Abstract
Objective:
Bilateral hypertrophic olivary degeneration on brain MRI has been reported in a few metabolic, genetic and neurodegenerative disorders, including mitochondrial disorders. In this report, we sought to analyse whether bilateral symmetrical inferior olivary nucleus hypertrophy is specifically associated with mitochondrial disorders in children.
Methods:
This retrospective study included 125 children (mean age, 7.6 ± 5 years; male:female, 2.6:1) diagnosed with various metabolic and genetic disorders during 2005–2012. The routine MRI sequences (T1 weighted, T2 weighted and fluid-attenuated inversion–recovery sequences) were analysed for the presence of bilateral symmetrical olivary hypertrophy and central tegmental tract or dentate nuclei signal changes. The other imaging findings and the final diagnoses were noted.
Results:
The cohort included patients with Leigh and Leigh-like syndrome (n = 25), other mitochondrial diseases (n = 25), Wilson disease (n = 40), Type 1 glutaric aciduria (n = 14), maple syrup urine disease (n = 13), giant axonal neuropathy (n = 5) and L-2 hydroxy glutaric aciduria (n = 3). Bilateral inferior olivary nucleus hypertrophy was noted in 10 patients, all of whom belonged to the Leigh and Leigh-like syndrome group.
Conclusion:
Bilateral hypertrophic olivary degeneration on MRI is relatively often, but not routinely, seen in children with Leigh and Leigh-like syndrome. Early detection of this finding by radiologists and physicians may facilitate targeted metabolic testing in these children.
Advances in knowledge:
This article highlights the occurrence of bilateral hypertrophic olivary nucleus degeneration on MRI in children with Leigh and Leigh-like syndrome, compared with other metabolic disorders.
Hypertrophic olivary degeneration is a rare form of neuronal degeneration that results from disruption of the afferent fibres to the inferior olive within the dentato-rubro-olivary tract, otherwise known as the triangle of Guillain–Mollaret.1 The first description of the enlargement of the inferior olivary nucleus was by Oppenheim, in Germany, in his post-mortem study.2 Since then, a number of reports describing the clinical, histopathological and MRI findings have been published.3–7
A variety of insults can lead to hypertrophic olivary degeneration, of which the most commonly reported are haemorrhage, infarct, trauma, surgery or tumour.8–13 The lesions result in loss of synaptic input to the inferior olivary nucleus and in trans-synaptic neuronal degeneration.5 This has been considered a unique morphological form of trans-synaptic degeneration because it is associated with hypertrophy rather than atrophy of the affected structure.14 Histopathologically, it is characterized by neuronal enlargement of the olivary neurons, neuronal loss, vacuolation, demyelination and marked astrogliosis.3,5,7 It is most often unilateral when it is secondary to a structural lesion, although bilateral changes are recognized8,15 and may occur if both the superior cerebellar peduncle and the central tegmental tracts are involved.16,17
Bilateral hypertrophic olivary degeneration has been reported in a few metabolic, genetic, neurodegenerative and toxic disorders.18–24 It has been previously noted that the presence of hypertrophic olivary degeneration in the appropriate clinical setting should alert the clinician to the possibility of a mitochondrial disorder.20 In addition, the reports on hypertrophic olivary degeneration in paediatric patients are limited.15,25–27 In this report, we sought to analyse whether bilateral symmetrical inferior olivary nucleus hypertrophy is specifically associated with mitochondrial disorders in children.
METHODS AND MATERIALS
This retrospective study was carried out in the departments of neurology and neuroimaging and interventional neuroradiology at a university hospital in South India. The study included 125 children [mean age: 7.6 ± 5 years; male:female (M:F), 2.6:1] diagnosed with various metabolic and genetic disorders seen over a period of 8 years (2005–2012). The cohort included three groups: (1) 25 children with Leigh and Leigh-like syndrome (mean age: 3.4 ± 2.6 years; M:F, 3:1); (2) 25 children with mitochondrial disorders other than Leigh syndrome (mean age: 8.8 ± 4.3 years); (3) 75 children with other metabolic and genetic disorders, which included Wilson disease, Type 1 glutaric aciduria, maple syrup urine disease, giant axonal neuropathy and L-2 hydroxy glutaric aciduria. These 75 patients were selected from a database of children with metabolic disorders and an MRI available for review. The inclusion of these disorders was based on their propensity to involve the brainstem, central tegmental tracts or dentate nuclei on MRI. This inclusion criterion was adopted to assess the presence of hypertrophic olivary degeneration in relation to the other components of the Guillain–Mollaret triangle. Metabolic disorders without propensity to involve the brainstem or dentate nuclei were excluded.
All patients with Leigh and Leigh-like syndrome fulfilled the criteria described by Rahman et al.28 All patients underwent sequencing of the complete mitochondrial genome and the SURF1, one of the most common nuclear genes consistently associated with Leigh syndrome and cytochrome c oxidase (COX) deficiency. Four of them showed mutations in SURF1. Three of these patients had COX deficiency in both histopathology and respiratory chain enzyme assays. The fourth patient did not have the test for COX. The clinical and imaging findings in these four patients have already been described.29,30 The diagnosis of other mitochondrial disorders were based on the modified Walker criteria for definite mitochondrial disorders by Bernier et al.31 All of them had clinical features consistent with a mitochondrial encephalomyopathy along with one or more of the following: (1) histopathological evidence of ragged red fibres or COX deficiency; (2) residual respiratory chain complex activity of <20% in muscle tissue compared with controls using citrate synthase as the reference enzyme; (3) known pathogenic mutations in the mitochondrial or nuclear genome. This group included polymerase gamma (POLG)-related disorder (n = 4); mitochondrial encephalomyopathy lactic acidosis and stroke syndrome-like episodes (n = 3); Lebers hereditary optic neuropathy plus syndrome (n = 1); myoclonic epilepsy with ragged red fibres syndrome (n = 2); and other non-syndromic mitochondrial disorders (n = 15).
The details of the non-mitochondrial group and the criteria for diagnosis in each disease group are as follows: Wilson disease (n = 40; age range: 6–16 years; M:F, 1.2:1)—the presence of Kayser–Fleischer rings by slit lamp, low serum caeruloplasmin and raised 24 h urinary copper; Type 1 glutaric aciduria (n = 14; age range: 8 months to 6 years; M:F, 1:1.3)—characteristic clinical and imaging findings with the presence of elevated glutaryl carnitine on tandem mass spectrometry and/or urinary organic acid estimation; maple syrup urine disease (n = 13; age range: 14 days to 11 years; M:F, 1:1.6)—confirmed by elevated branched chain amino acids on tandem mass spectrometry of dried blood spots and/or high-performance liquid chromatography; giant axonal neuropathy (n = 5; age range: 4–6 years; M:F, 4:1)—the presence of characteristic morphological features of frizzy hair, peripheral neuropathy and cerebellar signs along with giant axons on sural nerve biopsy; L-2 hydroxy glutaric aciduria (n = 3; age range: 16–18 years; M:F, 2:1)—elevated L-2 hydroxy glutaric acid on urinary organic acid estimation.
MRI was performed, after obtaining informed consent, using a Siemens Magnetom Vision 1.5-T MRI scanner (Erlangen, Germany) using standard procedures and protocols. Spin echo T1 weighted [repetition time (TR) = 650 ms and echo time (TE) = 14 ms] images in axial and sagittal planes were taken, with an acquisition time of 2.5 min, a matrix of 256 × 256 and a 230-mm field of view (FOV). T2 weighted images (TR = 4000 ms and TE = 120 ms) were acquired in axial and coronal planes. Fluid-attenuated inversion–recovery (FLAIR) sequences were obtained in the axial plane (TR = 9000 ms, TE = 119 ms, inversion time = 2457 ms). The slice thickness was 5 mm. MRI sequences included T1 weighted (with and without contrast), T2 weighted and FLAIR in all.
For those who underwent imaging in the Achieva 3.0-T MRI scanner (Philips Medical Systems, Best, Netherlands), MRI scans were obtained by first performing a three-plane localizer scan, followed by an axial turbo spin-echo T2 weighted scan (TR/TE = 3270/80 ms; flip angle = 90°; FOV = 250 × 250 mm; acquired voxel size = 0.89/1.21/5.00 mm; and slice thickness/gap = 5/1 mm); T2 weighted FLAIR sense (TR/TE = 11000/120 ms; flip angle = 90°; FOV = 250 × 250 mm; acquired voxel size = 0.96/1.79/5.00 mm; and slice thickness/gap = 5/1 mm). Three-dimensional magnetization-prepared rapid acquisition gradient recalled echo volumetric scans of the whole brain were acquired (TR/TE = 8.3/3.8 ms; sense factor = 1.5; flip angle = 8°; FOV = 256; acquired voxel size = 1/1/1 mm).
Findings on MRI at the level of the olives were evaluated by two neurologists (ABT and PSB) and one neuroradiologist (HRA) with emphasis on the lateral placement, signal intensity and hypertrophy of the lesions. If the oval shape of the olive had become round with an apparent bulge, it was classified as hypertrophic.5 Additional features noted include the presence of hyperintensity of the dentate nuclei and central tegmental tract and midbrain signal changes. The clinicians were not blinded to the diagnoses. The neuroradiologists reviewed the images independently. A consensus was reached when there was a disagreement.
RESULTS
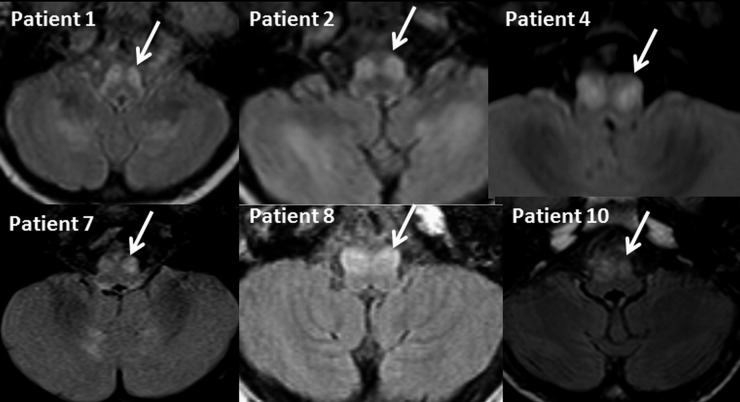
Of the 125 MRI scans reviewed, 10 showed bilateral hypertrophic olivary degeneration. The inferior olivary nuclei were demonstrated as areas of high signal intensity on the T2 weighted and FLAIR images in all of the patients (Figure 1). The shape of the olivary nucleus was round in all patients with loss of serrated appearance (Figure 1). On T1 weighted images, the nucleus appeared isointense. A focal enhancement of the hypertrophic olivary nuclei was noted in one patient. All the patients with an inferior olivary hypertrophy belonged to the mitochondrial disease group and had a diagnosis of Leigh and Leigh-like syndrome, as defined by the criteria of Rahman et al.28 The phenotypic features of these 10 patients are shown in Table 1. Of the 25 patients who fulfilled the diagnostic criteria for Leigh syndrome, there were 5 patients with Leigh syndrome secondary to SURF1 mutation (4 patients with mutation positive and the sibling of one of the patients with a mutation). All five showed inferior olivary hypertrophy. Two other patients with COX deficiency in the non-syndromic mitochondrial group, but without a Leigh phenotype, did not show evidence of hypertrophic olivary degeneration. Dentate nucleus hyperintensity was noted in five patients, lesions of the pontine tegmentum in two and central tegmental tract in four patients in this group. The olivary degeneration occurred with or without central tegmental tract and dentate nucleus hyperintensity (Figure 2). The duration of illness in these patients at the time of MRI ranged from 1 month to 5 years. In two patients with Leigh-like syndrome (Patients 9 and 10), the olivary degeneration was noted on follow-up images.
Figure 1.
MRI brain fluid-attenuated and inversion–recovery axial views in different patients showing bilateral symmetrical signal changes in the inferior olivary nucleus. The margins of the nuclei are round and an apparent bulge (indicated by arrows) is noted.
Table 1.
Clinical and laboratory features of patients with bilateral symmetrical hypertrophic olivary degeneration
| Patients | Age | Sex | Duration of illness at the time of MRI | Clinical features | Laboratory tests | MRI findings other than hypertrophic olivary degeneration | Final diagnosis |
|---|---|---|---|---|---|---|---|
| Patient 1 | 5 years | M | 2 years | Developmental delay, episodic neuroregression, hypotonia, sighing respiration, ophthalmoparesis, nystagmus, dystonia, and demyelinating neuropathy | Elevated serum lactate | Bilateral symmetrical signal changes in caudate putamen, central tegmental tract and dentate | Leigh syndrome |
| Patient 2 | 4 years | F | 6 months | Developmental delay, episodic neuro-regression, hypotonia, sighing respiration, tremor of hands, titubation, ataxia, ophthalmoparesis, and nystagmus | Elevated serum lactate and CSF lactate, elevated lactate–pyruvate ratio | Bilateral symmetrical signal changes in dentate nuclei, cerebellar white matter and putamen. Lactate peak on MR spectroscopy | Leigh syndrome |
| Patient 3 | 2.5 years | F | 2 years | Developmental delay, episodic neuroregression, hypotonia, sighing respiration, ophthalmoparesis, dystonia, hypertrichosis and family history of retinitis pigmentosa | Elevated serum lactate. Novel SURF1 mutation | Bilateral symmetrical signal changes in caudate, putamen thalamus, mid-brain and cerebellar white matter | Leigh syndrome |
| Patient 4 | 18 months | M | 6 months | Developmental delay, neuroregression, hypotonia, sighing respiration, ophthalmoparesis, nystagmus, ataxia, dystonia, hypertrichosis, family history of Leigh syndrome in the younger siblinga | Elevated serum lactate, COX deficiency on histopathology, <20% activity of complex IV on respiratory chain enzyme assays. Novel SURF1 mutation | Bilateral symmetrical signal changes in caudate, putamen, subthalamic nucleus, substantia nigra, central tegmental tract, periaqueductal grey matter | Leigh syndrome |
| Patient 5 | 3 years | F | 1 month | Developmental delay, neuroregression, ataxia, sighing respiration, hypertrichosis, family history of Leigh syndrome in elder sibling | Elevated serum lactate, COX deficiency on muscle biopsy. <20% activity of complex IV on respiratory chain assays. Novel SURF1 mutation | Bilateral symmetrical signal changes in substantia nigra and posterior periventricular white matter and speck of hyperintensity in putamen | Leigh syndrome |
| Patient 6 | 6 years | M | 3 years | Developmental delay, hypotonia, ataxia, ophthalmoparesis, sighing respiration hypertrichosis, hypopigmented hair and skin | Elevated lactate, COX deficiency on muscle biopsy, <20% activity of complex IV on respiratory chain enzyme assays. Novel SURF1 mutation | Bilateral symmetrical signal changes in dentate nuclei and central tegmental tract | Leigh syndrome |
| Patient 7a | 2 years | M | 9 months | Developmental delay, neuroregression, hypotonia, sighing respiration, ophthalmoparesis, nystagmus, ataxia, dystonia and hypertrichosis | Elevated serum lactate. Elder sibling has Leigh syndrome with COX deficiency and SURF1 mutation | Bilateral symmetrical signal changes in caudate putamen, subthalamic nucleus, substantia nigra | Leigh syndrome |
| Patient 8 | 9 years | M | 6 months | Episodic encephalopathy, tremor, ataxia and ocular bobbing | Normal serum lactate. Subsarcolemmal aggregation of reaction products on succinate dehydrogenase enzyme staining on muscle biopsy | Bilateral symmetrical signal changes in thalamus and central tegmental tract | Leigh-like syndrome |
| Patient 9 | 5 years | M | 1 year | Progressive extrapyramidal syndrome, generalized dystonia | Elevated serum lactate, multiple complex deficiency (<20% activity) on respiratory chain enzyme assays | Bilateral symmetrical signal changes in caudate and putamen | Leigh-like syndrome |
| Patient 10 | 5 years | M | 5 years | Progressive extrapyramidal syndrome, generalized dystonia | Elevated serum lactate, elevated serum alanine, subsarcolemmal accumulation of reaction products on succinate dehydrogenase enzyme staining on muscle biopsy | Bilateral symmetrical signal changes in caudate and putamen | Leigh-like syndrome |
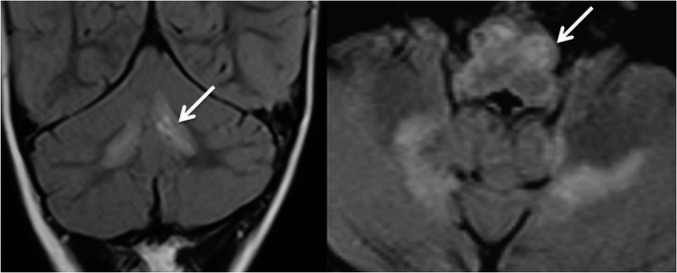
Figure 2.
MRI scan of the brain in Patient 6. Fluid-attenuated inversion–recovery coronal and axial views showing bilateral symmetrical signal changes in dentate nuclei and inferior olivary hypertrophy (arrows) in this patient with cytochrome c oxidase deficiency and SURF1 mutation.
In the Wilson disease group, although 10 patients had bilateral central tegmental tract involvement, neither dentate nucleus hyperintensity nor inferior olivary hypertrophy was present. Out of the 14 patients with Type 1 glutaric aciduria, 9 had both central tegmental tract and dentate nucleus hyperintensities and none had hypertrophic olivary degeneration. In the giant axonal neuropathy, three patients had dentate nucleus hyperintensity, but none had central tegmental tract or olivary nucleus hypertrophy. In maple syrup urine disease, there was extensive involvement of the brainstem, cerebellum and dentate nuclei in all patients associated with diffusion restriction. The medullary signal changes involved both the dorsal and ventral medulla in some patients, and the inferior olivary nucleus was not clearly visible. All patients with L-2 hydroxy glutaric aciduria had dentate nucleus hyperintensity but did not have central tegmental tract or olivary nucleus involvement.
DISCUSSION
This report describes the occurrence of hypertrophic olivary degeneration in a cohort of children with metabolic and genetic disorders. Our results suggest that this finding is most often associated with a mitochondrial disorder in children, especially Leigh and Leigh-like syndrome. Reports on bilateral hypertrophic olivary degeneration resulting from genetic or metabolic disorders are rare. There are a number of reports describing hypertrophy of the olivary nucleus in mitochondrial disorders.19,20 In a series of 26 children with POLG-related disorders, bilateral hypertrophic olivary degeneration was observed in 2 patients, and 1 of them had the clinical correlate of palatal myoclonus.19 It has also been described in Leigh syndrome secondary to SURF1 mutations.20 Review of the previously published MRI studies in Leigh syndrome patients identified few reports describing the involvement of the olives.32–34 It is noteworthy that all but one of the patients in these reports had COX deficiency and SURF1 mutations. The only exception was the patient with Leigh syndrome secondary to pyruvate dehydrogenase deficiency in the report by Medina et al.34 In this cohort, the hypertrophic olivary degeneration was seen in 40% of the children with Leigh and Leigh-like syndrome and was not observed in other groups of metabolic disorders. Although all patients with SURF1 mutation had hypertrophic degeneration, it was also seen in patients without the mutations, indicating that it is not exclusively seen in this subtype of children with Leigh syndrome. In addition, two children with cytochrome c deficiency but without the Leigh syndrome phenotype did not show this finding. Nevertheless, analysis of the whole cohort of children with metabolic and genetic disorders indicates that this imaging observation is more commonly seen in children with Leigh and Leigh-like syndrome. None of the patients with mutation-positive POLG-related disorder had inferior olivary hypertrophy in the present study.
The bilateral inferior olivary hypertrophy seen in these children was not always associated with lesions of the central tegmental tract or dentate nucleus, suggesting an independent involvement of these nuclei in mitochondrial disorders. Neuropathological studies in mitochondrial diseases have shown that the olivary cerebellar system is involved in a variety of mitochondrial disorders and plays an important role in the pathogenesis of ataxia seen in these patients.35 This particular study involved 14 patients with definite mitochondrial diseases. There was variable neuronal loss in the dentate nuclei, inferior olives and Purkinje cell system in these patients along with severe deficiency of respiratory chain enzymes in the remaining neurons. This shows that the inferior olivary nucleus is vulnerable to energy deficiency in patients with mitochondrial disorders. The MRI correlates of these patients are not provided in the report. Nevertheless, it will be interesting to see if the microscopic changes in these patients are associated with imaging changes.
Neuropathological studies in Leigh syndrome patients also have highlighted the occurrence of neuronal loss and vacuolar degeneration in inferior olives.35–38 The finding was most often noted in those patients who presented after the first year of life. This was attributed to the delayed maturation of the olivary–cerebellar system in children, which occurs most often after infancy. The fact that hypertrophic olivary degeneration is present even in the presence of neuronal loss is explained by pathological studies. The characteristic features that define hypertrophic olivary degeneration are overall enlargement of the olive in which convolutions are obscured.4 The neurons appear large and deformed and show marked astroglial reaction. Gautier and Blackwood3 had demonstrated that there is enlargement of both grey and white matter and the increased width of the grey lamina is in part due to hypertrophy of the nerve cell bodies and processes. They suggest that the eventual atrophy of the abnormal neurons is not accompanied by shrinkage of the glial mass so that the enlarged outline of the olive persists. This may be the explanation for hypertrophied olives even when there is neuronal loss in mitochondrial disorders.
The clinical correlates of the hypertrophic olivary degeneration in children and in patients with mitochondrial disorders have been variable. It was associated with palatal myoclonus in one of the two patients with POLG-related disorder described by Tzoulis et al.19 In the report by Kinghorn et al,20 a generalized tremor was noted in a child with SURF1 mutations but was not present in two other patients. In the report of Sanverdi et al,15 none of the four children with hypertrophic olivary degeneration had palatal myoclonus. In the current study, two patients had a generalized tremor associated with ocular bobbing. In two patients, the clinical picture was dominated by generalized dystonia refractory to treatment with most of the anti-dystonia medications. It is difficult to comment about this finding in other patients, because it was not systematically looked for in most of the young patients.
The triangle of nerve tracts involved in the genesis of hypertrophic olivary degeneration is referred to as the triangle of Guillain and Mollaret.1 These dentato-rubro-olivary fibres connect the ipsilateral red nucleus and inferior olive with the contralateral dentate nucleus. Efferent fibres from the dentate nucleus course through the superior cerebellar peduncle, decussate in the brachium conjunctivum and then connect to the contralateral red nucleus. The efferent fibres from the red nucleus are connected to the olive through the central tegmental tract. The inferior olive is then connected to the contralateral cerebellum via the inferior cerebellar peduncle thus forming a triangle. It is pointed out that deafferentiating the inferior olivary nucleus is the key factor and, typically, lesions disrupting the superior cerebellar peduncle or central tegmental tract lead to hypertrophic olivary degeneration. The localization of the olivary degeneration depends on the site of the lesion. Lesions of the red nucleus or central tegmental tract cause the hypertrophy of the ipsilateral nucleus and, alternatively, the lesions of the superior cerebellar peduncle or dentate nucleus cause hypertrophic olivary degeneration on the contralateral side.9,12 Bilateral hypertrophy occurs when the lesion involves the brachium conjunctivum or interrupts both the central tegmental tract and superior cerebellar peduncle.16
In the cohort of children in the present study, the hypertrophic olivary degeneration was associated with lesions of the central tegmental tract and dentate nucleus in most of the patients, but also occurred independently. In patients with Wilson disease, although 10 patients had involvement of the central tegmental tract, hypertrophy of the inferior olivary nucleus was not observed. Diffusion tensor imaging (DTI) studies in patients with inferior olivary nucleus hypertrophy have demonstrated signal changes in all anatomical components of the Guillain–Mollaret triangle even when conventional MRI did not show any signs of involvement in the other components of the Guillain–Mollaret triangle.39,40 It was also shown that the DTI parameters can reflect the spatiotemporal evolution of transneuronal degeneration associated with hypertrophic olivary degeneration (HOD) in a manner consistent with the known pathological stages of HOD.26 In addition, susceptibility-weighted imaging has been utilized to study the red nucleus degeneration and atrophy in children with HOD after posterior fossa tumour resection.27 This indicates that quantitative imaging studies may better define the extent of the lesions in these patients and may be a future area of research.
In conclusion, this study on children with various metabolic and genetic disorders suggests that bilateral hypertrophic olivary degeneration is relatively often, but not routinely, seen in children with Leigh and Leigh-like syndrome. Nevertheless, when compared with other metabolic disorders, this finding was more commonly seen in children with Leigh and Leigh-like syndrome. This occurred both independently and in association with central tegmental tract and dentate nuclei involvement. The findings suggest that bilateral hypertrophic olivary degeneration should prompt the physician to carry out investigations to rule out a mitochondrial disorder.
FUNDING
This study was supported by a grant (BT/PR7470/MED/14/1011/2006) from the Department of Biotechnology, Government of India.
REFERENCES
- 1.Guillian G, Mollaret P. Deux cas de myoclonies synchrones et rhythmes velopharyngolaryngo-oculo-diaphragmatiques. Rev Neurol 1931; 2: 545–66. [Google Scholar]
- 2.Oppenheim H. Uber oliven degeneration bei atheromatose der basalen hinarterien. Berl Klin Wochenschr 1887; 34: 638–9. [Google Scholar]
- 3.Gautier JC, Blackwood W. Enlargement of the inferior olivary nucleus in association with lesions of the central tegmental tract or dentate nucleus. Brain 1961; 84: 341–61 [DOI] [PubMed] [Google Scholar]
- 4.Anderson JR, Treip CS. Hypertrophic olivary degeneration and Purkinje cell degeneration in a case of long-standing head injury. J Neurol Neurosurg Psychiatry 1973; 36: 826–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, et al. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 1994; 192: 539–43. doi: 10.1148/radiology.192.2.8029428 [DOI] [PubMed] [Google Scholar]
- 6.Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 2000; 21: 1073–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Nishie M, Yoshida Y, Hirata Y, Matsunaga M. Generation of symptomatic palatal tremor is not correlated with inferior olivary hypertrophy. Brain 2002; 125: 1348–57. [DOI] [PubMed] [Google Scholar]
- 8.Salamon-Murayama N, Russell EJ, Rabin BM. Diagnosis please. Case 17: hypertrophic olivary degeneration secondary to pontine haemorrhage. Radiology 1999; 213: 814–17. doi: 10.1148/radiology.213.3.r99dc43814 [DOI] [PubMed] [Google Scholar]
- 9.Krings T, Foltys H, Meister IG, Reul J. Hypertrophic olivary degeneration following pontine haemorrhage: hypertensive crisis or cavernous haemangioma bleeding? J Neurol Neurosurg Psychiatry 2003; 74: 797–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki M, Takashima T, Ueda F, Fujinaga Y, Horichi Y, Yamashita J. Olivary degeneration after intracranial haemorrhage or trauma: follow-up MRI. Neuroradiology 1999; 41: 9–12. [DOI] [PubMed] [Google Scholar]
- 11.Hornyak M, Osborn AG, Couldwell WT. Hypertrophic olivary degeneration after surgical removal of cavernous malformations of the brain stem: report of four cases and review of the literature. Acta Neurochir (Wien) 2008; 150: 149–56. doi: 10.1007/s00701-007-1470-0 [DOI] [PubMed] [Google Scholar]
- 12.Choh NA, Choh SA, Jehangir M. Hypertrophic olivary degeneration: the forgottentriangle of Guillain and Mollaret. Neurol India 2009; 57: 507–9. doi: 10.4103/0028-3886.55587 [DOI] [PubMed] [Google Scholar]
- 13.Gatlin JL, Wineman R, Schlakman B, Buciuc R, Khan M. Hypertrophic olivary degeneration after resection of a pontine cavernous malformation: a case report. J Radiol Case Rep 2011; 5: 24–9. doi: 10.3941/jrcr.v5i3.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duchen LW. General pathology of neurons and neuroglia. In: Greenfield H, Corsellis JAN, Duchen LW, eds. Neuropathology. 4th edn. New York, NY: Wiley; 1984. p. 18–9. [Google Scholar]
- 15.Sanverdi SE, Oguz KK, Haliloglu G. Hypertrophic olivary degeneration in children: four new cases and a review of the literature with an emphasis on the MRI findings. Br J Radiol 2012; 85: 511–16. doi: 10.1259/bjr/60727602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conforto AB, Smid J, Marie SKN, Ciriaco JGM, Santoro PP, Leite CC, et al. Bilateral olivary hypertrophy after unilateral cerebellar infarction. Arq Neuropsiquiatr 2005; 63: 321–3. [DOI] [PubMed] [Google Scholar]
- 17.Gerace C, Fele MR, Luna R, Piazza G. Neurologial picture: bilateral hypertrophic-olivary degeneration. J Neurol Neurosurg Psychiatry 2006; 77: 73. doi: 10.1136/jnnp.2005.069831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulkarni PK, Muthane UB, Taly AB, Jayakumar PN, Shetty R, Swamy HS. Palatal tremor, progressive multiple cranial nerve palsies, and cerebellar ataxia: a case report and review of literature of palatal tremors in neurodegenerative disease. Mov Disord 1999; 14: 689–93 [DOI] [PubMed] [Google Scholar]
- 19.Tzoulis C, Engelsen BA, Telstad W, Aasly J, Zeviani M, Winterthun S, et al. The spectrum of clinical disease caused by the A467T and W748S POLG mutations: a study of 26 cases. Brain 2006; 129: 1685–92. doi: 10.1093/brain/awl097 [DOI] [PubMed] [Google Scholar]
- 20.Kinghorn KJ, Kaliakatsos M, Blakely EL, Taylor RW, Rich P, Clarke A, et al. Hypertrophic olivary degeneration on magnetic resonance imaging in mitochondrial syndromes associated with POLG and SURF1 mutations. J Neurol 2013; 260: 3–9. doi: 10.1007/s00415-012-6564-9 [DOI] [PubMed] [Google Scholar]
- 21.Otto J, Guenther P, Hoffmann KT. Bilateral hypertrophic olivary degeneration in Wilson disease. Korean J Radiol 2013; 14: 316–20. doi: 10.3348/kjr.2013.14.2.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanihara T, Amano N, Takahashi T, Itoh Y, Yagishita S. Hypertrophy of the inferior olivary nucleus in patients with progressive supranuclear palsy. Eur Neurol 1998; 39: 97–102 [DOI] [PubMed] [Google Scholar]
- 23.Cazals X, Omoumi P, Agnard P, Bibi R, Ferquel C, Cazeneuve N, et al. Reversible metronidazole-induced encephalopathy and hypertrophic olivary degeneration. J Radiol 2010; 91: 304–6. [DOI] [PubMed] [Google Scholar]
- 24.Seok JI, Yi H, Song YM, Lee WY. Metronidazole-induced encephalopathy and inferior olivary hypertrophy: lesion analysis with diffusion-weighted imaging and apparent diffusion coefficient maps. Arch Neurol 2003; 60: 1796–800. doi: 10.1001/archneur.60.12.1796 [DOI] [PubMed] [Google Scholar]
- 25.Phatouros CC, McConachie NS. Hypertrophic olivary degeneration: case report in a child. Pediatr Radiol 1998; 28: 830–1. [DOI] [PubMed] [Google Scholar]
- 26.Meoded A, Poretti A, Ilica AT, Perez R, Jallo G, Burger PC, et al. Diffusion tensor imaging in a child with hypertrophic olivary degeneration. Cerebellum 2013; 12: 469–74. doi: 10.1007/s12311-013-0448-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vossough A, Ziai P, Chatzkel JA. Red nucleus degeneration in hypertrophic olivary degeneration after pediatric posterior fossa tumor resection: use of susceptibility-weighted imaging (SWI). Pediatr Radiol 2012; 42: 481–5. doi: 10.1007/s00247-011-2330-x [DOI] [PubMed] [Google Scholar]
- 28.Rahman S, Blok RB, Dal HH, Danks DM, Kirby DM, Chow CW, et al. Leigh syndrome: clinical features and biochemical and DNA abnormalities. Ann Neurol 1996; 39: 343–51. doi: 10.1002/ana.410390311 [DOI] [PubMed] [Google Scholar]
- 29.Sonam K, Khan NA, Bindu PS, Taly AB, Gayathri N, Srinivas Bharath MM, et al. Clinical and magnetic resonance imaging findings in patients with Leigh syndrome and SURF1 mutations. Brain Dev Nov 2013. Epub ahead of print. doi: 10.1016/j.braindev.2013.10.012 [DOI] [PubMed] [Google Scholar]
- 30.Sonam K, Bindu PS, Gayathri N, Khan NA, Govindaraju C, Arvinda HR, et al. The double panda sign in Leigh disease. J Child Neurol Apr 2013. Epub ahead of print. doi: 10.1177/0883073813484968 [DOI] [PubMed] [Google Scholar]
- 31.Bernier FP, Boneh A, Dennett X, Chow CW, Cleary MA, Thorburn DR. Diagnostic criteria for respiratory chain disorders in adults and children. Neurology 2002; 59: 1406–11. [DOI] [PubMed] [Google Scholar]
- 32.Farina L, Chiapparini L, Uziel G, Bugiani M, Zeviani M, Savoiardo M. MR findings in Leigh syndrome with COX deficiency and SURF-1 mutations. AJNR Am J Neuroradiol 2002; 23: 1095–100. [PMC free article] [PubMed] [Google Scholar]
- 33.Savoiardo M, Uziel G, Strada L, Visciani A, Grisoli M, Wang G. MRI findings in Leigh’s disease with cytochrome-c-oxidase deficiency. Neuroradiology 1991; 33: 507–8. [Google Scholar]
- 34.Medina L, Chi TL, DeVivo DC, Hilal SK. MR findings in patients with subacute necrotizing encephalomyelopathy (Leigh syndrome): correlation with biochemical defect. AJR Am J Roentgenol 1990; 154: 1269–74 [DOI] [PubMed] [Google Scholar]
- 35.Lax NZ, Hepplewhite PD, Reeve AK, Nesbitt V, McFarland R, Jaros E, et al. Cerebellar ataxia in patients with mitochondrial DNA disease: a molecular clinicopathological study. J Neuropathol Exp Neurol 2012; 71: 148–61. doi: 10.1097/NEN.0b013e318244477d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leigh D. Subacute necrotizing encephalomyelopathy in an infant. J Neurol Neurosurg Psychiatry 1951; 14: 216–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dayan AD, Ockenden BG, Crome L. Necrotizing encephalomyelopathy of Leigh. Neuropathological findings in 8 cases. Arch Dis Child 1970; 45: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cavanagh JB, Harding BN. Pathogenic factors underlying the lesions in Leigh's disease. Tissue responses to cellular energy deprivation and their clinico-pathological consequences. Brain 1994; 117: 1357–76. [DOI] [PubMed] [Google Scholar]
- 39.Dinçer A, Ozyurt O, Kaya D, Koşak E, Ozturk C, Erzen C, et al. Diffusion tensor imaging of Guillain-Mollaret triangle in patients with hypertrophic olivary degeneration. J Neuroimaging 2011; 21: 145–51. doi: 10.1111/j.1552-6569.2009.00461.x [DOI] [PubMed] [Google Scholar]
- 40.Shah R, Markert J, Bag AK, Cur JK. Diffusion tensor imaging in hypertrophic olivary degeneration. AJNR Am J Neuroradiol 2010; 31: 1729–31 [DOI] [PMC free article] [PubMed] [Google Scholar]