Abstract
PIKE-A (PIKE-activating Akt), an isoform of PIKE GTPase that enhances phosphatidylinositol 3-kinase (PI3-kinase) activity, specifically binds to active Akt but not PI3-kinase. PIKE-A stimulates Akt activity in a GTP-dependent manner and promotes invasiveness of cancer cell lines. Here, we show that PIKE-A is amplified in a variety of human cancers and that amplified PIKE-A directly stimulates Akt and inhibits apoptosis compared to cells with normal PIKE-A copy number. Overexpression of PIKE-A wild-type but not dominant-negative mutant stimulates Akt activity and prevents apoptosis. Moreover, knockdown of PIKE-A diminishes Akt activity and increases apoptosis. Our findings suggest that PIKE-A amplification contributes to cancer cell survival and progression by inhibiting apoptosis through up-regulating Akt.
PIKE-S (PI3-kinase enhancer, short isoform), initially identified as a 4.1-N binding partner in a yeast two-hybrid screening, is a brain-specific nuclear GTPase that binds phosphatidylinositol 3-kinase (PI3-kinase) and stimulates its lipid kinase activity (1). Nerve growth factor treatment leads to PIKE-S activation by triggering the nuclear translocation of PLC-γ1, which acts as a physiologic guanine nucleotide exchange factor for PIKE-S through its Src homology 3 (SH3) domain (2). PIKE-L, a longer isoform of PIKE gene, differs from PIKE-S by the addition of a 40-kDa C-terminal extension containing Arf-GAP and two ankyrin-repeat domains. In contrast to the exclusive nuclear localization of PIKE-S, PIKE-L occurs in both the nucleus and the cytoplasm. Recently, we show that PIKE-L physiologically associates with Homer 1, an mGluR I-binding adaptor protein. The Homer/PIKE-L complex couples PI3-kinase to mGluR I and mediates a major action of group I mGluRs, prevention of apoptosis (3).
More recently, we have identified a third PIKE isoform, PIKE-A, in our analysis of human glioblastoma multiforme brain cancers. PIKE-A has been identified in two other independent studies (4, 5). Unlike the brain-specific PIKE-L and -S isoforms, PIKE-A distributes in various tissues with higher levels of expression in brain, spleen, thymus, small intestine, and peripheral blood leukocytes (4–6). We have shown that PIKE-A is coamplified with CDK4 on chromosome 12 in a variety of human cancers including sarcoma, neuroblastoma,and glioblastoma (32). PIKE-A is readily detected in 12q-amplified cell lines including RMS13 rhabdomyosarcoma and OSA osteosarcoma but not in normal muscle (6). PIKE-A contains the GTPase, PH, ArfGAP,and two Ankyrin repeats domains present in PIKE-L but lacks the N-terminal proline-rich domain, which binds protein 4.1N, PI3-kinase and PLC-γ1. PIKE-A specifically binds to active Akt and up-regulates its activity in a GTP-dependent manner, mediating human cancer cell invasion (32).
Akt/PKB is a crucial regulator of divergent cellular processes including apoptosis, proliferation, differentiation, and metabolism. Constitutive Akt activation has been observed in cancer cells as the result of Akt amplification and mutations in protein components of PI3-kinase signaling cascade. Akt was originally identified as a retroviral oncogene product, v-Akt, that can transform rodent cells (7, 8). Akt isoforms have been shown overexpressed in ovarian, breast, and pancreatic cancers (9–11). Overexpression of constitutively activated Akt mutants in many cell types promotes cellular transformation by stimulating proliferation and inhibiting apoptosis (12).
Here, we report that human cancer cells with PIKE-A amplification strongly resists apoptosis compared to cancer cells with normal PIKE-A copy number. Overexpression of wild-type PIKE-A but not dominant-negative mutant stimulates Akt activity and inhibits apoptosis. Moreover, knockdown PIKE-A diminishes Akt activity and enhances apoptosis. Our findings indicate that PIKE-A amplification promotes cancer cell growth by inhibiting apoptosis through stimulating Akt.
Materials and Methods
Cells and Reagents. HEK293 cells and human glioblastoma SF188, SF767, TP366, LN229, LN-Z308, and U87MG cells were maintained in DMEM, supplemented with 10% FBS/2 mg/ml glutamine and 100 units of penicillin-streptomycin at 37°C with 5% CO2 atmosphere in a humidified incubator. Mouse monoclonal anti-hemagglutinin–horseradish peroxidase (HRP), anti-Myc-HRP, and anti-GST antibodies were from Sigma. Mouse monoclonal anti-Ser 473, anti-Thr 308, anti-Akt, and anti-phospho-GSK3α/β (ser21/9), anti-Lamin A/C antibodies were from Cell Signaling Technologies (Beverly, MA). Anti-PIKE antibody was raised against GST–PIKE-L (amino acids 1095–1186) protein. Protein A/G-conjugated agarose beads were from Calbiochem. Glutathione Sepharose 4B was supplied by Amersham Pharmacia. GST–GSK3 fusion protein was from Cell Signaling Technologies. All of the chemicals not included above were from Sigma.
Fluorescent in Situ Hybridization (FISH) Staining. Experimental procedures for FISH staining on human cancer cell lines are as described (13).
Coimmunoprecipitation and in Vitro Binding Assays. Experimental procedures for coimmunoprecipitation and in vitro binding assays are as described (1).
Cytochemical Staining of Apoptotic Cells. Morphological changes in the nuclear chromatin of cells undergoing apoptosis were detected by staining with 4,6-diamidino-2-phenylindole (DAPI) as described (14). In brief, 0.5 × 106 to 3 × 106 cells were fixed with 4% glutaraldehyde/0.2% Triton X-100 in PBS, incubated at room temperature for 10 min, then centrifuged at 1,000 × g for 10 min and resuspended in 20 μl of 0.1% DAPI in ethanol. After a 15-min incubation at room temperature, 10 μl of resuspended cells were placed on a glass slide, and 400 cells per slide were scored for the incidence of apoptotic chromatin changes with fluorescence microscope.
Penetratin 1-Conjugated Oligonucleotide Preparation. Sense (5′-CTG GGC AAC ATG CAT GCC CAG-3′) and antisense (5′-CTG GGC ATG CAT GTT GCC CAG-3′) oligonucleotides of PIKE-A containing a Src homology group at the 5′ end were conjugated to Penetratin 1 as described (15). The oligonucleotides were resuspended in deionized water, an equal molar ratio of Penetratin 1 (Oncor) was added, and the mixture was incubated at 37°C for 1 h. The yield of the reaction, estimated by SDS/PAGE followed by Coomassie blue staining, was >50%. The conjugated Penetratin 1 was added into serum-starved cells 6 h before treatment.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide (MTT) Cell Viability Assay. Serum-starved U87MG and LN-Z308 cells were pretreated with penetratine 1-conjugated sense and antisense oligonucleotides for 6 h, then treated with 250 nM staurosporine in a 96-well plate for various time points, and medium was replaced by 100 μl per well normal medium containing 0.5 mg/ml MTT (Sigma). Cells were incubated at 37°C for 4 h. Then the medium containing MTT was removed and 100 μl of lysis buffer (50% DMF, 20% SDS) per well was added. After incubated at 37°C for another 4 h, the plates were placed on microplate reader (Bio-Rad, model 450) to measure optical density at 595 nm with reference wavelength at 655 nm.
Results
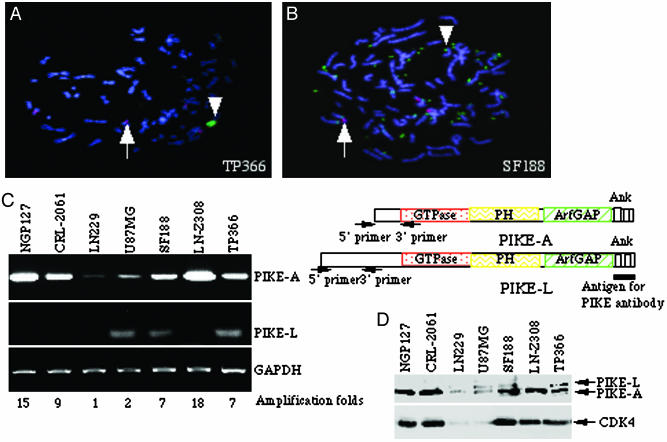
PIKE-A Amplification in Human Cancer Cells. Chromosome segment 12q13-q15 is altered in a variety of benign and malignant human tumors (16). We have shown previously that PIKE gene localizes in CDK4 amplicon at 12q13.3 and is amplified in human sarcoma cell line CRL-2061, neuroblastoma cell line NGP-127, and primary glioma cultures (32). Cytologically, amplified DNA may exist either extrachromosomally as double minutes or intrachromosomally as homogeneously staining region (6). To investigate PIKE gene amplification in glioblastoma cells, we performed fluorescent in situ hybridization using PIKE gene as a probe. PIKE is substantially amplified (arrowhead, green) in a non-chromosome-12 (red dot) region in TP366 cells (Fig. 1A), demonstrating that PIKE has also undergone rearrangement. In addition, PIKE is markedly amplified as double-minute chromatin bodies (arrowhead, green) in SF188 cell line (Fig. 1B). Thus, distinct genetic mechanisms appear to account for PIKE amplification in glioblastoma.
Fig. 1.
PIKE-A is amplified in human glioblastoma cells. (A and B) Fluorescent in situ hybridization staining of glioblastoma TP366 (A) and SF188 (B) cells. Red dot stains for the centromere of human chromosome 12, whereas the green stains for PIKE-A with the amplified PIKE (arrowhead) versus normal PIKE (arrow). Amplified PIKE-A translocates from chromosome 12 to another chromosome in TP366 cells (A). Amplified PIKE-A also forms double minute chromosomes in SF188 cells (B). (C) RT-PCR analysis of PIKE-A and -L mRNA expression in human cancer cell lines. PIKE-A mRNA is robustly expressed in NGP-127, CRL-2061, TP366, LN-Z308, and SF188 cells, but not in LN229 or U87MG cells (Top, left lane). By contrast, PIKE-L is weakly expressed in U87MG, SF188, and TP366 cells (left lane, Middle). Equal levels of GAPDH were monitored as control (Bottom, left lane). The relative gene amplification folds are labeled at the bottom. The primers for PIKE-A and -L used in RT-PCR analysis and the antigen used to raise anti-PIKE antibody are indicated on the right. (D) Western blotting analysis of PIKE-A protein expression. Cell lysate (50 μg) of a variety of human glioblastoma cell lines was used for immunoblotting analysis with rabbit polyclonal anti-PIKE and mouse monoclonal CDK4 antibodies.
To further examine amplification of PIKE in these cancer cell lines, we conducted RT-PCR using the 5′ sequence (amino acids 1–72) unique to PIKE-A isoform as primers (Fig. 1C Top, right lane). PIKE-A is robustly overexpressed in TP366, LN-Z308, and SF188, but not LN229 or U87MG glioblastoma cells, whereas NGP127 and CRL-2061 cells act as positive controls (Fig. 1C Top, left lane). RT-PCR analysis with specific primers (amino acids 120–225) for PIKE-L isoform reveals that modest PIKE-L is only selectively expressed in U87MG and SF188 cells (Fig. 1C Middle, left lane). Equal amount of GAPDH was amplified by PCR (Fig. 1C Bottom, left lane). Densitometrical analysis of RT-PCR bands upon normalizing GAPDH level reveals gene amplification folds for each cell line (Fig. 1C Bottom). Consistent with our observations in RT-PCR, Western blotting analysis with anti-PIKE antibody, raised against the C terminus of PIKE, demonstrates that PIKE-A are overexpressed in these cancer cells. Fitting with RT-PCR analysis, a faint amount of PIKE-L occurs in U87MG, TP366 and SF188 cells. The coamplified CDK4 is also selectively overexpressed in PIKE-A-enriched cells (Fig. 1D).
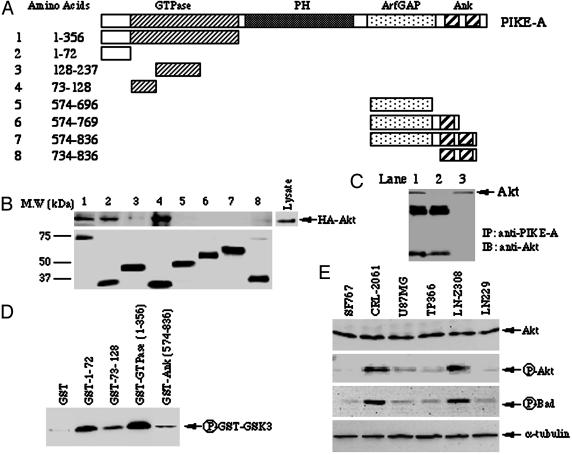
Akt Activity Correlates with PIKE-A Amplification. Our previous work demonstrated that interaction of PIKE-A with Akt requires the regulatory and partial catalytic domains from Akt and the N-terminal GTPase and C-terminal domains from PIKE-A (32). To better define the domain of PIKE-A required for Akt binding, we performed in vitro binding assay employing GST-recombinant proteins of various N- and C-terminal truncations of PIKE-A (Fig. 2A). Western blotting analysis reveals that Akt strongly associates with the N-terminal residues 1–72 and residues 73–128, a portion of GTPase domain. Akt interacted less strongly with residues 128–237 in the GTPase domain and residues 734–836 in C-terminal Ankyrin repeat domain (Fig. 2B Upper). Equal amount of GST-recombinant proteins were used (Fig. 2B Lower). To further examine the interaction of PIKE-A N terminus with native Akt in vivo, we conducted coimmunoprecipitation assay with anti-PIKE antibody from TP366 cell lysate in the presence of recombinant protein GST–PIKE-A-1–356 or GST alone. GST–PIKE-A-1–356 but not GST abolishes the interaction between endogenous PIKE-A and Akt (Fig. 2C), indicating that the N terminus of PIKE-A is required for binding to native Akt.
Fig. 2.
PIKE-A interacts with Akt via its N terminus. (A) Diagram of PIKE-A fragments. (B and C) The N terminus of PIKE-A is sufficient to bind to Akt. (B) In vitro binding assay: HEK293 cells were transfected with hemagglutinin (HA)-Akt. The transfected cells were serum-starved overnight, then stimulated with EGF for 5 min. Purified GST-PIKE-A recombinant proteins were conjugated to glutathione beads, and incubated 293 cell lysate at 4°C for 3 h. The glutathione bead-associated proteins were analyzed with anti-HA antibody (B Upper). GST-recombinant proteins were verified with anti-GST–HRP (B Lower). (C) In vivo coimmunoprecipitation. Cell lysate (1 mg) of TP366 cells was incubated with 25 μl of Protein A/G conjugated beads and 2 μg of rabbit polyclonal anti-PIKE antibody in the presence of GST–PIKE-A-1–356 (lane 2) or GST alone (lane 1). The coimmunoprecipitated proteins were analyzed with anti-Akt antibody. The input in lane 3 is whole cell lysate. Akt specifically binds to PIKE-A in the absence of its N-terminal fusion protein (lane 1). (D) PIKE-A stimulates Akt kinase activity. Purified recombinant GST–PIKE-A fragments incubated with in vitro kinase assay mixture containing purified Akt (1 μg) and GST–GSK3. GST alone was used as a negative control. The phosphorylation status was monitored by anti-phospho-GSK3-specific antibody. (E) Western blotting analysis of Akt, phospho-Akt, phospho-Bad, and α-tubulin in various cancer cells.
To assess whether these fragments from PIKE-A affect Akt kinase activity, we next conducted an in vitro kinase assay with purified Akt protein. GSK3 substrate is robustly phosphorylated in the presence of GST-GTPase, and marked phosphorylation is also observed with other the fragments from GTPase domain compared to the ankyrin repeat domain or GST alone, suggesting that PIKE-A interacting fragments from GTPase domain are sufficient to provoke Akt kinase activity, probably through stabilizing Akt in active conformation (Fig. 2D).
Akt is frequently constitutively active in many types of human cancers. Constitutive Akt activation can occur because of amplification of Akt genes or as a result of mutations in components of the signaling pathway that activates Akt (12). We have shown that PIKE-A specifically interacts with activated Akt (32). To examine whether PIKE-A amplification is accompanied with Akt activation, we monitored Akt phosphorylation and kinase activity in human glioblastoma cells. Western blotting analysis demonstrates that Akt is remarkably phosphorylated in some of the cells (Fig. 2 E Upper Middle), although approximately equal Akt levels are expressed in these cells (Fig. 2E Top). These observations are further confirmed by monitoring the phosphorylation of Bad, a physiological Akt substrate implicated in apoptosis (Fig. 2E Lower Middle). An equal amount of α-tubulin was verified as loading control (Fig. 2E Bottom).
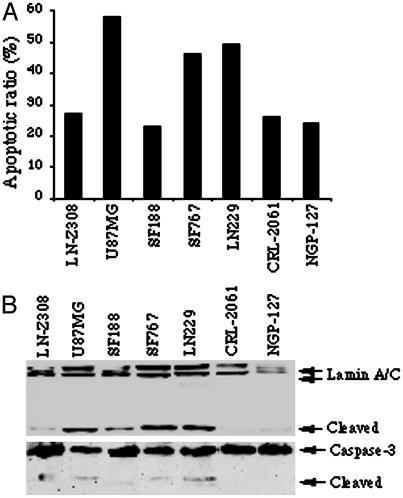
PIKE-A Amplification Is Accompanied by Increased Resistance to Apoptosis. Akt promotes cell survival by inhibiting proteins that mediate apoptosis. To investigate whether PIKE-A amplification in cancer cells leads to reduced apoptosis, we treated cells with the apoptosis-inducing drug Staurosporine (250 nM) for 16 h, and stained the cells with DAPI to assess chromatin condensation and fragmentation. Apoptotic activity is lower in PIKE-A amplified cells compared to cancer cells with normal copy number (Fig. 3A). Lamin A/C are nuclear membrane proteins. During apoptosis, they are cleaved into 40- to 45- and 28-kDa fragments by caspase, resulting in nuclear disregulation and cell death, and serve as a marker for apoptosis (17, 18). In agreement with the observations in DAPI staining, Western blotting analysis with antibody specific for the N terminus reveals that Lamin A/C is substantially cleaved in cells with normal copy of PIKE-A but not in amplified cells (Fig. 3B Upper). Consistently, the activation of Caspase-3 couples with Lamin A/C cleavage (Fig. 3B Lower). Thus, overexpression of PIKE-A through gene amplification appears to impart glioblastoma cells with resistance to apoptosis.
Fig. 3.
Cancer cells with PIKE-A amplification resist to apoptosis. (A) Apoptotic assay. Various human cancers were treated with 250 nM staurosporine for 16 h, and followed by chromatin condensation and fragmentation analysis with DAPI staining. PIKE-A amplified cells display lower apoptotic activity compared to cells with normal copy. (B) Western blotting analysis of Lamin A/C and Caspase-3 cleavage during apoptosis.
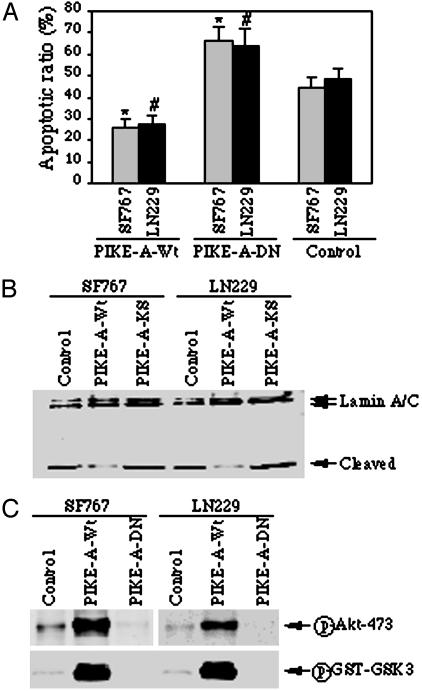
PIKE-A Overexpression Protects Cells from Apoptosis Through Up-Regulating Akt. To determine whether PIKE-A overexpression is sufficient to inhibit apoptosis, we infected SF763 and LN229 cells that lack endogenous PIKE-A amplification with control adenovirus and adenovirus overexpressing wild-type PIKE-A and dominant-negative PIKE-A, termed PIKE-A-DN. PIKE-A-DN with two amino acid substitutions (lysine-84 to alanine and serine-85 to asparagine) binds Akt and inhibits its activation (32). After 24-h infection, the infected cells were treated with Staurosporine for another 16 h and stained with DAPI. Wild-type PIKE-A remarkably inhibits apoptosis, whereas PIKE-A-DN overexpression increases apoptosis in these glioblastoma cells compared to the control (Fig. 4A). Analogous results are observed when Lamin A/C cleavage is used as a marker for apoptosis (Fig. 4B). Similar results also occur in PIKE-A amplified glioblastoma cell lines (data not shown). To examine whether PIKE-A inhibited apoptosis is through activating Akt, we monitored Akt phosphorylation and kinase activity. Compared to control cells, robust Akt phosphorylation is observed in wild-type PIKE-A infected cells. In contrast, Akt phosphorylation is completely inhibited in PIKE-A-DN infected cells (Fig. 4C). Moreover, an in vitro kinase assay with Akt immunocomplex displays that Akt activity is greatly enhanced after wild-type PIKE-A infection and completely abolished after PIKE-A-DN infection. Thus, PIKE-A overexpression appears to increase resistance to apoptosis by up-regulating Akt.
Fig. 4.
Overexpression of PIKE-A prevents apoptosis by stimulating Akt. (A) Apoptotic assay. Glioblastoma cells (SF767 and LN229) were infected with control adenovirus or adenovirus expressing wild-type PIKE-A or dominant-negative PIKE-A-DN, and treated with staurosporine for 16 h, and followed by chromatin condensation and fragmentation analysis with DAPI staining. Wild-type PIKE-A markedly prevents apoptosis compared to control cells (*, P < 0.005, Student's t test). Conversely, dominant-negative PIKE-A-DN increases the sensitivity of the infected cells to apoptosis (#, P < 0.01, Student's t test). (B) Western blotting analysis of Lamin A/C cleavage during apoptosis. Cell lysate (50 μg) from adenovirus-infected cells was separated by SDS/10% PAGE and analyzed by Western blotting with anti-lamin A/C antibody. Wild-type PIKE-A noticeably prevents lamin A/C cleavage compared to control cells. In contrast, dominant-negative PIKE-A-DN enhances lamin A/C cleavage. (C) PIKE-A stimulates Akt activity in glioblastoma cells. SF767 and LN229 cells were infected with adenovirus expressing PIKE-A-WT and PIKE-A-DN, respectively; after 24-h infection, the cell lysate was analyzed with phospho-serine-473 antibodies. Robust phosphorylation on S473 of Akt was observed in wild-type PIKE-A but not control adenovirus-infected cells. By contrast, the phosphorylation was completely inhibited by dominant-negative PIKE-A-DN (Upper). The in vitro Akt kinase assay was performed with the immunoprecipitated endogenous Akt, and GST–GSK3 was used as a substrate. GSK3 phosphorylation was analyzed by Western blotting with anti-phospho-GSK3-specific antibody (Lower).
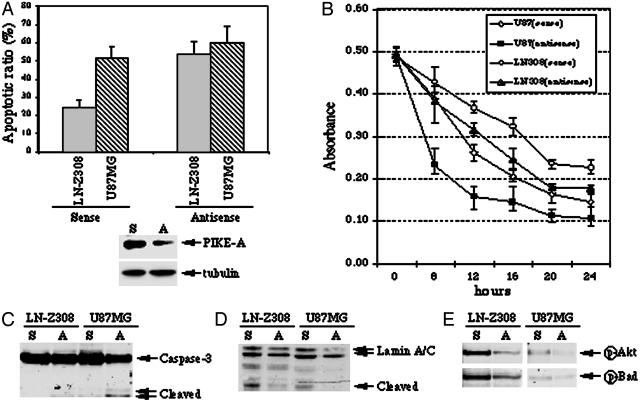
PIKE-A Knockdown Increases Apoptosis by Inhibiting Akt. To test further the idea that PIKE-A promotes cell survival through activating Akt, we chose cells with normal copy of PIKE-A (U87MG) and PIKE-A amplification (LN-Z308), and knocked down PIKE-A expression by using Penetratin 1-conjugated antisense oligonucleotide, an antennapedia peptide-mediated intracellular delivery technique that has been widely and successfully used for such purposes (15). Cells were incubated with Penetratin 1-conjugated sense or antisense oligonucleotide for 6 h, and treated with Staurosporine for 16 h. Chromatin condensation and fragmentation were analyzed with DAPI staining. Under the sense oligonucleotide control condition, ≈20% LN-Z308 and 50% U87MG cells are apoptotic, indicating that PIKE-A amplification protects cells from apoptosis. Antisense oligonucleotide treatment increases apoptosis ≈15% in U87MG cells and 50% in LN-Z308 cells compared to the sense oligonucleotide control (Fig. 5A Upper). PIKE-A expression level is selectively decreased >50% under these conditions, whereas control α-tubulin expression is not altered (Fig. 5A Lower). Similar results were observed in other cancer cells (data not shown). To further assess the effect of PIKE-A knockdown on cell viability, we conducted MTT-based colorimetric assay, a technique dependent on cleavage of the tetrazolium salt MTT by enzymes of the endoplasmic reticulum. Compared to the sense oligonucleotide control, knockdown PIKE-A by antisense oligonucleotide substantially decreases both U87MG and LN-Z308 cell viability upon staurosporine treatment. In agreement with DAPI staining analysis, MTT cell viability assay reveals U87MG cells are more sensitive than LN-Z308 cells in response to staurosporine treatment, underscoring that PIKE-A amplification protects cell from apoptosis (Fig. 5B). Consistent with these observations, caspase-3 and lamine A/C cleavage is noticeably stimulated under antisense condition compared to sense control (Fig. 5 C and D). These results show a further direct correlation between PIKE-A levels and apoptosis.
Fig. 5.
PIKE-A knockdown increase apoptosis by inactivating Akt. (A) Apoptotic assay. Serum-starved cells were treated with Penetratin 1-conjugated antisense and sense oligonucleotides of PIKE-A. After 6 h, the cells were treated with staurosporine for 16 h, and followed by chromatin condensation and fragmentation analysis with DAPI staining (Upper). PIKE-A expression is selectively decreased by antisense but not control sense oligonucleotide. By contrast, tubulin expression level is not changed (Lower). (B) MTT cell viability assay. Serum-starved cells were treated with Penetratin 1-conjugated antisense and sense oligonucleotides of PIKE-A. After 6 h, the cells were treated with staurosporine for various time points, and followed by MTT assay. (C and D) Western blotting analysis of Caspase-3 and Lamin A/C cleavage in sense and antisense oligonucleotide treated cells during apoptosis. (E) PIKE-A knockdown inhibits Akt activity. Western blotting analysis of Akt and Bad phosphorylation in sense and antisense oligonucleotide-treated cells. Consistent with PIKE-A knockdown, Akt phosphorylation is diminished in antisense but not sense oligonucleotide treated cells (Upper). The phosphorylation of Akt physiological substrate, Bad, in sense and antisense oligonucleotide-treated cells (Lower).
To explore whether decreased apoptosis as the result of PIKE-A knockdown is associated with reduced Akt activity, we conducted Western blotting analysis and in vitro kinase assay to monitor Akt activation. Akt phosphorylation is abrogated in both LN-Z308 and U87MG cells exposed to antisense oligonucleotide (Fig. 5E Upper). The phosphorylaton of BAD, is markedly diminished in both LN-Z308 and U87MG cells with PIKE-A knockdown (Fig. 5E Lower). It is known that phosphorylation of BAD by Akt enables it to interact with 14-3-3 protein and prevents it from binding to Bcl-XL, and thereby suppresses apoptosis (19). Therefore, these findings suggest that PIKE-A knockdown increases apoptosis by inhibiting Akt.
Discussion
Our experiments have demonstrated that PIKE-A is amplified via multiple genetic mechanisms in human glioblastoma cells. PIKE-A amplification up-regulates Akt activation and inhibits apoptosis. Amplification is a common mechanism to increase gene dosage and overexpression in cancer. Amplification of cellular oncogenes in tumor cell lines and primary tumor often provides tumor cells a selective growth advantage in vitro and in vivo (6). In our study, PIKE-A amplification correlated with constitutive Akt activation and prevented glioblastoma cells from apoptosis. We have provided consistent data showing that PIKE-A stimulates Akt activity through direct interaction with Akt, leading to resistance to apoptosis. Dominant-negative and knockdown PIKE-A reduced Akt activity and increased apoptosis, further supporting this model.
After the activation of PI3-kinase, Akt is recruited from the cytoplasm to the plasma membrane through the interaction with PI(3,4,5)P3 and/or PI(3,4)P2. PIP3 levels are tightly regulated by the action of phosphatases such as PTEN (phosphatase and tensin homolog deleted on chromosome 10; also called MMAC1 or TEP1), a tumor suppressor, which removes phosphate from the 3-OH position. Membrane translocated Akt undergoes a conformational change and becomes activated by phosphorylation of two residues, Thr-308 and Ser-473, a prerequisite for the activation of Akt (20). Akt is active in PIKE-A-amplified glioblastoma cells, but we also observe Akt activation in the cells without PIKE-A amplification. This could be due to the mutations in the machinery of PI3-kinase/Akt signaling cascade. For instance, Akt is phosphorylated in U87MG cells, although it does not amplify PIKE-A. Interestingly, it has been shown that U87MG lacks functional PTEN, an antagonizer of PI3-kinase, and this leads to hyperactivation of Akt (21), fitting with the previous report that in the absence of PTEN function, cells exhibit elevated Akt activity (22). Even though Akt is constitutively active in U87MG cell, but it lacks resistance to apoptosis compared to PIKE-A amplified cells (Fig. 3). Presumably, the indispensable antiapoptotic signaling pathways distinct from those mediated by Akt are abrogated in U87MG cells. AMP-activated protein kinase (AMPK) has been shown to activate Akt, and active Akt could use negative feedback to regulate AMPK (23, 24). It remains unclear whether other kinases including protein kinase A and AMPK could act as possible upstream activators of Akt in these glioblastoma cells.
Akt promotes cell survival by inhibiting proteins that mediate apoptosis and up-regulating proteins that inhibit apoptosis. For example, Akt phosphorylates the proapoptotic Bcl-2 family member BAD (25, 26), caspase 9 (27), and apoptosis signal-regulating kinase 1 (Ask1) (28), and inhibits their proapoptotic functions. In addition to its effects on the cytoplasmic apoptotic machinery, Akt both negatively regulates Forkhead family of transcription factors that promote the expression of death genes (29–31) and positively regulates cAMP-response element-binding protein transcription factors that induce survival genes (32). Our data demonstrate that PIKE-A promotes cell survival through up-regulating Akt (Figs. 4 and 5). This finding is consistent with our recent discovery that PIKE-L mediates the antiapoptotic action of mGluR I in hippocampal neurons through binding to Homer (3).
Because Akt promotes cell survival, specific inhibition of its activity is an attractive therapeutic strategy for cancer therapy. However, Akt also plays an essential role in various cellular processes under physiological conditions, unselective inhibition of Akt will block its normal functions and cause severe side effects (33). Up-regulation of Akt by PIKE-A provides a mechanism whereby growth factors (5) and other cellular stimuli regulate Akt. Like PIKE-A, both PIKE-L and -S isoforms contain Akt-interacting GTPase domain; thus, they could also bind and stimulate Akt. It is possible that these two isoforms are implicated in other human brain disorders. Our experiments demonstrate the N terminus of PIKE-A in this process and pharmacologic interventions that disrupt PIKE-A/Akt interaction might promote apoptosis with less toxicity than those that directly target Akt. Further investigation of this potential mechanism of glioblastoma chemotherapy seems to be warranted. An inhibitor disrupting PIKE/Akt interaction may be useful in combination therapy with other anticancer agents.
Acknowledgments
This work was supported by American Cancer Society Grant RSG-04-077-01-TBE and the Distinguished Scientist Award from The Sontag Foundation (to K.Y.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PI3-K, phosphatidylinositol 3-kinase; DAPI, 4,6-diamidino-2-phenylindole; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide.
References
- 1.Ye, K., Hurt, K. J., Wu, F. Y., Fang, M., Luo, H. R., Hong, J. J., Blackshaw, S., Ferris, C. D. & Snyder, S. H. (2000) Cell 103, 919-930. [DOI] [PubMed] [Google Scholar]
- 2.Ye, K., Aghdasi, B., Luo, H. R., Moriarity, J. L., Wu, F. Y., Hong, J. J., Hurt, K. J., Bae, S. S., Suh, P. G. & Snyder, S. H. (2002) Nature 415, 541-544. [DOI] [PubMed] [Google Scholar]
- 3.Rong, R., Ahn, J. Y., Huang, H., Nagata, E., Kalman, D., Kapp, J. A., Tu, J., Worley, P. F., Snyder, S. H. & Ye, K. (2003) Nat. Neurosci. 6, 1153-1161. [DOI] [PubMed] [Google Scholar]
- 4.Nagase, T., Seki, N., Ishikawa, K., Tanaka, A. & Nomura, N. (1996) DNA Res. 3, 17-24. [DOI] [PubMed] [Google Scholar]
- 5.Xia, C., Ma, W., Stafford, L. J., Liu, C., Gong, L., Martin, J. F. & Liu, M. (2003) Mol. Cell. Biol. 23, 2476-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkahloun, A. G., Krizman, D. B., Wang, Z., Hofmann, T. A., Roe, B. & Meltzer, P. S. (1997) Genomics 42, 295-301. [DOI] [PubMed] [Google Scholar]
- 7.Bellacosa, A., Testa, J. R., Staal, S. P. & Tsichlis, P. N. (1991) Science 254, 274-277. [DOI] [PubMed] [Google Scholar]
- 8.Staal, S. P. (1987) Proc. Natl. Acad. Sci. USA 84, 5034-5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, J. Q., Godwin, A. K., Bellacosa, A., Taguchi, T., Franke, T. F., Hamilton, T. C., Tsichlis, P. N. & Testa, J. R. (1992) Proc. Natl. Acad. Sci. USA 89, 9267-9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng, J. Q., Ruggeri, B., Klein, W. M., Sonoda, G., Altomare, D. A., Watson, D. K. & Testa, J. R. (1996) Proc. Natl. Acad. Sci. USA 93, 3636-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatani, K., Thompson, D. A., Barthel, A., Sakaue, H., Liu, W., Weigel, R. J. & Roth, R. A. (1999) J. Biol. Chem. 274, 21528-21532. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson, K. M. & Anderson, N. G. (2002) Cell Signalling 14, 381-395. [DOI] [PubMed] [Google Scholar]
- 13.Kroll, T. G. (2002) Am. J. Pathol. 160, 1941-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye, K., Ke, Y., Keshava, N., Shanks, J., Kapp, J. A., Tekmal, R. R., Petros, J. & Joshi, H. C. (1998) Proc. Natl. Acad. Sci. USA 95, 1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Troy, C. M. & Shelanski, M. L. (1994) Proc. Natl. Acad. Sci. USA 91, 6384-6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reifenberger, G., Reifenberger, J., Ichimura, K., Meltzer, P. S. & Collins, V. P. (1994) Cancer Res. 54, 4299-4303. [PubMed] [Google Scholar]
- 17.Oberhammer, F. A., Hochegger, K., Froschl, G., Tiefenbacher, R. & Pavelka, M. (1994) J. Cell Biol. 126, 827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao, L., Perez, D. & White, E. (1996) J. Cell Biol. 135, 1441-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downward, J. (1999) Nat. Cell Biol. 1, E33-E35. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor, M. A. & Alessi, D. R. (2001) J. Cell Sci. 114, 2903-2910. [DOI] [PubMed] [Google Scholar]
- 21.Maier, D., Jones, G., Li, X., Schonthal, A. H., Gratzl, O., Van Meir, E. G. & Merlo, A. (1999) Cancer Res. 59, 5479-5482. [PubMed] [Google Scholar]
- 22.Stambolic, V., Suzuki, A., de la Pompa, J. L., Brothers, G. M., Mirtsos, C., Sasaki, T., Ruland, J., Penninger, J. M., Siderovski, D. P. & Mak, T. W. (1998) Cell 95, 29-39. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., Funahashi, T. & Walsh, K. (2004) J. Biol. Chem. 279, 1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovacic, S., Soltys, C. L., Barr, A. J., Shiojima, I., Walsh, K. & Dyck, J. R. (2003) J. Biol. Chem. 278, 39422-39427. [DOI] [PubMed] [Google Scholar]
- 25.Datta, S. R., Dudek, H., Tao, X., Masters, S., Fu, H., Gotoh, Y. & Greenberg, M. E. (1997) Cell 91, 231-241. [DOI] [PubMed] [Google Scholar]
- 26.del Peso, L., Gonzalez-Garcia, M., Page, C., Herrera, R. & Nunez, G. (1997) Science 278, 687-689. [DOI] [PubMed] [Google Scholar]
- 27.Cardone, M. H., Roy, N., Stennicke, H. R., Salvesen, G. S., Franke, T. F., Stanbridge, E., Frisch, S. & Reed, J. C. (1998) Science 282, 1318-1321. [DOI] [PubMed] [Google Scholar]
- 28.Kim, A. H., Khursigara, G., Sun, X., Franke, T. F. & Chao, M. V. (2001) Mol. Cell. Biol. 21, 893-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biggs, W. H., III, Meisenhelder, J., Hunter, T., Cavenee, W. K. & Arden, K. C. (1999) Proc. Natl. Acad. Sci. USA 96, 7421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brunet, A., Bonni, A., Zigmond, M. J., Lin, M. Z., Juo, P., Hu, L. S., Anderson, M. J., Arden, K. C., Blenis, J. & Greenberg, M. E. (1999) Cell 96, 857-868. [DOI] [PubMed] [Google Scholar]
- 31.Kops, G. J., de Ruiter, N. D., De Vries-Smits, A. M., Powell, D. R., Bos, J. L. & Burgering, B. M. (1999) Nature 398, 630-634. [DOI] [PubMed] [Google Scholar]
- 32.Du, K. & Montminy, M. (1998) J. Biol. Chem. 273, 32377-32379. [DOI] [PubMed] [Google Scholar]
- 33.Hill, M. M. & Hemmings, B. A. (2002) Pharmacol. Ther. 93, 243-251. [DOI] [PubMed] [Google Scholar]