Abstract
Objective:
This study compared the dosimetry of volumetric-arc therapy (VMAT) and intensity-modulated radiotherapy (IMRT) with a dynamic multileaf collimator using the Monte Carlo algorithm in the treatment of prostate cancer with and without simultaneous integrated boost (SIB) at different energy levels.
Methods:
The data of 15 biopsy-proven prostate cancer patients were evaluated. The prescribed dose was 78 Gy to the planning target volume (PTV78) including the prostate and seminal vesicles and 86 Gy (PTV86) in 39 fractions to the intraprostatic lesion, which was delineated by MRI or MR-spectroscopy.
Results:
PTV dose homogeneity was better for IMRT than VMAT at all energy levels for both PTV78 and PTV86. Lower rectum doses (V30–V50) were significantly higher with SIB compared with PTV78 plans in both IMRT and VMAT plans at all energy levels. The bladder doses at high dose level (V60–V80) were significantly higher in IMRT plans with SIB at all energy levels compared with PTV78 plans, but no significant difference was observed in VMAT plans. VMAT plans resulted in a significant decrease in the mean monitor units (MUs) for 6, 10, and 15 MV energy levels both in plans with and those without SIB.
Conclusion:
Dose escalation to intraprostatic lesions with 86 Gy is safe without causing serious increase in organs at risk (OARs) doses. VMAT is advantageous in sparing OARs and requiring less MU than IMRT.
Advances in knowledge:
VMAT with SIB to intraprostatic lesion is a feasible method in treating prostate cancer. Additionally, no dosimetric advantage of higher energy is observed.
Randomized trials have shown a gain in biochemical relapse-free survival using dose escalation for prostate cancer.1 However, isolated local failure is still reported in nearly one-third of patients, even with higher radiotherapy (RT) doses.1 Local recurrence is of clinical importance because a relationship has been suggested between local control, distant metastasis and survival.2 It has also been demonstrated that intraprostatic failure mainly originates at the initial tumour location as a result of intrinsic resistance of a fraction of the tumour clones, which implies that selective dose escalation to the dominant intraprostatic lesion using simultaneous integrated boost (SIB) might be beneficial.3
With new RT techniques, such as intensity-modulated RT (IMRT) and volumetric-arc therapy (VMAT), SIB could be delivered without increasing acute toxicity.4–7 Several recent studies have performed dosimetric comparison of IMRT and VMAT plans in prostate cancer;8–10 however, dosimetric evaluation of IMRT and VMAT plans delivering SIB is rare. In these studies, target volume and organs at risk (OARs) doses may vary with different treatment planning systems. Another aspect not often addressed in these planning studies is the photon energy level.4,8,9,11 Although higher energy photons have the potential advantage of reduced attenuation with depth, this may in turn increase the risk of secondary malignancies because of the presence of neutrons generated in the accelerator head at treatment energies >8 MV.12
Functional imaging techniques can clearly demonstrate tumour within the prostate. MRI, MR spectroscopy (MRS) and positron emission tomography are capable of demonstrating intraprostatic lesions (IPLs).13 The advent of combined MRI with MRS or dynamic contrast enhanced (DCE)-MRI improves the detection rate of tumours within the prostate.13–15
The aim of the present study was to make dosimetric comparisons of VMAT and 7-field IMRT with dynamic multileaf collimators (MLCs) using the Monte Carlo algorithm with XVMC code in the treatment of prostate cancer with or without SIB, which can provide improved dose calculation accuracy and has been implemented successfully in the clinical setting.16,17 Additionally, the impact of three photon energies on target volumes, OARs and normal tissue was evaluated in IMRT and VMAT plans.
METHODS AND MATERIALS
The CT and MRI/MRS data of 15 consecutive intermediate risk prostate cancer patients were selected for the present study. The inclusion criterion was the presence of an MRI or MRS detected IPL, which was defined as an MRI- and/or MRS-detected prostate tumour with characteristics suggesting a high probability of malignancy according to the criteria of Cruz et al18 for MRI and Villeirs et al13 for MRS.
CT and MRI
All patients had undergone 2.5-mm slice thickness CT with a comfortably full bladder and empty rectum.19 MRI scans were acquired with the same conditions as CT. Because of the negative effects of androgen deprivation on the metabolism of prostate cancer cells, MRS examinations were performed in the absence of or before hormone therapy.20 The MRI scans used for image fusion and treatment planning were acquired on a 1.5 T Siemens Avanto® MRI System (Siemens Healthcare, Erlangen, Germany). T2 weighted (T2W) diffusion weighted images and DCE-MRI examinations were performed using an eight-element phased array coil during the scans without an endorectal coil. T1, T2, MRS, apparent diffusion coefficient (ADC) and DCE images of the prostate were reviewed by an experienced radiologist (GE). The IPLs identified on T2, ADC, DCE images or MRS were used for SIB planning.21 The CT and MRI data were digitally transferred to an Eclipse™ (Varian Medical Systems, Palo Alto, CA) workstation and coregistered to delineate the regions of interest. The CT and MRI fusion was done by automated computerized fusion and then checked manually, as described in other IPL boost studies.14,21,22
Clinical target volume (CTV) included the prostate and the entire seminal vesicles. The planning target volume for 78 Gy (PTV78) was defined as CTV with a margin of 5 mm posterior and 8 mm in other directions.19,23 The delineation of IPL was done together with a radiologist (Figure 1). The PTV for 86 Gy (PTV86) was created using a three-dimensional, isotropic, 4-mm margin around the IPL.6 The OARs included the rectum, sigmoid, bladder and femoral heads. The rectum was delineated from the anal verge to the recto-sigmoid junction.24 The femoral heads were contoured to the level of ischial tuberosities.
Figure 1.
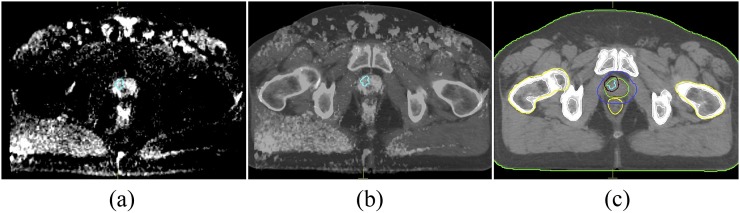
Representative image demonstrating intraprostatic lesion (a) in diffusion weighted MR scan and (b) coregistered MR and CT scans. (c) PTV86 and PTV78 are generated with given margins to intraprostatic lesion and prostate.
Treatment plans
The treatment plans were generated using IMRT and VMAT techniques. The IMRT plans consisted of seven coplanar fields, at gantry angles of 0°, 37°, 75°, 135°, 225°, 285° and 327°. The plans were calculated with Monaco treatment planning system (CMS; Elekta, Crawley, UK) using the Monte Carlo algorithm and a sliding window MLC delivery technique. The VMAT plans consisted of a single 360° arc. Gantry speed, MLC leaf position and dose rate varied continuously during VMAT delivery.25 For each patient, three different plans with 6, 10, and 15 MV energies were generated for both IMRT and VMAT techniques. Additionally, the same plans were made with SIB. All plans were created for delivery on an Elekta linear accelerator (Elekta) equipped with an MLC and designed for dynamic IMRT and VMAT. The leaf width of the Elekta accelerator used in the present study was 0.4 cm, and the leaves did not interdigitate.
Dose prescription
Two plans were generated and each plan was normalized to deliver 99% of CTV and 95% of PTV78 and PTV86 receiving at least 78 and 86 Gy, respectively. All treatments were planned to be delivered in 39 fractions. Dose constraints for the rectum and bladder were based on Radiation Therapy Oncology Group recommendations,26 where V50 and V70 for the rectum were 50% and 20% and V55 and V70 for the bladder were 50% and 30%, respectively. Normal tissue complication probability values for rectum and bladder were <10% and ≤5%, respectively.27,28 The femoral heads were limited to receive a maximum of 50 Gy.
Plan evaluation
We evaluated the treatment plans by comparing the planning results with the planning and physical indices (Table 1). D2 and D98 were used as surrogates for maximum and minimum doses for target volumes, respectively. Target dose homogeneity index (TDI) was calculated as: TDI = [(D2 – D98)/D50], where D50 is the minimal dose to 50% of target volume. Additionally, the heterogeneity index (HI) was defined as HI = D1/D95, where D1 and D95 are minimal dose to 1% and 95% of target volume, respectively. For the rectum, D2cc was defined as the minimum dose value in the 2-cc volume receiving the highest dose. To quantify the dose to normal tissues, relative volumes of the 50% isodose (V50%) was determined.
Table 1.
Dosimetric analysis of intensity-modulated radiotherapy (IMRT) and volumetric-arc therapy (VMAT) plans with different energy levels
| Parameters | 6 MV |
10 MV |
15 MV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IMRT | VMAT | p-value | IMRT | VMAT | p-value | IMRT | VMAT | p-value | |
| CTV | |||||||||
| D2 (Gy) | 81.860 ± 0.600 | 82.220 ± 0.900 | 0.020 | 81.470 ± 0.610 | 82.150 ± 1.000 | 0.001 | 81.520 ± 0.570 | 81.960 ± 0.900 | 0.030 |
| D98 (Gy) | 78.540 ± 0.370 | 78.580 ± 0.830 | 0.890 | 78.470 ± 0.290 | 78.540 ± 0.800 | 0.760 | 78.630 ± 0.310 | 78.550 ± 0.840 | 0.680 |
| D99 (Gy) | 78.320 ± 0.380 | 78.270 ± 0.900 | 0.840 | 78.230 ± 0.350 | 78.280 ± 0.870 | 0.860 | 78.390 ± 0.380 | 78.240 ± 0.940 | 0.490 |
| TDI (%) | 0.041 ± 0.006 | 0.045 ± 0.012 | 0.230 | 0.0370 ± 0.007 | 0.045 ± 0.013 | 0.020 | 0.036 ± 0.008 | 0.043 ± 0.013 | 0.050 |
| Dmean (Gy) | 80.170 ± 0.530 | 80.290 ± 0.610 | 0.500 | 79.990 ± 0.470 | 80.230 ± 0.610 | 0.190 | 80.190 ± 0.390 | 80.190 ± 0.700 | 0.670 |
| PTV (78 Gy) | |||||||||
| D2 (Gy) | 82.420 ± 0.730 | 82.890 ± 0.590 | 0.030 | 82.300 ± 0.610 | 82.920 ± 0.660 | 0.010 | 82.360 ± 0.750 | 82.770 ± 0.650 | 0.060 |
| D98 (Gy) | 77.320 ± 0.280 | 77.330 ± 0.290 | 0.920 | 77.230 ± 0.170 | 77.260 ± 0.240 | 0.740 | 77.130 ± 0.290 | 77.290 ± 0.260 | 0.770 |
| D99 (Gy) | 76.770 ± 0.310 | 76.770 ± 0.400 | 0.980 | 76.640 ± 0.260 | 76.710 ± 0.300 | 0.450 | 76.550 ± 0.300 | 76.680 ± 0.310 | 0.340 |
| Dmean (Gy) | 80.080 ± 0.350 | 80.400 ± 0.420 | 0.006 | 80.020 ± 0.390 | 80.410 ± 0.480 | 0.008 | 80.090 ± 0.350 | 80.35 ± 0.500 | 0.080 |
| TDI | 0.061 ± 0.008 | 0.045 ± 0.012 | <0.001 | 0.063 ± 0.009 | 0.045 ± 0.013 | <0.001 | 0.065 ± 0.012 | 0.043 ± 0.013 | <0.001 |
| HI | 1.040 ± 0.007 | 1.046 ± 0.013 | 0.080 | 1.038 ± 0.008 | 1.046 ± 0.014 | 0.060 | 1.037 ± 0.009 | 1.044 ± 0.014 | 0.070 |
| MU | 624.000 ± 70.000 | 573.000 ± 54.000 | 0.002 | 598.000 ± 73.000 | 554.000 ± 58.000 | 0.005 | 587.000 ± 66.000 | 544.000 ± 61.000 | 0.005 |
| PTV (86 Gy) | |||||||||
| D2 (Gy) | 87.980 ± 0.790 | 88.160 ± 0.900 | 0.140 | 87.860 ± 0.680 | 88.460 ± 0.660 | 0.130 | 87.940 ± 0.800 | 88.020 ± 0.590 | 0.500 |
| D98 (Gy) | 78.050 ± 0.700 | 77.740 ± 0.740 | 0.100 | 77.880 ± 0.660 | 77.610 ± 0.630 | 0.140 | 77.760 ± 0.760 | 77.590 ± 0.610 | 0.400 |
| D99 (Gy) | 77.410 ± 0.700 | 77.010 ± 0.680 | 0.030 | 77.240 ± 0.680 | 76.840 ± 0.600 | 0.040 | 77.060 ± 0.790 | 76.790 ± 0.610 | 0.240 |
| Dmean (Gy) | 82.290 ± 0.930 | 82.580 ± 1.230 | 0.180 | 82.240 ± 0.980 | 82.640 ± 1.000 | 0.070 | 82.160 ± 0.880 | 82.590 ± 1.010 | 0.030 |
| TDI | 0.046 ± 0.008 | 0.048 ± 0.011 | 0.001 | 0.044 ± 0.009 | 0.045 ± 0.011 | 0.030 | 0.041 ± 0.010 | 0.043 ± 0.011 | 0.010 |
| HI | 1.048 ± 0.009 | 1.049 ± 0.011 | 0.001 | 1.046 ± 0.010 | 1.047 ± 0.012 | 0.030 | 1.042 ± 0.011 | 1.044 ± 0.011 | 0.010 |
| MU | 604.000 ± 64.000 | 549.000 ± 47.000 | <0.001 | 554.000 ± 58.000 | 515.000 ± 53.000 | 0.003 | 551.000 ± 63.000 | 516.000 ± 47.000 | 0.020 |
CI, conformity index; CTV, clinical target volume; Dn, minimal dose to n% of target volume; HI, homogeneity index; MU, monitor unit; PTV, planning target volume; TDI, target dose homogeneity index.
Dose verification
Dose verification for the treatment plan was performed using a two-dimensional (2D) ion chamber array detector (IMRT MatriXX™; IBA Dosimetry, GmbH, Germany) that consists of 1020 ion chambers. For dose verification, the MatriXX was inserted into a MULTICube Plastic Water® phantom (CIRS, Norfolk, VA). All IMRT and VMAT plans were recalculated on the MatriXX phantom for each energy level, which was previously scanned and defined as a quality assurance phantom on the planning system. Dose map of the plan at effective 2D array depth was transferred to the dosimetry system. Dose verification used for calculation of 2D γ index, percentage of the γ < 1 averaged 96.87 ± 1.61 using 3% dose 3-mm distance criteria.
Statistical analysis
Statistical analysis was performed using SPSS® software v. 17.0 (SPSS Inc., Chicago, IL). The Wilcoxon's matched-pairs test was used to determine statistical differences between volumes and doses in IMRT vs VMAT plans. The dose–volume parameters of target volumes and OARs for each energy level were also measured and compared across energy levels. The comparison was also made for 78 and 86 Gy plans. The Mann–Whitney U-test was used to compare volumes or dose values in independent patient groups. All p-values reported are two-sided and a p < 0.05 was considered significant.
RESULTS
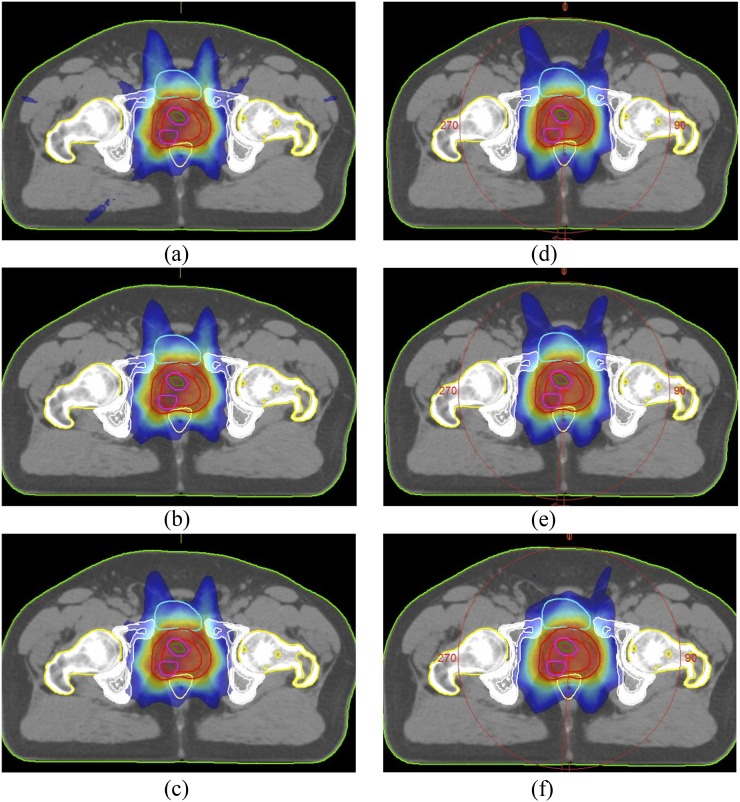
The mean CTV and PTV78 volumes were 34.2 cm3 (range, 19.8–74.6 cm3) and 148.3 cm3 (range, 98.6–225.8 cm3), respectively. The mean IPL and PTV86 volumes were 1.4 cm3 (range, 0.4–5.3 cm3) and 6.6 cm3 (range, 3.3–12.2 cm3), respectively. Figure 2 shows representative axial sections depicting dose distributions for IMRT and VMAT plans with different energy levels.
Figure 2.
Representative axial CT slices showing 50% of prescribed dose distributions for (a) 6 MV, (b) 10 MV, (c)15 MV energy intensity-modulated radiotherapy (IMRT) plans and (d) 6 MV, (e) 10 MV, (f) 15 MV energy volumetric-arc therapy (VMAT) plans.
Target volume doses
The dosimetric parameters for target volumes are summarised in Table 1. The average maximum doses for CTV and PTV78 (D2) were significantly higher in VMAT plans than IMRT plans for all energy levels; however, minimum doses (D98) did not differ significantly. No significant difference was found for D2 and D98 values between IMRT and VMAT plans at all energy levels for PTV86. The mean dose of PTV86 was significantly higher in VMAT plans than in IMRT plans at 15 MV, while the difference was close to the level of significance at 6 and 10 MV.
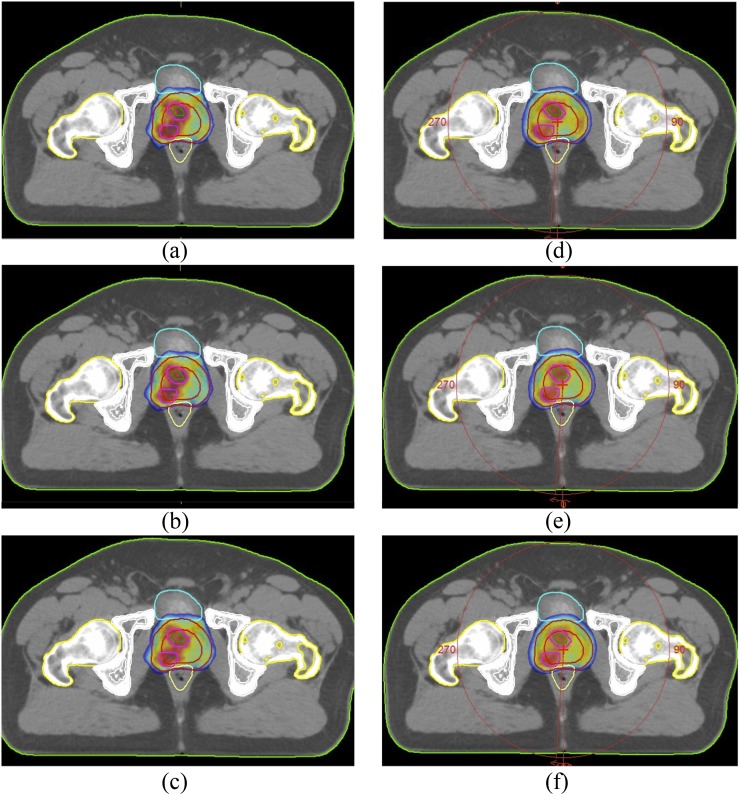
PTV dose homogeneity was better in IMRT plans than in VMAT plans at all energy levels for both PTV78 and PTV86. For PTV78, the HI had a borderline significance, whereas the HIs of PTV86 were significantly better in IMRT plans than in VMAT plans at all energy levels (Figure 3). No significant difference was observed in normal tissue doses between IMRT and VMAT plans. SIB technique did not cause a significant increase in normal tissue doses both with IMRT and VMAT techniques.
Figure 3.
Representative axial CT slices showing 95% of prescribed dose distributions for (a) 6 MV, (b) 10 MV, (c) 15 MV energy intensity-modulated RT (IMRT) plans and (d) 6 MV, (e) 10 MV, (f) 15 MV energy volumetric-arc therapy (VMAT) plans.
Organ at risk doses
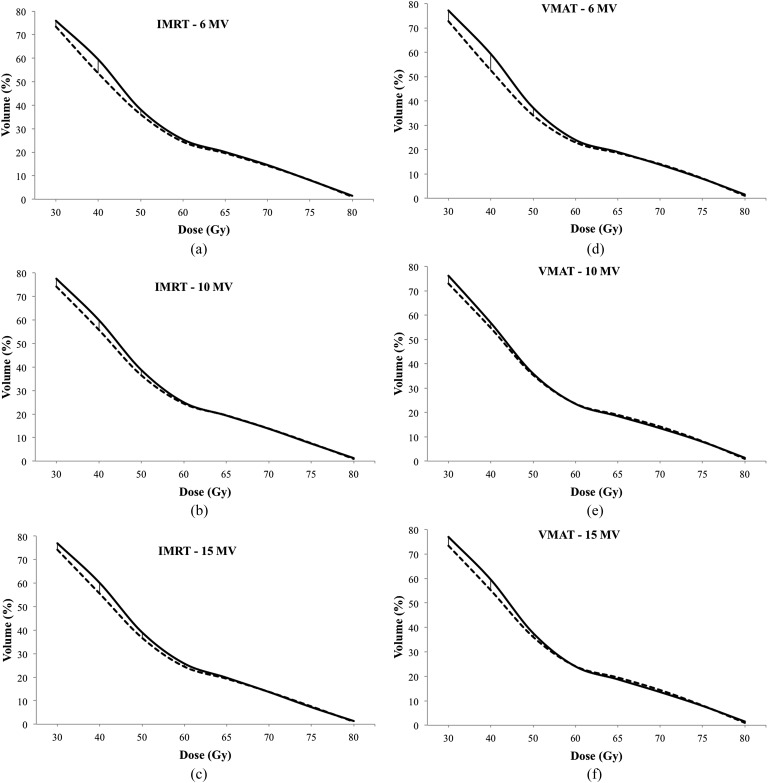
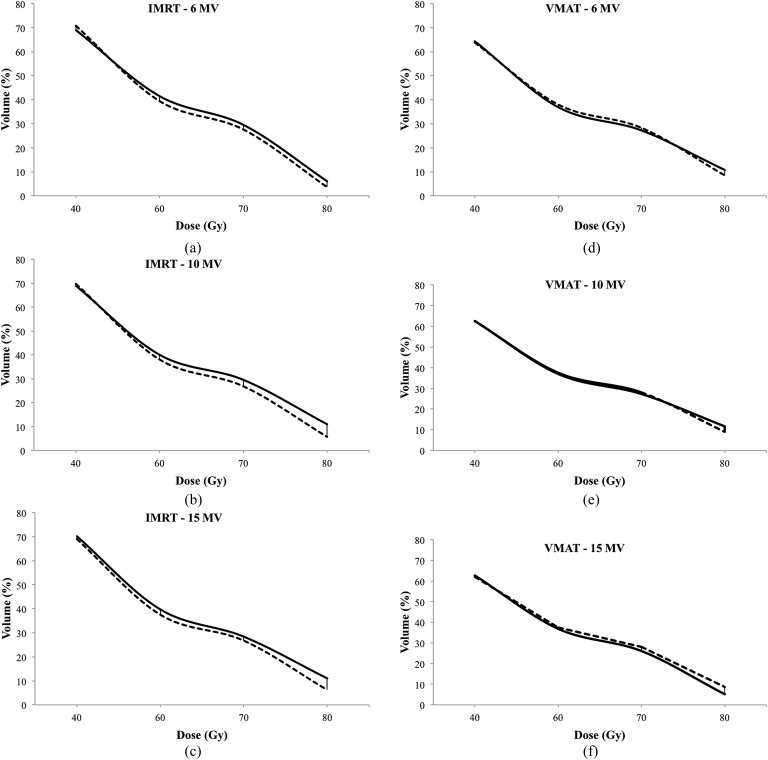
A comparison of the dosimetric parameters of OARs for each of the plan types is listed in Table 2. Compared with IMRT, VMAT plans achieved lower doses for all OARs for nearly all dosimetric end points. Only femur doses were higher in VMAT plans. Although lower rectum doses (V30–V50) were significantly higher in SIB plans than PTV78 plans in both IMRT and VMAT plans at all energy levels; there were no significant differences at high dose levels (Figure 4). The bladder doses at high dose levels (V60–V80) were significantly higher in IMRT plans with SIB at all energy levels compared with PTV78 plans (Figure 5). However, there was no significant difference in bladder doses between SIB plans and PTV78 plans with VMAT at all energy levels.
Table 2.
Dosimetric comparison of organs at risk doses of intensity-modulated radiotherapy (IMRT) and volumetric-modulated arc therapy (VMAT) plans with different energy levels for simultaneous integrated boost plan
| Organs at risk | 6 MV |
10 MV |
15 MV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| IMRT | VMAT | p-value | IMRT | VMAT | p-value | IMRT | VMAT | p-value | |
| Rectum | |||||||||
| V30 (%) | 75.92 ± 8.05 | 77.10 ± 8.34 | 0.470 | 77.52 ± 6.64 | 76.28 ± 9.99 | 0.580 | 77.25 ± 8.79 | 76.98 ± 9.79 | 0.910 |
| V40 (%) | 60.75 ± 9.96 | 59.27 ± 11.03 | 0.450 | 60.61 ± 8.87 | 56.78 ± 12.09 | 0.200 | 62.05 ± 11.65 | 59.50 ± 13.00 | 0.260 |
| V50 (%) | 38.86 ± 7.85 | 37.19 ± 8.65 | 0.150 | 38.73 ± 7.55 | 35.96 ± 8.98 | 0.090 | 40.40 ± 11.14 | 37.65 ± 10.13 | 0.130 |
| V60 (%) | 25.44 ± 7.29 | 23.96 ± 7.36 | 0.040 | 24.98 ± 6.59 | 23.52 ± 6.90 | 0.040 | 25.76 ± 8.13 | 24.03 ± 7.18 | 0.070 |
| V65 (%) | 20.09 ± 6.09 | 19.00 ± 5.97 | 0.050 | 19.39 ± 5.37 | 18.48 ± 5.97 | 0.060 | 19.75 ± 6.02 | 18.75 ± 6.02 | 0.060 |
| V70 (%) | 14.56 ± 5.08 | 13.78 ± 4.85 | 0.020 | 13.76 ± 4.57 | 13.50 ± 4.64 | 0.210 | 13.68 ± 4.64 | 13.55 ± 4.88 | 0.500 |
| V75 (%) | 8.51 ± 3.24 | 8.02 ± 2.74 | 0.110 | 7.85 ± 2.85 | 7.94 ± 3.18 | 0.670 | 7.57 ± 2.84 | 7.91 ± 3.21 | 0.430 |
| V80 (%) | 1.75 ± 0.34 | 1.53 ± 0.33 | 0.340 | 1.56 ± 0.30 | 1.77 ± 0.42 | 0.430 | 1.48 ± 0.25 | 1.77 ± 0.40 | 0.300 |
| D2cc (Gy) | 78.08 ± 1.98 | 77.74 ± 2.14 | 0.090 | 77.79 ± 2.17 | 77.62 ± 2.60 | 0.570 | 77.59 ± 2.45 | 77.87 ± 2.47 | 0.260 |
| Dmean (Gy) | 44.70 ± 3.46 | 44.46 ± 3.66 | 0.640 | 44.79 ± 2.60 | 43.89 ± 4.09 | 0.240 | 44.83 ± 3.72 | 44.55 ± 4.45 | 0.680 |
| Bladder | |||||||||
| V40 (%) | 69.03 ± 19.81 | 64.43 ± 22.92 | 0.100 | 68.94 ± 18.71 | 62.63 ± 23.51 | 0.030 | 70.19 ± 19.57 | 62.68 ± 22.31 | 0.007 |
| V60 (%) | 41.50 ± 17.93 | 36.90 ± 17.16 | 0.002 | 40.03 ± 17.20 | 36.95 ± 18.23 | 0.040 | 39.76 ± 16.73 | 36.85 ± 16.80 | 0.030 |
| V70 (%) | 29.56 ± 13.30 | 27.23 ± 13.48 | 0.020 | 28.44 ± 12.64 | 27.28 ± 14.24 | 0.270 | 12.44 ± 3.21 | 13.07 ± 3.37 | 0.120 |
| V80 (%) | 12.74±6.08 | 10.77 ± 4.71 | 0.090 | 10.99 ± 5.17 | 11.67 ± 6.34 | 0.580 | 4.87 ± 1.26 | 5.16 ± 1.33 | 0.910 |
| Dmean (Gy) | 51.16 ± 12.12 | 48.66 ± 11.21 | 0.001 | 50.60 ± 11.72 | 48.40 ± 11.90 | 0.004 | 50.48 ± 11.37 | 48.47 ± 10.99 | 0.020 |
| Sigmoid colon | |||||||||
| D50 (Gy) | 8.56 ± 6.73 | 8.66 ± 6.03 | 0.830 | 8.53 ± 7.04 | 9.21 ± 7.30 | 0.250 | 8.83 ± 7.81 | 9.59 ± 7.99 | 0.260 |
| Dmax (Gy) | 28.10 ± 26.16 | 32.75 ± 29.12 | 0.290 | 29.52 ± 26.57 | 34.58 ± 29.64 | 0.250 | 30.99 ± 26.66 | 35.35 ± 29.591 | 0.320 |
| Left femur | |||||||||
| D50 (Gy) | 10.25 ± 4.48 | 18.38 ± 4.32 | <0.001 | 11.04 ± 4.00 | 18.90 ± 3.32 | <0.001 | 11.04 ± 4.00 | 18.90 ± 3.32 | <0.001 |
| Dmax (Gy) | 39.84 ± 4.08 | 38.94 ± 6.07 | 0.460 | 40.01 ± 4.51 | 39.60 ± 5.56 | 0.710 | 40.73 ± 3.96 | 40.21 ± 5.89 | 0.630 |
| Right femur | |||||||||
| D50 (Gy) | 12.88 ± 6.35 | 17.65 ± 4.19 | 0.010 | 12.75 ± 5.41 | 18.46 ± 3.08 | 0.006 | 13.44 ± 5.37 | 18.46 ± 3.08 | 0.006 |
| Dmax (Gy) | 41.17 ± 3.74 | 38.72 ± 5.91 | 0.130 | 40.38 ± 4.20 | 39.34 ± 5.59 | 0.490 | 41.09 ± 4.05 | 38.99 ± 5.50 | 0.190 |
| Normal tissue | |||||||||
| V%50 (cc) | 2.88 ± 0.74 | 2.06 ± 0.65 | 0.001 | 2.46 ± 0.60 | 1.91 ± 0.63 | 0.001 | 2.36 ± 0.58 | 1.88 ± 0.61 | 0.001 |
Dn, minimal dose to n% of target volume; Vn, percentage organ volume receiving ≥n Gy.
Figure 4.
Rectum doses at different energy levels (a–c) in intensity-modulated RT (IMRT) plans and (d–f) volumetric-arc therapy (VMAT) plans. Solid lines represent plans with simultaneous integrated boost (SIB, PTV86). Dashed lines represent plans without SIB.
Figure 5.
Bladder doses at different energy levels (a–c) in intensity-modulated RT (IMRT) plans and (d–f) volumetric-arc therapy (VMAT) plans. Solid lines represent plans with simultaneous integrated boost (SIB, PTV86). Dashed lines represent plans without SIB.
Compared with IMRT plans, VMAT plans achieved 8.1%, 7.4% and 7.3% relative decreases in the mean number of monitor units (MUs) required for RT delivery in PTV78 plans at 6, 10 and 15 MV energy levels, respectively. VMAT plans with SIB achieved 9.1%, 7.0% and 6.3% decrease in the mean number of MUs at 6, 10 and 15 MV energy levels, respectively.
Dose verification
Dose verification used for calculation of 2D γ index, percentage of the γ <1 using 3% dose and 3-mm distance criteria averaged 97.86 ± 0.98 at 6 MV, 97.42 ± 1.11 at 10 MV and 96.83 ± 1.35 at 15 MV for SIB-IMRT plans. For SIB-VMAT plans, it was 97.89 ± 0.93, 97.73 ± 0.92 and 96.81 ± 0.91 at 6, 10, and 15 MV plans, respectively.
DISCUSSION
This study evaluated the dose distribution of target volumes, and OARs were assessed with two different RT techniques at three energy levels. Moreover, the SIB technique, where higher radiation doses were delivered to the IPL, did not cause any serious increment in OAR doses except for low-dose regions in the rectum and high-dose regions in the bladder. The most prominent advantage of VMAT is a significant decrease in treatment MUs, which means less radiation and shorter treatment time.
Dose escalation for prostate cancer causes improved biochemical control and reduced distant metastasis.1 However, local failure still occurs in one-third of patients after 78 Gy external RT (ERT).1 The original IPL is the most frequent location of relapse.3 Therefore, selectively boosting radiation to these lesions to a very high dose has been hypothesized to be a more effective method to improve the therapeutic ratio than a homogeneous, but more modest, dose escalation to the entire prostate.29
The accuracy of MRI and MRS in localizing prostate cancer was 82–85%, and the authors emphasized the importance of incorporating functional imaging techniques such as MRS for prostate cancer RT planning.30,31 Although MRI with endorectal coil has higher sensitivity for staging, this coil can distort the prostate.32 Therefore, appropriate image fusion was only possible in MRI and CT acquired using the same conditions. In this study, we preferred to use MRI or MRS in detecting the IPL for treatment planning. To obtain adequate images, MRI scans were acquired with the same conditions as CT. Additionally, one experienced radiologist who was aware of detailed histopathological findings contoured the tumour in coregistered CT and MRI images on an Eclipse work station.
Techniques such as VMAT and IMRT are able to generate conformal isodoses, which significantly reduce the OAR doses and normal tissue toxicity. There are numerous dosimetric studies comparing plans with and without SIB. Early results are encouraging, with patients who were treated even up to 94.5 Gy with acceptable early toxicity rates. In these studies, although the delivery techniques for IMRT and VMAT were similar, the results were conflicting. These heterogeneous results may be due to different target definitions and dose constraints. De Meerleer et al14 compared IMRT plans with 74 Gy prescribed to PTV with or without a simultaneous dose of 80 Gy to the gross tumour volume with no margin detected by MRI and found that the rectum dose levels (V70 and Dmax) were increased with SIB plans. Similarly, Pinkawa et al6 demonstrated improved dose escalation to the macroscopic tumour with minor rectum and bladder dose changes, similar to our findings. In this study, we escalated the IPL dose to 86 Gy with 78 Gy delivered to prostate and seminal vesicles. For estimated α/β ratios of 1.5 and 3.0, IPL dose corresponds to a total dose of 89 and 90 Gy, respectively, in conventional fractionation according to the linear-quadratic model.
There are few clinical studies assessing acute toxicity in patients treated with dose escalated SIB plans.5,29 Fonteyne et al5 did not find increased severity or incidence of acute toxicity in a group of 118 prostate cancer patients after dose escalation with SIB to an MRI/MRS-detected IPL (76 Gy median dose to PTV and 80 Gy median dose to gross tumour volume prescribed). In another study by Geier et al,29 acute side effects and dose–volume histogram data were evaluated in 40 intermediate risk prostate cancer patients treated with a definitive daily image-guided SIB-IMRT protocol via helical tomotherapy. The PTV was treated with 70 Gy in 35 fractions. The boost volume containing the prostate and 3-mm safety margins (5 mm craniocaudal) was treated as SIB to a total dose of 76 Gy (2.17 Gy per fraction) without increasing acute toxicity. In this study, we first evaluated the treatment plans dosimetrically. However, the acute toxicity with this escalated dose is a question for an ongoing study.
The techniques of IMRT and VMAT, planning strategies, optimization algorithm and beam angles affect the dosimetric outcome. With different dose calculation algorithms used in these studies, 3–4% deviations in target volume and OAR doses were seen.5 The Monte Carlo algorithm can improve dose calculation accuracy, which has been implemented successfully in clinical settings. Lafond et al33 recently compared the Monaco TPS and Pinnacle™ TPS (Philips Medical Systems, Madison, WI) for VMAT plans, and found that the bladder wall, femoral heads and healthy tissue doses were significantly lower in Monaco plans. For PTV doses, Monaco plans had higher conformity than Pinnacle plans. In this study, similar to previous studies, we compared the dose distributions of IMRT and VMAT plans using Monaco 3.2 TPS (CMS; Elekta, Crawley, UK). For normal tissues surrounding the target volume, VMAT is superior to IMRT, as was in our study.23
The photon energy may contribute to dose distribution. Recently, Ost et al4 compared IMRT and VMAT plans at 6 and 18 MV for primary prostate RT with SIB and showed no advantage of high-energy photons over low-energy photons. Pasler et al8 compared IMRT and VMAT plans with 6, 10 and 15 MV photon beams for prostate cancer involving pelvic lymph nodes and did not show advantages of 15 MV over 6 and 10 MV photon beams in large volume pelvic plans. In this study, we additionally investigated the effect of photon energy levels over dose distributions of IMRT and VMAT plans for only prostate and seminal vesicle, with or without SIB, which was not investigated before. We found no advantage of higher energy levels over lower energy levels both for target volume and OARs doses. Furthermore, we found no difference in low-dose irradiated volume in normal tissue surrounding the target with 6, 10 or 15 MV photons. However, concerning neutron generation for photon energies >8 MV, it was demonstrated that the neutron component is not negligible at 15 MV.34 Therefore, IMRT or VMAT delivery with low-energy photons may be adequate, since high-energy photons did not result in a significant benefit in terms of target coverage and OAR doses. Additionally, VMAT spares normal tissue surrounding the target volume better than IMRT; thus, rather than IMRT plans with higher energy, VMAT plans with lower energy may be a safe treatment strategy.
This study is not without limitations. First, with its retrospective nature, we only compared the dosimetric parameters of IMRT and VMAT plans in a limited number of patients. To make definitive conclusions, a large number of patients with different planning algorithms are essential. Additionally, the efficacy and toxicity of SIB plans with longer follow-up is required for making clinical decisions. Second, we prefer only single-arc plans in VMAT plans, rather than two or more arcs. Another study addressing the dose distributions of single and double arc VMAT plans with Monaco TPS could be considered.
CONCLUSION
Our study showed that dose escalation to the IPL is a safe technique without any serious increase in OAR doses. Additionally, although IMRT plans resulted in more homogenous dose distribution within the target volumes than VMAT plans, VMAT is better in sparing the rectum and bladder compared with IMRT plans. VMAT also had the added advantage of reducing the MUs required for treatment compared with seven-field IMRT. Moreover, high-energy photons had no advantage over low-energy photons. However, additional studies are needed to evaluate whether SIB plans result in improved biochemical control without increased toxicity and whether long-term follow-up is required to determine the potential effects of dose escalated SIB on local control and survival.
REFERENCES
- 1.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ Jr, Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. J Am Med Assoc 2005; 294: 1233–39. doi: 10.1001/jama.294.10.1233 [DOI] [PubMed] [Google Scholar]
- 2.Morgan PB, Hanlon AL, Horwitz EM, Buyyounouski MK, Uzzo RG, Pollack A. Radiation dose and late failures in prostate cancer. Int J Radiat Oncol Biol Phys 2007; 67: 1074–81. doi: 10.1016/j.ijrobp.2006.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cellini N, Morganti AG, Mattiucci GC, Valentini V, Leone M, Luzi S, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: implications for conformal therapy planning. Int J Radiat Oncol Biol Phys 2002; 53: 595–99. [DOI] [PubMed] [Google Scholar]
- 4.Ost P, Speleers B, De Meerleer G, De Neve W, Fonteyne V, Villeirs G, et al. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 MV: a planning comparison study. Int J Radiat Oncol Biol Phys 2011; 79: 920–26. [DOI] [PubMed] [Google Scholar]
- 5.Fonteyne V, Villeirs G, Speleers B, De Neve W, De Wagter C, Lumen N, et al. Intensity-modulated radiotherapy as primary therapy for prostate cancer: report on acute toxicity after dose escalation with simultaneous integrated boost to intraprostatic lesion. Int J Radiat Oncol Biol Phys 2008; 72: 799–807. [DOI] [PubMed] [Google Scholar]
- 6.Pinkawa M, Attieh C, Piroth MD, Holy R, Nussen S, Klotz J, et al. Dose-escalation using intensity-modulated radiotherapy for prostate cancer—evaluation of the dose distribution with and without 18F-choline PET-CT detected simultaneous integrated boost. Radiother Oncol 2009; 93: 213–19. [DOI] [PubMed] [Google Scholar]
- 7.Ishii K, Ogino R, Okada W, Nakahara R, Kawamorita R, Nakajima T. A dosimetric comparison of RapidArc and IMRT with hypofractionated simultaneous integrated boost to the prostate for treatment of prostate cancer. Br J Radiol 2013; 86: 20130199 doi: 10.1259/bjr.20130199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasler M, Georg D, Wirtz H, Lutterbach J. Effect of photon-beam energy on VMAT and IMRT treatment plan quality and dosimetric accuracy for advanced prostate cancer. Strahlenther Onkol 2011; 187: 792–98. doi: 10.1007/s00066-011-1150-0 [DOI] [PubMed] [Google Scholar]
- 9.Wolff D, Stieler F, Welzel G, Lorenz F, Abo-Madyan Y, Mai S, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiother Oncol 2009; 93: 226–33. doi: 10.1016/j.radonc.2009.08.011 [DOI] [PubMed] [Google Scholar]
- 10.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2008; 72: 996–1001. [DOI] [PubMed] [Google Scholar]
- 11.Hussein M, Aldridge S, Guerrero Urbano T, Nisbet A. The effect of 6 and 15 MV on intensity-modulated radiation therapy prostate cancer treatment: plan evaluation, tumour control probability and normal tissue complication probability analysis, and the theoretical risk of secondary induced malignancies. Br J Radiol 2012; 85: 423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider U, Lomax A, Pemler P, Besserer J, Ross D, Lombriser N, et al. The impact of IMRT and proton radiotherapy on secondary cancer incidence. Strahlenther Onkol 2006; 182: 647–52. doi: 10.1007/s00066-006-1534-8 [DOI] [PubMed] [Google Scholar]
- 13.Villeirs GM, De Meerleer GO, De Visschere PJ, Fonteyne VH, Verbaeys AC, Oosterlinck W. Combined magnetic resonance imaging and spectroscopy in the assessment of high grade prostate carcinoma in patients with elevated PSA: a single-institution experience of 356 patients. Eur J Radiol 2011; 77: 340–45. doi: 10.1016/j.ejrad.2009.08.007 [DOI] [PubMed] [Google Scholar]
- 14.De Meerleer G, Villeirs G, Bral S, Paelinck L, De Gersem W, Dekuyper P, et al. The magnetic resonance detected intraprostatic lesion in prostate cancer: planning and delivery of intensity-modulated radiotherapy. Radiother Oncol 2005; 75: 325–33. doi: 10.1016/j.radonc.2005.04.014 [DOI] [PubMed] [Google Scholar]
- 15.Jackson AS, Reinsberg SA, Sohaib SA, Charles-Edwards EM, Jhavar S, Christmas TJ, et al. Dynamic contrast-enhanced MRI for prostate cancer localization. Br J Radiol 2009; 82: 148–56. doi: 10.1259/bjr/89518905 [DOI] [PubMed] [Google Scholar]
- 16.Dogan N, Mihaylov I, Wu Y, Keall PJ, Siebers JV, Hagan MP. Monte Carlo dose verification of prostate patients treated with simultaneous integrated boost intensity modulated radiation therapy. Radiat Oncol 2009; 4: 18. doi: 10.1186/1748-717X-4-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang J, Li J, Chen L, Price R, McNeeley S, Qin L, et al. Dosimetric verification of IMRT treatment planning using Monte Carlo simulations for prostate cancer. Phys Med Biol 2005; 50: 869–78. [DOI] [PubMed] [Google Scholar]
- 18.Cruz M, Tsuda K, Narumi Y, Kuroiwa Y, Nose T, Kojima Y, et al. Characterization of low-intensity lesions in the peripheral zone of prostate on pre-biopsy endorectal coil MR imaging. Eur Radiol 2002; 12: 357–65. doi: 10.1007/s003300101044 [DOI] [PubMed] [Google Scholar]
- 19.Onal C, Topkan E, Efe E, Yavuz M, Arslan G, Yavuz A. The effect of concurrent androgen deprivation and 3D conformal radiotherapy on prostate volume and clinical organ doses during treatment for prostate cancer. Br J Radiol 2009; 82: 1019–26. doi: 10.1259/bjr/65939531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen M, Hricak H, Kalbhen CL, Kurhanewicz J, Vigneron DB, Weiss JM, et al. Hormonal ablation of prostatic cancer: effects on prostate morphology, tumor detection, and staging by endorectal coil MR imaging. AJR Am J Roentgenol 1996; 166: 1157–63. doi: 10.2214/ajr.166.5.8615261 [DOI] [PubMed] [Google Scholar]
- 21.Housri N, Ning H, Ondos J, Choyke P, Camphausen K, Citrin D, et al. Parameters favorable to intraprostatic radiation dose escalation in men with localized prostate cancer. Int J Radiat Oncol Biol Phys 2011; 80: 614–20. doi: 10.1016/j.ijrobp.2010.06.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sannazzari GL, Ragona R, Ruo Redda MG, Giglioli FR, Isolato G, Guarneri A. CT-MRI image fusion for delineation of volumes in three-dimensional conformal radiation therapy in the treatment of localized prostate cancer. Br J Radiol 2002; 75: 603–7. [DOI] [PubMed] [Google Scholar]
- 23.Tsai CL, Wu JK, Chao HL, Tsai YC, Cheng JC. Treatment and dosimetric advantages between VMAT, IMRT, and helical tomotherapy in prostate cancer. Med Dosim 2011; 36: 264–71. doi: 10.1016/j.meddos.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 24.Onal C, Topkan E, Efe E, Yavuz M, Sonmez S, Yavuz A. Comparison of rectal volume definition techniques and their influence on rectal toxicity in patients with prostate cancer treated with 3D conformal radiotherapy: a dose-volume analysis. Radiat Oncol 2009; 4: 14. doi: 10.1186/1748-717X-4-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008; 35: 310–17. [DOI] [PubMed] [Google Scholar]
- 26.Lawton CA, Michalski J, El-Naqa I, Buyyounouski MK, Lee WR, Menard C, et al. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int J Radiat Oncol Biol Phys 2009; 74: 383–87. doi: 10.1016/j.ijrobp.2008.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys 1989; 16: 1623–30. [DOI] [PubMed] [Google Scholar]
- 28.Burman C, Kutcher GJ, Emami B, Goitein M. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys 1991; 21: 123–35. [DOI] [PubMed] [Google Scholar]
- 29.Geier M, Astner ST, Duma MN, Jacob V, Nieder C, Putzhammer J, et al. Dose-escalated simultaneous integrated-boost treatment of prostate cancer patients via helical tomotherapy. Strahlenther Onkol 2012; 188: 410–16. doi: 10.1007/s00066-012-0081-8 [DOI] [PubMed] [Google Scholar]
- 30.Futterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology 2006; 241: 449–58. doi: 10.1148/radiol.2412051866 [DOI] [PubMed] [Google Scholar]
- 31.Speight JL, Roach M 3rd. Advances in the treatment of localized prostate cancer: the role of anatomic and functional imaging in men managed with radiotherapy. J Clin Oncol 2007; 25: 987–95. doi: 10.1200/JCO.2006.10.3218 [DOI] [PubMed] [Google Scholar]
- 32.Villeirs GM, L Verstraete K, De Neve WJ, De Meerleer GO. Magnetic resonance imaging anatomy of the prostate and periprostatic area: a guide for radiotherapists. Radiother Oncol 2005; 76: 99–106. doi: 10.1016/j.radonc.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 33.Lafond C, Gassa F, Odin C, Drean G, Even J, De Crevoisier R, et al. Comparison between two treatment planning systems for volumetric modulated arc therapy optimization for prostate cancer. Phys Med 2014; 30: 2–9. doi: 10.1016/j.ejmp.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 34.Wiezorek T, Voigt A, Metzger N, Georg D, Schwedas M, Salz H, et al. Experimental determination of peripheral doses for different IMRT techniques delivered by a Siemens linear accelerator. Strahlenther Onkol 2008; 184: 73–9. doi: 10.1007/s00066-008-1743-4 [DOI] [PubMed] [Google Scholar]