-dependent manner, suppressed cytokine-induced apoptosis, and attenuated H2O2-induced disruption of tight junction complex in the intestinal epithelial cells. These effects of LGG milk were blocked by the EGFR kinase inhibitor. LGG milk, but not non-LGG milk, contained two soluble proteins, p40 and p75, which have been reported to promote survival and growth of intestinal epithelial cells through activation of EGFR. Depletion of p40 and p75 from LGG milk abolished the effects of LGG milk on prevention of cytokine-induced apoptosis and H2O2-induced disruption of tight junctions.
Conclusions
These results suggest that LGG milk may regulate intestinal epithelial homeostasis and potentially prevent intestinal inflammatory diseases through activation of EGFR by LGG-derived proteins.
Keywords: Lactobacillus GG, fermented milk, colitis, epidermal growth factor receptor, apoptosis, tight junction, intestinal epithelial cells
Introduction
Probiotics are live microorganisms that confer health benefits to the host when consumed in adequate amounts [1]. Representative probiotics include lactic acid bacteria, particularly those of the genera Lactobacillus, Bifidobacterium, Escherichia coli strain Nissle 1917, and Saccharomyces. These microorganisms have exhibited various health promoting effects, such as prevention and/or treatment of diarrhea, pathological infection, allergy, and inflammatory bowel disease (IBD), in a strain-dependent manner in many animal studies and clinical trials [2–8]. As increasing evidence has revealed beneficial roles of probiotics on the host, many types of probiotic strains have been used in dairy foods, nutritional supplements, and infant formulas [9].
Food products or dietary supplements containing probiotic strains allow their efficient consumption. In fact, various probiotic product lines have been widely used, especially in fermented milk products. For example, yogurt is generally produced using lactic acid bacteria [10]. Recently, the consumption of fermented milk has rapidly increased even in Asian countries such as China, where dairy products were not widely consumed historically [11]. Therefore, the consumption of fermented milk is one of the best options for many people to promote good health using probiotics.
Lactobacillus rhamnosus GG (LGG), originally isolated from a healthy human [12], is widely used in probiotic foods such as yogurt and dietary supplements in many countries around the world. LGG has been reported for treating and/or preventing several disorders, including ulcerative colitis, diarrhea, and atopic dermatitis [13, 14]. Recent mechanistic studies have found that LGG prevents cytokine-induced apoptosis in intestinal epithelial cells through the activation of Akt and inhibition of p38 activation [15]. As important factors associated with the effect of LGG on cytokine-induced apoptosis, two soluble proteins, p75 and p40, have been isolated from the LGG culture supernatant [16]. These proteins are able to protect intestinal barrier function from hydrogen peroxide (H2O2)-induced insults [17]. Furthermore, specific delivery of p40 to the colon prevents and treats DSS-induced colonic epithelial cell injury and inflammation and ameliorates oxazolone-induced colitis in an epidermal growth factor receptor (EGFR)-dependent manner [18]. Thus, it is possible that consumption of fermented milk containing LGG may protect the intestine from inflammation and injury.
In the present study, we evaluated the effects of LGG-fermented milk (LGG milk), a widely available probiotic food, on dextran sulfate sodium (DSS)-induced intestinal injury and acute colitis in mice. In addition, we investigated whether LGG milk contained the functional proteins, p40 and p75, and examined LGG milk's effects on the regulation of intestinal epithelium homeostasis. Our results provided evidence that administration of probiotic fermented milk may exert beneficial effects on prevention and/or treatment of intestinal inflammatory disorders.
Materials and Methods
Milk products
LGG-fermented milk (LGG milk), a commercial product marketed under the name of LGG® (Valio Ltd., Helsinki, Finland) in Japan, was prepared by Takanashi Milk Products Co., Ltd. (Yokohama, Japan). LGG milk was fermented using only LGG. There are more than 14 billion viable cells in 100 ml of this product.
Non-LGG-fermented milk (Non-LGG milk) was prepared as an experimental control. Non-LGG milk was not fermented with LGG, so it did not contain LGG. The formulation of non-LGG milk was the same as that of LGG milk, except that the acidity was adjusted using lactic acid (Shin-shin Foods Co., Ltd., Tokyo, Japan).
The other 17 types of fermented milks tested in this study were randomly selected from commercially available fermented milk products in Japan. The probiotic species in these products are listed in Table 1. Some of these products contained more than one type of bacteria, such as L. delbreuckii subsp. bulgaricus and Streptococcus thermophillus, which are required for the fermentation of the products.
Table 1.
The fermented milks used in this study
| Product no. | Main species in productsa |
|---|---|
| 1 | Lactobacillus casei |
| 2 | L. brevis |
| 3 | Bifidobacterium breve |
| 4 | L. casei |
| 5 | Bifidobacterium |
| 6 | L. acidophilus |
| 7 | L. delbreuckii subsp. bulgaricus |
| 8 | L. gasseri |
| 9 | B. longum |
| 10 | L. gasseri |
| 11 | L. delbreuckii subsp. bulgaricus |
| 12 | Bifidobacterium |
| 13 | B. animalis |
| 14 | Unknown |
| 15 | L. gasseri |
| 16 | Unknown |
| 17 | L. helveticus |
| LGG milk | L. rhamnosus GG (LGG) |
a Fermented milk is generally produced by various bacterial species and strains. Since suppliers may not disclose all of bacteria used in their commercial products, it is possible that not all bacteria in these products are listed in this table. LGG-fermented milk contains only lgg
Induction of colitis by dextran sulfate sodium (DSS) in mice
Female 8- to 12-week-old C57BL/6 mice were purchased from The Jackson Laboratory (JAX® Mice & Services; Bar Harbor, ME). To induce acute colitis, 3% DSS (molecular weight; 36,000–50,000, MP Biomedicals, LLC) in drinking water was administered to mice for 4 days. To examine the effects of LGG milk treatment on DSS-induced colitis, 500 μl of LGG milk and non-LGG milk were gavaged to the mice once a day for 6 days before and during DSS treatment. Mice receiving water only were as control. The experimental design is shown in Fig. 1A. All the experiments were performed in accordance with the protocol approved by Vanderbilt's Institutional Animal Care and Use Committee.
Fig. 1.
LGG-fermented milk prevents DSS-induced colitis in mice. Female mice were treated with 3% DSS in drinking water for 4 d to induce colitis. Mice were gavaged with 500 μL of LGG milk or non-LGG mike once a day for 6 days before and during DSS treatment (A). Paraffin-embedded colon sections were stained with H&E for light microscopic assessment of epithelial damage (B). Colon inflammation/injury scores are shown (C). The length of colon was measured (D). Data presented as means ± SE. *P < 0.05 compared to the water group, #P < 0.05 compared to the DSS only group. Arrows indicate ulceration in the colon. Original magnification in (B), ×10.
Histological analysis
Mice were sacrificed at the end of DSS treatment. The entire colon was removed, and the length of the colon was measured. The colon was rinsed with PBS and fixed in 4% paraformaldehyde at 4°C overnight. Paraffin-embedded tissue sections were stained with hematoxylin and eosin for light microscopic examination. Inflammation and injury were assessed using a scoring system by a blinded pathologist [19]. Briefly, inflammation was scored as follows: grade 0, none; grade 1, slight; grade 2, moderate; grade 3, severe. The depth of inflammation was scored as follows: grade 0, normal; grade 1, mucosa; grade 2, submucosa; and grade 3, transmural. Crypt damage was scored as follows: grade 0, intact crypts; grade 1, loss of the bottom third of crypts; grade 2, loss of the bottom two thirds of crypts; grade 3, loss of the entire crypt with the surface epithelium remaining intact; and grade 4, loss of the entire crypt and surface epithelium. Inflammation and crypt damage were also quantified as to the percentage involvement: grade 1, 1% to 25%; grade 2, 26% to 50%; grade 3, 51% to 75%; and grade 4, 76% to 100%. Additive scores are between 0 (no colitis) and 15 (maximal colitis).
Preparation of milk supernatants and immunodepletion of p40 and p75 from milk supernatants
Fermented milk products were vigorously shaked to break the curd and centrifuged at 14,000 g for 15 min at 4°C. The supernatants were saved at 4°C.
Supernatants were incubated with anti-p75 antibody-conjugated beads for 4 h at 4°C. After removal of anti-p75 antibody-conjugated beads, supernatants were incubated with anti-p40 antibody-conjugated beads for another 4 h. The amounts of p75 and p40 present in the supernatant before and after immunodepletion were detected by immunoblot analysis. The p40 and p75 antibodies were generated as described previously [16]. The p40 and p75 antibody-conjugated beads were prepared by incubation of antibodies with protein A/G beads in PBS for 2 h at room temperature and washed with PBS twice.
Detection of p40 and p75 in the supernatants of milk products
The milk supernatants were mixed with Laemmli sample buffer and heated at 95°C for 10 min. The protein samples were separated by SDS-PAGE for Colloidal Blue Staining and for Western blot analysis using anti-p40 and anti-p75 antibodies prepared as described previously [16]. The purified p40 and p75 (1 μg in each lane) used as the standard controls were isolated from LGG culture broth as described previously [16]. The amount of p40 and p75 in the LGG milk supernatant was calculated by comparing the band density in Western blot to p40 and p75 standards using Image-J program.
Cell culture
Young adult mouse colon (YAMC) cell, a conditionally immortalized murine colonic epithelial cell line, was isolated from the mouse harboring a thermolabile mutation (tsA58) under the control of an interferon (IFN)-γ-inducible H-2Kb promoter and a temperature-sensitive simian virus 40 (SV40) large T antigen (Immotomouse). The functional expression of the SV40 large T antigen is induced by culturing the cells in vitro in medium containing (IFN)-γ at a temperature permissive for function of the tsA58 mutation (33°C). YAMC cells were maintained in RPMI 1640 media supplemented with 5% fetal bovine serum (FBS), 5 U/ml murine IFN-γ, 100 U/ml penicillin and streptomycin, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenous acid on collagen-coated plates and grown under at 33°C (permissive condition) with 5% CO2. Prior to the experiments, the YAMC cells were serum-starved for 16–18 h in IFN-γ-free RPMI 1640 medium containing 0.5% FBS and 100 U/ml penicillin and streptomycin at 37°C (non-permissive conditions).
The human colonic adenocarcinoma cell lines, HT-29 and Caco-2, were purchased from American Type Culture Collection (Rockville, MD). HT-29 and Caco-2 cells were was grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS and 100 U/ml penicillin and streptomycin at 37°C with 5% CO2. Cells were serum-starved in DMEM medium containing 0.5% FBS for 16–18 h before experiments.
Cell treatment
In each trial, the YAMC cells were treated with 100 ng/ml of TNF and 1 mg/ml of cycloheximide for 6 h to induce apoptosis, 20 μM H2O2 for 1–4 h to disrupt intestinal epithelial integrity, or 10 ng/ml EGF for 5 min to activate EGFR. The HT29 cells were treated with the cytokine combinations of 100 ng/ml TNF-α, 100 ng/ml IFN-γ and 10 ng/ml IL-1α for 6 h to induce apoptosis, as described previously [15]. Supernatants of LGG milk and non-LGG milk, and the LGG milk supernatant with p40 and p75 immunodepletion, were used to treat cells at indicated dilutions with 1-h pretreatment. 150 nM AG1478, an EGFR kinase inhibitor (Calbiochem, San Diego, CA) was also used for co-treatment with the supernatant of LGG milk.
Measurement of transepithelial resistance (TER)
Caco-2 cells were grown on polycarbonate membranes in Transwell inserts (6.5 mm, Costar). The experiments were conducted 11 – 13 days postseeding. TER was measured using a Millicell-ERS Electrical Resistance System (Millipore). The results were expressed as % of baseline value, which was calculated as {value in treated cells (Ω·cm2) - value in unseeded Transwell insert (Ω·cm2)} / {baseline value (Ω·cm2) - value in unseeded Transwell insert (Ω·cm2)} × 100.
Preparation of cellular lysates
After treatment, cell monolayers were rinsed twice with ice-cold PBS (pH 7.4) and then scraped into cell lysis buffer [50 mM Tris–HCl (pH 7.4), 120 mM NaCl, and 1% NP-40] with protease and phosphatase 1 and 2 inhibitor cocktails (Sigma-Aldrich Co. LLC, St. Louis, MO). The scraped suspensions were centrifuged (14,000 g for 10 min at 4°C) to remove cell debris, and the protein concentration of the supernatant was determined with the DC protein assay (Bio-Rad, Hercules, CA). Cellular lysate proteins were mixed with Laemmli sample buffer and separated by SDS-PAGE for Western blot analysis using anti-phospho-Tyr1068 EGFR (Cell Signaling Technology, Beverly, MA), anti-phospho-Akt (Cell Signaling Technology, Beverly, MA), or anti-PARP antibodies (Cell Signaling Technology, Beverly, MA). β-actin was detected using an anti-β-actin antibody (Sigma-Aldrich Co.) as a protein loading control.
Preparation of detergent-insoluble fractions
Cells were washed twice with ice-cold PBS and incubated for 15 min at 4°C in Tris buffer containing 1.0% Triton X-100 and protease and phosphatase 1 and 2 inhibitor cocktails (Sigma-Aldrich Co.). Cells were scraped from the substratum and passed through a needle for five times. It was then centrifuged at 15,600 g for 4 min at 4°C to sediment the high-density actin cytoskeleton. The pellet was suspended in cell lysis buffer. Protein contents were measured by the DC protein assay (Bio-Rad, Hercules, CA). The Triton-insoluble samples were mixed with Laemmli's sample buffer and heated at 95°C for 5 min for Western blot analysis using a rabbit anti-ZO-1 antibody (Invitrogen Corporation, Carlsbad, CA). Samples were also immunoblotted using an anti-β-actin antibody (Sigma-Aldrich Co.) as a protein loading control.
Apoptosis assay
Apoptosis in cell lines was detected by ApopTag In Situ Apoptosis Detection Kits (TUNEL; Intergen Company, Purchase, NY) according to the manufacturer's guidelines. The slides were observed using Differential interference contrast (DIC) microscopy. The number of positively stained cells within a population of at least 500 cells was counted in order to determine the proportion of apoptotic cells.
For Annexin V-FITC staining, attached cells were dissociated using Accutase (Innovative Cell Technologies, Inc. San Diego, CA) and double stained with Annexin V-FITC and propidium iodide (Calbiochem, San Diego, CA) according to the respective manufacturer's instructions. The percentage of cells positive for Annexin V and propidium iodide was determined by multi-color flow cytometry using BD LSRII system (BD Biosciences).
Immunostaining
Cultured cells were fixed with 4% paraformaldehyde for 15 min at room temperature, permeabilized with 0.2% Triton X-100 for 5 min, and blocked using 3% bovine serum albumin for 1 h. Then cells were incubated with an anti-ZO-1 antibody (Invitrogen Corporation, Carlsbad, CA) overnight at 4°C, followed by a Cy3-conjugated goat anti-rabbit IgG secondary antibody (Cell Signaling Technology) for 1 h at room temperature. The cells were counterstained with DAPI and observed using fluorescence microscopy. FITC and DAPI images are taken from the same field.
Statistical analyses
Statistical significance for comparisons in each study was determined by one-way ANOVA followed by Newman–Keuls analysis using Prism 5.0 (GraphPad Software, Inc., San Diego, CA). A P-value <0.05 was defined as statistically significant. All data are representative of at least three repeat experiments and are presented as mean ± the standard error of the mean (S.E.M).
Results
Orally administration of LGG milk prevents DSS-induced colitis in mice
Because the DSS mouse model of acute colitis is characterized well by increased epithelial injury and production of inflammatory cytokines [20], we investigated the preventive effects of LGG milk on DSS-induced colon epithelial injury and colitis in vivo. DSS treatment for 4 days induced injury and acute colitis with colon ulceration, crypt damage, and severe inflammation. These abnormalities were reduced by co-treatment with LGG milk, but not non-LGG milk (Fig. 1B). Compared to the intestinal injury and inflammation score in DSS-treated mice (score: 6.5 ± 0.9), LGG milk significantly decreased injury and inflammation score (score: 4.1 ± 2.9, P < 0.05), but not non-LGG milk (score: 8.2 ± 1.3) (Fig. 1C). In addition, the shortening of the colon induced by DSS is a marker for colitis. DSS treatment induced colon length shortening, (control: 8.4 ± 0.6 cm, DSS group: 6.6 ± 0.5 cm, P < 0.05), which was prevented by LGG milk treatment (7.5 ± 0.4 cm, P < 0.01), but not non-LGG treatment (6.4 ± 0.6 cm) (Fig. 1D). These results indicate that LGG milk exerts a preventive effect on DSS-induced colitis in mice.
LGG milk stimulates activation of EGFR and Akt in intestinal epithelial cells
Activation of EGFR in intestinal epithelial cells by probiotic LGG plays a protective role in tissue injury and inflammation [18]. Therefore, we detected the role of LGG milk on EGFR and Akt activation. YAMC cells were treated with LGG milk supernatant for 2 h, EGFR and Akt activation was examined by Western blot analysis. LGG milk supernatants activated EGFR and its down-stream target, Akt, in a concentration-dependent manner (Fig. 2). As appositive control, EGF stimulated EGFR and Akt activation in YAMC cells (Fig. 2). These results suggest that the LGG milk has the ability to activate EGFR signaling in intestinal epithelial cells.
Fig. 2.
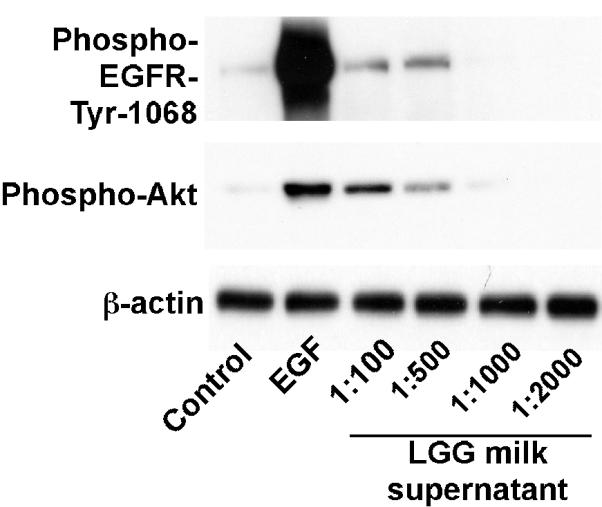
LGG milk supernatant stimulates EGFR and Akt activation in YAMC cells. YAMC cells were treated with supernatants of LGG milk at indicated dilutions for 2 h or EGF (10 ng/mL) for 5 min. Activation of EGFR and Akt was detected by Western blot analysis using anti-phospho-EGFR-Tyr1068 and anti-phospho-Akt antibodies, respectively. Anti-β-actin antibody was used as a loading control. The data is representative of at least two separate experiments.
LGG milk protects intestinal epithelial cells from cytokine-induced apoptosis
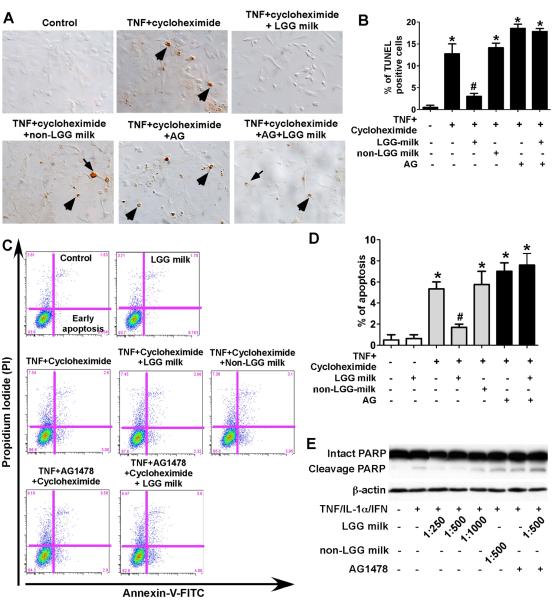
It is well known that activation of EGFR stimulates several signaling pathways, including PI3K/Akt, to promote proliferation and cell survival. Thus, we next evaluated the effect of LGG milk on apoptosis in colonic epithelial cells. We detected intestinal epithelial cell apoptosis and distinguished it from necrosis using two different methods: the TUNEL assay that measures apoptosis by specifically labeling fragmented genomic DNA with terminal deoxynucleatidyl transferase and Annexin V staining that detects apoptosis based on the specific binding of FITC-conjugated Annexin V to phosphatidylserine once it is exposed to the outer layer of the plasma membrane during the apoptotic process. TNF and cycloheximide-induced apoptosis detected by the TUNEL assay in the YAMC cells was inhibited by LGG milk supernatant, but not by non-LGG milk supernatant (Fig. 3A and B). These results were also confirmed by Annexin V staining (Fig. 3C and D). In addition, blocking EGFR activity by the EGFR kinase inhibitor AG1478 abolished the effects of LGG milk on the inhibition of apoptosis (Figures 3A–D), indicating that the transactivation of EGFR mediates the inhibitory effects of LGG milk on apoptosis in colonic epithelial cells.
Fig. 3.
LGG milk supernatant inhibits cytokine-induced apoptosis in intestinal epithelial cells. YAMC cells (A–D) were treated with TNF-α (100 ng/ml) and cycloheximide (1 μg/ml) for 6 h in the presence or absence of 1-h pretreatment of supernatants of LGG milk or non-LGG milk at 1:500 dilution, and/or AG1478 (150 nM). The TUNEL assay was performed to detect apoptosis in the YAMC cells. Arrows indicate representative apoptotic nuclei (A). The percentage of cells undergoing apoptosis is shown (B). The YAMC cells were dissociated and stained with Annexin V-FITC and propidium iodide and analyzed by flow cytometry, and results are shown as density plots with Annexin V-FITC vs propidium iodide (C). Viable cells have low Annexin V-FITC and low propidium iodide staining (lower left quadrant); early apoptotic cells have high Annexin V-FITC and low propidium iodide (lower right quadrant); late apoptotic cells have high Annexin V-FITC and high propidium iodide (upper right quadrant); and necrotic cells have low Annexin V-FITC and high propidium iodide staining (upper left quadrant). The early apoptotic cell populations in the lower right quadrant are shown in D. HT-29 cells (E) were treated with the “cytokine cocktail” combination of TNF-α (100 ng/ml), IL-1α (10 ng/ml), and IFN-γ (100 ng/ml) for 12 h in the presence or absence of supernatants of LGG milk or non-LGG milk at indicated dilutions, and/or AG1478 (150 nM). Total cellular lysates were subjected to Western blot analysis using an anti-PARP antibody which indentifies both intact and cleavage PARP. β-actin blot was performed for protein loading control. In B and D, * P < 0.05 compared to control, # P < 0.05 compared to TNF + cycleheximide treated group. The data from Western blot analysis are representative of two separate experiments.
The effect of LGG milk on the inhibition of apoptosis was also investigated in the human colonic epithelial carcinoma cell line HT-29 by detecting the cleavage of PARP, a marker of apoptosis. Treatment of HT-29 cells with combination of TNF, IFN-γ and IL-1α, induced PARP cleavage, which was inhibited by LGG milk supernatant but not non-LGG milk supernatant (Fig. 3E). This inhibitory effect by LGG milk was suppressed by the EGFR kinase inhibitor AG1478 (Fig. 3E). These results suggest that LGG in fermented milk plays an important role in prevention of cytokine-induced apoptosis through transactivation of EGFR in intestinal epithelial cells.
LGG milk prevents H2O2-induced disruption of intestinal epithelial cell integrity
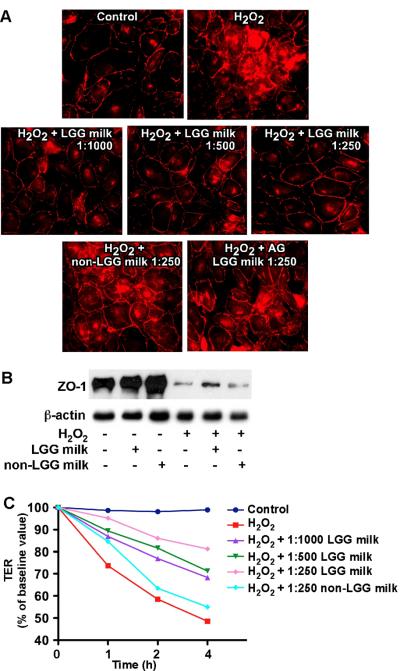
To determine the effects of LGG milk on preservation of intestinal barrier function, the distribution of a tight-junctional protein, ZO-1, was observed by immunostaining. H2O2-induced redistribution of this protein from apical tight junctional complexes to the cytoplasmic compartment of colon epithelial cells was prevented by LGG milk but not by non-LGG milk (Fig. 4A). In addition, the preventive effect was inhibited by the EGFR kinase inhibitor AG1478 (Fig. 4A).
Fig. 4.
LGG milk supernatant prevents H2O2-induced disruption of tight junction in intestinal epithelial cells. YAMC cells were treated with H2O2 (20 μM) for 3 h with or without 1-h pretreatment with LGG milk or non-LGG milk supernatants at the indicated dilutions and a EGFR kinase inhibitor AG1478 (150 nM). These co-treatments were present during H2O2 treatment. The cells were fixed and immunostaining of cells performed to localize ZO-1 using an anti-ZO-1 antibody and a Cy3-conjugated secondary antibody (red) (A). The Triton-insoluble samples were isolated from YAMC cells treated with H2O2 (20 μM) for 3 h with or without 1-h pretreatment with 1:250 dilution of LGG milk or non-LGG milk supernatants. Western blot analysis was performed using a rabbit anti-ZO-1 antibody and an anti-β-actin antibody as a protein loading control (B). Caco-2 cells were treated with H2O2 (20 μM) with or without 1-h pretreatment with LGG milk or non-LGG milk supernatants at the indicated dilutions. TER was measured at varying times after H2O2 treatment (C). The data is representative of three separate experiments.
Tight juntional protein complexes interact with the actin cytoskeleton to anchor these protein complexes at the apical end of the lateral membranes and the detergent-insoluble fraction of tight juntional proteins are more relevant to the integrity of junctional complexes than the membrane-associated or the soluble pools of these proteins [17]. Association of ZO-1 with the detergent insoluble fraction was reduced during the H2O2-induced disruption of tight junctions in YAMC cells, which was prevented by LGG milk supernatant, but not non-LGG milk supernatant (Fig. 4B).
Next, we evaluated the effect of LGG milk on the H2O2-induced increase in paracellular permeability. Incubation of Caco-2 cell monolayers with H2O2 reduced the TER in a time-dependent manner (Fig. 4C). Treatment of cells with LGG milk supernatant significantly reduced the H2O2-induced decrease in TER in a concentration-dependent manner, but not non-LGG milk (Fig. 4C).
These results suggest that the supernatan of LGG milk plays a role in preservation of epithelial integrity through the activation of EGFR.
p40 and p75 mediate LGG milk's protective effects on intestinal epithelial cells
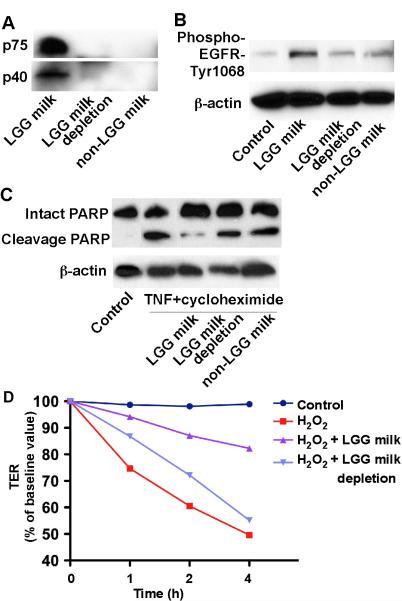
p40 and p75 are LGG derived soluble proteins. Both of them have been shown to prevent and treat intestinal injury and colitis, reduces apoptosis and preserves barrier function by activation of EGFR in intestinal epithelium [16, 18]. Thus, we determined whether p40 and p75 mediate LGG milk's protective role in intestinal epithelial cells. Western blot analysis using anti-p40 and anti-p75 antibodies showed that LGG milk supernatant contained p40 and p75, but non-LGG milk did not have these two proteins (Fig. 5A). Next, we immuno-depleted p40 and p75 from LGG milk supernatant. Incubation of LGG milk samples with anti-p40 and anti-p75 antibodies resulted in depletion of these proteins (Fig. 5A). The immunodepleted LGG milk supernatants failed to prevent TNF and cycloheximide-induced PARP cleavage in YAMC cells (Fig. 5C). Furthermore, H2O2-induced changes in TER in Caco-2 cells was preserved by LGG milk supernatant, but not immunodepleted LGG milk supernatants (Fig. 5D).
Fig. 5.
Immunodepletion of p75 and p40 blocks LGG milk's effects on intestinal epithelial cells. Immunodepletion of p75 and p40 was performed by sequential immunoprecipitation of LGG milk supernatant with anti-p75 and p40 antibodies, to remove both p75 and p40 from LGG milk supernatant. LGG milk with or without immunodepletion and non-LGG milk were separated by SDS-PAGE for Western blot analysis with anti-p75 and p40 antibodies (A). LGG milk, LGG milk depletion and non-LGG milk were used to treat YAMC cells to detect EGFR activation as shown in Figure 2 (B). YAMC cells were treated TNF-α (100 ng/ml) and cycloheximide (1 μg/ml) for 6 h in the presence or absence of 1-h pretreatment of supernatants of LGG milk, LGG milk depletion, or non-LGG milk at 1:250 dilution. Total cellular lysates were subjected to Western blot analysis using an anti-PARP antibody, which indentifies both intact and cleavage PARP. β-actin blot was performed for protein loading control (C). Caco-2 cells were treated with H2O2 (20 μM) with or without 1-h pretreatment with supernatants of LGG milk or LGG milk depletion at 1:250 dilution. TER was measured at varying times after H2O2 treatment (C). Data represent three independent experiments.
These results suggest that LGG in LGG milk secret p40 and p75, which mediate LGG milk's protective role in intestinal epithelial cells.
Detection of soluble proteins, p75 and p40, in other fermented milk products
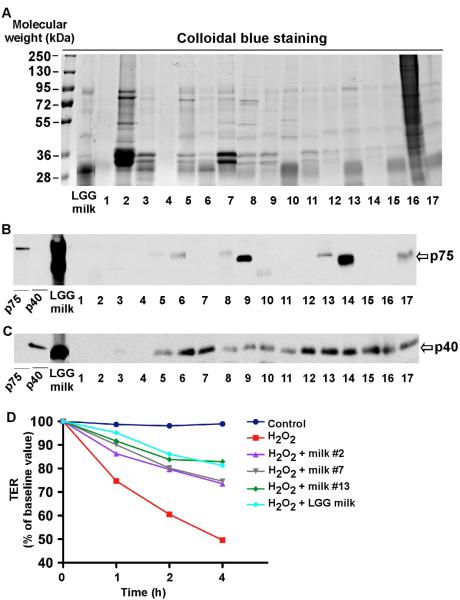
Secretion of p75 and p40 is strain specific, and LGG and L. casei have been shown to secret these two proteins [16, 21]. Therefore, we detected these two proteins in 17 commercially available fermented milk products and LGG milk. The probiotics in these milk products are shown in Table 1. 22.5 μl of the supernatants derived from these tested fermented milks were subjected to SDS-PAGE, and the band patterns were displayed in the stained gels (Fig. 6A). Western blot analysis showed that LGG and product #6, #8, #9, #13, #14, and #17 contained both p75 (Fig. 6B) and p40 (Fig. 6C). Product #5, #7, #10, #11, #12, #15 and #16 contained p40 (Fig. 6C), but not p75 (Fig. 6B). Product #1 to #4 did not contain either p40 or p75. These results further suggest that secretion of p40 and p75 by probiotics is strain specific.
Fig. 6.
Detection of p75 and p40 in other milk products. LGG-milk and other 17 milk products' supernatants were separated by SDS gel and stained with colloidal blue (A) and for Western blot analysis with anti-p75 (B) and anti-p40 antibodies (C). Purified p75 and p40 from LGG culture supernatant was used as the positive control. The probiotic species in these products are listed in Table 1. Caco-2 cells were treated with H2O2 (20 μM) with or without 1-h pretreatment of supernatants of LGG milk and product #2, #7, and #13 at 1:250 dilution. TER was measured at varying times after H2O2 treatment (D). The data were representative of three separate experiments.
Based on the p40 and p75 contents in the milk product supernatants, we chose product #2 (no p40, no p75), #7 (with p40, no p75), and #13 (with both p40 and p75) to detect their effects on preserving intestinal integrity. All of these 3 products exerted beneficial effects on H2O2-induced decreased of TER in Caco-2 cells. But the degree of their effects was different. Product #13 had more effects than products #3 and #7 did (Fig. 6D). These data suggest that, in addition to p40 and p75, other factors derived from probiotics may have beneficial effects on intestinal integrity. However, these factors may be less potent than p40 and p75 on regulation of intestinal homeostasis.
Discussion
In the present study, we demonstrated that fermented milk containing LGG prevented colon inflammation in a DSS-induced colitis mouse model. Currently, several animal studies using LGG have been conducted to evaluate the effects of LGG on the prevention and/or treatment of intestinal inflammatory disorders [22–26]. For example, the study using male Sprague–Dawley rats showed that the continuous intake of LGG during the experimental period was efficient in reducing mucosal damage, colonic prostaglandin E2 generation, and nitric oxidase synthase activity in indoacetmide-induced colitis. However, the LGG-intake had no effect on dinitrobenzene sulfuric acid-induce colitis [22]. In addition, another studies reported that LGG-treatment before the induction of colitis exacerbated disease activity and caused the death of treated C57BL/6 mice [23] and no effect of the administration of LGG on the prevention of DSS-induced colitis in C57BL/6 mice [25, 26]. Therefore, the effects of LGG may depend on colitis models, animals, and the approach for administering LGG.
It should be noted that we used LGG fermented milk in the present study, whereas most other studies used LGG suspended in drinking water or PBS [22, 23, 25]. The significant difference between them is that fermented milk generally contains not only ingredients but also some metabolites produced by fermentation. In addition, LGG may survive better in milk than in water or PBS. Considering the fact that orally administrated LGG can successfully colonize in the intestine of host animals [27, 28], living LGG delivered to the intestinal tract by LGG milk may act more efficiently on prevention of DSS-induced acute colitis.
Recent studies showed that microbial metabolites, such as proteins and polyphosphate, were important to alleviate DSS-induced intestinal injury and inflammation [18, 29]. Administration of these metabolites using indigestible beads or by intrarectally injection suppressed DSS-induced despite the absence of live LGG. In addition to the data from the experiments using LGG suspension in water or PBS [22, 23, 25], the delivery of living LGG to the intestine only might not be enough to suppress the experimental colitis. Therefore, we focused on studying soluble proteins in LGG milk. In fact, the present study showed that LGG milk contained the soluble proteins p40 and p75, which are known to promote intestinal epithelial homeostasis [16]. The soluble fraction derived from LGG milk exerted the activation of EGFR and Akt, suppression of cytokine-induced apoptosis, and prevention of H2O2-induced relocation of ZO-1 in cultured cells as the previous reports about p75 and p40 [16, 17]. In addition, a report that even N-terminal peptide of p40 can prevent DSS-induced colitis gave us a hypothesis that p40 may play a key role for the preventive effect after digestion in the gastrointestinal tract. Although we have not isolated the most important factor(s) from LGG milk, the soluble proteins p75 and p40 may be candidates for the preventive effects on DSS-induced injury and inflammation in mice.
It is known that soluble factors from LGG prevent the intestinal epithelium from stress, such as inflammatory cytokines or super oxides, through stimulation of different signal pathways [15–17, 30]. In fact, the activation of Akt through p40-induced activation of EGFR is considered as one of the most important mechanisms of the preventive/therapeutic actions of LGG against intestinal inflammation and injury [18]. The present study suggested that the soluble fraction recovered from LGG milk suppressed cytokine-induced apoptosis and H2O2-induced intestinal barrier disruption through the activation of EGFR because these effects were strongly inhibited by the EGFR kinase inhibitor AG1478. Given the fact that these effects were not found in non-LGG milk, there is no doubt that soluble factor(s) produced by fermentation with LGG stimulated EGFR, leading to the activation of Akt. Furthermore, our data suggested that p40 and p75 may be candidates for the effective soluble factor(s) because depeletion of p40 and p75 decreased LGG milk's effects on intestinal epithelial cells.
Genes encoding p40 and p75 homologues have been exclusively found in the genomes of L. rhamnosus and L. casei/paracasei, and several strains belonging to these species actually secreted the two proteins in culture broth [16, 21]. However, proteins reacted with anti-p75 and/or anti-p40 antibodies were also detected from various fermented milk products containing other species such as L. delbreuckii subsp. bulgaricus or B. longum but not L. casei. In a BLAST search, the amino acid sequence of p40 from LGG (GenBank accession number YP_003169777) also showed approximately 40–60% similarities with those from L. sakei subsp. sakei, Enterococcus, and Bifidobacteria (data not shown). Therefore, it is possible that the fermented milk with strains other than L. rhamnosus or L. casei/paracasei contains homologue proteins of p40 and/or p75. On the other hand, the L casei strains in fermented milks used for this study may not have an ability of secreting these soluble proteins. Since little information is available on manufacturing processes and conditions, bacterial strains used in those commercial fermented milk, the ability of production of p40, p75 production, and those homologues by each bacterial strain cannot be evaluated in the present study. However, the present study suggested that other fermented milks than LGG milk may also have the preventive effect on regulation of intestinal epithelial homeostasis.
Since fermented milk, such as yogurt, is a just food expanding in many countries. It can be consumed by a large number of people and give them its health promoting function. The present study demonstrates a possibility that the consumption of LGG milk is useful for preventing intestinal inflammatory disorders through regulating intestinal homeostasis. In the future, more scientific evidences, such as whether p40 and p75 can be recover from the gastrointestinal tract after consumption of LGG milk in humans, should be studies to clarify the mechanism of actions of probiotic fermented milk on regulation of intestinal health and inflammatory disorders.
Acknowledgements
We thank Ms. Ayako Kusanagi (Product Development Department at Takanashi Milk Products) for kindly providing non-LGG-milk, and Dr. M. Kay Washington (Department of Pathology, Microbiology, and Immunology at Vanderbilt University Medical Center) for analysis of colon inflammation and injury.
Financial Support This work was supported by Takanashi Milk Products Co., Ltd. and NIH grants R01DK081134 (F.Y.) and P30DK058404 (Vanderbilt University Digestive Disease Research Center).
Footnotes
Author disclosures K. Yoda, K. Miyazawa, M. Hosoda, M. Hiramatsu, and F. He are employees of Takanashi Milk Products Co., Ltd.; F. Yan declares no conflicts of interest.
References
- 1.Gilliland SE, Morelli L, Reid G. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food: Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria Report. Córdoba, Argentina: 2001. [Google Scholar]
- 2.Szajewska H, Mrukowicz JZ. Probiotics in the treatment and prevention of acute infectious diarrhea in infants and children: a systematic review of published randomized, double-blind, placebo-controlled trials. J Pediatr Gastroenterol Nutr. 2001;33(Suppl 2):S17–25. doi: 10.1097/00005176-200110002-00004. [DOI] [PubMed] [Google Scholar]
- 3.Kawase M, He F, Kubota A, Harata G, Hiramatsu M. Oral administration of lactobacilli from human intestinal tract protects mice against influenza virus infection. Lett Appl Microbiol. 2010;51:6–10. doi: 10.1111/j.1472-765X.2010.02849.x. [DOI] [PubMed] [Google Scholar]
- 4.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 5.Kawase M, He F, Kubota A, Hiramatsu M, Saito H, Ishii T, et al. Effect of fermented milk prepared with two probiotic strains on Japanese cedar pollinosis in a double-blind placebo-controlled clinical study. Int J Food Microbiol. 2009;128:429–434. doi: 10.1016/j.ijfoodmicro.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Kawase M, He F, Kubota A, Harata G, Hiramatsu M. Clinical effects of cell preparation of Lactobacillus GG and L. gasseri TMC0356 on perennial allergic rhinitis: a double-blind placebo-controlled trial. Int J Probio Prebio. 2009;4:241–248. [Google Scholar]
- 7.Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14:1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 8.Fedorak RN, Madsen KL. Probiotics and the management of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:286–299. doi: 10.1097/00054725-200405000-00018. [DOI] [PubMed] [Google Scholar]
- 9.Saxelin M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin Infect Dis. 2008;46(Suppl 2):S76–79. doi: 10.1086/523337. discussion S144–51. [DOI] [PubMed] [Google Scholar]
- 10.Blayney D, Gehlhar M, Hilda CH, Jones K, Langley S, Normile HA, et al. World Production and Trade by Country and Product USDA Economic Research Report Number 28. USDA; Washington, D.C.: 2006. Appendix A; pp. 30–44. [Google Scholar]
- 11.Hu D. Animal Production and Health Commission for Asia and The Pacific. FAO Regional Office for Asia and The Pacific; Bangkok, Thailand: 2009. China: Dairy product quality as the new industry driver; pp. 22–43. [Google Scholar]
- 12.Gorbach SL. The discovery of lactobacillus GG. Nutr Today. 1996;31:2S–4S. [Google Scholar]
- 13.Yan F, Polk DB. Probiotics as functional food in the treatment of diarrhea. Curr Opin Clin Nutr Metab Care. 2006;9:717–721. doi: 10.1097/01.mco.0000247477.02650.51. [DOI] [PubMed] [Google Scholar]
- 14.Goldin BR, Gorbach SL. Clinical indications for probiotics: an overview. Clin Infect Dis. 2008;46(Suppl 2):S96–100. doi: 10.1086/523333. discussion S144–51. [DOI] [PubMed] [Google Scholar]
- 15.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seth A, Yan F, Polk DB, Rao RK. Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1060–1069. doi: 10.1152/ajpgi.00202.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan F, Cao H, Cover TL, Washington MK, Shi Y, Liu L, et al. Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J Clin Invest. 2011;121:2242–2253. doi: 10.1172/JCI44031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–391. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 21.Bauerl C, Perez-Martinez G, Yan F, Polk DB, Monedero V. Functional analysis of the p40 and p75 proteins from Lactobacillus casei BL23. J Mol Microbiol Biotechnol. 2010;19:231–241. doi: 10.1159/000322233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibolet O, Karmeli F, Eliakim R, Swennen E, Brigidi P, Gionchetti P, et al. Variable response to probiotics in two models of experimental colitis in rats. Inflamm Bowel Dis. 2002;8:399–406. doi: 10.1097/00054725-200211000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Mileti E, Matteoli G, Iliev ID, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of Lactobacilli using complex culture systems: prediction for in vivo efficacy. PLoS One. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claes IJ, Lebeer S, Shen C, Verhoeven TL, Dilissen E, De Hertogh G, et al. Impact of lipoteichoic acid modification on the performance of the probiotic Lactobacillus rhamnosus GG in experimental colitis. Clin Exp Immunol. 2010;162:306–314. doi: 10.1111/j.1365-2249.2010.04228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Przybyszewski J, Mitra D, Becker C, Brehm-Stecher B, Tentinger A, et al. Soy protein diet, but not Lactobacillus rhamnosus GG, decreases mucin-1, trefoil factor-3, and tumor necrosis factor-alpha in colon of dextran sodium sulfate-treated C57BL/6 mice. J Nutr. 2011;141:1239–1246. doi: 10.3945/jn.110.137414. [DOI] [PubMed] [Google Scholar]
- 27.Saxelin M, Elo S, Salminen S, Vapaatalo H. Dose response colonisation of faeces after oral administration of Lactobacillus casei strain GG. Microb Ecol Health Dis. 1991;4:209–214. [Google Scholar]
- 28.Saxelin M, Ahokas M, Salminen S. Dose response on the faecal colonisation of Lactobacillus strain GG administered in two different formulations. Microb Ecol Health Dis. 1993;6:119–122. [Google Scholar]
- 29.Segawa S, Fujiya M, Konishi H, Ueno N, Kobayashi N, Shigyo T, et al. Probiotic-derived polyphosphate enhances the epithelial barrier function and maintains intestinal homeostasis through integrin-p38 MAPK pathway. PLoS One. 2011;6:e23278. doi: 10.1371/journal.pone.0023278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]