Abstract
Breast cancer constitutes about one-quarter of all cancers and is the leading cause of cancer death in women. To reduce breast cancer mortality, mammographic screening programmes have been implemented in many Western countries. However, these programmes remain controversial because of the associated radiation exposure and the need for improvement in terms of diagnostic accuracy. Phase-contrast imaging is a new X-ray-based technology that has been shown to provide enhanced soft-tissue contrast and improved visualization of cancerous structures. Furthermore, there is some indication that these improvements of image quality can be maintained at reduced radiation doses. Thus, X-ray phase-contrast mammography may significantly contribute to advancements in early breast cancer diagnosis. Feasibility studies of X-ray phase-contrast breast CT have provided images that allow resolution of the fine structure of tissue that can otherwise only be obtained by histology. This implies that X-ray phase-contrast imaging may also lead to the development of entirely new (micro-) radiological applications. This review provides a brief overview of the physical characteristics of this new technology and describes recent developments towards clinical implementation of X-ray phase-contrast imaging of the breast.
CHALLENGES IN CURRENT BREAST CANCER DIAGNOSIS AND SCREENING
Breast cancer is the most common cancer in women in both the developed and the developing world.1 In the USA and European countries about one in eight women will develop invasive breast cancer during the course of her lifetime.2 Although mortality has continuously declined over the past decades, probably as a consequence of cancer screening programmes and improved systemic therapy, breast cancer continues to be the most common cause of cancer death in women.3
Since the disease stage at the time of diagnosis appears to be one of the most critical factors for survival, early detection by breast cancer screening remains one of the cornerstones of breast cancer control. It is estimated that 2–7 of 15 expected breast cancer deaths can be avoided per 1000 screening participants, thus achieving a mortality reduction of 20–25%.4 Nevertheless, the rate of missed lesions and the rate of false-positive recalls in the existing screening programmes remain crucial. In recent years, digital full-field mammography has significantly improved mammography image quality and increasingly replaced conventional film mammography. However, the sensitivity of digital screening mammography for lesion detection remains fairly low at about 62–88%.5 In specific subgroups, the sensitivity is even lower, e.g. 60% in females younger than 50 years, and as low as 33% in women at elevated familial risk for breast cancer.6 Hence, the early and reliable detection of breast carcinoma remains a challenge.
Tomosynthesis is a procedure developed from digital mammography for slice examination of breasts, which limits the effects of overlapping tissue. Recent screening studies have shown moderate superiority of two-view tomosynthesis to conventional mammography.7–10 Recent research efforts have furthermore focused on the development of CT imaging of the breast.11,12 Breast CT meets many demands as an ideal tool for breast examination, such as full three-dimensional capability, quantitative tissue density assessment, high isotropic spatial resolution, reproducibility and standardization, low patient dose and acceptable costs. However, like mammography and tomosynthesis, the technology is limited by the intrinsically low soft-tissue contrast, which poses limits on the accurate differentiation of glandular and tumour tissue.
Alternative techniques for breast cancer diagnosis include ultrasound and MRI. In recent years, ultrasound has become an outstanding method to investigate breast disease in young females13 but remains time consuming, operator dependent and of limited value in breast involution. Contrast-enhanced breast MRI has been shown to be highly sensitive for the detection of cancer lesions.6,14 However, the specificity of breast MRI is still relatively low, leading to possible overdiagnosis and unnecessary biopsy.15 Moreover, breast MRI requires exclusion of patients with contraindications for MR examination or administration of intravenous contrast agent. Thus, these techniques are time consuming and cost intensive and remain not applicable to a breast cancer screening setting.
Phase-contrast imaging
Phase-contrast imaging is fundamentally a new X-ray-based imaging method that exploits the phase shift that occurs when X-ray waves pass through different components of a material.16 Recent experimental studies have shown that phase-contrast imaging yields improved soft-tissue contrast and can reveal the fine structure of tissue that is invisible to absorption-based imaging. If successfully transferred to a clinical setting, the technology may therefore have a significant impact on breast cancer diagnosis, characterization and treatment. This article briefly summarizes the different technical approaches for the implementation of this novel method and reviews recent advances towards clinical implementation in breast imaging.
PHASE-CONTRAST TECHNOLOGY
When X-rays penetrate matter, both attenuation and phase-shift effects of the X-ray wave are defined by the complex index of refraction n. The refractive index can be written as  , where δ is related to the phase shift and iβ determines the attenuation. The propagation of an electromagnetic wave through a medium with refractive index n is described by
, where δ is related to the phase shift and iβ determines the attenuation. The propagation of an electromagnetic wave through a medium with refractive index n is described by
which can be expanded to
The first and second exponential terms describe the shift in phase and decrease in amplitude of a wave penetrating a medium of refractive index n, respectively.
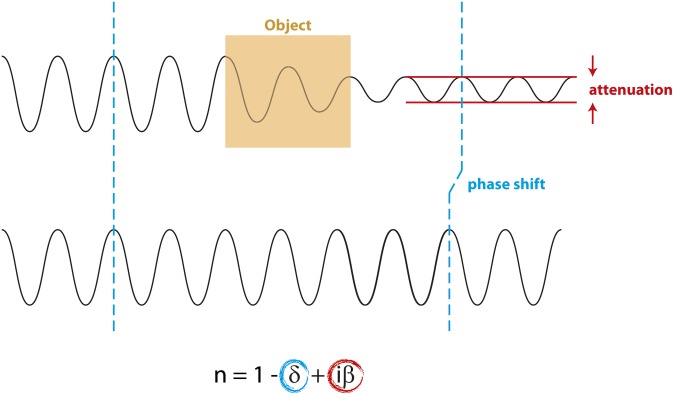
Conventional X-ray-based imaging technology relies entirely on the detection of the attenuation (i.e. the decrease in amplitude) of electromagnetic X-ray waves (Figure 1). The differences in attenuation, and thus image contrast, depend on tissue density: the denser a certain type of tissue, the less radiation will penetrate. Additionally, the X-ray wave penetrating a medium with refractive index n gets phase shifted relative to the original X-ray wave (Figure 1). This phase shift is detected in phase-contrast imaging. For more detail, also see Als-Nielsen and McMorrow.16
Figure 1.
X-rays encounter changes in intensity and phase when traversing an object. Compared with an X-ray wave bypassing the object (bottom), an X-ray wave travelling through an object (top) experiences changes in amplitude (attenuation) and phase. The degree of attenuation roughly depends on material density; the strength of the phase shift on the electron density. The complex index of refraction n for X-ray waves can be expressed as  , where δ is proportional to the phase shift and iβ is proportional to absorption. For biological tissues and the X-ray energies typically used in breast imaging, δ is significantly larger than iβ.
, where δ is proportional to the phase shift and iβ is proportional to absorption. For biological tissues and the X-ray energies typically used in breast imaging, δ is significantly larger than iβ.
The tissue density differences in breast tissue are small, and the contrast obtained by attenuation (absorption) is thus limited. Therefore, the X-ray energies used in mammographic imaging are typically low (20–30 kV) compared with conventional X-ray imaging. At these energies, small differences in the refractive index have a higher impact on the phase than on the absorption (Figure 1). Thus, phase-shift-based imaging has the potential to improve image contrast compared with pure absorption-based imaging. However, just like absorption, phase-shift effects are smaller at higher X-ray energies and whether phase-contrast imaging can be realistically performed at very high energies (i.e. very low mean glandular doses) is still a matter of debate.17,18 Nevertheless, studies at synchrotron sources suggest that phase-contrast imaging can be performed at radiation doses compatible with clinical imaging.19,20 Thus, in a clinical setting, phase-contrast imaging could be exploited either to yield images of enhanced contrast at equal dose or to provide images of equal quality at reduced dose.
DETECTING PHASE INFORMATION
Several methods have been described to effectively extract X-ray phase information for phase-contrast imaging. The most common techniques include propagation-based imaging (PBI), analyser-based imaging, crystal interferometry and grating interferometry. A brief description of these methods is provided below; for a more detailed review that exceeds the scope of this article, see Momose21 and Bravin et al.22
Crystal interferometry
Crystal interferometry is one of the oldest methods of X-ray phase detection (Figure 2a).23,24 It requires a perfect silicon crystal that splits the X-ray beam, with only one-half of the beam traversing the object. Both beams are reunited in front of the detector and phase shifts within the object can be detected based on their interferences.23 The technique is extremely sensitive to the smallest phase shifts induced in the object, but also to environmental disturbances, and is characterized by a small field of view. These difficulties lead to the fact that it is practically no longer widely used in experimental breast imaging. Furthermore, crystal interferometry requires a monochromatic parallel X-ray beam, hampering future clinical implementation.
Figure 2.
Examples of phase detection techniques. (a) Crystal interferometry. A monochromatic X-ray beam is split by a silicon crystal. One-half of the beam traverses the object before both beams are reunited in front of the detector. (b) Propagation-based imaging. Partially coherent (polychromatic) X-rays pass through the object before meeting the detector. If the distance between object and detector is small (d1), only absorption information is recorded in the resulting image. Increasing the distance between object and detector (d2) yields a composite image that contains both absorption and phase information. (c) Analyser-based imaging. Phase information is extracted by a silicon analyser positioned in the X-ray beam. (d) Grating-based interferometry set-up with a conventional X-ray tube source. X-rays originating from the tube source are masked by a source grating before traversing the object. Subsequent phase and analyser gratings allow detection of pure phase, dark-field and absorption information, which can be calculated from the intensities recorded by the detector.
Propagation-based imaging
PBI requires the simplest set-up and is therefore considered a very robust method suitable for clinical implementation (Figure 2b).25,26 Indeed, all published patient trials of phase-contrast imaging of the breast to date have been performed using this technique, which is able to detect X-ray phase shifts by simply increasing the distance between object and detector20,27–29 (Figure 2b). However, the method requires a large degree of coherence in the X-ray beam and typically produces a small field of view, as it requires detectors with pixel sizes in the few microns range (or alternatively sources with a source size in the few microns range).
Analyser-based imaging/diffraction-enhanced imaging
Analyser-based imaging (ABI)/diffraction-enhanced imaging (DEI) yields relatively large fields of view (in one direction), but depends on an analyser crystal for effective phase detection, which renders the set-up very sensitive to vibrations and other disturbances, and requires monochromatic and parallel X-ray radiation (Figure 2c).30,31 Using synchrotron radiation, the technology has been successfully used to perform a number of important feasibility studies of phase-contrast breast imaging.18,32–35
Grating interferometry
Grating interferometry with synchrotron radiation requires two gratings placed between an object and detector to extract X-ray phase information.21 Implementation of a third grating between the X-ray source and object has allowed the technique to be successfully applied to conventional clinical X-ray tubes36 (Figure 2d). Grating-based interferometry can cope with the relatively broad spectra provided by conventional X-ray sources and yields fields of view of up to several centimetres in diameter. For these reasons, it is currently the most efficient phase-contrast imaging method when applied to clinical X-ray sources. Furthermore, the technique allows for quantitative imaging.37–39 Also, grating interferometry intrinsically provides access to a third contrast modality, X-ray dark-field imaging, which enhances microstructures in a sample due to X-ray scattering.40
CURRENT DIRECTIONS IN PHASE-CONTRAST IMAGING OF THE BREAST
Given the additional contrast provided by phase-contrast imaging, the technology may improve current diagnostic technologies or may allow the development of novel modalities for breast cancer characterization. Consequently, recent research efforts in the field can be roughly classified into (1) studies aiming to improve soft-tissue contrast and hence clinical performance in mammography and (2) feasibility analyses exploring maximum achievable results in tissue fine-structure characterization using phase-contrast CT on ex vivo breast specimens.
Phase-contrast mammography
Studies on excision specimens recorded with ABI at a synchrotron radiation source suggest that phase-contrast mammography allows better visualization of structural details than conventional digital mammography.34,35 In particular, the images in these analyses accurately reveal fine collagen strands, and observers were able to precisely delineate boundaries between glandular and adipose tissue in phase contrast, but not in conventional digital specimen radiography.34 These studies furthermore suggest that remodelling of collagen during cancer growth can be correlated to contrast changes in ABI.35 However, the individual contributions of the high-quality X-ray beam and the phase-contrast signal to the improved mammographic images could not be distinguished in these analyses. Furthermore, PBI at synchrotron sources shows that phase-contrast mammographic images of breast tumour excision samples are able to outperform corresponding absorption-based images, suggesting that implementation of the technology could allow a certain dose reduction in clinical mammography.17
Williams et al41 assessed the performance of PBI mammography of excision samples and mastectomies taken from 72 patients applying a conventional microfocus X-ray tube. Evaluation of image quality by blinded scorers showed that the propagation-based images in this trial resulted in higher scoring outcomes than corresponding absorption-based images recorded by the same system.41 Furthermore, image analysis of five mastectomy samples by grating interferometry on a conventional X-ray tube source suggests that combined phase-contrast, absorption and dark-field images may improve diagnostic capabilities, including refined visualization of small tumours and tumour boundaries.42
Initial patient trials of phase-contrast mammography have been devised to assess whether the improved image quality suggested by studies on ex vivo specimens translates to improved clinical performance.20,27–29,43 A recent clinical trial of phase-contrast mammography with synchrotron radiation was performed at the SYRMEP beamline of the Elettra synchrotron in Trieste, Italy.20,43 All 47 patients who completed this trial had previously undergone digital mammography and ultrasound examinations at a local hospital but received an unclear diagnosis. In this patient population, the reference clinical mammograms had a specificity and sensitivity of 52% and 69%, respectively, whereas PBI mammography at the synchrotron resulted in specificity and sensitivity values of 94% and 81%.20 In each case, either biopsy or 1-year follow-up was used as the standard of reference. This outcome suggests that PBI mammography with synchrotron radiation may increase the number of true negatives and may therefore be particularly suitable as a second-level examination following clinical mammography. However, a more careful clinical evaluation of a population with lower prevalence and including independent readings and receiver operating characteristic analyses will be necessary to fully judge the technology's added value. Furthermore, improved image quality in this trial (Figure 3) can be partially attributed to improved X-ray beam characteristics at the synchrotron, such as monochromaticity, non-divergence and spatial coherence, rather than the phase effect itself. This is in line with clinical trials performed with a prototype digital full-field phase-contrast mammography system using a conventional tube X-ray source and PBI technology.27–29 Initially, receiver operating characteristics curves for the detection of microcalcifications or masses in a pilot trial of 38 patients suggested improved performance compared with screen–film mammograms.29 Furthermore, the system appeared to improve visualization of subtle density variations or abnormal fibrous structures in highly dense breasts.28 However, when applied to an asymptomatic screening population of >3000 patients, digital full-field phase-contrast mammography did not result in statistically different recall or cancer detection rates.27 It will be interesting to evaluate the performance of other phase detection techniques, such as grating interferometry, in a clinical non-synchrotron setting. A first prototype system for potential clinical trials, using coded aperture detection in combination with grating interferometry, is currently under development.44 An example of a differential phase image of an ablated breast recorded by grating-based mammography is shown in Figure 4. The image highlights the power of phase-contrast imaging in enhancing the outlines of certain tissue structures, such as collagen strands (K Scherer and S Grandl, 2013, personal communication).
Figure 3.
Comparison of clinical mammograms with propagation-based imaging mammograms recorded at a synchrotron source. (a) Mediolateral oblique digital mammography image and (b) corresponding digital zoom image, showing a suspicious mass (arrow). (c) Findings on the synchrotron radiation mammographic image (recorded with propagation-based imaging technology) and (d) corresponding digital zoom image confirm and better depict the spiculated mass. Reproduced with permission from the Radiological Society of North America.20
Figure 4.
Absorption (a), phase-contrast (b) and dark-field (c) grating-based mammography images (craniocaudal) of an ablated breast recorded with a conventional X-ray tube source. The images are of a 52-year-old patient and show the complete remission of an angiosarcoma after neoadjuvant chemotherapy. The differential phase image enhances the edges of tissue structures and thus depicts collagen strands more clearly. The dark-field image is particularly sensitive for calcifications.
Phase-contrast breast CT
CT studies on isolated tumour-bearing breast tissue samples and recorded with synchrotron radiation yield images with excellent soft-tissue contrast compared with absorption-based images of the same specimens.32,33 The improved visibility of tissue morphology and collagen architecture provides evidence that phase-contrast imaging can obtain radiological images with excellent correspondence to histology, suggesting that phase-contrast CT may allow deeper insight into the fine structure of tissue than conventional imaging modalities. This was confirmed by a study that compared the grating interferometry-derived phase-contrast signal in a sample containing invasive ductal carcinoma with that of ductal carcinoma in situ (DCIS). Phase-contrast imaging of this specimen revealed a high-contrast signal outlining the intraductal components and coinciding with the walls of the dilated ductules of the DCIS, as judged by comparison with histopathology.45 Corresponding absorption-based images were unable to resolve these structures (Figure 5). Taken together, these studies imply that phase-contrast CT could assist ex vivo tissue analysis following surgery, when rapid evaluation of tumour boundaries is needed for surgical quality control. Furthermore, if successfully translated to in vivo applications, it may assist breast cancer diagnosis by providing advanced insight into tumour morphology and differentiation between cancerous and unaffected tissues.
Figure 5.
Direct comparison of absorption and phase-contrast breast CT. Experimental absorption-contrast (a) and phase-contrast (b) tomographic images recorded at the European synchrotron radiation facility. The grey-scale bars represent Hounsfield units (absorption contrast) and phase-contrast Hounsfield units (phase contrast), respectively. The sample presented here contains invasive ductal carcinoma, as well as ductal carcinoma in situ. Phase-contrast images reveal contrast differences within the tumour that are not resolved by absorption-based imaging. In particular, circular structures of high phase contrast within ductal carcinoma in situ are better resolved in phase-contrast images and coincide with ductal walls and the basement membrane. Conversely, calcifications are better seen in absorption-based images, underlining the complementary nature of the methods. For more details, see Sztrokay et al.45
Indeed, first steps have been taken towards clinical implementation of dedicated phase-contrast breast CT. For example, Pani et al19 have evaluated the feasibility of breast tomography at the Elettra synchrotron radiation facility, using the same in vivo breast imaging set-up that was used to complete the clinical mammography trial mentioned above. Their analysis of fresh post-mortem whole breast samples from healthy donors shows that phase-contrast breast CT can be recorded at clinically accepted radiation doses.19 More recently, it was shown that ABI/DEI performed at the European synchrotron radiation facility is capable of imaging large whole-breast samples at energies as high as 70 keV.18 Advanced image reconstruction algorithms that can cope with a significantly reduced number of recorded projections allowed further dose reduction in phase-contrast breast CT data sets, down to levels approaching those of current clinical dual-view mammography without loss of phase-contrast image quality.46 Furthermore, detailed theoretical considerations suggest that breast CT applications may indeed benefit from implementation of phase-contrast technology.47 Thus, future patient trials of phase-contrast breast tomography appear feasible. It should be noted, however, that the phase-contrast breast tomography studies mentioned above were carried out at synchrotron X-ray sources, where rotating gantries cannot be implemented. It will thus be exciting to see the results of the first phase-contrast breast CT studies using conventional X-ray tubes. A first study of this kind has recently been published by Grandl et al.48 Based on selected samples, the authors show that grating-based phase-contrast CT at a conventional X-ray source provides complementary information to conventional absorption contrast, albeit at radiation doses far exceeding those deemed clinically acceptable.
DARK-FIELD IMAGING OF BREAST SAMPLES
As briefly mentioned above, in addition to absorption and phase images, grating interferometry also yields a dark-field image. This dark-field image is sensitive to the small-angle scattering ability of the sample and can reveal microstructures within specimens that are smaller than the pixel pitch of the detector.40 Indeed, high contrast in the dark-field signal of mastectomy specimens has been shown to correlate with tumour regions containing microcalcifications.49 In some instances, dark-field imaging was able to reveal micrometre-sized calcifications that were invisible in corresponding absorption and phase-contrast images and in clinical mammography.50 Figure 4 shows an example of a dark-field image of an ablated breast. Calcifications cause a particularly strong signal in this image. Potential future applications of in vivo dark-field mammography may include diagnosing calcified breast tumours, and it may be particularly advantageous for accurate diagnosis of multifocal invasive tumours in dense breasts. However, the diagnostic value of such images still needs to be investigated.
CONCLUSIONS
Despite great improvements, established breast imaging techniques continue to suffer from shortcomings. Mammography faces the problem of tissue superposition and low tumour–tissue contrast, ultrasonography highly depends on the experience of the examiner and MRI requires the application of intravenous contrast agents. The studies above have demonstrated the feasibility and added value of phase-contrast and dark-field imaging of the breast. A clinical trial of phase-contrast mammography at a synchrotron radiation source showed better sensitivity and specificity than reference clinical mammograms. Phase-contrast breast CT analyses have provided images with improved delineation of tumour boundaries and enhanced intratumour soft-tissue contrast compared with conventional images.
Thus, implementation of phase-contrast technology in existing breast imaging set-ups might provide additional information, especially in equivocal findings. Phase-contrast technology might depict tumour spread as well as extension of intraductal carcinoma more precisely, which often exceed the mammographically depicted microcalcifications. So far, improved patient outcome of phase imaging in breast cancer screening has been demonstrated at a synchrotron source.20 Access to synchrotron sources, however, is highly restricted and thus the use of synchrotron radiation does not appear feasible for comprehensive screening programmes or routine analyses. Research within the next decade will thus have to reveal whether improved clinical outcome in phase-contrast breast cancer screening can be maintained at conventional X-ray tubes. Studies using conventional X-ray tubes that suggest added clinical value have so far largely been performed at X-ray doses and measurement times that far exceed accepted clinical values.42,48 However, technical advancements (e.g. optimized detectors, X-ray tubes or image reconstruction algorithms) of the existing highly experimental systems may lead to significant improvement of these parameters. Future research efforts aiming at in vivo clinical implementation of phase-contrast imaging of the breast should thus focus on transferring the technology from synchrotron to conventional X-ray sources and on optimizing set-ups for minimal radiation exposure.
Furthermore, phase-contrast imaging might serve as a tool for the examination of ex vivo specimens. The re-excision rate after breast cancer surgery as a result of incomplete excision can reach 40%.51–53 Since tumour involvement of resection margins is associated with an elevated risk of local recurrence, intraoperative histological analysis of frozen sections for fast evaluation of margin involvement is the current diagnostic tool. However, diagnostic accuracy is inferior to the analysis of permanent sections.54 In trials assessing the value of micro-CT as a complementary method to histopathology in evaluation of resection margin involvement, margin involvement was slightly overestimated.55 The improved soft-tissue contrast and visibility of fine structures as offered by phase-contrast CT might provide additional information in ex vivo diagnosis of breast tissue.
FUNDING
This work was supported by the DFG Cluster of Excellence, Munich Centre for Advanced Photonics (MAP, EXC158).
ACKNOWLEDGMENTS
The authors acknowledge the support by the DFG Cluster of Excellence, Munich Centre for Advanced Photonics (MAP, EXC158), the DFG Gottfried Wilhelm Leibniz programme and the European Research Council (ERC, FP7, StG 240142). Part of this work (Figures 4 and 5) was carried out with the support of the Karlsruhe Nano Micro Facility (KNMF, www.kit.edu/knmf), a Helmholtz Research Infrastructure at Karlsruhe Institute of Technology (KIT).
REFERENCES
- 1.GLOBOCAN. Cancer fact sheets: breast cancer. Lyons, France: International Agency for Research on Cancer; 2012. Available from:http://globocan.iarc.fr/pages/fact_sheets_cancer.aspx [Google Scholar]
- 2.National Cancer Institute Surveillance epidemiology and end results (SEER) stat fact sheets: breast cancer. 2009. Bethesda, MD: NCI; 2012. Available from: http://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
- 3.Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med 2005; 353: 1784–92. doi: 10.1056/NEJMoa050518 [DOI] [PubMed] [Google Scholar]
- 4.Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer 2013; 108: 2205–40. doi: 10.1038/bjc.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisano ED, Gatsonis C, Hendrick E, Yaffe M, Baum JK, Acharyya S, et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med 2005; 353: 1773–83. doi: 10.1056/NEJMoa052911 [DOI] [PubMed] [Google Scholar]
- 6.Kuhl C, Weigel S, Schrading S, Arand B, Bieling H, Konig R, et al. Prospective multicenter cohort study to refine management recommendations for women at elevated familial risk of breast cancer: the EVA trial. J Clin Oncol 2010; 28: 1450–7. doi: 10.1200/JCO.2009.23.0839 [DOI] [PubMed] [Google Scholar]
- 7.Teertstra HJ, Loo CE, van den Bosch MAAJ, van Tinteren H, Rutgers EJT, Muller SH, et al. Breast tomosynthesis in clinical practice: initial results. Eur Radiol 2010; 20: 16–24. doi: 10.1007/s00330-009-1523-2 [DOI] [PubMed] [Google Scholar]
- 8.Gennaro G, Toledano A, di Maggio C, Baldan E, Bezzon E, La Grassa M, et al. Digital breast tomosynthesis versus digital mammography: a clinical performance study. Eur Radiol 2010; 20: 1545–53. doi: 10.1007/s00330-009-1699-5 [DOI] [PubMed] [Google Scholar]
- 9.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. [DOI] [PubMed] [Google Scholar]
- 10.Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14: 583–9. doi: 10.1016/S1470-2045(13)70134-7 [DOI] [PubMed] [Google Scholar]
- 11.Boone JM, Lindfors KK. Breast CT: potential for breast cancer screening and diagnosis. Future Oncol 2006; 2: 351–6. doi: 10.2217/14796694.2.3.351 [DOI] [PubMed] [Google Scholar]
- 12.Kalender WA, Beister M, Boone JM, Kolditz D, Vollmar SV, Weigel MC. High-resolution spiral CT of the breast at very low dose: concept and feasibility considerations. Eur Radiol 2012; 22: 1–8. doi: 10.1007/s00330-011-2169-4 [DOI] [PubMed] [Google Scholar]
- 13.Clevert DA, Jung EM, Jungius KP, Ertan K, Kubale R. Value of tissue harmonic imaging (THI) and contrast harmonic imaging (CHI) in detection and characterisation of breast tumours. Eur Radiol 2007; 17: 1–10. doi: 10.1007/s00330-006-0325-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardanelli F, Giuseppetti GM, Panizza P, Bazzocchi M, Fausto A, Simonetti G, et al. Sensitivity of MRI versus mammography for detecting foci of multifocal, multicentric breast cancer in fatty and dense breasts using the whole-breast pathologic examination as a gold standard. AJR Am J Roentgenol 2004; 183: 1149–157. doi: 10.2214/ajr.183.4.1831149 [DOI] [PubMed] [Google Scholar]
- 15.Kuhl CK, Schrading S, Bieling HB, Wardelmann E, Leutner CC, Koenig R, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007; 370: 485–92. doi: 10.1016/S0140-6736(07)61232-X [DOI] [PubMed] [Google Scholar]
- 16.Als-Nielsen J, McMorrow D. Elements of modern X-ray physics. 2nd edn. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 17.Olivo A, Rigon L, Vinnicombe SJ, Cheung KC, Ibison M, Speller RD. Phase contrast imaging of breast tumours with synchrotron radiation. Appl Radiat Isot 2009; 67: 1033–41. doi: 10.1016/j.apradiso.2009.01.075 [DOI] [PubMed] [Google Scholar]
- 18.Sztrokay A, Diemoz PC, Schlossbauer T, Brun E, Bamberg F, Mayr D, et al. High-resolution breast tomography at high energy: a feasibility study of phase contrast imaging on a whole breast. Phys Med Biol 2012; 57: 2931–42. doi: 10.1088/0031-9155/57/10/2931 [DOI] [PubMed] [Google Scholar]
- 19.Pani S, Longo R, Dreossi D, Montanari F, Olivo A, Arfelli F, et al. Breast tomography with synchrotron radiation: preliminary results. Phys Med Biol 2004; 49: 1739–54. [DOI] [PubMed] [Google Scholar]
- 20.Castelli E, Tonutti M, Arfelli F, Longo R, Quaia E, Rigon L, et al. Mammography with synchrotron radiation: first clinical experience with phase-detection technique. Radiology 2011; 259: 684–94. doi: 10.1148/radiol.11100745 [DOI] [PubMed] [Google Scholar]
- 21.Momose A. Recent advances in X-ray phase imaging. Jpn J Appl Phys 2005; 44: 6355–67. [Google Scholar]
- 22.Bravin A, Coan P, Suortti P. X-ray phase-contrast imaging: from pre-clinical applications towards clinics. Phys Med Biol 2013; 58: R1–35. doi: 10.1088/0031-9155/58/1/R1 [DOI] [PubMed] [Google Scholar]
- 23.Bonse U, Hart M. Principles and design of Laue-case X-ray interferometers. Z Physiother 1965; 188(2): 154–164. [Google Scholar]
- 24.Bonse U, Hart M. An X-ray interferometer with Bragg case beam splitting and beam recombination. Z Phys 1966; 194: 1–17. [Google Scholar]
- 25.Wilkins SW, Gureyev TE, Gao D, Pogany A, Stevenson AW. Phase-contrast imaging using polychromatic hard X-rays. Nature 1996; 384: 335–8. [Google Scholar]
- 26.Snigirev A, Snigireva I, Kohn V, Kuznetsov S, Schelokov I. On the possibilities of x-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev Sci Instrum 1995; 66: 5486–92. [Google Scholar]
- 27.Morita T, Yamada M, Kano A, Nagatsuka S, Honda C, Endo T. A comparison between film-screen mammography and full-field digital mammography utilizing phase contrast technology in breast cancer screening programs. Lect Notes Comput Sci. 2008; 5116: 48–54. [Google Scholar]
- 28.Morita T, Yamada M, Kano A, Nagatsuka S, Honda C, Endo T. Subtle abnormalities in highly dense breasts detected by use of a digital phase contrast mammography system: a report of three invasive cancer cases in the early stage. Lect Notes Comput Sci 2008; 5116: 228–34. [Google Scholar]
- 29.Tanaka T, Honda C, Matsuo S, Noma K, Oohara H, Nitta N, et al. The first trial of phase contrast imaging for digital full-field mammography using a practical molybdenum x-ray tube. Invest Radiol 2005; 40: 385–96. [DOI] [PubMed] [Google Scholar]
- 30.Davis TJ, Gao D, Gureyev TE, Stevenson AW, Wilkins SW. Phase-contrast imaging of weakly absorbing materials using hard X-rays. Nature 1995; 373: 595–8. [Google Scholar]
- 31.Ingal VN, Beliaevskaya EA. X-ray plane-wave topography observation of the phase-contrast from a noncrystalline object. J Phys D Appl Phys 1995; 28: 2314–17. [Google Scholar]
- 32.Bravin A, Keyrilainen J, Fernandez M, Fiedler S, Nemoz C, Karjalainen-Lindsberg ML, et al. High-resolution CT by diffraction-enhanced x-ray imaging: mapping of breast tissue samples and comparison with their histo-pathology. Phys Med Biol 2007; 52: 2197–211. doi: 10.1088/0031-9155/52/8/011 [DOI] [PubMed] [Google Scholar]
- 33.Fiedler S, Bravin A, Keyrilainen J, Fernandez M, Suortti P, Thomlinson W, et al. Imaging lobular breast carcinoma: comparison of synchrotron radiation DEI-CT technique with clinical CT, mammography and histology. Phys Med Biol 2004; 49: 175–88. [DOI] [PubMed] [Google Scholar]
- 34.Keyrilainen J, Fernandez M, Fiedler S, Bravin A, Karjalainen-Lindsberg ML, Virkkunen P, et al. Visualisation of calcifications and thin collagen strands in human breast tumour specimens by the diffraction-enhanced imaging technique: a comparison with conventional mammography and histology. Eur J Radiol 2005; 53: 226–37. doi: 10.1016/j.ejrad.2004.03.015 [DOI] [PubMed] [Google Scholar]
- 35.Fernandez M, Keyrilainen J, Serimaa R, Torkkeli M, Karjalainen-Lindsberg ML, Leidenius M, et al. Human breast cancer in vitro: matching histo-pathology with small-angle x-ray scattering and diffraction enhanced x-ray imaging. Phys Med Biol 2005; 50: 2991–3006. doi: 10.1088/0031-9155/50/13/002 [DOI] [PubMed] [Google Scholar]
- 36.Pfeiffer F, Weitkamp T, Bunk O, David C. Phase retrieval and differential phase-contrast imaging with low-brilliance X-ray sources. Nat Phys 2006; 2: 258–61. [Google Scholar]
- 37.Herzen J, Donath T, Pfeiffer F, Bunk O, Padeste C, Beckmann F, et al. Quantitative phase-contrast tomography of a liquid phantom using a conventional x-ray tube source. Opt Express 2009; 17: 10010–18. [DOI] [PubMed] [Google Scholar]
- 38.Qi Z, Zambelli J, Bevins N, Chen GH. Quantitative imaging of electron density and effective atomic number using phase contrast CT. Phys Med Biol 2010; 55: 2669–77. doi: 10.1088/0031-9155/55/9/016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donath T, Pfeiffer F, Bunk O, Grunzweig C, Hempel E, Popescu S, et al. Toward clinical X-ray phase-contrast CT: demonstration of enhanced soft-tissue contrast in human specimen. Invest Radiol 2010; 45: 445–52. doi: 10.1097/RLI.0b013e3181e21866 [DOI] [PubMed] [Google Scholar]
- 40.Pfeiffer F, Bech M, Bunk O, Kraft P, Eikenberry EF, Bronnimann C, et al. Hard-X-ray dark-field imaging using a grating interferometer. Nat Mater 2008; 7: 134–7. doi: 10.1038/nmat2096 [DOI] [PubMed] [Google Scholar]
- 41.Williams IM, Siu KK, Gan R, He X, Hart SA, Styles CB, et al. Towards the clinical application of X-ray phase contrast imaging. Eur J Radiol 2008; 68(Suppl. 3): S73–7. doi: 10.1016/j.ejrad.2008.04.042 [DOI] [PubMed] [Google Scholar]
- 42.Stampanoni M, Wang Z, Thuring T, David C, Roessl E, Trippel M, et al. The first analysis and clinical evaluation of native breast tissue using differential phase-contrast mammography. Invest Radiol 2011; 46: 801–6. doi: 10.1097/RLI.0b013e31822a585f [DOI] [PubMed] [Google Scholar]
- 43.Dreossi D, Abrami A, Arfelli F, Bregant P, Casarin K, Chenda V, et al. The mammography project at the SYRMEP beamline. Eur J Radiol. 2008; 68(Suppl. 3): S58–62. doi: 10.1016/j.ejrad.2008.04.038 [DOI] [PubMed] [Google Scholar]
- 44.Munro PR, Ignatyev K, Speller RD, Olivo A. Design of a novel phase contrast x-ray imaging system for mammography. Phys Med Biol 2010; 55: 4169–85. doi: 10.1088/0031-9155/55/14/014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sztrokay A, Herzen J, Auweter SD, Liebhardt S, Mayr D, Willner M, et al. Assessment of grating-based X-ray phase-contrast CT for differentiation of invasive ductal carcinoma and ductal carcinoma in situ in an experimental ex vivo set-up. Eur Radiol 2013; 23: 381–7. doi: 10.1007/s00330-012-2592-1 [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y, Brun E, Coan P, Huang Z, Sztrokay A, Diemoz PC, et al. High-resolution, low-dose phase contrast X-ray tomography for 3D diagnosis of human breast cancers. Proc Natl Acad Sci U S A 2012; 109: 18290–4. doi: 10.1073/pnas.1204460109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raupach R, Flohr T. Performance evaluation of x-ray differential phase contrast computed tomography (PCT) with respect to medical imaging. Med Phys 2012; 39: 4761–74. doi: 10.1118/1.4736529 [DOI] [PubMed] [Google Scholar]
- 48.Grandl S, Willner M, Herzen J, Mayr D, Auweter SD, Hipp A, et al. Evaluation of phase-contrast CT of breast tissue at conventional X-ray sources—presentation of selected findings. Z Med Phys 2013; 23: 212–21. doi: 10.1016/j.zemedi.2013.02.005 [DOI] [PubMed] [Google Scholar]
- 49.Anton G, Bayer F, Beckmann MW, Durst J, Fasching PA, Haas W, et al. Grating-based darkfield imaging of human breast tissue. Z Med Phys 2013; 23: 228–35. doi: 10.1016/j.zemedi.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 50.Michel T, Rieger J, Anton G, Bayer F, Beckmann MW, Durst J, et al. On a dark-field signal generated by micrometer-sized calcifications in phase-contrast mammography. Phys Med Biol 2013; 58: 2713–32. doi: 10.1088/0031-9155/58/8/2713 [DOI] [PubMed] [Google Scholar]
- 51.Park CC, Mitsumori M, Nixon A, Recht A, Connolly J, Gelman R, et al. Outcome at 8 years after breast-conserving surgery and radiation therapy for invasive breast cancer: influence of margin status and systemic therapy on local recurrence. J Clin Oncol 2000; 18: 1668–75. [DOI] [PubMed] [Google Scholar]
- 52.Chagpar AB, Martin RC 2nd, Hagendoorn LJ, Chao C, McMasters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg 2004; 188: 399–402. doi: 10.1016/j.amjsurg.2004.06.020 [DOI] [PubMed] [Google Scholar]
- 53.Cao D, Lin C, Woo SH, Vang R, Tsangaris TN, Argani P. Separate cavity margin sampling at the time of initial breast lumpectomy significantly reduces the need for reexcisions. Am J Surg Pathol 2005; 29: 1625–32. [DOI] [PubMed] [Google Scholar]
- 54.Noguchi M, Minami M, Earashi M, Taniya T, Miyazaki II, Mizukami Y, et al. Pathologic assessment of surgical margins on frozen and permanent sections in breast conserving surgery. Breast Cancer 1995; 2: 27–33. [DOI] [PubMed] [Google Scholar]
- 55.Fernandez LJ, Buckley JM, Aftreth OP, Tang R, Saksena M, Yagi Y, et al. Breast excision specimens evaluated by micro-computed tomography (micro-CT) with histopathological correlations. Lab Invest 2012; 92: 39a. [Google Scholar]