Summary
A total of 105 patients (age ≥ 18 years) with newly diagnosed low or intermediate risk acute promyelocytic leukaemia (APL) were treated with a standard induction and consolidation regimen including arsenic trioxide (ATO). Sixty-eight patients who were polymerase chain reaction (PCR) negative for PML-RARA post-consolidation were randomized to either one year of maintenance with tretinoin, mercaptopurine and methotrexate, or observation. Enrollment in this non-inferiority trial was stopped prematurely due to slow accrual. With a median follow up of 36.1 months, the overall survival of the 105 patients was 93%, and there have been no relapses in the patients randomized to maintenance or observation. These results demonstrate that cures can be expected in >90% of patients with low and intermediate risk APL and suggest that maintenance therapy may not be needed if patients are treated with an intensive post-remission regimen including ATO. This trial was registered at clinicaltrials.gov as #NCT00492856.
Keywords: 1. Acute Promyelocytic Leukaemia, 2. Malignant Haematology, 3. Leukaemia Trials, 4. Leukaemia Clinical
Introduction
The outcome of treatment of acute promyelocytic leukaemia (APL) has significantly improved over the last two decades with the recognition of the importance of anthracyclines, the introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO), and improved supportive care measures.(Sanz and Lo-Coco, 2011; Wang and Chen, 2008) Following anthracycline-based chemotherapy and ATRA, remission rates exceed 90%, and primary resistance is extremely unusual.(Sanz et al., 2004) Most early mortality is due to bleeding complications related to coagulopathy. ATO is very active in APL, both in relapsed and newly diagnosed disease.(Shen et al., 2004; Soignet et al., 2001) The North American Intergroup protocol C9710 recently demonstrated improved disease-free (DFS) and overall survival (OS) when ATO was added as a first consolidation therapy after remission induction.(Powell et al., 2010)
Unlike in the treatment of other subtypes of acute myeloid leukaemia, post-consolidation maintenance therapy is often used in the treatment of APL. The studies demonstrating a benefit for maintenance were conducted before the introduction of ATO as consolidation therapy, and thus the need for maintenance in the setting of ATO consolidation is unknown. The current randomized non-inferiority study was designed to compare DFS among patients with previously untreated low and intermediate risk APL who were polymerase chain reaction (PCR)-negative for PML-RARA after consolidation therapy and received maintenance therapy versus patients who received no maintenance therapy.
Methods
Study population
Study S0521 accrued patients between June 2007 and May 2010 from three North American Cooperative Groups (Southwest Oncology Group [SWOG], Eastern Cooperative Oncology Group [ECOG], and Cancer and Leukemia Group B [CALGB]). It was closed to new patients prematurely due to slow accrual after 105 of a planned 400 patients were entered. Approval by the Institutional Review Board of each participating institution was obtained. Written informed consent was obtained from each participant. The trial is registered at ClinTrial.gov (NCT00492856). Eligibility criteria included a morphologically confirmed diagnosis of low or intermediate risk APL(Sanz et al., 2000) based on bone marrow or peripheral blood examination performed within 14 days before registration. The initial white blood cell (WBC) count had to be ≤10 × 109/l. ATRA was allowed at a dose of ≤ 45 mg/m2/day for ≤ 5 days before registration. No other prior therapy was allowed. Subsequent confirmation of diagnosis by reverse transcription polymerase chain reaction (RT-PCR) for PML-RARA was required.
Data collection and analyses were performed by the SWOG Statistical Center. The Statistical Center staff and the principal investigator reviewed the data for each patient. An independent Data Safety and Monitoring Committee reviewed the trial every 6 months.
Study design
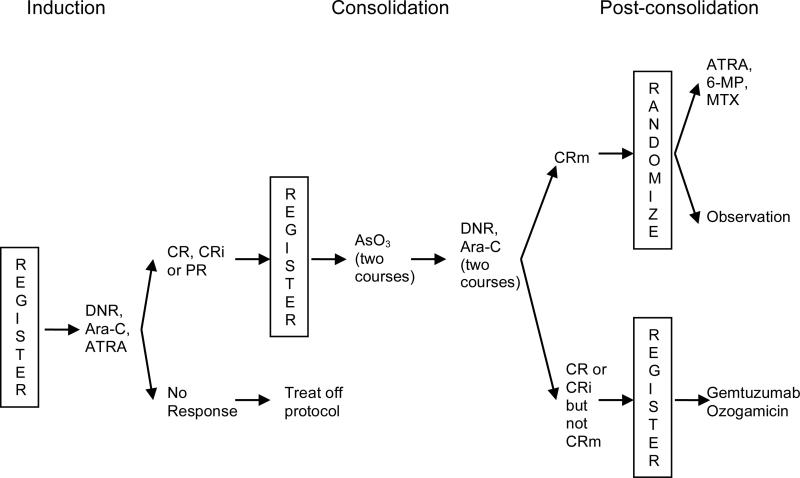
Patients aged 18 years and older were treated as per the C9710 regimen as shown in Figure 1.(Powell et al., 2010) After completion of consolidation, all patients whose bone marrow specimen was negative for PML-RARA by RT-PCR were randomized to one year of maintenance with ATRA (45 mg/m2/day orally given in divided doses twice daily for 7 days every other week), 6-mercaptopurine (60 mg/m2/day orally) and methotrexate (20 mg/m2 orally once per week) or observation with no maintenance. Patients were stratified at the time of post-consolidation randomization by age (18-60 versus >60 years), APL risk group (low [platelet counts >40 × 109/l] versus intermediate [platelet counts ≤40 × 109/l]), and whether or not the non-ATO consolidation was given (yes versus no). When the study was closed to accrual, all patients were non-randomly assigned to receive the maintenance treatment because this was the standard of care at the time. Maintenance therapy was started no earlier than 14 days and no later than 30 days after recovery of peripheral blood counts.
Figure 1. Treatment schema.
CR, complete remission; CRi, incomplete remission; CRm, molecular complete remission; PR, partial remission; DNR, daunorubicin; Ara-C, cytarabine; ATRA, all trans retinoic acid; AsO3, arsenic trioxide; 6-MP, 6-mercaptopurine.
Patients who were still PML-RARA positive after consolidation were eligible to receive gemtuzumab ozogamicin (6 mg/m2/day IV) on days 1 and 15 with one additional dose if they became PCR-negative or up to 4 additional doses if they were still PCR-positive.
Study endpoints
The primary endpoint was the 3-year DFS among patients who were PCR-negative for PMLRARA after consolidation therapy and received maintenance therapy versus patients who received no maintenance therapy. DFS was measured from the date of post-consolidation randomization until relapse of any kind or death from any cause. Observation was censored at the date of last follow-up for patients last known to be alive without report of any relapse. Survival was measured from the time of enrollment on the study.
A secondary endpoint was the assessment of toxicities of induction, consolidation and maintenance therapies. The study utilized the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0 (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) for toxicity and Serious Adverse Event reporting.
Statistical analyses
Descriptive statistics were tabulated for patient characteristics. Exact binomial confidence intervals were calculated for the response rate. OS and DFS were estimated using the Kaplan-Meier method. Regression analyses comparing the randomized arms were not possible due to the small number of events. All analyses were done using R. (R Core Team (2013).
Results
Induction
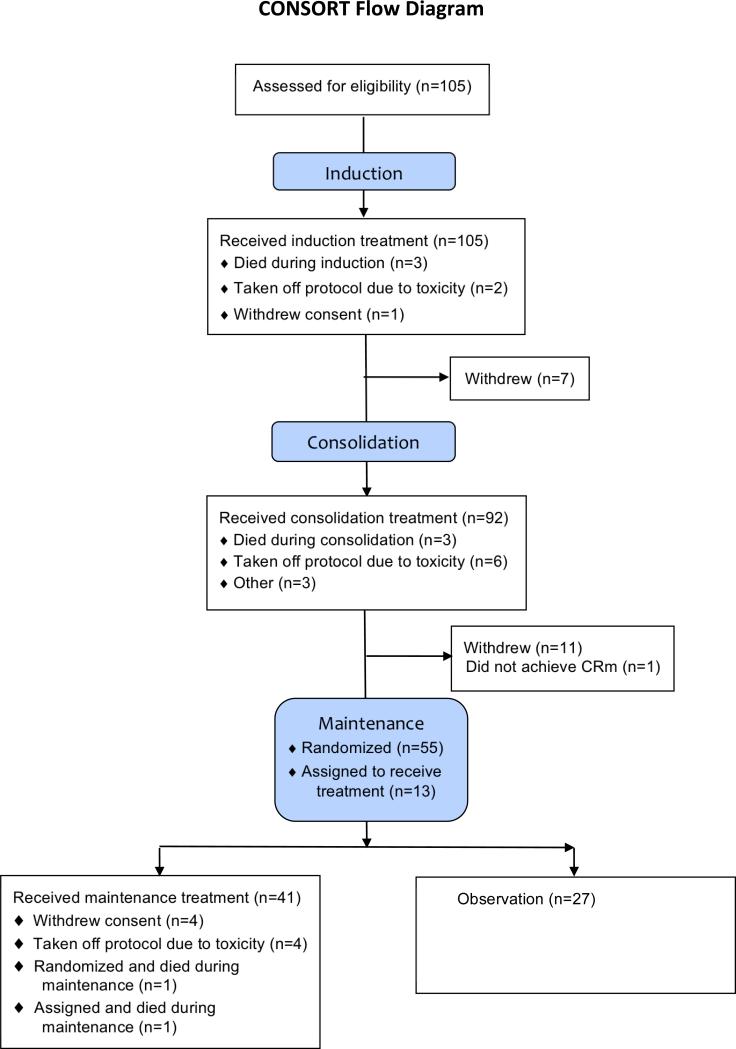
A total of 105 patients were accrued to the induction registration. All were eligible. Patient characteristics are shown in Table I. The Consolidated Standards of Reporting Trials (Schulz et al., 2010) flow diagram is shown in Figure 2. Two patients were taken off protocol induction therapy due to unresolved renal failure and to pneumonia with Grade 4 neutropenia. Both achieved a complete remission. One patient withdrew consent on day 30 of induction and was not assessed for response.
Table I.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Age (median 49, range 20-82 years) | |
| 18-60 years | 84 (80) |
| >60 years | 21 (20) |
| Sex | |
| Male | 61 (58) |
| Female | 44 (42) |
| Race | |
| White | 83 (79) |
| Black | 7 (7) |
| Asian | 7 (7) |
| Hispanic | 5 (5) |
| Native American | 1 (1) |
| Unknown | 2 (2) |
| Performance status | |
| 0 | 35 (33) |
| 1 | 52 (50) |
| 2 | 15 (14) |
| 3 | 2 (2) |
| 4 | 1 (1) |
| Risk group* | |
| Low | 38 (36) |
| Intermediate | 67 (64) |
Low risk group, white blood cell count ≤ 10 × 109/l and platelet count > 40 × 109/l; Intermediate, white blood cell count ≤ 10 × 109/l and platelet count ≤ 40 × 109/l.
Figure 2. Consolidated Standards of Reporting Trials flow diagram.
CRm, molecular complete remission.
All 105 patients were evaluated for induction toxicities. Three patients suffered fatal toxicities: infection, typhlitis and arrhythmia. Grade 4 non-haematological toxicities were reported for another 22 patients, including three with APL differentiation syndrome, one with disseminated intravascular coagulation (DIC) and one with both APL differentiation syndrome and DIC.
Ninety-nine of the 105 patients achieved complete response (94%, 95% confidence interval (CI) 88-98%), one of which was with incomplete blood count recovery. Two patients achieved partial remission. No patient had resistant disease.
Consolidation
Ninety-two patients were registered for consolidation therapy. Seven patients withdrew from the study after induction but prior to consolidation, but only one was due to prior toxicity. There were three consolidation deaths. One patient died from respiratory failure, one from pneumonia and one experienced sudden death. One major protocol deviation was reported for a patient who received extra ATRA and daunorubicin in error for the third consolidation cycle. This patient continued on study and completed maintenance. Before starting daunorubicin, another patient was taken off consolidation therapy by the treating physician due to poor performance status. Two patients did not complete consolidation for unknown reasons. Six additional patients came off consolidation therapy due to toxicity: one for pericardial effusion with pericarditis during the first consolidation cycle, one for sepsis after the first cycle, one each for thrombocytopenia and reduced ejection fraction after the second cycle, one for severe headache during the third cycle and one for neuropathy after the third cycle. Grade 4 nonhaematological toxicities were reported for five additional patients during consolidation: one each with infection and pericardial effusion during the first consolidation cycle, one with infection and elevated hepatic transaminases during the second cycle, one with infection during the third cycle and one with cardiac dysfunction during the fourth cycle.
Maintenance
Sixty-eight patients were registered to post-consolidation therapy, 55 were randomized and 13 were non-randomly assigned to maintenance chemotherapy after the study was revised. Eleven patients withdrew from the study after consolidation but prior to the maintenance randomization. One patient did not achieve molecular remission after completing consolidation and thus was registered to post-consolidation gemtuzumab ozogamicin therapy. Three major protocol deviations were reported for patients who were randomized to receive maintenance therapy due to treatment refusal. A fourth patient refused after starting maintenance therapy. Four patients discontinued maintenance therapy due to toxicity: one due to severe headache and fatigue, one due to headache, fever, nausea, vomiting and dyspnea, one due to headache and cardiac dysfunction, and one due to pneumonia and sinusitis.
Of the 37 patients evaluated for toxicity on maintenance therapy, no fatal toxicities have been reported. Grade 4 non-haematological toxicities were reported for two patients during maintenance therapy: one had elevated hepatic transaminases and one had cardiac dysfunction. One patient randomized to maintenance therapy died from sepsis without a report of relapse.
Outcomes
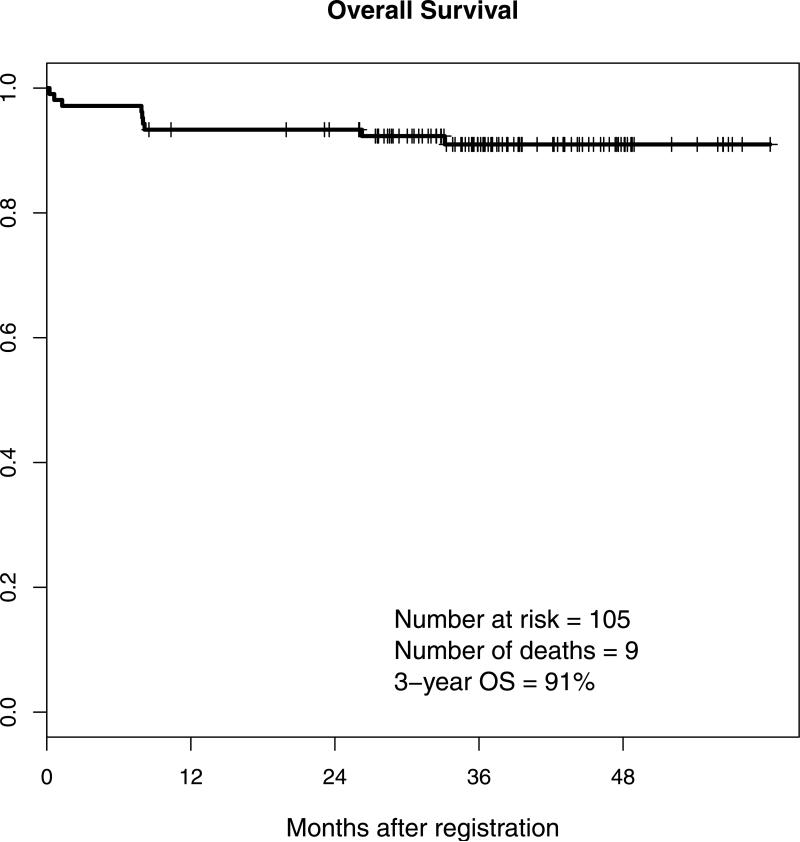
Two additional patients have died without evidence of relapse. With a median follow up of 3.1 years, a total of 9 of the 105 patients have died, to give a 3-year OS rate of 91% (Figure 3A; 95% CI 88%-98%). One patient relapsed from complete remission after completing consolidation therapy but before registration to maintenance. No other relapses have been reported.
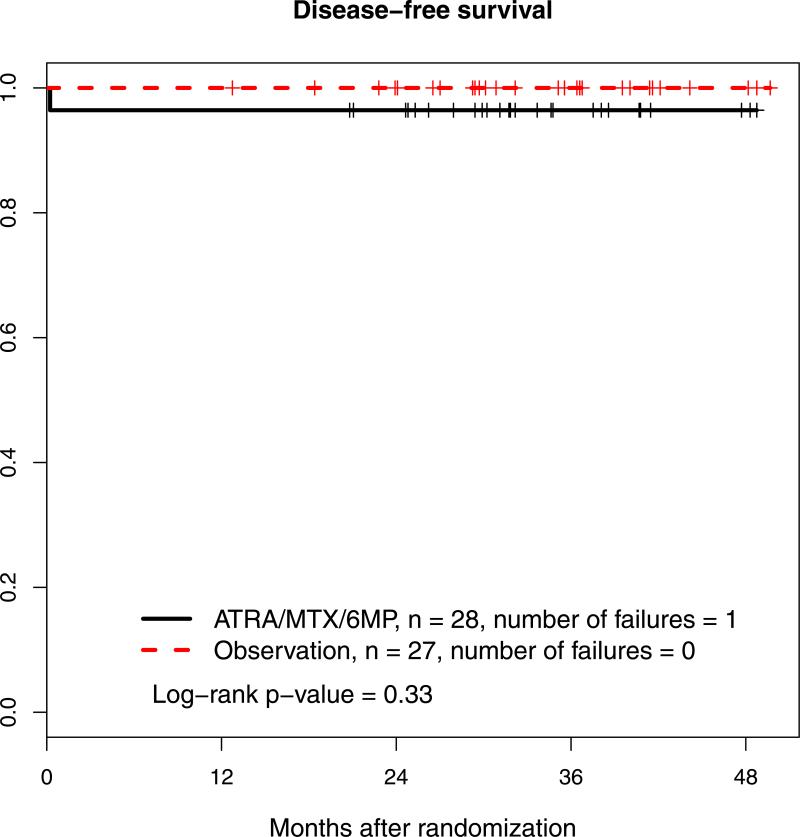
Figure 3. Overall survival and disease-free surviva.
A: Overall survival (OS)
B: Disease-free survival.
ATRA, all trans retinoic acid; MTX, methotrexate; 6-MP, 6-mercaptopurine
DFS by randomized post-consolidation treatment arm is shown in Figure 3B. With a median follow-up of 2.7 years, 1 patient in the maintenance therapy arm and no patient in the observation arm have died. The patient who died was still in remission and died of sepsis. Three-year DFS in the maintenance therapy arm was 96% (95% CI 90%-100%).
Only one patient was not in molecular remission after receiving consolidation and no patient received gemtuzumab ozogamicin as part of protocol treatment. Therefore, the predictive value of pre-treatment gene expression profiling could not be determined for post-consolidation minimal residual disease or gemtuzumab treatment. Similarly, there have been too few relapses to correlate with such profiles.
Discussion
The early results of this study confirm the excellent DFS and OS reported in the C9710 trial for APL patients in the low and intermediate risk groups (Powell et al 2010), and confirm the value of post-remission induction ATO using this treatment regimen. As in C9710, ATO was safe and well tolerated. Virtually all patients achieved a molecular complete response following consolidation, minimizing the need for extensive and sequential molecular testing after this point. The early death rate was also quite low, consistent with prior studies in low or intermediate risk groups.(de la Serna et al., 2008) Most importantly, survival with a median follow up of 3.1 years was 91% with no late relapses.
There have been no fatal toxicities in patients treated with maintenance therapy and only two patients have died (in continued remission). Only one patient (who was not randomized to maintenance or observation) has relapsed. There have been no relapses among the randomized patients. This observation argues that there is no need for APL disease monitoring once patients have completed effective consolidation therapy. As the study closed prior to enrolling the planned 400 patients, it will not be possible to answer the question conclusively whether maintenance therapy is required in this group of APL patients. However, the lack of late relapses with a median follow-up of 2.6 years together with the expense and toxicity associated with maintenance therapy argues against this treatment.
Although two earlier, randomized studies have reported benefit of ATRA-based maintenance therapy(Fenaux et al., 1999; Tallman et al., 1997), the recently reported long term follow up of the Gruppo Italiano Malattie Ematologiche dell‘Adulto (GIMEMA) AIDA (ATRA plus idarubicin) 0493 protocol did not demonstrate a benefit in 12-year DFS from any maintenance when compared to observation alone.(Avvisati et al., 2011) Further, the North American Intergroup trial C9710 compared ATRA alone to ATRA plus chemotherapy and found no advantage for the addition of chemotherapy.(Powell et al., 2011). There are several distinctions between the two earlier studies showing a need for maintenance and the more recent C9710 and GIMEMA AIDA trials. The GIMEMA study required PML-RARA PCR negativity post-consolidation, prior to maintenance randomization. In addition, the induction and consolidation therapies in the North American Intergroup C9710 and the GIMEMA AIDA trial were probably more effective than those used in the earlier studies where maintenance was beneficial. C9710 incorporated ATO in one consolidation arm of the trial and in that arm there were only seven (4%) relapses among the 196 patients who received the ATO; there were no relapses after 36 months. The GIMEMA trial did not use ATO, but did use idarubicin rather than daunorubicin as the anthracycline. This may have resulted in a better treatment effect with more effective eradication of minimal residual disease after remission was achieved. A similar lack of benefit from maintenance was reported in a Japan Adult Leukaemia Study Group protocol that also used idarubicin and required molecular negativity for PML-RARA post-consolidation.(Asou et al., 2007) Finally, a small study of 38 patients that did not include any maintenance therapy showed 5-year OS and leukaemia-free survival of 82% and 78%, respectively.(Gupta et al., 2005) Thus, at least four other studies suggest a lack of benefit for maintenance in APL. These results are entirely consistent with those reported here.
Toxicities are associated with maintenance therapy with either ATRA or chemotherapy, as seen in this study and others (Ades et al., 2010; Fenaux et al., 1999; Nagai et al., 2009). For example, in a series of trials reported by the European APL Group (Ades et al., 2010), 13% of patients who received ATRA, chemotherapy or both during maintenance stopped treatment after less than one year of the planned two year course. Fatal infections were observed in 2.5% of patients randomized to maintenance, and 2.5% of patients were hospitalized for febrile neutropenia, including three non-fatal cases of Pneumocystis carinii. Forty-five percent of the patients experienced either hepatic and/or haematological toxicity during maintenance, leading to dose reductions of their therapy. The omission of maintenance would obviously eliminate these side effects.
The cure of the great majority of patients with APL has been one of the true success stories in the treatment of adult acute myeloid leukaemia. As we try to refine treatment further by reducing or eliminating some therapy for certain groups of APL patients, it is becoming increasingly difficult to accrue adequate numbers of patients to non-inferiority trials, for which large numbers of patients are required to ensure that treatment reductions do not unacceptably impact outcomes. This is especially true in the lower risk groups where relapse is uncommon. Despite the lower-than-anticipated enrollment in S0521, the lack of relapses to date, along with existing data from other trials is enough to support the omission of maintenance therapy in low or intermediate risk APL patients, provided they receive intensive post-remission therapy with either idarubicin or ATO as used in the GIMEMA and C9710 studies, respectively.
Acknowledgments
Support: This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA46282, CA11083, CA41287, CA35431, CA27057, CA45807, CA20319, CA45808, CA76132, CA86780, CA58861, CA14028 (SWOG); CA31946 (CALGB); CA21115, CA17145 and CA16116(ECOG)
Footnotes
Author contributions
S.E.C., M.S.T., F.R.A., R.A.S., B.P., and J.K.A. conceived and designed the study; M.W., E.C.A., J.K.A., S.D.G., S.E.C., M.S.T., F.R.A., and B.P. provided patients; C.L.W., W.S., and E.P provided laboratory support; M.O., T.M., and K.J.K. performed the statistical analysis; S.E.C. wrote the manuscript; and all authors analysed and interpreted the data, critically revised the report for intellectual content, and provided final approval of the submitted version.
Disclosures of conflicts of interest
None
References
- Ades L, Guerci A, Raffoux E, Sanz M, Chevallier P, Lapusan S, Recher C, Thomas X, Rayon C, Castaigne S, Tournilhac O, de Botton S, Ifrah N, Cahn J, Solary E, Gardin C, Fegeux N, Bordessoule D, Ferrant A, Meyer-Monard S, Vey N, Dombret H, Degos L, Chevret S, Fenaux P, for the European APL Group Very long-term outcome of acute promyelocytic leukemia after treatment with all-trans retinoic acid and chemotherapy: the European APL Group experience. Blood. 2010;115:1690–1696. doi: 10.1182/blood-2009-07-233387. [DOI] [PubMed] [Google Scholar]
- Asou N, Kishimoto Y, Kiyoi H, Okada M, Kawai Y, Tsuzuki M, Horikawa K, Matsuda M, Shinagawa K, Kobayashi T, Ohtake S, Nishimura M, Takahashi M, Yagasaki F, Takeshita A, Kimura Y, Iwanaga M, Naoe T, Ohno R, for the Japan Adult Leukemia Study Group A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110:59–66. doi: 10.1182/blood-2006-08-043992. [DOI] [PubMed] [Google Scholar]
- Avvisati G, Lo-Coco F, Paoloni FP, Petti MC, Diverio D, Vignetti M, Latagliata R, Specchia G, Baccarani M, Di Bona E, Fioritoni G, Marmont F, Rambaldi A, Di Raimondo F, Grazia Kropp M, Pizzolo G, Pogliani E, Rossi G, Cantore N, Nobile F, Gabbas A, Ferrara F, Fazi P, Amadori S, Mandelli F, for the GIMEMA, AIEOP, and EORTC Cooperative Groups AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716–4725. doi: 10.1182/blood-2010-08-302950. [DOI] [PubMed] [Google Scholar]
- de la Serna J, Montesinos P, Vellenga E, Rayon C, Parody R, Leon A, Esteve J, Bergua JM, Milone G, Deben G, Rivas C, González M, Tormo M, Díaz-Mediavilla J, González JD, Negri S, Amutio E, Brunet S, Lowenberg B, Sanz MA. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–3402. doi: 10.1182/blood-2007-07-100669. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Chastang C, Chevret S, Sanz M, Dombret H, Archimbaud E, Fey M, Rayon C, Huguet F, Sotto JJ, Gardin C, Cony Makhoul P, Travade P, Solary E, Fegueux N, Bordessoule D, San Miguel J, Link H, Desablens B, Stamatoullas A, Deconinck E, Maloisel F, Castaigne S, Preudhomme C, Degos L, for the European APL Group A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- Gupta V, Yi QL, Brandwein J, Lipton JH, Messner HA, Schuh AC, Wells RA, Minden MD. Role of all-trans-retinoic acid (ATRA) in the consolidation therapy of acute promyelocytic leukaemia (APL). Leuk Res. 2005;29:113–114. doi: 10.1016/j.leukres.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Nagai S, Nannya Y, Hangaishi A, Takahashi T, Kurokawa M. The race and dose of chemotherapy should be considered for optimizing maintenance therapy for acute promyelocytic leukemia. Leuk Res. 2009;33:1427–1429. doi: 10.1016/j.leukres.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban S, Appelbaum FR, Tallman MS, Larson RA. Arsenic trioxide improves event-free and overall survival for adults with acute promyelocytic leukemia: North American Leukemia Intergroup Study C9710. Blood. 2010;116:3751–3757. doi: 10.1182/blood-2010-02-269621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BL, Moser B, Stock W, Gallagher RE, Willman CL, Stone RM, Rowe JM, Coutre S, Feusner JH, Gregory J, Couban S, Appelbaum FR, Tallman MS, Larson RA. Adding mercaptopurine and methotrexate to alternate week ATRA maintenance therapy does not improve the outcome for adults with acute promyelocytic leukemia (APL) in first remission: results from North American Leukemia Intergroup Trial C9710. Blood (ASH Annual Meeting Abstracts) 2011;118:258. [Google Scholar]
- R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2013. URL http://www.R-project.org/ [Google Scholar]
- Sanz MA, Lo-Coco F. Modern approaches to treating acute promyelocytic leukemia. J Clin Oncol. 2011;29:495–503. doi: 10.1200/JCO.2010.32.1067. [DOI] [PubMed] [Google Scholar]
- Sanz MA, Lo Coco F, Martin G, Avvisati G, Rayon C, Barbui T, Diaz-Mediavilla J, Fioritoni G, Gonzalez JD, Liso V, Esteve J, Ferrara F, Bolufer P, Bernasconi C, Gonzalez M, Rodeghiero F, Colomer D, Petti MC, Ribera JM, Mandelli F, for the Spanish PETHEMA and the Italian GIMEMA Cooperative Groups Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–1253. [PubMed] [Google Scholar]
- Sanz MA, Martin G, Gonzalez M, Leon A, Rayon C, Rivas C, Colomer D, Amutio E, Capote FJ, Milone GA, De La Serna J, Román J, Barragán E, Bergua J, Escoda L, Parody R, Negri S, Calasanz MJ, Bolufer P, Programa de Estudio y Traitmiento de las Hemopatías Malignas Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103:1237–1243. doi: 10.1182/blood-2003-07-2462. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Open Med. 2010;4:e60–68. [PMC free article] [PubMed] [Google Scholar]
- Shen ZX, Shi ZZ, Fang J, Gu BW, Li JM, Zhu YM, Shi JY, Zheng PZ, Yan H, Liu YF, Chen Y, Shen Y, Wu W, Tang W, Waxman S, De Thé H, Wang ZY, Chen SJ, Chen Z. All-trans retinoic acid/As2O3 combination yields a high quality remission and survival in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:5328–5335. doi: 10.1073/pnas.0400053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soignet SL, Frankel SR, Douer D, Tallman MS, Kantarjian H, Calleja E, Stone RM, Kalaycio M, Scheinberg DA, Steinherz P, Sievers EL, Coutre’ S, Dahlberg S, Ellison R, Warrell RP., Jr United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, Wiernik PH. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Chen Z. Acute promyelocytic leukemia: from highly fatal to highly curable. Blood. 2008;111:2505–2515. doi: 10.1182/blood-2007-07-102798. [DOI] [PubMed] [Google Scholar]