Abstract
Imaging plays an important role in the assessment of colorectal cancer, including diagnosis, staging, selection of treatment, assessment of treatment response, surveillance and investigation of suspected disease relapse. Anatomical imaging remains the mainstay for size measurement and structural evaluation; however, functional imaging techniques may provide additional insights into the tumour microenvironment. With dynamic contrast-enhanced CT techniques, iodinated contrast agent kinetics may inform on regional tumour perfusion, shunting and microvascular function and provide a surrogate measure of tumour hypoxia and angiogenesis. In colorectal cancer, this may be relevant for clinical practice in terms of tumour phenotyping, prognostication, selection of individualized treatment and therapy response assessment.
Colorectal cancer is one of the commonest of cancers, accounting for 10% of all cancers, with approximately 1.2 million new cases each year. Colorectal cancer remains a major cause of morbidity and mortality worldwide, with approximately 609 000 deaths per annum.1 Since a radical abdominopelvic resection approach for rectal cancer was described in 1908,2 significant inroads have been made into its treatment, including surgery, radiotherapy and chemotherapy, which have all improved morbidity and local recurrence rates, and also had some impact on the overall survival rate. These have included the introduction of surgical techniques such as total mesorectal excision,3,4 neoadjuvant radiotherapy prior to surgery to reduce the risk of local recurrence and an increase in the likelihood of resectability,5–7 as well as a more aggressive treatment of oligometastatic disease. Trialling of novel targeted therapies such as bevacizumab, a recombinant humanized monoclonal antibody against the vascular endothelial growth factor (VEGF), and the selective use of epidermal growth factor receptor inhibitors, such as cetuximab and panitumumab, have also led to improvements in outcome in the metastatic setting.8–10 These approaches have had a “knock-on” effect on imaging, requiring more accurate delineation of locoregional tumour extent and distant spread, and on the development of more sophisticated methods of tumour profiling to direct therapy and for assessing the therapy response and efficacy of the particular agent.
This article will highlight our current understanding of the molecular characterization of colorectal cancer, the architectural and physiological aspects of the vascular network in colorectal cancer, and discuss how dynamic contrast-enhanced CT (DCE-CT; perfusion CT), one of the increasing number of functional imaging techniques available in the clinic, may assist the management of colorectal cancer.
MOLECULAR CLASSIFICATION OF COLORECTAL CANCER
Traditionally, colorectal cancers have been classified by clinicopathological features, including tumour location, TNM stage, differentiation and grade. However, this may not provide sufficient information with respect to tumour profiling towards a more targeted treatment approach. Colorectal cancers are heterogeneous with respect to genetic and epigenetic mutations and may be classified by molecular characteristics.11,12 Chromosomal instability (CIN), which reflects the tendency for chromosome breakage; microsatellite instability (MSI), which reflects defective DNA repair; and frequent CpG island hypermethylation (CIMP), which reflects gene silencing owing to methylation of the promoter gene sequence, are three common classifiers. CIMP-high colorectal tumours have a distinct clinical, pathological and molecular profile, such as associations with proximal tumour location, female sex, poor differentiation, MSI and high BRAF and low TP53 mutation rates. CIN is present in the majority of sporadic cancers (85%) and may occur through different mechanisms, including whole chromosomal loss of heterozygosity, mitotic recombination and mitotic gene conversion. Loss of 18q heterozygosity is thought to reflect a worse prognosis13 and may be a factor for selecting adjuvant therapy in Stage II cancers. MSI is present in approximately 15% of sporadic cancers. Functional loss of MLH1 as a result of promoter methylation and gene silencing is the most common cause of MSI, particularly in sporadic MSI-high (MSI-H) cancer. MSI is typically assessed by analysing five microsatellite markers (D2S123, D5S346, D17S250, BAT25 and BAT26), referred to as the National Cancer Institute consensus panel. MSI status may also be of relevance in selecting Stage II patients to omit adjuvant therapy.13 A systematic review of 32 studies, including 7642 colorectal cancer patients of whom 1277 had MSI-H tumours, showed that MSI-H tumours were associated with a better prognosis than MSS tumours [hazard ratio for overall survival 0.65 (95% confidence interval: 0.59 to 0.71].14
THE ARCHITECTURE OF THE VASCULAR NETWORK IN COLORECTAL CANCER
Angiogenesis is an important aspect of tumorigenesis. Neovascularization arises early in the adenoma–carcinoma sequence, via upregulation of VEGF, probably related to the K-RAS mutation, which is found in 24% of adenomas.15 Vascular sprouting and de novo vascular formation from precursor endothelial cells from bone marrow are the main mechanisms by which neovascularization occurs in colorectal cancer. Tumour angiogenesis is characterized structurally by abnormal blood vessels that are thin, fragile, tortuous and hyperpermeable because of an incomplete endothelium and a relative absence of smooth muscle and pericyte coverage. Hence, the VEGF signalling pathway represents a suitable target for anticancer agents, because it is involved in tumour angiogenesis, stimulating tumour neovascularization and promoting endothelial cell survival, migration and permeability, which in turn leads to a higher risk of relapse and a worse overall prognosis.
Architecturally unlike normal colonic mucosa, in which the capillary plexus is arranged in a hexagonal pattern around the mucosal glands, and supplied by subepithelial arteries that divide within the submucosa, the microcirculation in colorectal tumours lacks a regular pattern and vessel hierarchy.16 The vascular architecture appears to be tumour type specific and consistent irrespective of tumour size. In colorectal carcinomas, there is a chaotic intratumoral distribution with areas of low vascular density mixed with regions of high angiogenic activity, but with a tendency for a decline in vessel density towards the tumour centre.16 Vessel diameters in general are increased, but with an increased number of blind-ending vessels. Vessel diameters are typically <200 μm in diameter; capillary diameters are typically <10 μm. Towards the centre of the tumour, where there are a higher number of elongated compressed vessels, the intervessel distance and interbranch distances are generally higher.
PHYSIOLOGICAL ASPECTS OF THE VASCULAR NETWORK IN COLORECTAL CANCER
Tumours require an adequate blood supply to deliver oxygen and nutrients for growth and to remove waste products. Functionally, tumour vessels differ from normal vessels with evidence of arteriovenous shunting, intermittent flow or even reversal of flow. There may be acute vascular collapse where there are areas with raised tumour interstitial pressure, particularly towards the centre of the tumour. Higher haematocrit in cancer patients also contributes to altered flow characteristics. The normal vessel wall typically consists of a single layer of endothelial cells with supporting smooth muscle and pericytes. In tumour vessels, vascular hyperpermeability occurs as a result of looser endothelial connections, larger fenestrations and a relative lack of endothelium, smooth muscle and pericyte coverage. A secondary effect of vascular hyperpermeability is raised intratumoral interstitial pressure.
IMAGING THE VASCULAR NETWORK IN COLORECTAL CANCER
Quantitative DCE-CT (perfusion CT) based on standard low-molecular-weight, iodinated contrast agents (<1 kDa) may be incorporated easily into clinical imaging protocols.17,18 Such an approach reflects the vascular delivery to the tumour, accumulation of contrast agent within the tumour interstitium and recirculation, and allows clinicians to combine functional assessment of the vasculature with anatomical assessment. In oncology, this is clinically relevant as it may provide an indirect measure of hypoxia19 and angiogenesis20–22 with data from a variety of cancers. Nevertheless, the data for colorectal cancer remain conflicted (Table 1).
Table 1.
Radiological–pathological correlative studies: colorectal cancer
| Tumour type | Perfusion CT parameter/method | Pathological correlate/method | Findings | Study |
|---|---|---|---|---|
| Angiogenesis | ||||
| Colorectal, n = 23 | Blood volume Permeability surface Whole tumour cross-sectional area Johnson–Wilson 1 s interval Limited coverage | CD34 Random field | Moderate correlations BV: r = 0.59a, p = 0.002 PS: r = 0.46a, p = 0.03 with CD34 expression | Goh et al23 |
| Colorectal, n = 29 | Blood flow Blood volume Permeability Surface Two selected areas: Luminal and invasive edge Johnson–Wilson 1 s interval Limited coverage | Factor VIII CD105 Focused region | Variable correlations, some significant for BV and PS: BF: r = 0.05 to 0.19; p = 0.98 to 0.32 BV: r = 0.02 to 0.55a; p = 0.91 to 0.003a PS: r = 0.09 to 0.43a; p = 0.96 to 0.023a | Dighe et al24 |
| Colorectal, n = 37 | Perfusion Whole tumour cross-sectional area Slope method 2 s interval Limited coverage | CD34 Hot spot (3) | No correlation between perfusion and CD34 r = 0.18, p = 0.29 Decrease in perfusion and CD34 expression with stage | Li et al25 |
| Colorectal, n = 32 | Blood flow Blood volume Permeability surface Whole tumour cross-sectional area Johnson–Wilson 1 s interval Limited coverage | CD34 Hot spot (3) | No correlations with CD34 BF: r = −0.14, p > 0.45 BV: r = 0.11, p > 0.51 PS: r = 0.28, p > 0.12 | Feng et al26 |
| Colorectal, n = 27 | Blood flow Blood volume Permeability surface Whole tumour cross-sectional area Johnson–Wilson 1 s interval Limited coverage | CD34 Hotspot (3) | No correlations with CD34 | Kim et al27 |
BF, regional blood flow; BV, regional blood volume; PS, permeability surface area product.
Significant correlations.
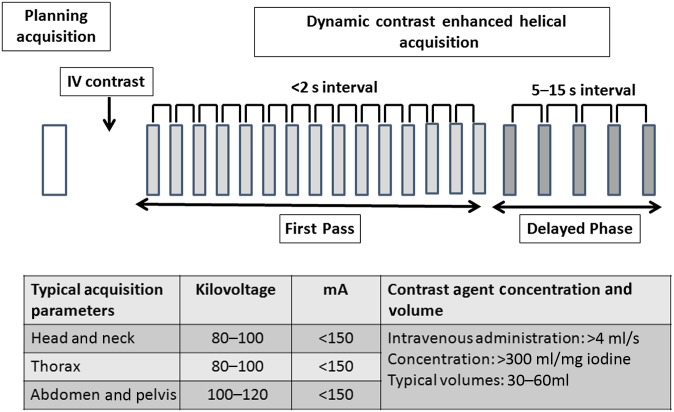
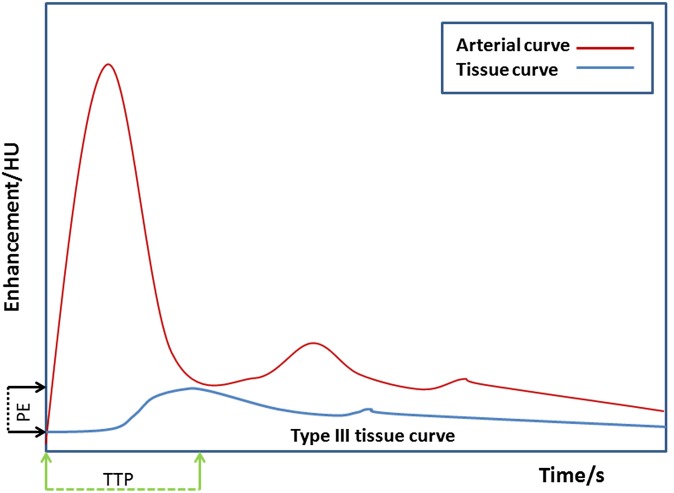
A typical acquisition and contrast administration protocol is shown in Figure 1. With current state-of-the-art technology, a z-axis coverage of up to 28 cm may be achieved depending on the required temporal sampling rate using helical techniques or up to 16 cm with non-table-moving techniques. The dynamic acquisition allows the changes in contrast enhancement within the tumour and adjacent vessels to be plotted against time. From the tissue enhancement curve, qualitative and model-free semiquantitative information may be derived. This includes the tissue curve shape (Type I: slow rising curve; Type II: initial rapid uptake with plateau; and Type III: initial rapid uptake with washout), time to peak enhancement, peak enhancement and area under the enhancement time curve (Figure 2). Tumours typically demonstrate an initial rapid uptake of contrast agent and washout (although some tumours also demonstrate a plateau), a shorter time to peak enhancement and a higher peak enhancement than normal tissue.
Figure 1.
Typical perfusion CT acquisition protocol for cancer. IV, intravenous.
Figure 2.
Typical enhancement time curves. PE, peak enhancement; TTP, time to peak enhancement.
More complex kinetic modelling may also be applied to obtain more physiologically based parameters (Table 2).
Table 2.
Kinetic models used for perfusion CT analysis
| Kinetic model | Compartments | Parameter measured | Assumptions |
|---|---|---|---|
| Maximum slope | Single | BF | No venous outflow |
| Johnson–Wilson | Dual | BF, BV, MTT, PS | Constrained IRF |
| Patlak | Dual | EF, BV | One way transfer Well-mixed compartments |
| Distributed parameter | Dual | BF, BV, PS, Ve | Constrained IRF |
BF, regional blood flow; BV, regional blood volume; EF, extraction fraction; IRF, impulse residual function; MTT, mean transit time; PS, permeability surface area product, Ve, extravascular extracellular volume.
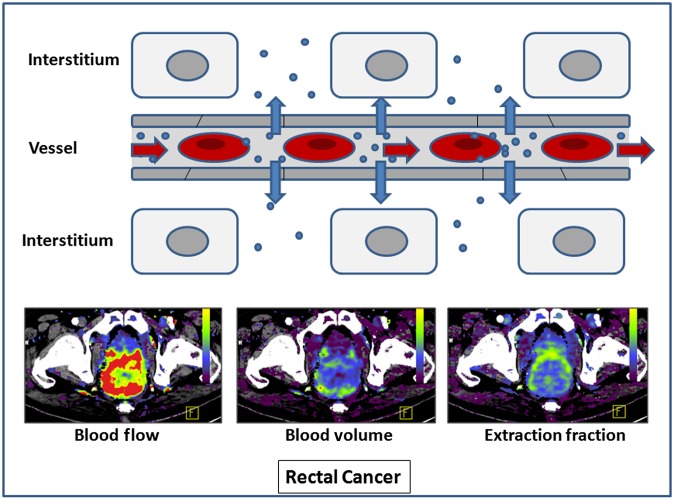
These parameters include regional blood flow (BF; blood flow per unit volume or mass of tissue); regional blood volume (BV; the proportion of tissue that comprises flowing blood); and the flow–extraction product (the rate of transfer of contrast agent from the intravascular to extravascular space), from which the permeability–surface area product (PS; the product of permeability and total surface area of capillary endothelium in a unit mass of tissue) may be derived. BF reflects the rate of delivery of oxygen and nutrients to the tumour, BV reflects the functioning vascular volume and the flow–extraction product or PS reflects the vascular leakage rate of the microcirculation (Figure 3). Extravascular extracellular volume (Ve; %) may also be estimated.
Figure 3.
Parameters obtained from kinetic modelling. F, front.
VASCULARIZATION OF TUMOUR COMPARED WITH THAT OF NORMAL COLON
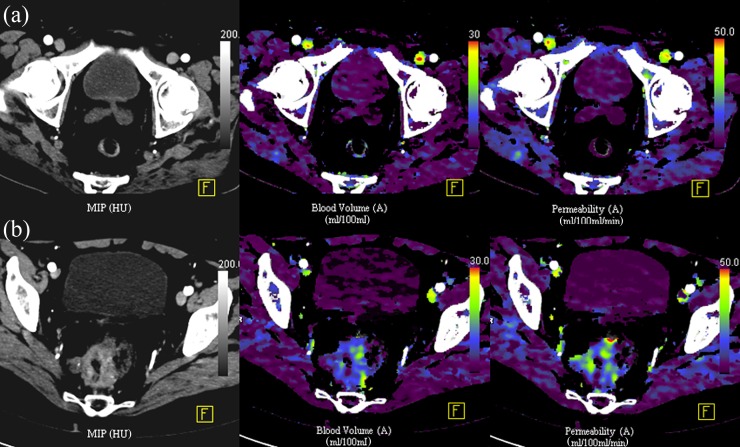
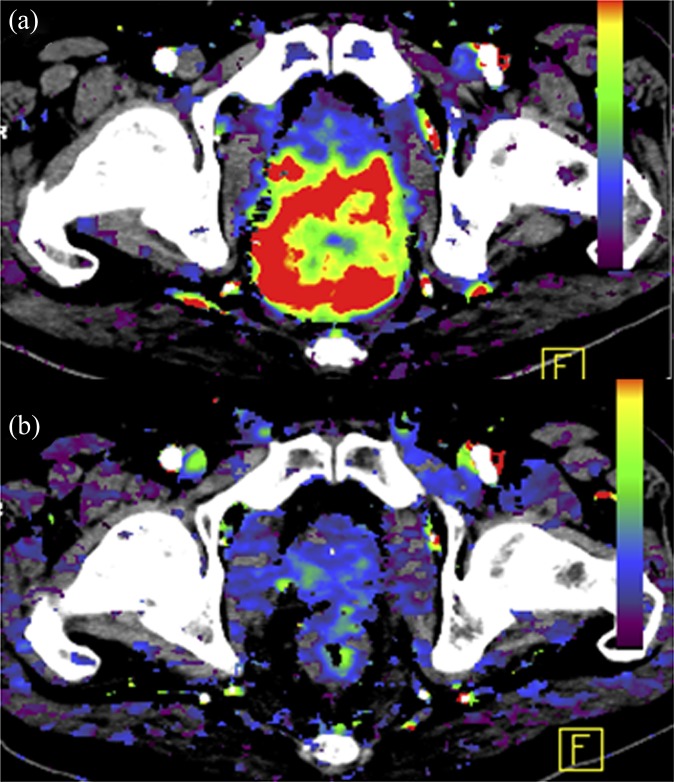
As a result of the differences in the architecture of the vascular network between normal colonic mucosa and colorectal cancer, there are differences also in the imaging characteristics. Tumour BF, BV and vascular permeability are higher than in the normal bowel wall (Figure 4). A typical range of BF values for colorectal cancer is 50–200 ml min−1 100 g−1 tissue vs 10–40 ml min−1 100 g−1 tissue for the normal bowel wall. There are regional differences in normal bowel wall perfusion, which may be related to the underlying function of the bowel, the vascular architecture and underlying supply (superior mesenteric artery, inferior mesenteric artery or other branches); BF is generally lower in the distal than in the proximal large bowel.28 With respect to inflammation, there may be an overlap in vascular parameters between inflammation and tumour. This is to be expected given the underlying pathophysiology: an increase in vascular flow, vessel dilatation, increase in permeability, increase in vascularization (neoangiogenesis) and shunting are seen with acute inflammation. For example, a study of patients with diverticular disease, acute diverticulitis or cancer confirmed that there is a trend for higher blood flow in cancer (80 ml min−1 100 g−1 tissue vs 52 ml min−1 100 g−1 tissue for cancer and diverticulitis, respectively) but with clear overlap in parameter values for these two conditions.29
Figure 4.
Perfusion CT characteristics of the normal rectum (a) compared with a cancer (b).
TUMOUR PHENOTYPING WITH PERFUSION CT IMAGING
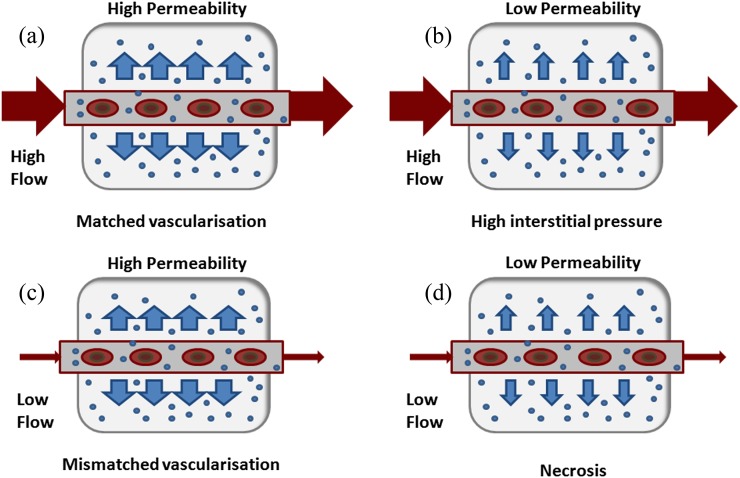
The downstream physiological effects of the underlying molecular biology of tumours may be apparent with imaging. Perfusion CT techniques may provide a global overview of the degree of vascularization within the tumour as well as the associations between individual parameters, BF, BV and vascular leakage, which are inter-related. Different intratumoural patterns may be present (Figure 5). Areas of high blood flow, blood volume and leakage may reflect well-perfused areas, with the presence of shunting and areas of angiogenesis; areas of low blood flow and blood volume and low leakage areas may represent areas of poor vascularization ± necrosis; areas of low blood flow and blood volume and high leakage areas may represent poor perfusion areas with a high degree of angiogenesis. It is hypothesized that this may lead to clonal adaptation with a selection of more aggressive clones. These patterns may coexist within the tumour, reflecting the spatial and functional heterogeneity of the tumour vasculature.
Figure 5.
Different patterns of vascularization within the tumour.
In terms of clinical translation, small clinical studies have shown that more poorly perfused tumours have a poorer outcome. Hayano et al30,31 have shown in rectal cancers (n = 44) and oesophageal cancers (n = 31) that patients with poorly perfused tumours (<40 and <50 ml min−1 100 g−1 tissue, respectively) are more likely to have a poorer overall survival (p ≤ 0.001). Similarly, we have shown that colorectal tumours with a lower perfusion at staging and planned for curative surgery have a greater tendency for subsequent metastatic disease.32 Patients with these tumours may also have a poorer overall survival. In this scenario, extravascular extracellular volume may also be a relevant measure, as demonstrated by Koh et al.33 A hypothesis for why lower extravascular extracellular volume tumours have a poorer prognosis relates to the higher grade, differentiation and larger cellular volume these tumours are likely to have.
The generalizability of these findings to more widespread clinical practice is an important issue. With respect to the prognostic value of perfusion CT in colorectal cancer, this is currently undergoing evaluation as part of the National Institute for Health Research Health Technology Assessment-funded PROSPeCT study, which is in progress and aims to recruit 370 patients with primary colorectal cancer without metastatic disease at staging. To date, there have been few data from multicentre studies of perfusion CT in oncology outside of the therapy response setting, and this will provide invaluable information.
A further area of development is the integration of perfusion CT with positron emission tomography (PET) imaging, which has been facilitated by current generation integrated PET-CT scanners that allow helical volumetric perfusion CT imaging. This provides an opportunity to assess different physiological aspects, e.g. glucose metabolism [18F-fludeoxyglucose (18F-FDG)], integrin expression [18F-labelled arginine–glycine–aspartic acid peptides (18F-RGD-peptides)], hypoxia [18F-labelled fluoromisonidazole (18F-FMISO) or 64Cu-labelled diacetyl-bis (N4-methylthiosemicarbazone) (64Cu-ATSM)], cellular proliferation [18F-labelled fluoro-3′-deoxy-3′-l-thymidine (18F-FLT)] and lipid metabolism (11C-acetate) alongside perfusion, and to explore the alongside perfusion, and to explore the inter-relationships between these physiological features both at staging and in response to therapies that may produce discordant effects.
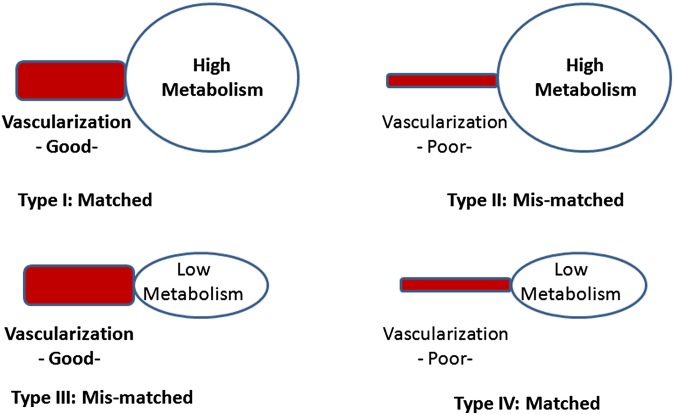
To date, most studies have focused on the relationship between tumour vascular parameters and glucose metabolism. The norm is for delivery and utilization of oxygen and nutrients to be matched, with physiological feedback mechanisms in place to promote this. However in tumours, there may be different scenarios. Vascularization and metabolism may not necessarily be matched (Figure 6), and it has been hypothesized that mismatch between vascularization and metabolism may be an indicator of a more aggressive phenotype. Tumours that are poorly perfused but with high metabolism may reflect an adaptation to intratumoral hypoxia and may be more resistant to treatment or belie a poorer prognosis.34
Figure 6.
Different patterns of vascularization and metabolism within the tumour.
In support of this, in colorectal cancer, a negative association between BF/maximum standardized uptake value (SUVmax) and higher hypoxia-inducible factor 1 (HIF-1) and VEGF expression has been shown, i.e. tumours with a lower BF/SUVmax ratio are more likely to express HIF-1 and VEGF.35 Preliminary studies have also suggested that the relationship between vascularization and metabolism is complex depending on the tumour stage and tumour type. In colorectal cancer, vascularization and metabolism are more likely to be matched in higher than in lower stage cancers, unlike lung cancers where mismatch occurs with increasing stage.
ASSESSING THERAPY RESPONSE WITH PERFUSION CT
Quantitative parameters derived from perfusion CT have a role in monitoring the effects of a variety of treatments that affect the tumour vasculature. These include chemotherapy with standard and novel agents (antiangiogenic drugs, vascular disrupting agents and immunotherapy), radiotherapy and interventional oncological procedures. These therapies typically result in a reduction in measured vascular parameters as a consequence of treatment (Figure 7). During therapy or in the immediate post-therapy period, there may be a more variable vascular effect, depending on the therapeutic mechanism of action (Table 3).
Figure 7.
Decrease in vascularization of the primary tumour before (a) and after (b) chemoradiation. F, front.
Table 3.
Vascular effects of systemic and locoregional therapies
| Therapy | Perfusion CT parameter |
||
|---|---|---|---|
| Blood flow | Blood volume | Vascular leakage | |
| Systemic therapies | |||
| Cytotoxic chemotherapy | |||
| Acute effects | Unchanged | Unchanged | Unchanged |
| Chronic effects | Decrease | Decrease | Decrease |
| Unchanged | |||
| Antiangiogenics | |||
| Acute effects | Increase | Increase | Decrease |
| Unchanged | Unchanged | ||
| Chronic effects | Decrease | Decrease | Decrease |
| Locoregional therapies | |||
| Radiotherapy | |||
| Acute effects | Increase | Increase | Increase |
| Chronic effects | Decrease | Decrease | Decrease |
| Interventional | |||
| Radiofrequency ablation | Decrease | Decrease | Decrease |
| Transarterial chemoembolization | Decrease | Decrease | Decrease |
| Absent | Absent | Absent | |
With standard chemotherapy, which affects actively replicating cells via DNA damage or interruption of DNA repair, this effect is thought to reflect the loss of angiogenic cytokine support following cell death. With antiangiogenic therapies, differing vascular effects may be seen depending on the mechanism of action of the drug under investigation and the timing of the scan. An initial effect may be a decrease in vascular permeability and a reduction in interstitial fluid pressure, with normalization of function of the vasculature resulting in a transient increase in tumour BF.36 In the longer term, with subsequent pruning of the vasculature, a reduction in BF, BV and vascular permeability may be elicited. With vascular disrupting agents, which target the proliferating immature vasculature ± the mature vasculature, a rapid shutdown in tumour vascularization may occur that is usually transient and reversible within 24–48 h. This may be followed by a rebound revascularization.37 With radiotherapy, the acute effects are related to an initial inflammatory effect; the permeability is related to microvascular damage, which can lead to tumour shrinkage.38 With interventional procedures, perfusion CT parameters may provide evidence of effective treatment or the need for further procedural attempts for optimal therapeutic effect.39
With respect to primary colorectal cancer, there have been a few published studies. In the neoadjuvant setting, chemoradiation has been shown to decrease BF, BV and PS. The degree of reduction in blood flow has typically been >40%.40–42 Similarly, for the antiangiogenic agent, bevacizumab, a monoclonal antibody targeted at VEGF, a reduction of up to 40% has been seen in vascularization.43
ASSESSMENT OF TUMOUR VASCULAR HETEROGENEITY
It is recognized that the tumour vasculature is architecturally and functionally heterogeneous. Although the vascular volume is typically <10% of the total tumour volume, changes in vascularization that occur spatially and temporally are relevant particularly with respect to quantification, where a change in quantified parameters is used to determine a vascular response/non-response. One of the limitations of current software platform methods is the reliance on a global mean value for BF, BV or vascular leakage. This clearly underestimates the extent of spatial heterogeneity. While histogram analysis can provide some information regarding the spread of data, it does not provide spatial information.44 There has been an increasing interest in modelling methods such as fractal analysis that may better describe the spatial pattern of vascularization. Fractal dimension (FD) refers to how an object fills space. Proof of principle studies have indicated the feasibility of using two-dimensional and three-dimensional techniques for perfusion CT maps and have shown that the technique is reproducible45 and that the FD is higher for tumours than for normal bowel.46 To date, there have been limited data on its performance in therapy response settings. Temporal changes in vascularization may also occur related to fluctuations in vascular function. Assessment of baseline reproducibility, where two scans are performed prior to therapy and the variations in vascular parameters between the two scans are assessed, remains a way of demonstrating how much this variation is on a per patient basis.47 This is particularly relevant in therapy response settings.
CONCLUSION
Perfusion CT is one of a number of functional imaging techniques available to us in clinics that allows us to quantify tumour vascularization. The technique is robust and, with the current state-of-the-art technology, whole tumour BF, BV and vascular leakage can be investigated. As we move towards the future, it may allow us to better phenotype the tumour and combined with PET imaging may be a more powerful tool. As technological improvements in CT continue to evolve, this will further extend clinical applications.
FUNDING
The authors hold a research grant from the National Institute for Health Research Health Technology Assessment Programme (PROSPeCT: NIHR HTA 09/22/49). The authors also acknowledge financial support from the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust and from the King's College London/University College London Comprehensive Cancer Imaging Centre funded by Cancer Research UK and the Engineering and Physical Sciences Research Council in association with the Medical Research Council and Department of Health.
REFERENCES
- 1.Globocan 2008. Colorectal cancer fact sheet. Lyons, France: International Agency for Research on Cancer [updated 6 December 2013; cited 10 December 2013]. Available from:http://globocan.iarc.fr/factsheets/cancers/colorectal.asp [Google Scholar]
- 2.Miles WE. A method of performing abdominoperineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon. Lancet 1908; 2: 1812–13. [Google Scholar]
- 3.Heald RJ, Ryall RDH. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–82. [DOI] [PubMed] [Google Scholar]
- 4.Martling AL, Holm T, Rutqvist LE, Moran BJ, Heald RJ, Cedermark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000; 356: 93–6. [DOI] [PubMed] [Google Scholar]
- 5.Improved survival with preoperative radiotherapy in respectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med 1997; 336: 980–87. doi: 10.1056/NEJM199704033361402 [DOI] [PubMed] [Google Scholar]
- 6.Kapiteijn E, Kranenbarg EK, Steup WH, Taat CW, Rutten HJ, Wiggers T, et al. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg 1999; 165: 410–20. doi: 10.1080/110241599750006613 [DOI] [PubMed] [Google Scholar]
- 7.Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008; 26: 3687–94. doi: 10.1200/JCO.2007.15.3858 [DOI] [PubMed] [Google Scholar]
- 8.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350: 2335–42. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 2011; 29: 2011–19. doi: 10.1200/JCO.2010.33.5091 [DOI] [PubMed] [Google Scholar]
- 10.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013; 369: 1023–34. doi: 10.1056/NEJMoa1305275 [DOI] [PubMed] [Google Scholar]
- 11.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319: 525–32. doi: 10.1056/NEJM198809013190901 [DOI] [PubMed] [Google Scholar]
- 12.Shen L, Toyota M, Kondo Y, Lin E, Zhang L, Guo Y, et al. Integrated genetic and epigenetic analysis identifies three different subclasses of colon cancer. Proc Natl Acad Sci U S A 2007; 104: 18654–9. doi: 10.1073/pnas.0704652104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bertagnolli MM, Redston M, Compton CC, Niedzwiecki D, Mayer RJ, Goldberg RM, et al. Microsatellite instability and loss of heterozygosity at chromosomal location 18q: prospective evaluation of biomarkers for Stage II and Stage III colon cancer—a study of CALGB 9581 and 89803. J Clin Oncol 2011; 29: 3153–62. doi: 10.1200/JCO.2010.33.0092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005; 23: 609–18. doi: 10.1200/JCO.2005.01.086 [DOI] [PubMed] [Google Scholar]
- 15.Ajiki T, Fujimori T, Ikehara H, Saitoh Y, Maeda S. K-ras gene mutation related to histological atypias in human colorectal adenomas. Biotech Histochem 1995; 70: 90–4. [DOI] [PubMed] [Google Scholar]
- 16.Konerding MA, Fait E, Gaumann A. 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer 2001; 84: 1354–62. doi: 10.1054/bjoc.2001.1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goh V, Padhani AR, Rasheed S. Functional imaging of colorectal cancer angiogenesis. Lancet Oncol 2007; 8: 245–55. doi: 10.1016/S1470-2045(07)70075-X [DOI] [PubMed] [Google Scholar]
- 18.Miles KA, Lee TY, Goh V, Klotz E, Cuenod C, Bisdas S, et al. ; Experimental Cancer Medicine Centre Imaging Network Group. Current status and guidelines for the assessment of tumour vascular support with dynamic contrast-enhanced computed tomography. Eur Radiol 2012; 22: 1430–41. [DOI] [PubMed] [Google Scholar]
- 19.Mandeville HC, Ng QS, Daley FM, Barber PR, Pierce G, Finch J, et al. Operable non-small cell lung cancer: correlation of volumetric helical dynamic contrast-enhanced CT parameters with immunohistochemical markers of tumor hypoxia. Radiology 2012; 264: 581–9. doi: 10.1148/radiol.12111505 [DOI] [PubMed] [Google Scholar]
- 20.Tateishi U, Kusumoto M, Nishihara H, Nagashima K, Morikawa T, Moriyama N. Contrast-enhanced dynamic computed tomography for the evaluation of tumor angiogenesis in patients with lung carcinoma. Cancer 2002; 95: 835–42. doi: 10.1002/cncr.10730 [DOI] [PubMed] [Google Scholar]
- 21.Sauter AW, Winterstein S, Spira D, Hetzel J, Schulze M, Mueller M, et al. Multifunctional profiling of non-small cell lung cancer using 18F-FDG PET/CT and volume perfusion CT. J Nucl Med 2012; 53: 521–9. doi: 10.2967/jnumed.111.097865 [DOI] [PubMed] [Google Scholar]
- 22.Reiner CS, Roessle M, Thiesler T, Eberli D, Klotz E, Frauenfelder T, et al. Computed tomography perfusion imaging of renal cell carcinoma: systematic comparison with histopathololgical angiogenic and prognostic markers. Invest Radiol 2013; 48: 183–91. doi: 10.1097/RLI.0b013e31827c63a3 [DOI] [PubMed] [Google Scholar]
- 23.Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT—do tumor perfusion measurements reflect angiogenesis? Radiology 2008; 249: 510–17. doi: 10.1148/radiol.2492071365 [DOI] [PubMed] [Google Scholar]
- 24.Dighe S, Blake H, Jeyadevan N, Castellano I, Koh DM, Orton M, et al. Perfusion CT vascular parameters do not correlate with immunohistochemically derived microvessel density count in colorectal tumors. Radiology 2013; 268: 400–10. doi: 10.1148/radiol.13112460 [DOI] [PubMed] [Google Scholar]
- 25.Li ZP, Meng QF, Sun CH, Xu DS, Fan M, Yang XF, et al. Tumor angiogenesis and dynamic CT in colorectal carcinoma: radiologic-pathologic correlation. World J Gastroenterol 2005; 11: 1287–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng ST, Sun CH, Li ZP, Mak HK, Peng ZP, Guo HY, et al. Evaluation of angiogenesis in colorectal carcinoma with multidetector-row CT multislice perfusion imaging. Eur J Radiol 2010; 75: 191–6. doi: 10.1016/j.ejrad.2009.04.058 [DOI] [PubMed] [Google Scholar]
- 27.Kim JW, Jeong YY, Chang NK, Heo SH, Shin SS, Lee JH, et al. Perfusion CT in colorectal cancer: comparison of perfusion parameters with tumor grade and microvessel density. Korean J Radiol 2012; 13(Suppl. 1): S89–97. doi: 10.3348/kjr.2012.13.S1.S89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan S, Goh V, Tam E, Wellsted D, Halligan S. Perfusion CT assessment of the colon and rectum: feasibility of quantification of bowel wall perfusion and vascularization. Eur J Radiol 2012; 81: 821–4. doi: 10.1016/j.ejrad.2011.02.033 [DOI] [PubMed] [Google Scholar]
- 29.Goh V, Halligan S, Taylor SA, Burling D, Bassett P, Bartram CI. Differentiation between diverticulitis and colorectal cancer: quantitative CT perfusion measurements versus morphologic criteria—initial experience. Radiology 2007; 242: 456–62. [DOI] [PubMed] [Google Scholar]
- 30.Hayano K, Shuto K, Koda K, Yanagawa N, Okazumi S, Matsubara H. Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis Colon Rectum 2009; 52: 1624–9. doi: 10.1007/DCR.0b013e3181afbd79 [DOI] [PubMed] [Google Scholar]
- 31.Hayano K, Okazumi S, Shuto K, Matsubara H, Shimada H, Nabeya Y, et al. Perfusion CT can predict the response to chemoradiation therapy and survival in esophageal squamous cell carcinoma: initial clinical results. Oncol Rep 2007; 18: 901–8. [PubMed] [Google Scholar]
- 32.Goh V, Halligan S, Wellsted DM, Bartram CI. Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol 2009; 19: 79–89. doi: 10.1007/s00330-008-1128-1 [DOI] [PubMed] [Google Scholar]
- 33.Koh TS, Ng QS, Thng CH, Kwek JW, Kozarski R, Goh V. Primary colorectal cancer: use of kinetic modeling of dynamic contrast-enhanced CT data to predict clinical outcome. Radiology 2013; 267: 145–54. doi: 10.1148/radiol.12120186 [DOI] [PubMed] [Google Scholar]
- 34.Padhani AR, Miles KA. Multiparametric imaging of tumor response to therapy. Radiology 2010; 256: 348–64. doi: 10.1148/radiol.10091760 [DOI] [PubMed] [Google Scholar]
- 35.Goh V, Engledow A, Rodriguez-Justo M, Shastry M, Peck J, Blackman G, et al. The flow-metabolic phenotype of primary colorectal cancer: assessment by integrated 18F-FDG PET/perfusion CT with histopathologic correlation. J Nucl Med 2012; 53: 687–92. doi: 10.2967/jnumed.111.098525 [DOI] [PubMed] [Google Scholar]
- 36.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 2005; 307: 58–62. doi: 10.1126/science.1104819 [DOI] [PubMed] [Google Scholar]
- 37.Hinnen P, Eskens FA. Vascular disrupting agents in clinical development. Br J Cancer 2007; 96: 1159–65. doi: 10.1038/sj.bjc.6603694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. doi: 10.1126/science.1082504 [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Ma DQ, He W, Zhang BF, Zhao LQ. Computed tomography perfusion in evaluating the therapeutic effect of transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2008; 14: 5738–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Curvo-Semedo L, Portilha MA, Ruivo C, Borrego M, Leite JS, Caseiro-Alves F. Usefulness of perfusion CT to assess response to neoadjuvant combined chemoradiotherapy in patients with locally advanced rectal cancer. Acad Radiol 2012; 19: 203–13. doi: 10.1016/j.acra.2011.10.019 [DOI] [PubMed] [Google Scholar]
- 41.Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology 2007; 244: 486–93. doi: 10.1148/radiol.2442061189 [DOI] [PubMed] [Google Scholar]
- 42.Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 2005; 234: 785–92. doi: 10.1148/radiol.2343040286 [DOI] [PubMed] [Google Scholar]
- 43.Willett CG, Boucher Y, Di Tomaso E, Duda DG, Munn LL, Tong RT, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 2004; 10: 145–7. doi: 10.1038/nm988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, et al. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging 2012; 3: 573–89. doi: 10.1007/s13244-012-0196-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanghera B, Banerjee D, Khan A, Simcock I, Stirling JJ, Glynne-Jones R, et al. Reproducibility of 2D and 3D fractal analysis techniques for the assessment of spatial heterogeneity of regional blood flow in rectal cancer. Radiology 2012; 263: 865–73. doi: 10.1148/radiol.12111316 [DOI] [PubMed] [Google Scholar]
- 46.Goh V, Sanghera B, Wellsted DM, Sundin J, Halligan S. Assessment of the spatial pattern of colorectal tumour perfusion estimated at perfusion CT using two-dimensional fractal analysis. Eur Radiol 2009; 19: 1358–65. doi: 10.1007/s00330-009-1304-y [DOI] [PubMed] [Google Scholar]
- 47.Goh V, Halligan S, Hugill JA, Bartram CI. Quantitative assessment of tissue perfusion using MDCT: comparison of colorectal cancer and skeletal muscle measurement reproducibility. AJR Am J Roentgenol 2006; 187: 164–9. doi: 10.2214/AJR.05.0050 [DOI] [PubMed] [Google Scholar]